Abstract
Introduction
Growth hormone (GH) is an essential regulator of growth, body composition and fuel metabolism and, consequently, GH secretion is under the feedback control of numerous nutritional and endocrine mediators. Glucagon-like peptide 1 receptor agonists (GLP-1RAs) have been shown to exert pleiotropic effects, including stimulation of the activity of the hypothalamic-pituitary-adrenal axis. As GLP-1RAs exert multiple metabolic effects, we hypothesised that they may also affect the secretion of GH and examined the effect of a short-acting and a long-acting GLP-1 RA on GH secretion.
Methods
This is a post hoc analysis of data from clinical trials. Two separate single-group open-label clinical trials were carried out in the ambulatory care setting with a duration of 1 and 21 days, respectively. Healthy adult male and female volunteers with no chronic illnesses or use of daily medicines were recruited for the study. The two interventions were: study 1, single dose of 10 µg exenatide administered subcutaneously (s.c.); study 2, 0.6 mg liraglutide administered s.c. once daily for 21 days.
Results
Administration of a single dose of exenatide (study 1) caused a clear increase in GH levels, peaking between 60 and 120 min post-administration. There was also a small but statistically significant decrease in luteinising hormone and testosterone levels 120 min after exenatide dosing. Administration of the long-acting GLP-1RA liraglutide daily for 21 days (study 2) elicited an increase in GH levels with no change in insulin-like growth factor-1 (IGF-1) concentrations after 3 weeks of treatment.
Conclusions
The results show that the administration of GLP-1RAs may elicit an increase in growth hormone levels. GLP-1 signalling may be a novel mechanism of regulation of GH secretion. This finding needs to be replicated in the placebo-controlled trial.
Clinical Trial Registration Numbers
NCT02089256 and NCT03160261.
Keywords: Exenatide, GLP-1 receptor agonist, Growth hormone
Key Summary Points
| Why carry out this study? |
| Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) may affect the regulation of other hormonal systems. |
| The aim of this study was to investigate whether GLP-1RAs play a role in the regulation of secretion of growth hormone (GH). |
| What was learned from the study? |
| Preliminary evidence from our post hoc analysis of two clinical trials suggests that GLP-1RAs may lead to an increase in GH levels. |
| This effect may have implications in the long-term effects of the drug class. |
Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced and released in the distal gut by enteroendocrine L-cells in response to food intake [1]. It reduces postprandial glucose excursions through multiple mechanisms, namely, by stimulating insulin release in a glucose-dependent manner, inhibiting glucagon secretion and delaying gastric emptying [2, 3]. GLP-1 receptor agonists (GLP-1RAs) are a relatively novel class of antidiabetic drugs that have gained popularity in recent years due to a number of associated benefits, such as a very low risk of hypoglycaemia, concomitant weight loss and cardiovascular benefits [4, 5]. GLP-1RAs mimic the effects of native GLP-1, but as the resulting pharmacological concentrations reach supraphysiological levels, GLP-1 RAs have been shown to exert pleiotropic effects beyond glucose control [6], such as stimulation of the activity of the hypothalamic-pituitary- adrenal axis [7–10]. Specifically, we and another research group have shown that acute administration of exenatide moderately stimulated the release of adrenocorticotrophic hormone (ACTH) and cortisol but inhibited the renin-aldosterone system in healthy volunteers [11, 12].
Growth hormone (GH) is an essential regulator of growth, body composition and fuel metabolism. As such, the secretion of GH is under the feedback control of numerous nutritional and endocrine mediators, including ghrelin, leptin, glucose and certain amino acids, among others [13].
As GLP-1 RAs exert multiple metabolic effects, including the regulation of glucose homeostasis, it is logical to assume that they may also affect the secretion of GH. The authors of a very recent review proposed that GH and insulin-like growth factor-1 (IGF-1) may mediate some of the effects of GLP-1RAs.
Since GLP-1 RAs stimulate ACTH release and decrease glucose levels we hypothesised that they may increase the secretion of GH. We report the results of two clinical trials on the effects of GLP-1 RAs in healthy volunteers.
Methods
Two single-group open-label clinical trials were conducted to examine the effects of GLP-1RAs on healthy volunteers. Details on the design and primary outcomes of the trials have been published previously [11, 14]. The key characteristics of the trials are presented in Table 1.
Table 1.
Design of clinical trials and baseline characteristics of subjects
| Baseline parameters | Studies | |
|---|---|---|
| Exenatide 10 µg s.c | Liraglutide 0.6 mg s.c. QD | |
| Dosing | Acute (single dose) | Chronic (21 days) |
| Blood sampling | 0, 60, 120, 180, 240 min after dosing | Before graded glucose infusion test at treatment days-7 (no drug); 1 and 21 (both 12 h after Liraglutide) |
| Age in years, mean (SD) | 33.0 (7.6) | 28.2 (1.9) |
| Sex, n (males/females) | 9/1 | 7/3 |
| Weight in kg, mean (SD) | 84.7 (8.9) | 77.0 (2.6) |
Study 1
Ten healthy volunteers aged 18–50 years and with a body weight > 65 kg were recruited. Tests were carried out in the morning between 08.00 and 11.00 a.m. after a 12-h fast. A peripheral venous catheter was placed in one arm 20 min before the first blood sample was collected, and blood samples were taken at baseline (i.e. 0 min) and at 30, 60, 90, 120 and 150 min after administration of the drug. After the collection of the first blood sample, 10 µg of exenatide solution was administered subcutaneously (s.c.).
Study 2
Ten healthy volunteers aged between 18 and 50 years and with body weight > 50 kg were recruited. Subjects received treatment with liraglutide at a dose of 0.6 mg for 21 consecutive days. Liraglutide was self-administered s.c. in the stomach area once daily between 9.00 a.m. and 11.00 p.m. The graded glucose infusion test (GGIT) was used to determine the primary outcome, and testing was carried out 7 days before the initiation of treatment, 12 h after the first liraglutide injection and 12 h after the last liraglutide injection (21 days on treatment). The tests were carried out in the morning between 8.00 and 10.00 a.m. after 12-h fast. Blood samples were taken to determine the baseline levels of glucose and hormones before the start of the GGIT.
Compliance with Ethics Guidelines
The studies were performed in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments. Approval for the trials was granted by the Research Ethics Committee of the University of Tartu (270/T-13 and 236/T-10) and the Estonian Agency of Medicines (RKU-4/28 and RKU-4/18) and registered at Clinicaltrials.gov (https://clinicaltrials.gov/; NCT02089256 and NCT03160261). Each volunteer read and signed a written informed consent form.
Main Outcome Measure(s)
The results of the post hoc analysis of the original studies were evaluated. In study 1, we analysed the peak level of GH after the administration of exenatide. In study 2, we analysed the change in GH and IGF-1 levels after acute and sub-chronic treatment with liraglutide. In study 1, we also measured the levels of other hypothalamic/pituitary hormones at 0, 60 and 120 min post-administration of exenatide: prolactin, copeptin, luteinising hormone (LH) and testosterone.
All laboratory analyses were conducted at the accredited laboratory of Tartu University Hospital using standard techniques. Chemiluminescence technology was used to measure GH levels (IDS-iSYS system, Human Growth Hormone [hGH] assay; Immunodiagnostic Systems, East Bolden, UK) and IGF-1 (IDS-iSYS, Insulin like Growth Factor-I Assay; Immunodiagnostic Systems), LH (Elecsys LH assay; Roche Diagnostics, Indianapolis, IN, USA), prolactin (Elecsys Prolactin II assay; Roche Diagnostics) and testosterone (Elecsys Testosterone II assay; Roche Diagnostics). TRACE technology (Time-Resolved Amplified Cryptate Emission) was used for measuring copeptin (B.R.A.H.M.S™ Copeptin proAVP; Thermo Fisher Scientific, Waltham, MA, USA). All reference values are given in the figure legends.
Statistical Analyses
Mean values with the standard deviation (SD) or median values with range are presented in the figures. All data were analysed using Statistica version 12 (StatSoft, Inc., College Station, TX, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The D’Agostino-Pearson normality test was used to verify a normal distribution. Data that did not have a normal distribution were analysed using the Wilcoxon signed-rank test. Time-series data were statistically examined using a repeated-measures analysis of variance (ANOVA) or the Friedman repeated-measures ANOVA when appropriate. Tukey’s and Dunn’s tests were used for post hoc analysis. Covariance was examined using Pearson correlation. The level of statistical significance was set as p < 0.05.
Results
The baseline characteristics of the study participants are given in Table 1.
Effects of Exanatide Dose on GH and IGF-1 Levels
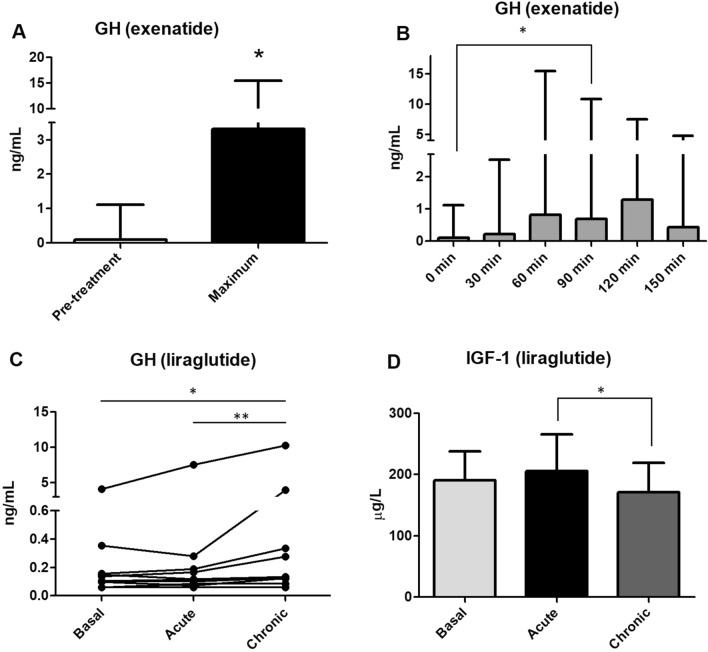
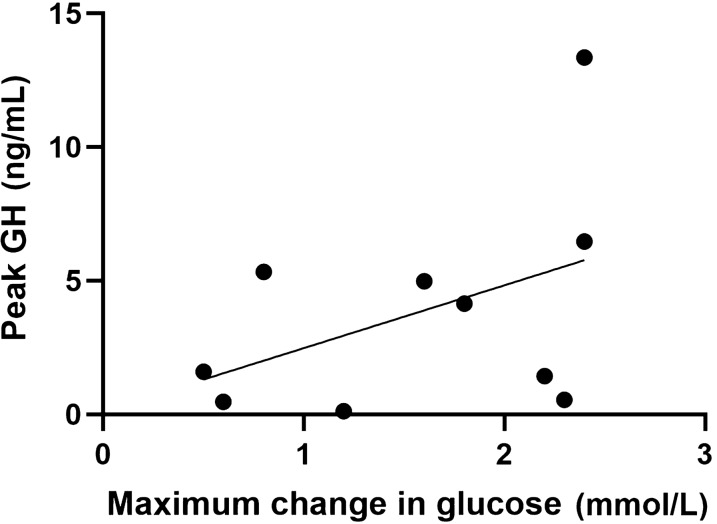
Following the administration s.c. of a single dose of 10 µg exenatide we observed a statistically significant peak in GH levels compared to pre-treatment values (p < 0.05; Fig. 1A). The GH peak occurred most frequently between 60 and 90 min post-administration of exanatide (Fig. 1B). There was no correlation between change in glucose levels and GH peak values following the administration of exenatide (Pearson correlation coefficient 0.437, 95% confidence interval [CI] − 0.266 to 0.835; p = 0.207 (Fig. 2).
Fig. 1.
A Growth hormone (GH) pre-treatment and peak value after administration of 10 µg exenatide subcutaneously (s.c.) Asterisk indicates a significant difference at *p < 0.05 (Wilcoxon signed-rank test). B GH values at pre-specified sampling points depicted as timeline. Asterisk indicates a significant difference at *p < 0.05 (Dunn’s test). C GH levels at baseline and after acute and chronic treatment with 0.6 mg liraglutide s.c. Asterisks indicate a significant difference at *p < 0.05, **p < 0.01 (Dunn’s test). D Insulin-like growth factor 1 (IGF-1) levels at baseline, and after acute and chronic treatment with 0.6 mg liraglutide s.c. Asterisk indicates a significant difference at *p < 0.05 (Tukey test). GH data are depicted as the median with range; IGF-1 data are given as the mean with standard deviation (SD). Reference values: GH < 5 ng/mL; IGF-1 116–353 µg/L
Fig. 2.
Correlation between GH peak values and change in glucose levels in the exenatide experiment (study 1). Pearson correlation, p = 0.207
GH levels were significantly higher after chronic administration of 0.6 mg liraglutide compared with baseline values (Fig. 1C). After administration of 0.6 mg liraglutide, IGF-1 levels did not change compared to baseline but were slightly lower after chronic treatment when compared with the acute administration of the drug (p < 0.05; Fig. 1D).
Effects of Exenatide Dose on LH and Testosterone Levels
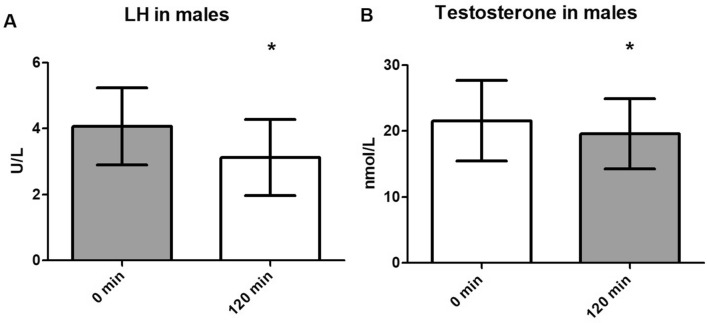
The analysis of LH and testosterone levels included only male subjects (n = 8). Testosterone was significantly suppressed 120 min after the administration of 10 µg exenatide (p < 0.05) (Fig. 3B). LH levels followed a similar trend: LH levels were significantly lower at 120 min compared to baseline (p = 0.02) (Fig. 3A).
Fig. 3.
A Luteinising hormone (LH) levels at baseline and at 120 min after administration of 10 µg exenatide. B Testosterone levels at baseline and at 120 min after administration of 10 µg exenatide. Asterisk indicates a significant difference at *p < 0.05 (Tukey test). Data are depicted as the mean with SD. Reference values: LH 1.7–8.6 U/L; testosterone 6.68–29.0 nmol/L
Effects of Exenatide Dose on Prolactin Levels
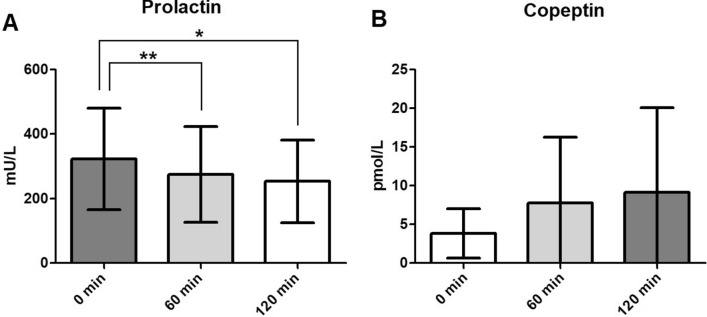
Administration of a single dose of 10 µg exenatide resulted in a statistically significant decrease in prolactin levels both 60 and 120 min post-administration compared to baseline (p < 0.05; Fig. 4A).
Fig. 4.
Change in prolactin (A) and copetine (B) levels. Asterisks indicate a significant difference at *p < 0.05 and **p < 0.01 (Tukey test). Data are depicted as mean with SD. Reference values: prolactin (males) 86–324 mU/L, (females) 102–496 mU/L; copeptine 1.0–28.2 pmol/L
Effects of Exenatide Dose Copeptin Levels
Blood samples were available for six subjects. The change in the concentration of copeptin after the administration of 10 µg exenatide was not significant (p = 0.22) (Fig. 4B).
Discussion
The principal finding of the present study is that acute administration of 10 µg exenatide s.c. elicited a clear peak in GH levels in 8 out of 10 healthy volunteers. The peak level of GH was observed between 60 and 120 min after injection, peaking most frequently at 90 min post-injection. To our knowledge, this effect of GLP-1 RAs on GH secretion has not been described previously. However, there is only limited indirect evidence on the possible interaction of incretin hormones and GH secretion. The dipeptidyl peptidase-4 inhibitor sitagliptin, which increases endogenous levels of both GLP-1 and gastric inhibitory polypeptide (GIP), has been shown to potentiate stimulated GH secretion in women [15]. On the other hand, Teti et al. [16] reported that dipeptidyl-peptidase 4 (DPP-4) inhibition did not alter the GH/IGF-1 axis in adults with type 2 diabetes. In addition, GLP-RAs do not interfere with the secretion of hypoglycaemia-induced counter-regulatory hormones, including GH. Intravenous exenatide infusion did not alter the secretion of GH during hyperinsulinaemic hypoglycaemic clamp in healthy male volunteers [17]. Similarly, Almby et al. [18] investigated the effects of GLP-1 receptor activation on counter-regulatory hormones (i.e. glucagon, catecholamines, cortisol, GH) during hyperinsulinaemic hypoglycaemic clamp in patients who had undergone a gastric bypass surgery. These authors concluded that neither the basal levels nor the rise in GH during hypoglycaemia differed between groups receiving placebo or infusion with GLP-1RA [18].
It is currently unclear whether GLP-1RAs act directly on the release of GH, GH-releasing hormone (GHRH) or somatostatin or whether they have an indirect effect mediated by some of the known regulators of the GH axis. There is electrophysiological evidence that GLP-1 can act on ghrelin-sensitive neurons in the nucleus arcuatus (ARC) [19]; specifically, ghrelin-excited ARC neurons were concordantly stimulated by GLP-1 and, to a lesser degree, inhibitory effects were also observed in this population of cells [19]. Accordingly, GLP-1 RAs may at least partially share the pathway with ghrelin in stimulating GHRH/GH release. Regarding possible indirect mechanisms of action, a change in metabolites (amino acids, free fatty acids) seems an unlikely mediator as our experiment was conducted under fasting conditions. Glucose levels decreased but no hypoglycaemic values were detected, and the change in glucose levels did not correlate with the observed GH release. GLP-1RAs induce insulin secretion [20], but insulin itself seems to have a neutral or inhibitory effect in healthy subjects [21, 22]. Ghrelin is known to be a potent stimulant of GH secretion. However, distinct GLP-1RAs have been reported to exert a neutral or inhibitory effect on the release of ghrelin after acute or chronic administration [23–26].
Taken together, the direct effect of exenatide on the hypothalamic/GH axis seems to be the most likely mechanism underlying the stimulation of GH release. Thus, GLP-1 signalling may represent a novel mechanism of regulation of GH secretion.
Interestingly, the administration of liraglutide (0.6 mg s.c.) did not change GH levels significantly after acute administration. There are several explanations for the differences in the effect of the two GLP-1RAs tested in the present study. Firstly, 10 µg of exenatide is the full therapeutic dose of the drug; in contrast, 0.6 mg of liraglutide is considered to be a subtherapeutic dose for adults aiming to minimise its gastrointestinal side effects [27]. Secondly, liraglutide is a long-acting GLP-1RA. As a result of modifications in its structure, its absorption and half-life are enhanced, which allows once-daily administration. These pharmacokinetic properties lead to smaller fluctuations in plasma concentrations and also improved tolerability [28]. Following s.c. administration of liraglutide, the maximum concentrations are achieved in 9–14 h [29, 30]. Accordingly, in our trial, liraglutide was administered 12 h before testing, i.e. the preceding evening. On the contrary, exenatide is rapidly absorbed and reaches its peak concentrations in approximately 2 h [31].
GH levels were clearly elevated after chronic treatment with liraglutide, adding validity to the findings of the exenatide trial. In contrast, IGF-1 values did not reflect the changes in GH secretion and were somewhat lower after chronic treatment. Acute GLP-1 infusion has been demonstrated to decrease insulin-like growth factor-binding protein-3 (IGFBP-3) levels in humans, and this effect may explain the observed decrease in total IGF-1 levels [32]. Alternatively, changes in GH and IGF-1 levels may also reflect the decrease in body weight in the liraglutide trial. Taken together these results provide support for the hypothesis that GLP-1RAs interfere with the functioning of the GH/IGF-1 axis.
We also tested whether exenatide may change the levels of other pituitary hormones. Interestingly, LH and testosterone levels decreased slightly following the administration of a single dose of exenatide. Although these changes were statistically significant, they were small and their functional significance remains to be determined. Prolactin levels fell during the study as well, but this decrease reflects the circadian rhythm and stress response during the initiation of blood sampling.
We also observed an increase in copeptin levels, which did not reach statistical significance. However, data were only available for six subjects, and thus the possible effect must be scrutinised in further studies. A recent study showed that nausea and vomiting, which are common side effects of GLP-1RAs, cause an increase in copeptin levels. Consequently, these side effects should be taken into account in further studies [33].
There are important limitations of our studies that should be taken into account. Firstly, the studies reported represent a post hoc analysis of original studies with a small number of subjects. We were able to measure only a limited number of parameters, and the possible links between GLP-1 signalling and GH and glucose changes need further molecular level characterisation. Also, the studies did not have a placebo arm. Thus, despite the clear peak observed in the exenatide trial, we cannot rule out the possibility that the increase of the GH levels is related to the stress reaction or represents a pulsatile nature of hormone secretion. Thus, the findings must be replicated in a properly designed placebo controlled clinical trials. Secondly, the subjects were young healthy volunteers who were predominantly male.
Conclusions
In conclusion, we demonstrated that the administration of GLP-1 RAs may elicit an increase in GH levels. GLP-1 signalling may be a novel mechanism of regulation of GH secretion. This finding needs to be replicated in a placebo-controlled trial.
Acknowledgements
Funding
The study was supported by the Estonian Research Council (grant number IUT20-41) and EU Horizon 2020 research and innovation programme under grant agreement no. 668989. No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were involved in the conception and design of the studies. Vallo Volke and Tuuli Sedman were involved in subject recruitment and data collection. Vallo Volke, Keiu Heinla and Ingrid Reppo were involved in data analysis, interpretation and statistical analysis. All authors were involved in the writing of the manuscript. Tuuli Sedman and Vallo Volke share the last authorship.
Compliance with Ethics Guidelines
The studies were performed in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments. Approval for the trials was granted by the Research Ethics Committee of the University of Tartu (270/T-13 and 236/T-10) and the Estonian Agency of Medicines (RKU-4/28 and RKU-4/18) and registered at Clinicaltrials.gov (NCT02089256 and NCT03160261). Each volunteer read and signed a written informed consent form.
Prior Publication
Part of the study has been presented at the European Congress of Endocrinology, Milan, 21–24 May 2022: Endocrine Abstracts (2022) 81:P406 (https://doi.org/10.1530/endoabs.81.P406).
Disclosures
Vallo Volke has served at speakers’ bureaus or received travel grants from Astra Zeneca, Eli Lilly and Novo Nordisk. Ingrid Reppo has served at speakers’ bureaus and advisory panels for Novo Nordisk. All other authors declare that they have no conflicts of interest in the authorship or publication of this contribution.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Eissele R, Göke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 2.Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marathe CS, Pham H, Wu T, et al. Acute administration of the GLP-1 receptor agonist lixisenatide diminishes postprandial insulin secretion in healthy subjects but not in type 2 diabetes, associated with slowing of gastric emptying. Diabetes Ther. 2022;13:1245–1249. doi: 10.1007/s13300-022-01258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroda VR. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 5.Nair R, Mody R, Yu M, et al. Real-world treatment patterns of glucose-lowering agents among patients with type 2 diabetes mellitus and cardiovascular disease or at risk for cardiovascular disease: an observational, cross-sectional. Retrospect Study Diabet Ther. 2022;13:1921–1932. doi: 10.1007/s13300-022-01320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Malendowicz LK, Neri G, Nussdorfer GG, Nowak KW, Zyterska A, Ziolkowska A. Prolonged exendin-4 administration stimulates pituitary-adrenocortical axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med. 2003;12(4):593–596. [PubMed] [Google Scholar]
- 8.Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, et al. Effects of prolonged exendin-4 administration on hypothalamic-pituitary-adrenal axis activity and water balance. Am J Physiol Endocrinol Metab. 2013;304(10):E1105–E1117. doi: 10.1152/ajpendo.00529.2012. [DOI] [PubMed] [Google Scholar]
- 9.Krass M, Volke A, Rünkorg K, et al. GLP-1 receptor agonists have a sustained stimulatory effect on corticosterone release after chronic treatment. Acta Neuropsychiatr. 2015;27(1):25–32. doi: 10.1017/neu.2014.36. [DOI] [PubMed] [Google Scholar]
- 10.Krass M, Rünkorg K, Vasar E, Volke V. Acute administration of GLP-1 receptor agonists induces hypolocomotion but not anxiety in mice. Acta Neuropsychiatr. 2012;24(5):296–300. doi: 10.1111/j.1601-5215.2012.00648.x. [DOI] [PubMed] [Google Scholar]
- 11.Heinla K, Vasar E, Sedman T, Volke V. A GLP-1 receptor agonist inhibits aldosterone release in healthy volunteers. Horm Metab Res. 2021;53(6):402–407. doi: 10.1055/a-1498-7098. [DOI] [PubMed] [Google Scholar]
- 12.Baretić M, Kušec V, Pavlić-Renar I. Glucagon-like peptide-1 infusion suppresses aldosterone levels in healthy normal-weight individuals: double-blind Placebo-controlled crossover study. Diabetes Ther. 2018;9(6):2315–2324. doi: 10.1007/s13300-018-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernabeu I, Marazuela M, Casanueva FF. General concepts of hypothalamus-pituitary anatomy. Oxford Textb Endocrinol Diabetes. 2011;2:71–81. doi: 10.1093/med/9780199235292.003.2004. [DOI] [Google Scholar]
- 14.Sedman T, Heinla K, Vasar E, Volke V. Liraglutide treatment may affect renin and aldosterone release. Horm Metab Res. 2017;49(1):5–9. doi: 10.1055/s-0042-109065. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JR, Brown NJ, Nian H, Yu C, Bidlingmaier M, Devin JK. Dipeptidyl peptidase-4 inhibition potentiates stimulated growth hormone secretion and vasodilation in women. J Am Heart Assoc. 2018;7(5):e008000. doi: 10.1161/JAHA.117.008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teti C, Talco M, Albertelli M, et al. Dipeptidyl peptidase-4 inhibitors do not alter GH/IGF-I axis in adult diabetic patients. J Endocrinol Invest. 2020;43(3):389–393. doi: 10.1007/s40618-019-01106-6. [DOI] [PubMed] [Google Scholar]
- 17.Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes. 2004;53(9):2397–2403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- 18.Almby KE, Abrahamsson N, Lundqvist MH, et al. Effects of GLP-1 on counter-regulatory responses during hypoglycemia after GBP surgery. Eur J Endocrinol. 2019;181(2):161–171. doi: 10.1530/EJE-19-0171. [DOI] [PubMed] [Google Scholar]
- 19.Riediger T, Eisele N, Scheel C, Lutz TA. Effects of glucagon-like peptide 1 and oxyntomodulin on neuronal activity of ghrelin-sensitive neurons in the hypothalamic arcuate nucleus. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1061–R1067. doi: 10.1152/ajpregu.00438.2009. [DOI] [PubMed] [Google Scholar]
- 20.Sedman T, Vasar E, Volke V. Tolerance does not develop toward liraglutide’s glucose-lowering effect. J Clin Endocr. 2017;102(7):2335–2339. doi: 10.1210/jc.2017-00199. [DOI] [PubMed] [Google Scholar]
- 21.Sharp PS, Foley K, Vitelli F, Maneschi F, Kohner EM. Growth hormone response to hyperinsulinaemia in insulin-dependent diabetics Comparison of patients with and without retinopathy. Diabet Med. 1984;1(1):55–58. doi: 10.1111/j.1464-5491.1984.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 22.Lanzi R, Manzoni MF, Andreotti AC, et al. Evidence for an inhibitory effect of physiological levels of insulin on the growth hormone (GH) response to GH-releasing hormone in healthy subjects. J Clin Endocrinol Metab. 1997;82(7):2239–2243. doi: 10.1210/jcem.82.7.4071. [DOI] [PubMed] [Google Scholar]
- 23.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept. 2007;143(1–3):64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Farr OM, Tsoukas MA, Triantafyllou G, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism. 2016;65(7):945–953. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beti C, Stratmann B, Bokman G, et al. Exenatide delays gastric emptying in patients with Type 2 diabetes mellitus but not in those with gastroparetic conditions. Horm Metab Res. 2019;51(4):267–273. doi: 10.1055/a-0818-6374. [DOI] [PubMed] [Google Scholar]
- 26.Garg M, Ghanim H, Kuhadiya ND, et al. Liraglutide acutely suppresses glucagon, lipolysis and ketogenesis in type 1 diabetes. Diabetes Obes Metab. 2017;19(9):1306–1311. doi: 10.1111/dom.12944. [DOI] [PubMed] [Google Scholar]
- 27.novoMEDLINK. Dosing and administering Victoza®. https://www.victozapro.com/dosing-and-prescribing.html. Accessed 4 June 2021.
- 28.Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35(1):e3070. doi: 10.1002/dmrr.3070. [DOI] [PubMed] [Google Scholar]
- 29.Elbrønd B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25(8):1398–1404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 30.Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45(2):195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 31.European Medicines Agency. European public assessment report (EPAR) for Byetta. Discussion. https://www.ema.europa.eu/en/documents/scientific-discussion/byetta-epar-scientific-discussion_en.pdf. Accessed 4 June 2021.
- 32.Skov J, Frystyk J, Christiansen JS. GLP-1 infusion reduces IGFBP-1 serum level in humans. Growth Horm IGF Res. 2014;24(2–3):67–70. doi: 10.1016/j.ghir.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Brooks E, Bachmeier C, Vorster J, et al. Copeptin is increased by nausea and vomiting during hypertonic saline infusion in healthy individuals. Clin Endocrinol (Oxf) 2021;94(5):820–826. doi: 10.1111/cen.14417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.