Highlights
-
•
Rubiscolin-6 is a linear peptide isolated by the spinach Rubisco.
-
•
New analogues have been prepared via SPPS with high purity and good overall yields.
-
•
Their activity on opioid receptors has been detected in vitro and in vivo.
-
•
Some of them possess a strong antioxidant and tyrosinase inhibitor activity.
-
•
Peptide LMAS6 exerts also a significant anti-inflammatory in vivo.
Keywords: Rubiscolin-6, Rubisco, Antioxidants, Anti-browning agent, Nutraceuticals
Abstract
Rubiscolin-6 (amino acid sequence: YPLDLF) is a selective δ-opioid receptor peptide isolated from spinach Rubisco. Its synthetic analogue, peptide YPMDIV is the most potent described so far for its increased opioid activity, thus in this work it was considered as lead compound for the design of twelve new analogues e.g.LMAS1-12. Firstly all the novel compounds have been tested for their antinociceptive and anti-inflammatory capacity in vitro and in vivo in order to evaluate their ability to maintain or loss the original activity. Among them peptides LMAS5-8 gave the best results, thus their antioxidant properties have been investigated along with their enzymatic inhibitory ability. Peptide LMAS6 shows a strong antioxidant (154.25 mg TE/g CUPRAC) and inhibitor activity on tyrosinase (84.49 mg KAE/g), indicating a potential role in food industry as anti-browning agent, while peptides LMAS5 and LMAS7 possess a modest cholinesterase inhibitory activity suggesting a conceivable use for nutraceuticals production.
1. Introduction
Plant and animal food proteins are considered rich sources of bioactive peptides, which are classified on the basis of their biological effects, e.g., antithrombotic, antimicrobial, antihypertensive, opioid, immunomodulatory and antioxidant (Sánchez and Vázquez, 2017, Udenigwe and Aluko, 2012). Given their small size, sample preparation’s process, high potency and selectivity, they are an excellent example of tools to inhibit protein-protein interactions. Food derived bioactive peptides may play an important role in the regulation of various human functions acting directly through food’s consumption or after in vivo or in vitro hydrolysis of the proteins in which they are encrypted (Hartmann & Meisel, 2007). In fact they are generally a mixture of small size-peptides because of the high cost of purification and low yield of extraction; after purification, some of them may lose their additive or synergistic activity with polyphenols or other food components, resulting in inactive compounds that are useless as nutraceuticals. However, the literature reports examples of isolated bioactive compounds with proven physiological effects that can be added to food products (Day, Seymour, Pitts, Konczak, & Lundin, 2009). For instance, antioxidant peptides derived from the digestion process of different proteins may be added to food products to provide them antioxidant benefits (Lafarga & Hayes, 2014). Indeed, bioactive peptides with antioxidant properties have been used as free radical scavengers in meat products (Sohaib et al., 2017, Tkaczewska, 2020). The antioxidant effect of protein hydrolysates is related to their amino acids content (Sánchez & Vázquez, 2017).
Rubiscolin-6 (amino acid sequence: YPLDLF) is an interesting natural peptide recently evaluated for its antioxidant activity; it was identified in the spinach Rubisco large subunit (residues 103–108) (Stefanucci et al., 2020, Yang et al., 2001). Rubisco (d-ribulose-1,5-bisphosphate carboxylase/oxygenase; EC 4.1.1.39) represents the 10–30% of the whole amount of leaves proteins. It is composed of two subunits A and B, combined in a type-structure A8B8 (Kobbi et al., 2017, Taylor and Andersson, 1997, Yoshikawa, 2015). This enzyme plays an important role in carbon dioxide fixation and photorespiration (Yoshikawa, 2015), and it is involved in food’s production for the whole biosphere. A recent study showed that Rubisco hydrolysate from alfa-lfa green juice has significant antioxidant properties preventing linoleic acid oxidation, decreasing ferric ion and inducing stable ABTS*+ compared to the unhydrolysate protein (Kobbi et al., 2017).
Rubiscolin-6 is a selective δ-opioid receptor peptide agonist highly conserved in a huge variety of plants (Yang et al., 2001). It exhibits several effects, e.g., antinociceptive activity, anxiolytic-like effect, orexigenic action, enhancement of glucose uptake in skeletal cell lines, memory consolidation and reduction of skin inflammation (Chajra et al., 2015, Hirata et al., 2007, Kairupan et al., 2019, Kaneko et al., 2012, Miyazaki et al., 2014, Yang et al., 2001, Yang et al., 2003a). Numerous rubiscolin-6 analogues have been already synthesized to evaluate its structure-activity relationships in order to improve its δ-opioid activity (Yang, Sonoda, Chen, & Yoshikawa, 2003). The structural features of rubiscolin-6 analogues required to guarantee a high δ-opioid activity have been also determined through 3D quantitative structure-relationship (QSAR) analysis (Caballero, Saavedra, Fernández, & González-Nilo, 2007). Thus researchers have considered the hypothesis of using rubiscolin-6 and its derivatives for the production of functional foods and as lead compounds for the development of new analogues (Stefanucci et al., 2020).
Rubiscolin-6 and its C-terminal amide analogue have been recently tested in vitro for their antioxidant activity, however rubiscolin-6C-amide showed low DPPH radical scavenging activity (2.72 mg TE/g), while rubiscolin-6 gave the best result in ABTS assay (8.86 mg TE/g) (Stefanucci et al., 2020). Among the novel analogues, YPMDIV showed an increased δ-opioid activity on mouse vas deferens (MVD) assay in comparison with rubiscolin-6 (Yang et al., 2003). In the current study, YPMDIV has been assumed as lead compound for the design of twelve new analogues named LMAS1-12 (Fig. 1), with the aim to find bioactive peptides endowed with antioxidant and enzyme inhibitory activities potentially useful as food ingredients or in the development of new nutraceuticals.
Fig. 1.
Structure of the lead compound (YPMDIV) and peptides LMAS1-12.
2. Materials and methods
2.1. Design
A rational structure-based design approach has been applied to the development of novel peptide analogues of rubiscolin-6. Considering the multi-residue replacement described by a SAR study in literature (Yang et al., 2003), we selected the synthetic peptide YPMDIV as the lead compound for further structural modifications. The following findings have been taken in consideration in order to design the novel bioactive peptides: i) the protonated Tyr at the N-terminus fits the essential requirement of message domain for opioid receptor binding; ii) the Pro2 residue contributes to the restriction of peptide conformation and stability; iii) the presence of Met3 enhances the opioid activity in MVD about four times, while Ala3 and Val3, Phe3 and Trp3 decreased the activity in MVD assay; iv) Asp4 is the ideal residue for high activity and selectivity on δ-opioid receptor (DOR); v) in the fifth position of Rubiscolin-6, hydrophobic residues increase the potency at DOR, the preferential order is Leu > Ile > Met > Val > Ala; vi) the Phe6 in rubiscolin-6 can be replaced by aliphatic residue without losing δ -opioid activity. In a first attempt we explored the substitution of Met3 in the lead compound with an unnatural residue of (2S,4S)-4-(methylthio)pyrrolidine-2-carboxylic acid (Proline-Methionine chimeras) prepared in laboratory following the procedure previously described by Mollica et al. (Mollica, Paradisi, Varani, Spisani, & Lucente, 2006) linked to both D/L-Pro2 (compounds LMAS1-4), with the aim to improve the metabolic stability of the former peptides as C-terminal acids and amides. Since methionine is one of the most documented amino acid responsible of antioxidant activity in several peptides (Lorenzo et al., 2018), the replacement with Cys and the shortening of the Met3 side chain have been also investigated in peptides of the series LMAS5-8 and LMAS9-12 respectively. According to the SAR studies (Yang et al., 2003), all the other amino acids in position first, fourth, fifth and sixth have been conserved. These modifications are intended to understand the role exerted by this amino acid in varying the biological activity of such modified analogues of rubiscolin-6. The lead compound has been prepared as reference for the biological assays (Fig. 1).
2.2. Chemistry
Coupling reagents and solvents have been purchased by VWR (Radnor, PN, USA). Fmoc-Tyr(t-Bu)–OH, Fmoc-Pro-OH, Fmoc-d-Pro-OH, Fmoc-Met-OH, (S)-2-amino-3-(methylthio)propanoic acid, Fmoc-Cys(Trt)–OH, Fmoc-Asp(t-Bu)–OH, Fmoc-Ile-OH and Fmoc-Val-OH by Chem-Impex (Wood Dale, IL, USA). The synthetic amino acid residue (2S,4S)-4-(methylthio)pyrrolidine-2-carboxylic acid has been synthesized as previously reported in literature (Mollica et al., 2006). Commercial amino acid (S)-2-amino-3-(methylthio)propanoic acid has been protected with fluorenylmethoxycarbonyl protecting group (Fmoc) before its use (Carpino & Han, 1979). Fmoc-Rink amide and 2-chlorotrityl chloride resins (Loading coefficient: 0.74 mmol/g and 1.60 mmol/g respectively) by IRIS Biotech GmbH (Marktredwitz, DH, Germany). Peptides have been prepared by manual solid-phase synthesis (SPPS) using TBTU/HOBt/DIPEA and TBTU/2,4,6-trimethylpyridine for Fmoc-Cys(Trt)–OH reaction, as coupling mixture; a solution of piperidine 20%/DMF for Fmoc-deprotection. A strong cleavage has been applied to the peptide-resin system using TFA/TIPS/H2O for LMAS1-4,9–12 or TFA/TES/H2O for LMAS5-8 as cocktails (95:2.5:2.5) (Scheme S1, see SI). The final peptides as TFA salts have been purified by RP-HPLC system equipped with Waters XBridge Prep BEH130 C18, 5.0 µM, 250–10 mm, 7.0 mL/min, Waters Binary 1525 pump, H2O + 0.1% TFA/ACN + 0.1% TFA gradient from 5% to 95% of ACN in 35 min (wavelengths: 213 nm, 254 nm and 275 nm). Peptides purity as TFA salts was assessed using 1H NMR (Varian Inova 300 MHz) in DMSOd6 and RP-HPLC analytic column C-18, 4.6–150 mm, 1 mL/min, H2O + 0.1% TFA/ACN + 0.1% TFA gradient from 5% to 95% of ACN in 30 min. Mass spectrometer LCQ (Thermo Finnigan, San Jose, CA, USA) with capillary temperature 300 °C, electrospray ion Source (ESI), 4.00 Kv, auxiliary and preservative gas N2 has been used to confirm the compound’s identity. The purity of all the final peptides resulted to be ≥95% after purification (see SI).
2.3. Opioid binding assay and GTP stimulation
The opioid receptor binding affinity has been calculated performing displacement assay on μ-opioid receptor (MOR), δ-opioid receptor (DOR) and κ-opioid receptor (KOR) on rat and guinea pig brain membrane homogenates. For the detailed procedures see the supporting information.
2.4. In vitro antioxidant assay
Methodologies applied in our previous literature have been used (Uysal, Zengin, Locatelli, Bahadori, Mocan, Bellagamba, & Aktumsek, 2017), considering the following parameters: mg Trolox equivalents (TE)/g extract in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging, cupric reducing antioxidant capacity (CUPRAC) and ferric reducing antioxidant power (FRAP) tests, and mmol TE/g extract in phosphomolybdenum assay.
2.5. In vitro enzymatic inhibitory assay
The enzyme inhibitory assays were performed following the procedure reported in literature (Zengin, 2016). The following parameters have been choosen: acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition in mg galanthamine equivalents (GALAE)/g extract; tyrosinase inhibition in mg kojic acid equivalents KAE/g extract; amylase and glucosidase inhibition in mmol acarbose equivalents (ACAE)/g extract.
2.6. In vivo antinociceptive assays
Tail flick and formalin tests, and zymosan-induced edema formation have been carried out following the procedures previously described (Della Valle et al., 2021, Pieretti et al., 2022) using groups of 7–8 animals. The experimental protocols performed in the present study were in accordance with Italian Legislative Decree 27/92 and approved by the local ethics committee (approval number: 198/2013-B). Detailed procedures are described in the Supporting Information.
2.7. Data analysis and statistics
Data obtained from in vivo experiments are reported as mean ± s.e.m. Data analysis of tail flick and formalin test was carried out using one-way ANOVA followed by Dunnett’s multiple comparisons test. Data from zymosan-induced paw edema experiments were analysed using two-way ANOVA followed by Dunnett’s multiple comparisons test. GraphPad Prism 9.0 software (San Diego, CA, USA) for data elaboration. Means are considered statistically significant at P ≤ 0.05.
3. Results and discussion
3.1. Chemical synthesis
The novel peptides as TFA salts were prepared following a straightforward and highly efficient solution phase peptide synthesis protocol, both as C-terminal amides and acids (Table S1, Scheme S1 see SI). Their amino acid sequences and overall yields are shown in Table S1 (see SI). All peptides contain a L or d-proline in position 2 in combination with (2S,4S)-4-(methylthio)pyrrolidine-2-carboxylic acid (LMAS1-4), cysteine (LMAS5-8) or (S)-2-amino-3-(methylthio)propanoic acid (LMAS9-12) in third position. The synthetic procedure applied in this work allowed us to optimize the reactions time, reagent/solvents costs and the purification steps since the crude final products present less detectable by-products in RP-HPLC chromatographic traces, they are completely soluble in water/methanol medium and they are easier to purify than those obtained by solution phase peptide synthesis.
3.2. Opioid binding assay
All the final peptides and the lead compound have been tested for their ability to bind the MOR, DOR and KOR in presence of the reference ligands [3H]DAMGO, [3H]Ile5,6-delthorphin II and [3H]HS665 respectively (Fig. S1, Table S2, see SI). Peptide LMAS4 seems to be selective for MOR with a moderate binding affinity (Ki: 137.4±0.15 nM). All the other compounds are not able to bind the three opioid receptors at 10 μM concentration. It’s worth to note that none of them exhibits a significant binding affinity for δ -opioid receptor, for which the lead compound shows a IC50 value of 0.12 μM in [3H]DPDPE binding assay (Yang et al., 2003).
This result is not surprising since the sequence d-Pro-(2S,4S)-4-(methylthio)pyrrolidine-2-carboxylic acid (proline-methionine chimera) contained in LMAS4 has been already reported to confer an increased metabolic stability to plasma and enzyme’s degradation (Mollica et al., 2012), as well as to be responsible of an improved μ-opioid receptor selectivity, thanks to the ability of such sequence to assume a well-defined 3D structure (Stefanucci et al., 2011).
3.3. GTP stimulation assay
In the GTP stimulation assay, all the peptides were tested to evaluate their capacity to stimulate the G-protein coupled receptor (Fig. S2, Table S2 see SI). Their efficacy almost stayed around the basal activity, with the only exception of compound LMAS4 (Emax: 139.8±2.8%). In agreement with the binding data, this analogue of rubiscolin-6 is more efficacious and potent in GTP stimulation assay than the lead compound, which is in turn about 20 times more potent than rubiscolin-6 in MVD assay (EC50 value of 5.65 μM) (Yang et al., 2003). The novel analogue shows a diverse affinity/selectivity profile in vitro and a more potent antinociceptive activity than rubiscolin-6 and its analogue previously described by Yang et al.
3.4. In vivo experiments
3.4.1. Tail flick test
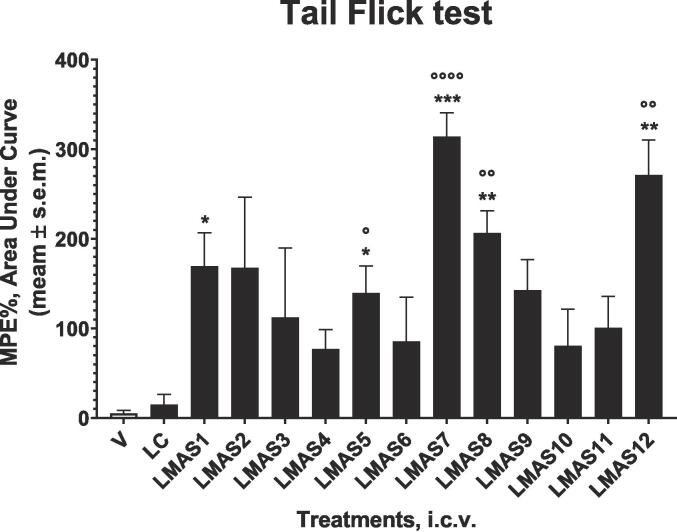
In the first series of experiments, the effects of the lead compound and LMAS1-12 peptides were investigated in an animal model of acute nociception induced by thermal stimuli as the tail flick tests. Peptides were injected via intracerebroventricular route (i.c.v.) at the dose of 10 μg/mouse (Fig. 2). Peptides LMAS1-12 induce a greater antinociceptive effect than vehicle-treated animals, which reaches statistical significance for LMAS1,5,7,8,12. All of them also increased antinociceptive response times in comparison with lead compound-treated animals. Statistical analysis reveals that this effect is significant in LMAS5,7,8,12 treatment groups. All of them exert an antinociceptive effect in this assay higher than that of rubiscolin-6 and its C-terminal amide derivative previously described by us (Stefanucci et al., 2020), which could be due to an improved enzymatic stability at a central level.
Fig. 2.
Antinociceptive effects induced by the lead compound (LC) and LMAS1-12 peptides in the tail flick test. Peptides were administered i.c.v. at the dose of 10 μg/mouse. Tail flick data were reported as area under %MPE curve (AUC). * is for P < 0.05, ** is for P < 0.01 and *** is for P < 0.001 vs vehicle-treated animals (V, DMSO 0.1% in saline); ° is for P < 0.05, °° is for P < 0.01 and °°°° is for P < 0.0001 vs LC-treated animals. N = 7.
3.4.2. Formalin test
In the formalin test, the lead compound and LMAS1-12 peptides were administered subcutaneously (s.c.) into the mice hind paw at a dose of 100 μg/mouse, 15 min before formalin (Fig. 3). In the early phase of the formalin test, LMAS3,4,5,6,7,9,11 reduced the licking behaviour induced by formalin, although only the antinociceptive effects of LMAS5-7 peptides reach statistical significance in comparison with vehicle treated animals (Fig. 3, left panel). In the late phase of the formalin test, LMAS1,2,4–9 reduced formalin-induced nociceptive effect, even if only the LMAS4 peptide is able to significantly reduce the licking activity induced by the aldehyde (Fig. 3, right panel). These results partially support our findings described in paragraph 3.2, where peptide LMAS4 exerted the best binding affinity for μ-opioid receptor probably due to an improved plasma or metabolic stability. On the contrary, the antinociceptive effect of this peptide and analogues LMAS5-7 is lower than that of rubiscolin-6C-terminal amide, previously described by us (Stefanucci et al., 2020) in the early phase of the formalin test, while it appears to be stronger than the parent compound in the late phase.
Fig. 3.
Antinociceptive effects induced by the lead compound (LC) and LMAS1-12 peptides in the formalin test. In the left panel, the result obtained in the early phase of the test are reported. The right panel reports the licking activity recorded in the late phase of the formalin test. Peptides were administered s.c. into the hind paw at the dose of 100 μg/mouse. * is for P < 0.05, ** is for P < 0.01 and **** is for P < 0.0001 vs vehicle-treated animals (V, DMSO:saline, ratio 1:3 v/v). N = 7.
3.4.3. Zymosan-induced edema formation
Since some of the tested peptides show the best in vivo antinociceptive profile, we decided to study the possible anti-inflammatory effects of LMAS6,7 and lead compound (Fig. 4). All of them induced an anti-inflammatory effect, although a significant reduction of edema volume for the lead compound was observed after 2 h and for the LMAS7 peptide, 1 and 2 h after zymosan administration. The LMAS6 peptide appears to be the most active, as it was able to significantly reduce the formation of edema for the entire duration of the observation period. This result pairs with the data obtained by the formalin test in the early phase after subcutaneous administration, leading us to suppose a possible analgesic activity at the periphery, however further investigation is required to support such hypothesis raising by an embryonic stage of the work.
Fig. 4.
Anti-inflammatory effects induced by the lead compound (LC) and LMAS6,7 peptides. Inflammatory paw edema was induced by zymosan, and peptides were administered s.c. at the dose of 100 μg/mouse 30 min before. The increase in paw volume was evaluated as the percentage difference between the paw volume at each time point and the basal paw volume. * is for P < 0.05 and **** is for P < 0.0001 vs vehicle-treated animals (V, DMSO:saline, ratio 1:3 v/v). N = 8.
Overall, the cluster of peptides LMAS5-8 exhibits the best in vivo antinociceptive results. Surprisingly they are not able to bind opioid receptors neither to stimulate G protein coupled to them, thus their activity in vivo should be related to the activation of other systems involved in nociceptive stimuli control. It is well known that several non-opioid peptides, some of them recently marketed, are powerful antinociceptive agents as evidenced in pre-clinical studies. Non-opioid peptides acting directly or indirectly at different ion channels or non-opioid G-protein coupled receptors (GPCRs) localized in the nociceptive pathways include peptides targeting Ca2+, Na+ and K+ voltage-gated ion channels, the neuronal nicotinic receptors (nAChR), transient receptor potential channels (TRP), the calcitonin gen-related peptide (CGRP), cannabinoid, bradykinin and neurotensin receptors. (Dimmito et al., 2021, Mollica et al., 2015, Mollica et al., 2017, Pérez de Vega et al., 2018). These are all targets involved in the mechanism of pain through interaction with peptidic endogenous ligands, also showing a certain overlapping positioning with opioid system in the central nervous system (CNS) (Dvoracsko, Stefanucci, Novellino, & Mollica, 2015). Thus we cannot exclude the possibility of a single or multitarget interaction with some of them located at central level or periphery. These peptides are characterized by the presence of a cysteine residue in position 3 in place of methionine contained in the lead compound. In light of these data, we decided to test the most active compounds, e.g. LMAS5-8 in a battery of antioxidant and enzyme inhibition assays.
3.5. Antioxidant activity
Peptides LMAS5-8 have been evaluated for their antioxidant properties using radical scavenging assays (DPPH and ABTS), reducing power assays (CUPRAC and FRAP) and Phosphomolybdenum assay (Table 1).
Table 1.
Antioxidant activities of lead compound and LMAS5-8 in DPPH, ABTS, FRAP, CUPRAC and Phosphomolybdenum assays.*
| Compounds | DPPH mg TE/g | ABTS mg TE/g | FRAP mg TE/g | CUPRAC mg TE/g | Phosphomolybdenum mg TE/g |
|---|---|---|---|---|---|
| Lead compound | na | 8.69±0.64 | 14.21±0.64 | 19.35±0.64 | 0.02±0.00 |
| LMAS5 | 88.84±2.25 | 136.03±0.61 | 24.71±0.37 | 96.79±0.98 | 1.67±0.03 |
| LMAS6 | 91.02±0.74 | 154.25±0.21 | 27.01±0.11 | 156.91±2.16 | 2.18±0.01 |
| LMAS7 | 62.43±0.46 | 139.45±1.58 | 18.53±0.86 | 99.67±0.97 | 1.72±0.15 |
| LMAS8 | 75.75±2.65 | 131.28±1.78 | 19.71±0.17 | 99.64±1.02 | 1.61±0.03 |
Values are reported as mean ± SD of three parallel experiments. TE: Trolox Equivalent; na: not active.
Peptide LMAS6 was the most remarkable antioxidant compound showing the best activity in DPPH and ABTS assays, among the other analogues. Furthermore, it shows a good activity in FRAP, CUPRAC and phosphomolybdenum assays. Interestingly its antioxidant potential is higher than that of the lead compound, which seems to be only slightly active. The improved antioxidant activity of LMAS6 could be due to its amino acid sequence, in particular the presence of cysteine in position 3. Indeed, sulfur-containing amino acids already showed a paramount effect in the reduction of Fe3+-ferricyanide complex (Nwachukwu and Aluko, 2019, Udenigwe and Aluko, 2011). Sulphur group contained in cysteine may neutralize free radicals forming cysteine sulfoxide, a stable oxidation compound (He et al., 2012). Furthermore, proline and tyrosine could be involved in direct electrons transfer causing the enhancement of free radical scavenging activity (Ketnawa et al., 2018, Nwachukwu and Aluko, 2019).
3.6. Enzyme inhibitory activity
Oxidative stress is the principle cause of a huge number of medical diseases like neurological disorders, inflammatory processes, ischemic diseases, hypertension etc (Lobo, Patil, Phatak, & Chandra, 2010). Bioactive agents able to reduce the oxidative damages simultaneously inhibiting the main enzymes involved in this kind of diseases could be useful for the production of functional foods or nutraceuticals in combination with commercial drugs. For example, J. acutus, J. maritimus and J. inflexus leaves and roots extracts have been tested as acetylcholinesterase and butyrylcholinesterase inhibitors and for their antioxidant activity; the results suggest their potential role as sources of bioactive compounds useful for the production of nutraceuticals with cognitive improvement properties or food additives (Rodrigues et al., 2017).
For this reason, peptides LMAS5-8 have been studied to investigate their in vitro inhibitory activity against acetylcholinesterase, butyrylcholinesterase, amylase, glucosidase and tyrosinase (Table 2). Acetylcholinesterase (AChe) and butyrylcholinesterase (BChE) inhibition causes the increase of acetylcholine levels enhancing cognitive functions (Greig et al., 2005, Rodrigues et al., 2017), representing a promising approach for the management of Alzheimer disease. Peptides LMAS5 and LMAS7 exhibit an increased inhibitory activity against acetylcholinesterase compared to the lead compound. Peptide LMAS5 is more effective against butirylcholinesterase than the reference compound which is not active. Hyperglycaemia-induced reactive oxygen species has widely described in literature (Brownlee, 2001, Vanessa Fiorentino et al., 2013). The inhibition of carbohydrate hydrolysing enzymes α-amylase and α-glucosidase, is important in the management of hyperglycaemia (Cardullo et al., 2020, Hakamata et al., 2009). For instance, C-glucosidic ellagitannins and some galloylated glucopyranosis have been recently evaluated for their potential use as food ingredients with anti-diabetic effect due to their inhibitor action on amylase and glucosidase (Cardullo et al., 2020). We tested the lead compound and LMAS5-8 for their amylase and glucosidase inhibitory activities (Table 2). Surprisingly the lead compound resulted to be the only active glycosidase inhibitor, all the other analogues show low inhibitory activity against amylase. Tyrosinase inhibitory activity has been checked for LMAS5-8 (Table 2). Tyrosinase is involved in browning reactions in food causing the variation of aspect and organoleptic properties of food products reducing their shelf-life and market value (Chazarra, Escribano, & Cabanes, 2001). The use of tyrosinase inhibitors is a promising strategy to prevent browning phenomenon in food industry (Zheng, Cheng, To, Li, & Wang, 2008). In contrast to the lead compound, peptides LMAS5-8 are active against tyrosinase; among them LMAS6 gives the best result, suggesting a possible use as anti-browning agent in food industry.
Table 2.
Cholinesterase, tyrosinase, amylase and glucosidase inhibitory activity of lead compound and LMAS5-8.*
| Compounds | AChE mg GALAE/g | BChE mg GALAE/g | Tyrosinase mg KAE/g | Glucosidase mmol ACAE/g | Amylase mmol ACAE/g |
|---|---|---|---|---|---|
| Lead compound | 3.17±0.09 | na | na | 1.57±0.03 | 0.08±0.00 |
| LMAS5 | 6.24±0.03 | 9.57±0.08 | 59.84±2.28 | na | 0.09±0.01 |
| LMAS6 | 2.73±0.05 | na | 84.49±1.27 | na | 0.09±0.02 |
| LMAS7 | 5.87±0.04 | na | 39.49±2.22 | na | 0.09±0.00 |
| LMAS8 | 2.94±0.03 | 0.48±0.02 | 49.94±2.28 | na | 0.11±0.01 |
Values are reported as mean ± SD of three parallel experiments. AChE: Acetylcholinesterase; BChE: butyrylcholinesterase; GALAE: galantamine equivalents; KAE: kojic acid equivents; ACAE: acarbose equivalents; na: not active.
Overall the combined in vitro and in vivo data confirm the group of synthetic peptides LMAS5-8 as the most active antinociceptive and anti-inflammatory agents, antioxidants and enzyme inhibitors. These peptides possess a molecular weight very close each other’s and a chemical structure characterized by common amino acids in several positions of the primary sequence. The C-terminal acid and amide functions don’t seem to be responsible of any changes in biological activity, while the presence of a D-Pro2 is crucial to guarantee efficient antinociceptive and anti-inflammatory activity in vivo, as well as the best antioxidant power and anti-tyrosinase activity (e.g. LMAS6). This could be due to a stronger metabolic or enzymatic stability in vivo or in vitro, as also recently reported by us for their parent compounds (Stefanucci et al., 2020). Furthermore Cys3 is responsible of a strong effect encountered by CUPRAC, DPPH, and ABTS assays, being also involved in several neuroprotective roles at the CNS (Su, Han, Cao, & Xu, 2020). It’s interesting to note that all these described features are not present in the lead compound, which resulted to be less active than LMAS6 in all the performed assays.
4. Conclusion
Isolation and identification of bioactive peptides require high-cost equipment to elucidate their secondary and tertiary structures. This aspect is fundamental to correlate the specific 3D-structure with their biological effects. Regarding the antioxidant peptides, their application as prophylactic agents should be emphasized in order to improve the quality of life. In this work, three series of rubiscolin-6 analogues have been designed following modifications in position 2 and 3 of a previously described rubiscolin-6 analogue YPLDLF. The synthetic procedure is easy and straightforward allowing to obtain high purity compounds through a simple isolation technique. The peptides LMAS5-8 give the best results in vivo for their antinociceptive and anti-inflammatory effect. Among them, peptide LMAS6 shows the best antioxidant and tyrosinase inhibitory activities suggesting a possible use in food industry as preservatives or/and anti-browning agents. In addition, data obtained for the anti-cholinesterase peptides LMAS5 and LMAS7 indicate their potential development in new nutraceuticals with cognitive-enhancing properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to all our collaborators for their efforts. We thank PON Ricerca e innovazione 2014-2020 for supporting PhD. program of L.M.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100640.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Caballero J., Saavedra M., Fernández M., González-Nilo F.D. Quantitative structure–activity relationship of rubiscolin analogues as δ opioid peptides using Comparative Molecular Field Analysis (CoMFA) and Comparative Molecular Similarity Indices Analysis (CoMSIA) Journal of agricultural and food chemistry. 2007;55(20):8101–8104. doi: 10.1021/jf071031h. [DOI] [PubMed] [Google Scholar]
- Cardullo N., Muccilli V., Pulvirenti L., Cornu A., Pouységu L., Deffieux D.…Tringali C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food chemistry. 2020;313 doi: 10.1016/j.foodchem.2019.126099. [DOI] [PubMed] [Google Scholar]
- Carpino, L.; Han, G. The 9-Fluorenylmethoxycarbonyl Amino-Protecting Group. J. Org. Chem.1979, 44 (21), 3739–3739. https://doi.org/10.1021/jo01335a600.
- Chajra H., Amstutz B., Schweikert K., Auriol D., Redziniak G., Lefevre F. Opioid receptor delta as a global modulator of skin differentiation and barrier function repair. International journal of cosmetic science. 2015;37(4):386–394. doi: 10.1111/ics.12207. [DOI] [PubMed] [Google Scholar]
- Chazarra S., Escribano J., Cabanes J. F. Garc? ia-Carmona, “Competitive Inhibition of Mushroom Tyrosinase by 4-Substituted Benzaldehydes”. Journal of agricultural and food chemistry. 2001;49:4060–4063. doi: 10.1021/jf010194h. [DOI] [PubMed] [Google Scholar]
- Day L., Seymour R.B., Pitts K.F., Konczak I., Lundin L. Incorporation of functional ingredients into foods. Trends in Food Science & Technology. 2009;20(9):388–395. [Google Scholar]
- Della Valle A., Stefanucci A., Scioli G., Szűcs E., Benyhe S., Pieretti S.…Zengin G. Selective MOR activity of DAPEA and Endomorphin-2 analogues containing a (R)-γ-Freidinger lactam in position two. Bioorganic Chemistry. 2021;115 doi: 10.1016/j.bioorg.2021.105219. [DOI] [PubMed] [Google Scholar]
- Dimmito M.P., Stefanucci A., Della Valle A., Scioli G., Cichelli A., Mollica A. An overview on plants cannabinoids endorsed with cardiovascular effects. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111963. [DOI] [PubMed] [Google Scholar]
- Dvoracsko S., Stefanucci A., Novellino E., Mollica A. The design of multitarget ligands for chronic and neuropathic pain. Future Medicinal Chemistry. 2015;7:2469–2483. doi: 10.4155/fmc.15.156. [DOI] [PubMed] [Google Scholar]
- Greig N.H., Utsuki T., Ingram D.K., Wang Y., Pepeu G., Scali C.…Giordano T. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proceedings of the National Academy of Sciences. 2005;102(47):17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata W., Kurihara M., Okuda H., Nishio T., Oku T. Design and screening strategies for α-glucosidase inhibitors based on enzymological information. Current Topics in Medicinal Chemistry. 2009;9(1):3–12. doi: 10.2174/156802609787354306. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Meisel H. Food-derived peptides with biological activity: From research to food applications. Current opinion in biotechnology. 2007;18(2):163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- He R., Ju X., Yuan J., Wang L., Girgih A.T., Aluko R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Research International. 2012;49(1):432–438. [Google Scholar]
- Hirata H., Sonoda S., Agui S., Yoshida M., Ohinata K., Yoshikawa M. Rubiscolin-6, a δ opioid peptide derived from spinach Rubisco, has anxiolytic effect via activating σ1 and dopamine D1 receptors. Peptides. 2007;28(10):1998–2003. doi: 10.1016/j.peptides.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Kairupan T.S., Cheng K.-C., Asakawa A., Amitani H., Yagi T., Ataka K.…Inui A. Rubiscolin-6 activates opioid receptors to enhance glucose uptake in skeletal muscle. journal of food and drug analysis. 2019;27(1):266–274. doi: 10.1016/j.jfda.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K., Lazarus M., Miyamoto C., Oishi Y., Nagata N., Yang S.…Narumiya S. Orally administered rubiscolin-6, a δ opioid peptide derived from Rubisco, stimulates food intake via leptomeningeal lipocallin-type prostaglandin D synthase in mice. Molecular nutrition & food research. 2012;56(8):1315–1323. doi: 10.1002/mnfr.201200155. [DOI] [PubMed] [Google Scholar]
- Ketnawa S., Wickramathilaka M., Liceaga A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food chemistry. 2018;254:36–46. doi: 10.1016/j.foodchem.2018.01.133. [DOI] [PubMed] [Google Scholar]
- Kobbi S., Bougatef A., Balti R., Mickael C., Fertin B., Chaabouni S.…Nedjar N. Purification and recovery of RuBisCO protein from alfalfa green juice: Antioxidative properties of generated protein hydrolysate. Waste and biomass valorization. 2017;8(2):493–504. [Google Scholar]
- Lafarga T., Hayes M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat science. 2014;98(2):227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.M., Munekata P.E., Gomez B., Barba F.J., Mora L., Perez-Santaescolastica C., Toldra F. Bioactive peptides as natural antioxidants in food products–A review. Trends in Food Science & Technology. 2018;79:136–147. [Google Scholar]
- Miyazaki Y., Kaneko K., Iguchi S., Mizushige T., Kanamoto R., Yoshikawa M.…Ohinata K. Orally administered δ opioid agonist peptide rubiscolin-6 stimulates food intake in aged mice with ghrelin resistance. Molecular nutrition & food research. 2014;58(10):2046–2052. doi: 10.1002/mnfr.201400100. [DOI] [PubMed] [Google Scholar]
- Mollica A., Costante R., Novellino E., Stefanucci A., Pieretti S., Zador F., Samavati R., Borsodi A., Benyhe S., Vetter I., Lewis R.J. Design, Synthesis and Biological Evaluation of Two Opioid Agonist and Cav2.2 Blocker Multitarget Ligands. Chem Biol Drug Des. 2015;86:156–162. doi: 10.1111/cbdd.12479. [DOI] [PubMed] [Google Scholar]
- Mollica A., Paradisi M.P., Varani K., Spisani S., Lucente G. Chemotactic peptides: fMLF-OMe analogues incorporating proline–methionine chimeras as N-terminal residue. Bioorganic & medicinal chemistry. 2006;14(7):2253–2265. doi: 10.1016/j.bmc.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Mollica A., Pelliccia S., Famiglini V., Stefanucci A., Macedonio G., Chiavaroli A., Orlando G., Brunetti L., Ferrante C., Pieretti S., Novellino E., Benyhe S., Zador F., Erdei A., Szucs E., Samavati R., Dvrorasko S., Tomboly C., Ragno R., Patsilinakos A., Silvestri R. Exploring the first Rimonabant analog-opioid peptide hybrid compound, as bivalent ligand for CB1 and opioid receptors. J Enzyme Inhib Med Chem. 2017;32(1):444–451. doi: 10.1080/14756366.2016.1260565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A., Pinnen F., Stefanucci A., Mannina L., Sobolev A.P., Lucente G., Davis P., Lai J., Ma S.W., Porreca F., Hruby V.J. cis-4-amino-L-proline residue as a scaffold for the synthesis of cyclic and linear endomorphin-2 analogues: Part 2. Journal of Medicinal Chemistry. 2012;55(19):8477–8482. doi: 10.1021/jm300947s. [DOI] [PubMed] [Google Scholar]
- Nwachukwu I.D., Aluko R.E. Structural and functional properties of food protein-derived antioxidant peptides. Journal of Food Biochemistry. 2019;43(1):e12761. doi: 10.1111/jfbc.12761. [DOI] [PubMed] [Google Scholar]
- Pérez de Vega M.J., Ferrer-Montiel A., González-Muñiz R. Arch Biochem Biophys. 2018;660:36–52. doi: 10.1016/j.abb.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Pieretti S., Saviano A., Mollica A., Stefanucci A., Aloisi A.M., Nicoletti M. Calceolarioside A, a Phenylpropanoid Glycoside from Calceolaria spp., Displays Antinociceptive and Anti-Inflammatory Properties. Molecules. 2022;27(7):2183. doi: 10.3390/molecules27072183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M.J., Gangadhar K.N., Zengin G., Mollica A., Varela J., Barreira L., Custódio L. Juncaceae species as sources of innovative bioactive compounds for the food industry: In vitro antioxidant activity, neuroprotective properties and in silico studies. Food and Chemical Toxicology. 2017;107:590–596. doi: 10.1016/j.fct.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Sánchez A., Vázquez A. Bioactive peptides: A review. Food Quality and Safety. 2017;1(1):29–46. [Google Scholar]
- Sohaib M., Anjum F.M., Sahar A., Arshad M.S., Rahman U.U., Imran A., Hussain S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. International Journal of Food Properties. 2017;20(11):2581–2593. [Google Scholar]
- Stefanucci A., Dimmito M.P., Tenore G., Pieretti S., Minosi P., Zengin G.…Cichelli A. Plant-derived peptides rubiscolin-6, soymorphin-6 and their c-terminal amide derivatives: Pharmacokinetic properties and biological activity. Journal of Functional Foods. 2020;73 [Google Scholar]
- Stefanucci A., Pinnen F., Feliciani F., Cacciatore I., Lucente G., Mollica A. Conformationally Constrained Histidines in the Design of Peptidomimetics: Strategies for the χ-Space Control. International Journal of Molecular Science. 2011;12:2853–2890. doi: 10.3390/ijms12052853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Han M., Cao D., Xu M. Molecular and Biological Properties of Snakins: The Foremost Cysteine-Rich Plant Host Defense Peptides. J. Fungi. 2020;6:220. doi: 10.3390/jof6040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.C., Andersson I. Structure of a product complex of spinach ribulose-1, 5-bisphosphate carboxylase/oxygenase. Biochemistry. 1997;36(13):4041–4046. doi: 10.1021/bi962818w. [DOI] [PubMed] [Google Scholar]
- Tkaczewska J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends in Food Science & Technology. 2020;106:298–311. [Google Scholar]
- Udenigwe C.C., Aluko R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. International Journal of Molecular Sciences. 2011;12(5):3148–3161. doi: 10.3390/ijms12053148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Journal of food science. 2012;77(1):R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- Uysal, S., Zengin, G., Locatelli, M., Bahadori, M. B., Mocan, A., Bellagamba, G., . . . Aktumsek, A. (2017). Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Frontiers in pharmacology, 8, 290. [DOI] [PMC free article] [PubMed]
- Vanessa Fiorentino T., Prioletta A., Zuo P., Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Current pharmaceutical design. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- Yang S., Kawamura Y., Yoshikawa M. Effect of rubiscolin, a δ opioid peptide derived from Rubisco, on memory consolidation. Peptides. 2003;24(2):325–328. doi: 10.1016/s0196-9781(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Yang S., Sonoda S., Chen L., Yoshikawa M. Structure–activity relationship of rubiscolins as δ opioid peptides. Peptides. 2003;24(4):503–508. doi: 10.1016/s0196-9781(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Yang S., Yunden J., Sonoda S., Doyama N., Lipkowski A.W., Kawamura Y., Yoshikawa M. Rubiscolin, a δ selective opioid peptide derived from plant Rubisco. FEBS letters. 2001;509(2):213–217. doi: 10.1016/s0014-5793(01)03042-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M. Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides. 2015;72:208–225. doi: 10.1016/j.peptides.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Zengin G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Industrial Crops and Products. 2016;83:39–43. [Google Scholar]
- Zheng Z.P., Cheng K.W., To J.T.K., Li H., Wang M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Molecular nutrition & food research. 2008;52(12):1530–1538. doi: 10.1002/mnfr.200700481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.