Abstract
Uptake of human papillomavirus (HPV) vaccine in the United States (U.S.) is far below the Healthy People 2020 goal of 80% coverage among adolescents. In rural communities, HPV vaccination coverage is low, yet incidence and mortality rates of HPV-associated cancer are high. Much of the research focused on HPV vaccination in rural U.S. communities has involved qualitative investigations, observations, survey research, and secondary data analysis with limited implementation of interventional study designs. The purpose of this narrative review was to examine intervention studies to increase HPV vaccination in rural settings and to summarize study characteristics and associated outcomes. PubMed, PsycINFO, CINAHL, and Web of Science were searched utilizing systematic narrative review methodology for studies describing implementation of HPV vaccination interventions in rural U.S. settings from January 2006–December 2019. Using specific search criteria, 991 studies were identified. After abstract review, 30 full-text articles were assessed for eligibility, and 15 met the inclusion criteria. The 15 articles – published from 2011–2019 – described HPV vaccination interventions in rural settings of six states, including communities, health clinics, and schools. A range of primary and secondary outcomes were reported, including HPV vaccine receipt (series initiation, continuation, and/or completion); HPV vaccine knowledge; and/or cervical cancer knowledge. Across the studies, there was an absence of the description of rural context. As compared to the broader HPV vaccination intervention literature, interventions in rural settings were limited. More interventional research is needed in rural communities given the elevated rates of HPV-related cancer and low rates of HPV vaccine uptake.
Keywords: United States, Rural Populations, Vaccination, Papillomavirus Vaccines, Neoplasms
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the United States (U.S.) (Centers for Disease Control and Prevention (CDC), 2017a; Chesson et al., 2014). Surveillance data suggest 45,300 cases of HPV-associated cancer, including cervical, vaginal, vulvar, oropharyngeal, anal, and penile, occur annually in the U.S. (Centers for Disease Control and Prevention 2020). People living in rural areas are disproportionately impacted by HPV-associated cancers (Zahnd et al., 2019b, 2018). A recent analysis of rural-urban differences in HPV-associated cancers showed rural females had significantly higher rates of cervical, vaginal, vulvar, oropharyngeal, and anal cancer when compared with urban counterparts (Zahnd et al., 2019b). Rural males had higher rates of penile cancer as compared with urban counterparts (Zahnd et al., 2019b). Additional disparities in HPV-associated cancers in rural settings by race and ethnicity were found; for example, non-Hispanic Black women residing in rural areas had higher rates of cervical cancer than non-Hispanic White and Hispanic women (Zahnd et al., 2019b). These findings underscore the significant burden of HPV-related cancers in rural communities and the tremendous opportunity for intervention to prevent these cancers (Vanderpool et al., 2019).
Despite the significant cancer burden associated with HPV and the advent of a safe and effective prophylactic vaccine, HPV vaccination rates remain below national goals for both adolescents and young adults (Meites et al., 2016). For 14 years, vaccination has been available to protect against HPV types that lead to cancer in males and females. Current HPV vaccine recommendations target 11–12-year-olds starting as early as age 9 and up to age 26; shared clinical decision-making has been recommended for some adults aged 27 to 45 who are not adequately vaccinated (Meites et al., 2019). The focus on adolescents remains a priority for optimizing the benefits of HPV prevention, particularly in rural communities. National Immunization Survey-Teen (NIS-Teen) data (2019) showed HPV vaccination uptake was lower among rural-dwelling adolescents (non-metropolitan statistical areas [MSAs]) as compared to those in urban settings (MSAs) with rural up-to-date rates of 47.3% (an increase of 6.6% from 2018) compared to 54.2% in the U.S. overall (an increase of 3.1% from 2018) and 57.1% in MSA principal cities (an increase of 1% from 2018) (Elam-Evans et al., 2020).
Rural residents in the U.S. face multiple and unique barriers accessing health care, and specifically related to HPV vaccination (Newcomer et al., 2020; Peterson et al., 2020; U.S. Department of Health and Human Services, 2020). Rural residents have demonstrated lower knowledge of HPV vaccination, which may result in lower rates of HPV vaccination uptake (Boyd et al., 2018; Mohammed et al., 2018). Healthcare provider shortages in rural settings may also lead to fewer access points for HPV vaccination (Shipman et al., 2011). Notably, rural areas are recognized for poor health outcomes, including lower childhood and adult immunization rates (Elam-Evans et al., 2020; Hill et al., 2018; Hughes et al., 2019; McLaughlin et al., 2019; Walker et al., 2018; Walker et al., 2019), limited access to healthcare services (Centers for Disease Control and Prevention, 2017b; Jones et al., 2009), and lower socioeconomic status (Centers for Disease Control and Prevention, 2017b; Jones et al., 2009). These factors provide the impetus for research designed to understand and address barriers and facilitators of HPV vaccination across multiple levels of influence in rural communities (Newcomer et al., 2020; Peterson et al., 2020; Vanderpool et al., 2019).
To date, the majority of HPV vaccination research in rural U.S. communities has used non-interventional study methodologies, such as qualitative research, observations, surveys, and secondary data analyses to assess HPV and vaccine-related knowledge and attitudes (Blake et al., 2015; Kepka et al., 2011; Luque et al., 2011; Merzouk et al., 2011; Mohammed et al., 2018; Vanderpool et al., 2015b), vaccine acceptability (Cates et al., 2009; Reiter et al., 2014; Tiggelaar et al., 2014), intention to vaccinate (Fazekas et al., 2008; Reiter et al., 2013; Sperber et al., 2008; Spleen et al., 2012), vaccine recommendations / communication (Bednarczyk et al., 2017; Bhatta and Phillips, 2015; Moss et al., 2016), barriers and facilitators to HPV vaccination uptake and completion (Boyd et al., 2018; Cartmell et al., 2018; Head et al., 2013), feedback on HPV vaccination messaging (Cates et al., 2015, 2012; Shafer et al., 2011), predictors of HPV vaccination (Casey et al., 2013; Gerend et al., 2019; Lai et al., 2016), and HPV vaccination rates (Barefoot et al., 2017; Henry et al., 2017; Vielot et al., 2017). These studies have been conducted among adolescents and young adults, parents/guardians, and healthcare providers in rural communities across the U.S., including foci on Latinx, African American, and Appalachian populations. This research has been important to understand the landscape of factors associated with HPV vaccination and non-vaccination for descriptive purposes, but limits guidance on how to increase uptake of HPV vaccination through interventions.
In comparison, interventional HPV vaccination study designs, such as randomized control trials (RCT), quasi-experimental studies, and pragmatic trials, focused on changing HPV vaccination-related outcomes have been less commonly conducted in rural U.S. communities. To reduce the burden of HPV-associated cancers in rural settings and improve low HPV vaccination uptake, investigation of efforts to intervene to increase participation in HPV prevention is needed. Therefore, the purpose of this narrative review was to examine the body of interventional HPV vaccination research conducted in rural U.S. areas since the introduction of the first HPV vaccine in 2006 and to assess study populations, geographic locations, intervention components, intervention design, outcome measure(s), and primary study findings. Commonalities and differences among the identified studies, scientific gaps, and recommendations to increase HPV vaccination interventional research in rural communities are reported herein.
METHODS
Search Strategy
In this review, we aimed to provide an overview of the current state of HPV vaccination interventions in rural U.S. communities by employing a narrative review methodology (Green et al., 2006; Gregory and Denniss, 2018). The process involved systematically searching four electronic databases with the most relevant HPV vaccination intervention publications. We searched PubMed, PsycINFO, the Cumulative Index for Nursing and Allied Health Literature (CINAHL), and Web of Science for articles from January 2006-December 2019. With the assistance of a health sciences research librarian, the research team developed search criteria for each database (Table 1). Relevant key terms, such as immunization, vaccination, rural, and papillomavirus, used in each database were identified and applied. In PubMed, key terms were searched for as medical subject heading (MeSH) and text words (tw) fields; in PsycINFO, subject (DE), title (TI), and abstract (AB) fields; in CINAHL, exact subject heading (MH), title (TI), and abstract (AB); and in Web of Science, exact terms. The code in Table 1 was used to identify articles meeting the specified criteria.
Table 1:
Search criteria for each database accessed for the systematic narrative review of HPV vaccination interventions in rural U.S. communities
| Database | Search Criteria |
|---|---|
| PubMed | ((((((((((immunization programs[MeSH]) OR immunization[MeSH]) OR immunization*[tw]) OR immunize*[tw]) OR vaccin*[tw])) AND ((((papillomavirus infections[MeSH]) OR papillomaviridae[MeSH]) OR HPV*[tw]) OR papilloma*[tw]))) OR (((papillomavirus vaccines[MeSH]) OR cervarix[tw]) OR gardasil[tw]))) AND (((((((appalachian region[MeSH]) OR hospitals, rural[MeSH]) OR rural health[MeSH]) OR rural health services[MeSH]) OR rural population[MeSH])) OR (((((((((appalachia*[tw]) OR frontier[tw]) OR geographically isolated[tw]) OR non-metropolitan[tw]) OR nonmetropolitan[tw]) OR remote[tw]) OR RUCA[tw]) OR RUCC[tw]) OR rural*[tw])) AND (“2006/01/01”[PDAT]: “2019/12/31”[PDAT]) |
| PsycINFO | DE “Rural Environments” OR [(TI Appalachia* OR frontier OR “geographically isolated” OR “non-metropolitan” OR nonmetropolitan OR remote OR RUCA OR RUCC OR rural*) OR (AB TI Appalachia* OR frontier OR “geographically isolated” OR “non-metropolitan” OR nonmetropolitan OR remote OR RUCA OR RUCC OR rural*)] AND [[(TI Cervarix OR Gardasil) OR (AB Cervarix or Gardasil)] OR [[DE immunization OR [(TI immunization* OR immunize* OR vaccine*) OR (AB immunization* OR immunize* OR vaccine*)]] OR [DE “human papillomavirus” OR [(TI HPV* OR papilloma*) OR (AB HPV* OR papilloma*)]]]] AND (DT 20060101–20191231) |
| CINAHL | [[(MH “Appalachian region+”) OR (MH “rural health personnel”) OR (MH “rural health centers”) OR (MH “hospitals, rural”) OR (MH “rural population”) OR (MH “rural health services”) OR (MH “rural health nursing”) OR (MH “rural areas”) OR (MH “rural health”) OR (MH “frontier nursing service”)] OR [(TI Appalachia* OR frontier OR “geographically isolated” OR “non-metropolitan” OR nonmetropolitan OR remote OR RUCA OR RUCC OR rural*) OR (AB Appalachia* OR frontier OR “geographically isolated” OR “non-metropolitan” OR nonmetropolitan OR remote OR RUCA OR RUCC OR rural*)]] AND [[MH “human papillomavirus” OR [(TI Cervarix OR Gardasil) OR (AB Cervarix OR Gardasil)]] OR [[(MH “immunization programs” OR MH “immunization”) OR [(TI immunization* OR immunize* OR vaccine*) OR (AB immunization* OR immunize* OR vaccine*)]] AND [(MH “papillomavirus infections+” OR MH papillomaviruses) OR [(TI HPV* OR papilloma*) OR (AB HPV* OR papilloma*)]]]] AND (DT 20060101 −20191231) |
| Web of Science | (Appalachia* OR frontier OR “geographically isolated” OR “non-metropolitan” OR nonmetropolitan OR remote OR RUCA or RUCC or rural*) AND [(Gardasil or Cervarix) OR (immunization* OR immunize* OR vaccine* AND HPV* OR papilloma*)] |
For inclusion in our review, articles had to meet the definition of “intervention”, which was defined as “…any activity undertaken with the objective of improving human health by preventing disease, by curing or reducing the severity or duration of an existing disease, or by restoring function lost through disease or injury” (Smith et al., 2015). In addition, we used the following inclusion criteria: (a) focused on a rural U.S. setting (i.e., using the word rural to describe the setting and/or referencing an official definition of rural); (b) focused on HPV vaccination receipt, including first dose, series continuation (as applicable), and/or series completion, as an outcome; and (c) contained full article access in English, including study description and results. No restrictions on target population of the intervention were included in the inclusion criteria.
Screening Procedures
One research team member reviewed the citation details of identified records to remove duplicate records. Two research team members screened the title and abstracts of non-duplicative records to identify articles for full-text review. Titles, abstracts, and full articles were reviewed by the same two members of the research team who then met to compare decisions and reconcile disagreement by consensus to determine article classification. This screening process continued until the final set of articles was determined.
Data Extraction
The search yielded 15 intervention articles that were analyzed for content based on study and intervention characteristics and results. Two members of the research team systematically abstracted the following information from each article: geographic location of the intervention, target population(s), sample size, intervention setting, intervention and control characteristics, theoretical underpinnings, barriers and facilitators, outcome measures, data sources, and results. In addition, each study design was categorized based on the International Agency for Research on Cancer classification system for study designs (dos Santos Silva, 1999). Study design types were then expanded to include quasi-experimental designs. The abstracted data were compiled into tables and reviewed by each research team member. Modifications were made following research team discussion, when needed.
RESULTS
The narrative review process is depicted in Figure 1. One research team member reviewed the citation details of the 991 records (417 PubMed; 73 PsycINFO; 194 CINAHL; 307 Web of Science) identified through the study selection process to remove duplicates resulting in 518 records to be screened further. Two research team members screened the titles and abstracts of these 518 non-duplicative records (417 PubMed; 3 PsycINFO; 39 CINAHL; 59 Web of Science) resulting in the identification of 30 records for full-text review. The study selection process resulted in 15 records to be included in the synthesis process. Fifteen records were excluded from further review due to the following reasons: secondary analysis (n = 6), rural setting unknown (n = 5), absence of an HPV vaccination outcome (n = 2), protocol paper describing a study to be conducted (n = 1), and inability to access the full text of the article (n = 1) (Figure 1).
Figure 1:
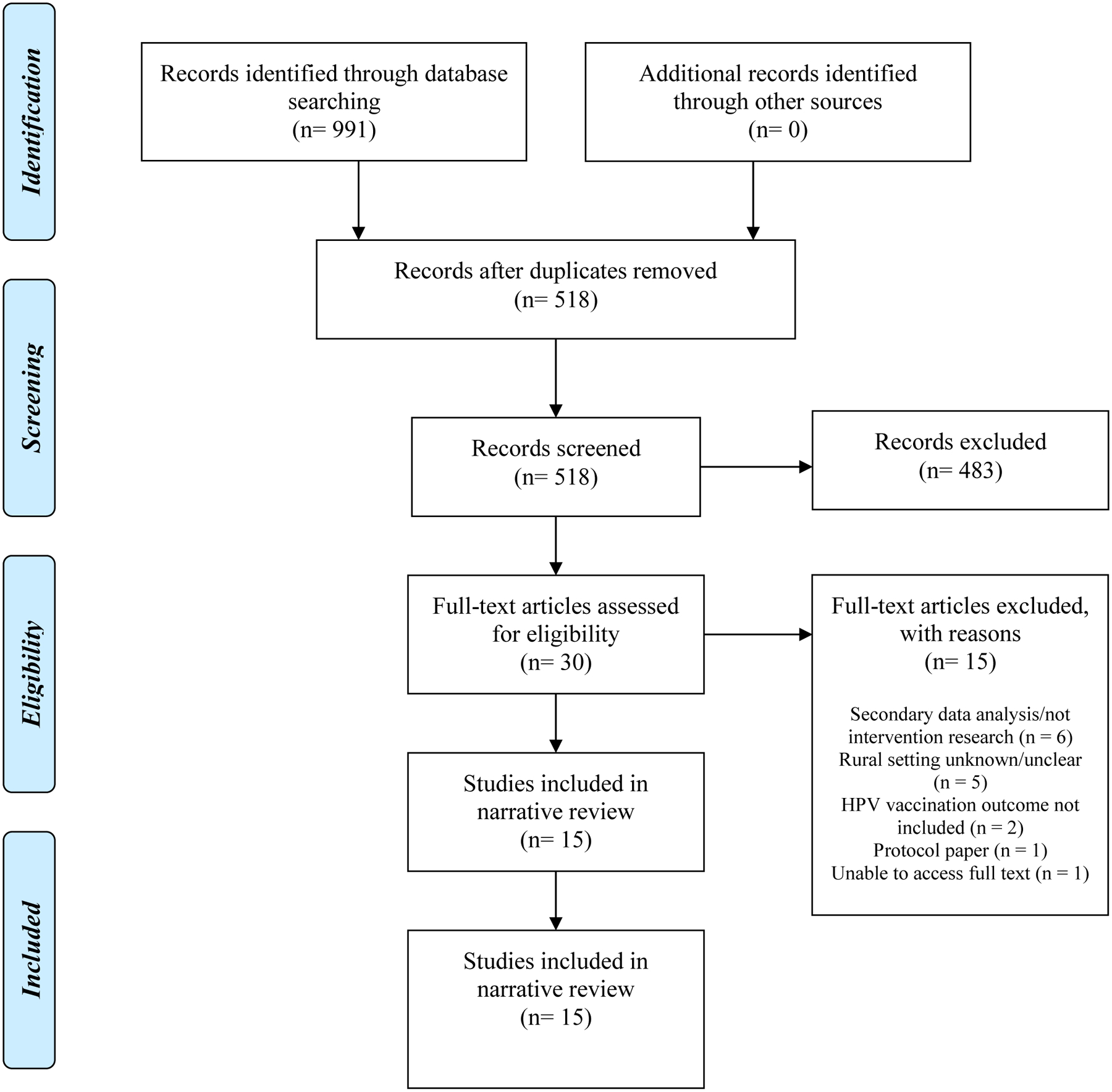
PRISMA flow chart of the narrative review process
Our narrative literature review identified 15 articles focused on HPV vaccination interventions in rural U.S. settings published from 2011–2019 (Brewer et al., 2017; Carman et al., 2015; Cates et al., 2018, 2011; Chung et al., 2015; Crosby et al., 2011; Kaul et al., 2019; Paskett et al., 2016; Richman et al., 2019, 2016; Szilagyi et al., 2015; Underwood et al., 2015; Vanderpool et al., 2015a, 2013; Vogel et al., 2018), which are summarized in Tables 2 and 3.
Table 2:
Characteristics of included studies (n=15)
| Author, year | Study design | Description of intervention (I) and control (C) conditions | Target population, sample size, setting | Intervention target | Setting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinic/Practice | Health Care Providers | Staff | Parents | Vaccine-eligible population± | Clinic | Health Department | Community | K-12 School | College | ||||
| Brewer NT, et al., 2017 (Brewer et al., 2017) | Randomized controlled trial | I (1): Clinic-level/provider-level: 1-hour in-clinic, physician-led training on announcement recommendations I (2): Clinic-level/provider-level: 1-hour in-clinic, physician-led training on conversational recommendations C: No activities |
I (1): 9 clinics I (2): 10 clinics C: 10 clinics Overall: 17,173 adolescents (aged 11–12) across 29 clinics in North Carolina |
X | X | ||||||||
| Carman AL, et al., 2015 (Carman et al., 2015) | Intervention only | I: Individual-level: “1–2-3 Pap” educational video disseminated via one of 3 modes of delivery: 1) local health department website; 2) play on loop in local health department lobby; or 3) present to parent/patient at time of first dose at local health department C: N/A (intervention only design) |
I: 18 local health departments in Kentucky C: N/A (intervention only design) |
X | X | X | X | ||||||
| Cates JR, et al., 2011 (Cates et al., 2011) | Field trial | I: Community-level: Social marketing campaign (posters, brochures, website, hotline, newspapers, radio/tv) Provider-level: Social marketing campaign (posters, brochures, campaign buttons, reminder sticky notes) C: No activities |
I: 225 mothers of girls aged 9–13 and 35 health care providers serving pre-teen girls in 4 counties in rural North Carolina C: 96 counties |
X | X | X | X | ||||||
| Cates JR, et al., 2018 (Cates et al., 2018) | Field trial | I: Clinic-level: “Protect Them” practice-based communication information; also includes provider, parent, and pre-teen C: No activities |
I: 14 clinical practices in North Carolina C: 161 clinical practices |
X | X | X | X | X | |||||
| Chung RJ, et al., 2015 (Chung et al., 2015) | Quasi-experimental | I: Provider-level: Educational meetings with physicians and nurses; web-based North Carolina Immunization Registry trainings; reminder postcards and promotional posters; monetary incentives for using reminder postcards Parent-level: 2 informational telephone messages from providers C: No activities |
I: 7 medical practices in 1 county in rural North Carolina C: 4 counties |
X | X | X | |||||||
| Crosby RA, et al., 2011 (Crosby et al., 2011) | Intervention only | I: Individual-level: Voucher for free HPV vaccination C: N/A (intervention only design) |
I: Unvaccinated women aged 18–26 in Kentucky (246 in rural clinics; 251 in rural community college, and 209 in urban university health clinic) C: N/A (intervention only design) |
X | X | X | |||||||
| Kaul S, et al., 2019 (Kaul et al., 2019) | Quasi-experimental | I: Community-level: Community-based education provided by physicians School-level: On-site HPV vaccination program plus educational programs by physicians and project staff C: Community-level: Comparison schools received the community-based education only; no in-school programming |
I: 1 school in Rio Grande City Consolidated Independent School District in Texas C: 2 schools in same district |
X | X | X | X | ||||||
| Paskett ED, et al., 2016 (Paskett et al., 2016) | Randomized controlled trial | I: HPV vaccination focus Clinic-level: HPV vaccination print materials Provider-level: 1-hour educational session Parent-level: Print material, video, magnet by mail and telephone education session C: Influenza vaccination |
I: 10 clinics, 174 parents in 6 Appalachian Ohio counties C: 14 clinics, 163 parents in 6 Appalachian Ohio counties Note: 119 providers across 24 clinics participated but intervention and control participation was not reported |
X | X | X | X | X | |||||
| Richman AR, et al., 2016 (Richman et al., 2016) | Randomized controlled trial | I: Individual-level: Electronic HPV vaccine intervention (text/email appointment reminders and education) C: Standard-of-care |
I: 130 college students initiating HPV vaccination at college health center in rural North Carolina C: 134 college students initiating HPV vaccination at college health center in rural North Carolina |
X | X | X | |||||||
| Richman AR, et al., 2019 (Richman et al., 2019) | Randomized controlled trial | I: Individual-level: Electronic HPV vaccine intervention (text/email appointment reminders and education) C: Standard-of-care |
I: 129 parent-child dyads C: 128 parent-child dyads Note: Total sample consisted of 257 parent-child dyads in two clinics in eastern North Carolina |
X | X | X | |||||||
| Szilagyi PG, et al., 2015 (Szilagyi et al., 2015) | Randomized controlled trial | I: Clinic-level and provider-level: EHR-based prompts, nurse/staff prompts, follow-up telephone calls C: Standard-of-care |
I: 11 primary care practices; 11–17 year old adolescents C: 11 primary care practices; 11–17 year adolescents Note: 10 primary care practices were in a practice-based research network in New York; 12 were part of a national pediatric network |
X | X | ||||||||
| *Underwood NL, et al., 2015 (Underwood et al., 2015) | Randomized controlled trial | I (1): School-level/individual-level: Curriculum delivered by science teachers to students Parent-level: Mailed educational brochure I (2): Parent-level: Mailed educational brochure C: No activities |
I (1): 4 schools; 225 parents (Underwood et al., 2016) I (2): 4 schools; 251 parents (Underwood et al., 2016) C: 3 schools (Underwood et al., 2016); 210 parents Note: Parents (n=686) of adolescents enrolled in one of 11 middle or high schools in Georgia |
X | X | X | |||||||
| Vanderpool RC, et al., 2013 (Vanderpool et al., 2013) | Randomized controlled trial | I: Individual-level: 13-minute educational DVD, follow-up telephone call C: Follow-up telephone call only |
I: 178 newly vaccinated women aged 18–26 in 8 counties in Appalachian Kentucky C: 166 newly vaccinated women aged 18–16 in 8 counties in Appalachian Kentucky |
X | X | ||||||||
| Vanderpool RC, et al., 2015 (Vanderpool et al., 2015a) |
Intervention only | I: School-level: Increasing awareness through school-related outlets (e.g. newspaper, classrooms, football games) Parent-level: Including HPV educational materials in back-to-school packets; Sending reminder phone call to complete consent form; Sending reminder phone calls for doses 2 and 3 Individual-level: Increasing awareness through school-related outlets (e.g. newspaper, classrooms, football games) C: N/A (intervention only design) |
I: 447 HPV vaccine naïve students in 2 high schools in rural south-central Kentucky C: N/A (intervention only design) |
X | X | X | |||||||
| Vogel EP, et al., 2018 (Vogel et al., 2018) | Field trial | I: Clinic-level: Quality improvement Provider-level: Provider recommendation Parent- and individual-level: Additional HPV vaccination information (i.e., brochure and recommendation) during patient visit C: No additional HPV vaccination information during patient visit. |
I: 191 male patients aged 11–18 and their parent/guardian in a rural North Carolina clinic C: 226 male patients aged 11–18 and their parent/guardian in a rural North Carolina clinic. |
X | X | X | X | X | |||||
Key: I = Intervention; C = Control condition (as applicable)
Additional methodological details of the Underwood et al., 2015 study were extracted from Underwood et al., 2016 to aid in data completeness.
Vaccine-eligible population intervention target includes adolescents and adults of vaccination age, per current recommendations.
Table 3.
Results of included studies (n = 15)
| Author, year | Primary Outcome (PO) and Secondary Outcome (SO) | Data Source(s) | Selected results |
|---|---|---|---|
| Brewer NT, et al., 2017 (Brewer et al., 2017) | PO: ≥1 dose HPV vaccination at 6 months for 11–12 year olds (HPV initiation) SO: Changes in vaccine coverage from baseline to 3 months; HPV completion (3 doses); 13–17 year olds |
North Carolina Immunization Information System | PO: 5.4% increase in HPV vaccination initiation among 11–12 year olds at 6 months among adolescents in announcement training (I1) clinics as compared to control clinics SO: Observable positive increases at 3 months from baseline in I (1); Conversation intervention I (2) clinics did not differ from control clinics; Both I (1) and I (2) did not differ from control for 13–17 year olds or completion |
| Carman AL, et al., 2015 (Carman et al., 2015) | PO: Changes in HPV vaccination rates over a 6-month period (baseline, 3 months, 6 months) SO: Organizational readiness |
Each clinic reported total number of HPV vaccine doses | PO: No statistically significant differences in HPV vaccine uptake at designated time points or by intervention implementation strategy (no data provided) SO: Organizational readiness data |
| Cates JR, et al., 2011 (Cates et al., 2011) | PO: HPV vaccination rates SO: Awareness and use of campaign, website visits, hotline calls, media placement |
North Carolina Immunization Information System | PO: 2% increase in HPV vaccination rates within 6 months of campaign launch for 9–13 year old girls in 2 of 4 intervention counties compared to girls in non-intervention counties SO: 82% of participating mothers were aware of campaign; 94% of providers used campaign materials; 1,312 website visits (including 55 visits from 4 intervention counties); 2 hotline calls; 4 newspaper articles and 4 announcements on radio/television |
| Cates JR, et al., 2018 (Cates et al., 2018) | PO: HPV vaccination initiation and completion rates SO: None reported |
North Carolina Immunization Information System | PO: Individuals in intervention practices were 17% more likely to initiate HPV vaccination as compared to those in control practices during the active intervention period; completion rate was 18% higher in intervention practices |
| Chung RJ, et al., 2015 (Chung et al., 2015) | PO: 1st dose HPV vaccine receipt and vaccine completion SO: Number of vaccination reminders sent |
North Carolina Immunization Information System | PO: Increase in 1st dose HPV vaccine for girls (27–43%) and boys (14–32%) in 11–12 year olds; HPV completion in boys (1.6–4%) in 13–18 year olds SO: Registry reminder usage varied between the intervention practices, ranging from 0% to 100%; the health department accounted for 69% of all reminders sent |
| Crosby RA, et al., 2011 (Crosby et al., 2011) | PO: 1st, 2nd, and 3rd dose HPV vaccine receipt SO: None reported |
Coupon redemption for HPV vaccine | PO: 45.1% 1st dose, 13.8% 2nd, 4.5% 3rd in rural clinic; 6.8% 1st dose, 2.8% 2nd dose, 1.6% 3rd dose in rural community college; 50.7% 1st dose, 39.7% 2nd dose, 28.2% 3rd dose in urban clinic; statistically significant difference between urban clinic and rural community college only; rural clinic participants were seven times more likely than urban clinic to not return for follow-up doses |
| Kaul S, et al., 2019 (Kaul et al., 2019) | PO: HPV vaccination initiation and completion rates SO: None reported. |
School records | PO: HPV vaccine initiation rate increased by 34% from baseline in the intervention school compared to 13% in the comparison schools and completion rate increased by 20% for the intervention school compared to 6% in the control schools, both of which were statistically significant |
| Paskett ED, et al., 2016 (Paskett et al., 2016) | PO: 1st dose receipt within 3 months SO: 1st dose receipt within 6 months; changes in provider knowledge |
EMR and parent self-report | PO: 7.7% of daughters of intervention participants received the first dose of the HPV vaccine within 3 months of being sent the intervention materials compared to 3.2% of daughters of comparison participants SO: 13.1% of daughters of intervention participants received the first HPV vaccine shot within 6 months compared to 6.5% of daughters of comparison participants; Increase provider knowledge after intervention |
| Richman AR, et al., 2016 (Richman et al., 2016) | PO: HPV vaccine completion SO: HPV knowledge |
School records | PO: Intervention did not increase HPV vaccine completion as compared to control group SO: Mean knowledge scores were significantly higher in the intervention group post-intervention compared to baseline. No other changes observed. |
| Richman AR, et al., 2019 (Richman et al., 2019) | PO: HPV vaccine completion SO: HPV knowledge |
EMR | PO: Intervention did not increase HPV vaccine completion as compared to control group SO: Knowledge scores were higher among the intervention group as compared to the control group but not statistically significant |
| Szilagyi PG, et al., 2015 (Szilagyi et al., 2015) | PO: Receipt of HPV vaccine (also other adolescent vaccinations) SO: Time to vaccination since 11th birthday; missed opportunities |
EMR | PO: Intervention had no effect on receipt of HPV vaccine SO: Intervention had no effect on time to vaccination since 11th birthday; intervention improved missed opportunities for HPV vaccination by 18% |
| *Underwood NL, et al., 2015 (Underwood et al., 2015) | PO: Receipt of HPV vaccine SO: HPV vaccination attitudes |
Parent self-report | PO: 48.7% of parents reported child received at least one dose; of those, 61.9% reported child received 3 doses; no intervention effect SO: Parents with more positive attitudes had higher odds of reporting child had received initial dose of HPV vaccine Note: PO was not presented by intervention type or control condition |
| Vanderpool RC, et al., 2013 (Vanderpool et al., 2013) | PO: HPV vaccination completion SO: Intention to complete HPV vaccine series |
EMR | PO: 43.3% of intervention participants completed HPV vaccine series; 31.9% of control participants completed HPV vaccine series; women assigned to the intervention were 2.44 times more likely than women in the usual care group to complete the series |
| Vanderpool RC, et al., 2015 (Vanderpool et al., 2015a) |
PO: 1st dose HPV vaccine receipt SO: HPV vaccine series completion |
School records | PO: 70.5% of HPV vaccine naïve students initiated the HPV vaccine series SO: 88% of students who initiated the HPV vaccine series completed the series |
| Vogel EP, et al., 2018 (Vogel et al., 2018) | PO: Clinic-level pre- and post-intervention HPV vaccination rate SO: HPV education to adults and their male adolescents, healthcare provider HPV vaccine recommendation |
North Carolina Immunization Information System | PO: HPV vaccination rates for patients who received additional education were higher than for patients who did not. However, the difference was not statistically significant. SO: 191 patients and their parent/guardian received HPV vaccination education |
Key: PO = Primary Outcome; SO = Secondary Outcome
Additional methodological details of the Underwood et al., 2015 study were extracted from Underwood et al., 2016 to aid in data completeness.
Study Characteristics
Table 2 provides characteristics of included studies, such as study design, intervention and control conditions, target population, intervention target, and study setting. The most commonly reported study design was a RCT (Brewer et al., 2017; Paskett et al., 2016; Richman et al., 2019, 2016; Szilagyi et al., 2015; Underwood et al., 2015; Vanderpool et al., 2013).
Intervention types included patient, parent, and/or provider interventions in healthcare settings (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Crosby et al., 2011; Paskett et al., 2016; Richman et al., 2019, 2016; Szilagyi et al., 2015; Vogel et al., 2018) and educational interventions, including social marketing and communication campaigns, in communities and high schools (Carman et al., 2015; Cates et al., 2011; Kaul et al., 2019; Underwood et al., 2015; Vanderpool et al., 2015a, 2013). Eight of 15 studies used multilevel approaches focusing on at least two levels (Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Underwood et al., 2015; Vanderpool et al., 2015a; Vogel et al., 2018), such as organizational (e.g., clinic, school) or community, parent, and/or individual levels. Intervention content was primarily educational and delivered via training or instruction, print materials, social marketing campaigns, reminders and informational telephone calls, and/or DVD. One study used free HPV vaccination in the form of a voucher as the main intervention (Crosby et al., 2011).
Twelve studies reported intervention and control or comparison conditions employing an experimental design (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Richman et al., 2019, 2016; Szilagyi et al., 2015; Underwood et al., 2015; Vanderpool et al., 2013; Vogel et al., 2018). Seven of these studies randomized to either the intervention or control / comparison condition, with four of these randomized at the group level (either clinic or school) (Brewer et al., 2017; Paskett et al., 2016; Szilagyi et al., 2015; Underwood et al., 2015) and three at the individual level (Richman et al., 2019, 2016; Vanderpool et al., 2013). In addition, seven of these studies reported multilevel interventions (Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Underwood et al., 2015; Vogel et al., 2018), while five targeted one level of intervention (Brewer et al., 2017; Richman et al., 2019, 2016; Szilagyi et al., 2015; Vanderpool et al., 2013). Three studies reported no control or comparison condition and were intervention-only study designs (Carman et al., 2015; Crosby et al., 2011; Vanderpool et al., 2015a). Two of these three studies were singular in level of focus (Carman et al., 2015; Crosby et al., 2011) as compared to one that was multilevel (Vanderpool et al., 2015a).
We examined articles for theoretical underpinnings and formative research leading to the intervention research reported in the included studies (data not shown). Chung et al. (2015) did not provide information on theory or formative research as part of intervention development (Chung et al., 2015). In seven of the studies, formative research building on the work of the authors and/or previously published research was reported to have informed intervention development (Brewer et al., 2017; Cates et al., 2018; Crosby et al., 2011, Kaul et al., 2019; Richman et al., 2016; Richman et al., 2019; Vanderpool et al., 2015a). The remaining seven studies reported or described theoretical underpinnings (Carman et al., 2015; Cates et al., 2011; Paskett et al., 2016; Szilagyi et al., 2015; Underwood et al., 2015; Vanderpool et al., 2013; Vogel et al., 2018). Most theories were individual-level health behavior theories, such as the health belief model (HBM) and theory of reasoned action (TRA). In Carman et al. (2015) and Paskett et al. (2016), organizational theory was applied (Carman et al., 2015; Paskett et al., 2016). Paskett et al. (2016) also included organizational theory with the HBM, TRA, and extended parallel process model (Paskett et al., 2016). In Vanderpool et al. (2013), diffusion of innovation coupled with theory of planned behavior was used (Vanderpool et al., 2013).
The target population and setting for the 15 studies included a rural focus. Seven studies were conducted in rural North Carolina or included regions of North Carolina considered rural (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Richman et al., 2019, 2016; Vogel et al., 2018). Of those in North Carolina, Brewer et al. (2017) was conducted in 29 healthcare clinics (Brewer et al., 2017); Cates et al. (2011) was in four rural counties (Cates et al., 2011); Cates et al. (2018) was in rural primary care practices (Cates et al., 2018); Chung et al. (2015) was in one rural county (Chung et al., 2015); Richman et al. (2016) was in the rural eastern part of the state in a university setting (Richman et al., 2016); Richman et al. (2019) in two rural healthcare clinics (Richman et al., 2019); and Vogel et al. (2018) was in a rural healthcare clinic (Vogel et al., 2018). Four studies were conducted in rural Kentucky, specifically the 54 counties designated as Appalachian (Carman et al., 2015; Crosby et al., 2011; Vanderpool et al., 2015a, 2013). Of those in Kentucky, Carman et al. (2015) was in rural local health departments (Carman et al., 2015); Crosby et al. (2011) was in rural healthcare clinics, a rural community college, and in an urban university health clinic (Crosby et al., 2011); Vanderpool et al. (2013) was conducted in eight rural counties in community settings (Vanderpool et al., 2013); and Vanderpool et al. (2015) was conducted in two rural high schools in southeastern Kentucky (Vanderpool et al., 2015a). One additional study was conducted in Appalachia; Paskett et al. (2016) was conducted in six Appalachian Ohio counties (Paskett et al., 2016). Additional rural settings included Texas (Kaul et al., 2019), New York (Szilagyi et al., 2015) and Georgia (Underwood et al., 2015). Although all studies included in the narrative review met the inclusion criterion of rural U.S. setting, we note none of the studies explicitly defined rural nor provided additional information on the rural setting beyond the ‘rural’ descriptor.
Study Results
Table 3 summarizes the primary and secondary outcomes, data sources, and selected results from the 15 included studies. In terms of primary and secondary outcomes, all 15 studies included receipt of an HPV vaccine dose(s), either initiation (first dose), continuation (second dose), and/or completion (third dose), as a primary outcome, . Primary outcomes were measured in terms of clinic-level data change over time or individual-level data (e.g., percentage, cumulative percentage, total number of doses) from a range of data sources (e.g., medical records, self-report, immunization registries).
Among the 12 studies using experimental designs, HPV vaccination outcomes were positive in eight studies (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Vanderpool et al., 2013; Vogel et al., 2018); intervention effects were not observed in four studies (Richman et al., 2019, 2016; Szilagyi et al., 2015; Underwood et al., 2015). Among the three studies not using experimental designs, two had positive HPV vaccination outcomes (Crosby et al., 2011; Vanderpool et al., 2015a), and one study showed no effect (Carman et al., 2015). Secondary outcomes included: number of vaccination reminders sent, assessment of organizational readiness, missed opportunities for HPV vaccination, educational campaign awareness, provider knowledge, provision of educational materials to parents and adolescents, and HPV vaccine beliefs, attitudes, and decision-making.
Variability in data source, measurement timing, and target age group existed across nine of the studies demonstrating statistically significant increases in HPV vaccination (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Crosby et al., 2011; Kaul et al., 2019; Paskett et al., 2016; Vanderpool et al., 2015a, 2013). Vogel et al. (2018) showed positive, but not statistically significant, results (Vogel et al., 2018). Brewer et al. (2017), Cates et al. (2011), Cates et al. (2018), and Chung et al. (2015) used a state immunization registry for HPV vaccination rates (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015). Brewer et al. (2017) used 11–12 and 13–17 as the two age groups with HPV vaccination assessed at baseline, 3 months, and 6 months (Brewer et al., 2017); Cates et al. (2011) calculated a monthly cumulative rate of HPV vaccination for girls aged 9–13 and 14–19 for one year (Cates et al., 2011); Cates et al. (2018) focused on HPV vaccination initiation and completion 9 months pre-intervention, 9 months during the intervention, and 9 months post-intervention for 11–12 year olds (Cates et al., 2018); and Chung et al. (2015) examined HPV vaccination rates at baseline, 6 months, and 12 months for 11–12 year olds and 13–18 year olds (Chung et al., 2015). Kaul et al. (2019) and Vanderpool et al. (2015a) relied on school medical records to assess HPV vaccination rates (Kaul et al., 2019; Vanderpool et al., 2015a). Kaul et al. (2019) examined rates among sixth to eighth grade students at baseline and then 12, 18, and 20 months (Kaul et al., 2019). Vanderpool et al. (2015a) monitored uptake and completion rates during one school year (Vanderpool et al., 2015a). Crosby et al. (2011) relied on voucher redemption to determine dose received by vaccine-eligible women aged 18–26 (Crosby et al., 2011). Paskett et al. (2016) used medical record data and parent self-report to measure HPV vaccine initiation 3 months post-intervention and completion 6 months post-intervention among girls aged 9–17 (Paskett et al., 2016). Vanderpool et al. (2013) examined medical records of women aged 18–26 up to 9 months after the initial dose was received (Vanderpool et al., 2013). Data issues were also observed throughout the included studies. There was difficulty in obtaining needed data, including missing, limited, or incomplete data (Carman et al., 2015; Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016) and challenges accessing data through immunization registries (Chung et al., 2015); issues due to validity of self-reported data were also noted (Crosby et al., 2011). In addition, there were differences in baseline HPV coverage between intervention arms (Brewer et al., 2017; Cates et al., 2018).
Barriers and Facilitators
Many of the included studies also noted barriers and facilitators to implementing HPV vaccination interventions among rural populations (Table 4). These are organized by major domains of study design and measurement, intervention characteristics, implementation processes, and HPV vaccination access. A range of barriers were reported, most reflecting implementation challenges. Facilitators showed more congruence in attributes of the intervention and implementation processes enhancing the study and observed outcomes. Additionally, there was heterogeneity across the studies related to specific facilitators. For example, several studies determined that incentives or providing the HPV vaccine at no cost was not enough to increase HPV vaccination rates among their target population (Chung et al., 2015; Crosby et al., 2011), while others found these strategies to be beneficial (Carman et al., 2015; Vanderpool et al., 2013; Vanderpool et al., 2015a). Studies also found different age groups to be more effective for targeting. Chung et al. (2015) found younger age groups to be more effective due to existing vaccination requirements (Chung et al., 2015) while Underwood et al. (2015) found high school-aged students to be a better target population (Underwood et al., 2015). In addition, females and those with private health insurance were found to have higher odds of completing the HPV vaccine series (Underwood et al., 2015).
Table 4.
Barriers and facilitators reported in included studies
| Author, year | Barriers | Facilitators | ||||||
|---|---|---|---|---|---|---|---|---|
| Study Design and Measurement | Intervention Characteristics | Implementation Processes | HPV Vaccination Access | Study Design and Measurement | Intervention Characteristics | Implementation Processes | HPV Vaccination Access | |
| Brewer NT, et al., 2017 (Brewer et al., 2017) |
|
|
|
|
||||
| Carman AL, et al., 2015 (Carman et al., 2015) |
|
|
|
|
||||
| Cates JR, et al., 2011 (Cates et al., 2011) |
|
|
|
|
|
|||
| Cates JR, et al., 2018 (Cates et al., 2018) |
|
|||||||
| Chung RJ, et al., 2015 (Chung et al., 2015) |
|
|
|
|||||
| Crosby RA, et al., 2011 (Crosby et al., 2011) |
|
|
|
|||||
| Kaul S, et al., 2019 (Kaul et al., 2019) |
|
|
|
|||||
| Paskett ED, et al., 2016 (Paskett et al., 2016) |
|
|
|
|
||||
| Richman AR, et al., 2016 (Richman et al., 2016) |
|
|
|
|||||
| Richman AR, et al., 2019 (Richman et al., 2019) |
|
|
|
|
||||
| Szilagyi PG, et al., 2015 (Szilagyi et al., 2015) |
|
|
|
|||||
| *Underwood NL, et al., 2015 (Underwood et al., 2015) |
|
|
|
|||||
| Vanderpool RC, et al., 2013 (Vanderpool et al., 2013) |
|
|
|
|||||
| Vanderpool RC, et al., 2015 (Vanderpool et al., 2015a) |
|
|
|
|
||||
| Vogel EP, et al., 2018 (Vogel et al., 2018) |
|
|
|
|||||
Synthesis
In terms of intervention effects on HPV vaccine outcomes, nine of the 15 included studies demonstrated a positive, statistically significant increase in HPV vaccination post-intervention (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Crosby et al., 2011; Kaul et al., 2019; Paskett et al., 2016; Vanderpool et al., 2015a, 2013). Among these nine studies, six included a focus on the clinical setting and/or healthcare providers (Brewer et al., 2017; Cates et al., 2011, 2018; Chung et al., 2015; Crosby et al., 2011; Paskett et al., 2016) and specifically on provider recommendation for HPV vaccination. The other three studies showing positive effects were in schools (n = 2) (Kaul et al., 2019; Vanderpool et al., 2015a) and the community (n = 1) (Vanderpool et al., 2013). Overall, the majority of interventions described herein proved successful in increasing HPV vaccination behaviors, overcoming different barriers and capitalizing on facilitators.
DISCUSSION
As compared to the broader HPV vaccination intervention literature (Francis et al., 2017; Fu et al., 2014; Jacobson et al., 2016; Niccolai and Hansen, 2015; Paul and Fabio, 2014; Smulian et al., 2016; Walling et al., 2016), there is a paucity of intervention studies based in rural U.S. settings. Thus, narrative review methodology was a more suitable approach as compared to other types of review. Due to the limited HPV vaccination intervention literature in rural U.S. communities, we are somewhat limited in our ability to draw conclusions and identify recommendations. However, the impetus for increasing HPV vaccination in rural settings to decrease HPV-associated cancer disparities is well documented and recognized on a national level (Blake et al., 2017; Implementation Science Working Group, n.d.; National Cancer Institute, 2019, 2018, 2016). Rural populations represent a key population in which to address low HPV vaccination uptake and prevention of HPV-associated cancers (Williams et al., 2020; Zahnd et al., 2019b, 2018). We used this premise as a means for discussing the implications of the narrative review findings.
Twelve of the 15 identified studies used experimental designs to test intervention conditions in comparison to control condition(s) (Brewer et al., 2017; Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Richman et al., 2019, 2016; Szilagyi et al., 2015; Underwood et al., 2015; Vanderpool et al., 2013; Vogel et al., 2018). The remaining three studies used intervention-only designs (Carman et al., 2015; Crosby et al., 2011; Vanderpool et al., 2015a). Of the 12 studies using experimental designs, five used comparison conditions (Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Vogel et al., 2018) as opposed to usual care or standard-of-care or no intervention control groups. To determine the most effective intervention approaches, studies employing experimental designs – with and without randomization – in rural settings are needed to more comprehensively ascertain how intervention approaches are and are not successful in overcoming barriers in rural settings. This may be particularly important for elucidating the best implementation strategies for intervention research in rural contexts. Experimental designs in rural settings may pose challenges that must be overcome through use of an expanded collection of study designs, such as the application of “n-of-1” designs, wait-list control designs, stepped-wedge designs, and sequential multiple assignment randomized trial (SMART) designs, to allow for effective comparison of intervention and control conditions. Research on increasing HPV vaccination in rural U.S. communities should include robust study designs to allow for a greater understanding of effective implementation approaches suitable for replication and scaling to other rural settings. In cases where experimental designs may not be suitable or appropriate, future research should report the rationale for choosing non-experimental designs to aid in understanding important contextual factors in rural communities.
Eight studies used multilevel intervention conditions – seven with an experimental design (Cates et al., 2018, 2011; Chung et al., 2015; Kaul et al., 2019; Paskett et al., 2016; Underwood et al., 2015; Vogel et al., 2018) and one with an intervention-only design (Vanderpool et al., 2015a). Multilevel interventions were most often conducted at the organizational/clinic-level, provider-level, and/or individual/parent-level. Previous research has shown multilevel approaches are more effective than singular level approaches in increasing HPV vaccination outcomes (National HPV Vaccination Roundtable, 2019; Rodriguez et al., 2020). Additionally, few studies employed multi-component interventions. Multi-component, multilevel interventions allow for implementation of myriad strategies to overcome barriers and enhance facilitators to HPV vaccination in rural settings. A recent scoping review determined that barriers and facilitators to HPV vaccination series uptake and completion in rural populations have been heavily documented at the individual level (e.g., caregiver knowledge, HPV vaccination beliefs), yet further research is needed on these factors at other levels of influence (Peterson et al., 2020). In the included studies, several barriers across multiple levels were reported. Use of multi-component, multilevel approaches require sophisticated evaluation plans to ensure measurement of key variables can result in identifying correlates of favorable and unfavorable outcomes. We found limited evidence of theory-guided interventions. Approximately half of the included studies referenced theoretical underpinnings in intervention design and even fewer in measurement and results. In combination with multi-component, multilevel interventions, theoretically-informed interventions and measures may result in more effectively managing barriers and capitalizing on facilitators.
Among the eight studies using multilevel interventions, the levels targeted were easily discernable aiding in interpretation of the approaches; however, in most cases, corresponding primary outcomes were at only one level as compared to each level at which intervention occurred. While multi-component, multilevel intervention approaches are recommended to increase HPV vaccination, particularly in rural settings, ensuring measurement across components and levels is needed in future research. For HPV vaccination intervention research, the desired behavioral change most appropriate to include is an HPV vaccination outcome, which was the case for all 15 studies in this review. However, greater consistency in measurement and reporting of HPV vaccination is needed. In the included studies, different ages (e.g., 9–13, 11–12, 13–17, 18–26 years), one or both sexes (e.g., females only, both females and males), data sources (e.g., medical chart, parent self-report, school nurse records, state immunization registries), time points (e.g., 3 6, 12, 18, 20 months), and values (e.g., initiation rate, completion rate, cumulative percentages, total number of doses, missed opportunities) were reported. The variability in measurement and reporting created challenges in comparing outcomes across studies demonstrating statistically significant results. According to the most recent HPV vaccine recommendations, for 9–14 year old youth, a 2-dose regimen is recommended with doses at 0 (day of first dose) and 6–12 months after the first dose, and for 15–45 year old individuals, a 3-dose regimen is recommended at 0, 1–2 months, and 6 months (Meites et al., 2019). Some included studies were published prior to the 2016 change to two doses for those who initiate before age 15. However, going forward, utilizing consistent time points and values, at a minimum, will be important for assessing comparability and discerning effective approaches to increase HPV vaccination outcomes. Such consistency may also allow for comparisons to NIS-TEEN data, for example. Consistent measurement and reporting across multiple levels and for multiple components will aid in understanding the most effective approaches to increase HPV vaccination in rural settings.
There has been ample discussion about the definition of rural in the U.S. (Gessert et al., 2015). This discussion extends beyond mere definitions and implies a need for greater measurement and description of rural context (Do et al., 2020; Zahnd et al., 2019a). National reports (American Hospital Association, 2019; Harvard T.H. Chan School of Public Health, 2018; National Quality Forum, 2018; U.S. Department of Health and Human Services, 2020) have identified numerous strengths of rural communities, such as willingness to focus on capacity building efforts building on existing resources and addressing disparate health indicators and outcomes (Appalachian Regional Commission, 2018), including lower HPV vaccination uptake and higher rates of HPV-associated cancers. Across the 15 included studies, no study explicitly defined rural and few provided contextual information on the rural setting. Perhaps authors assumed use of the descriptor ‘rural’ and/or the location (e.g., Appalachia) would convey the rural setting. Few articles included the rural locale as part of the discussion section. A clear basis for how and why a target population and setting is ‘rural’ would allow for future research to understand how a proposed rural setting may or may not align with previous research in rural communities. Providing a documented definition of rural will serve as a starting point for understanding HPV vaccination approaches in settings described as rural. More detailed reporting on contextual factors is needed as well. For example, we were unable to discern how intersectionality was considered in the studies. By this, we mean a rural, uninsured, racial or ethnic minority parent may face different challenges to HPV vaccination for a child as compared to a rural, insured, White parent. Reporting on contextual factors in rural communities, such as healthcare provider shortage areas, proximity to primary care/medical home, and availability of HPV vaccination access points (e.g., clinics, pharmacies, urgent care), will strengthen the ability to apply approaches in other rural communities.
To improve reporting of geographic definitions and contextual information, at a minimum, studies should report the geographic location(s) of the study using an existing rural-urban metric or classification code such as MSA / non-MSA, Rural-Urban Continuum Codes (RUCC), or Rural-Urban Commuting Areas (RUCA). Recently, the National Cancer Institute’s (NCI) Division of Cancer Control and Population Sciences has called on investigators to clearly define rural in their grant applications using RUCC, RUCA, or Frontier and Remote Area Codes (Kennedy et al., 2018; US Department of Health and Human Services, n.d.). Moreover, existing reporting guidelines aimed at promoting consistency in intervention reporting and replication and translating evidence into practice could promote more detailed geographic descriptions in their requirements. We reviewed the AIMD framework, Standards for Reporting Implementation Studies (StaRI) checklist, Template for Intervention Description and Replication (TIDieR), and Consolidated Standards of Reporting Trials (CONSORT) (Bragge et al., 2017; Pinnock et al., 2017; Hoffmann et al., 2014; Welch et al., 2017). Context (e.g., description of who, what, where, when, and how) is included as part of reporting, yet there is less explicit direction on providing context about the rural setting, rural-residing populations, and logistical and practical considerations of working in rural communities.
Notably, the geographic distribution of the study locations was limited as most were based in Kentucky and North Carolina. We propose additional research be conducted across the U.S. Midwest, Southwest, West, and the Delta and Blackbelt regions of the South to further explore the impact of interventional research on HPV vaccination outcomes in rural communities – particularly those with increased HPV-related disease burden – and among specific sub-populations (e.g., American Indians, African Americans, Hispanics) to assess cultural-specific aspects that may intersect with geography. There has been important HPV vaccination-related formative, survey, and descriptive research conducted in these regions (Boyd et al., 2018; Inguva et al., 2020; Kepka et al., 2011; Koskan et al., 2019; Luque et al., 2011; Newcomer et al., 2020; Vickers et al., 2019) indicating both the need and capacity for interventional studies focused on improving HPV vaccination outcomes.
In some regards, our findings are not unexpected. There has been a limited focus on cancer prevention and control, including HPV vaccination, in rural settings (Blake et al., 2015; Vanderpool et al., 2019). When examining NCI’s Evidence-Based Cancer Control Programs (National Cancer Institute, n.d.), only two of the six evidence-based interventions on HPV vaccination included a rural setting, one of which is included in our narrative review (Vanderpool et al., 2013) and one of which is identified as having a rural focus but with no rural content in the article itself (Perkins et al., 2015). The absence of federally-funded cancer research in rural settings (Blake et al., 2017; Weaver et al., 2020) has led to an increased focus on cancer prevention in rural settings (Implementation Science Working Group, n.d.; National Cancer Institute, 2019, 2018; Weaver et al., 2020), especially in light of documented disparities in cancer outcomes. National initiatives, such as the Cancer Moonshot Blue Ribbon Panel prioritization of evidence-based HPV vaccination interventions (National Cancer Institute, 2016) and NCI-designated cancer centers convening to prevent HPV-associated cancers through increased vaccination, offer a means to augment these activities in rural settings.
Study Limitations and Strengths
Our study has notable strengths. To our knowledge, this is the first study to review the existing literature on HPV vaccination interventions in rural U.S. settings. In addition, our search utilized a wide scope of articles from four major databases. All authors engaged in review of procedures at each stage of the identification, review, and analysis process to allow for multiple reviewers determining inclusion and assessing study characteristics. However, our study has limitations given the paucity of published articles on HPV vaccination interventions in rural settings. The inclusion criteria allowed for only 15 studies to be fully examined, thereby limiting our ability to characterize intervention approaches and examine HPV vaccination outcomes beyond providing description and a summary. Further, our review was limited to interventions conducted in the U.S. and without representation across rural communities in the U.S. There was an over-representation of studies from certain regions and with limited sub-populations, as noted previously. Some articles were excluded because of a lack of a discernable, clear focus on intervention efforts to increase HPV vaccination in rural areas. The exclusion may have derived from a lack of an explicit intervention design while focusing on HPV vaccination in rural areas; a lack of clarity about a focus on receipt of one or more doses of HPV vaccine as opposed to HPV vaccination intention in combination with other vaccines (e.g., influenza) even if situated in rural areas; and/or HPV vaccination knowledge rather than behavior.
CONCLUSION
Our narrative review revealed few HPV vaccination intervention studies have been conducted in rural U.S. settings given available published articles. This is concerning given the low uptake of HPV vaccination and high burden of HPV-associated disease in rural settings. During the COVID-19 pandemic, early evidence has shown a decrease in vaccination coverage, which could further impact existing HPV-related disparities (Bramer et al., 2020; Hoffman, 2020; Santoli et al., 2020). Future research is needed to expand on the literature of HPV vaccination in rural settings to identify evidence-based interventions and implementation approaches unique to rural communities. This includes a need for rigorous methods and experimental designs to compare interventions and components of interventions; theory-guided intervention development and related metrics; consistency in examining behavioral outcomes (i.e., initiation, continuation, and/or completion of HPV vaccination), age groups, and timeframes; and the effectiveness of multi-component, multilevel interventions in rural settings. We also identified a need to define ‘rural’ and ensure reporting of contextual factors to better understand study results and application to other rural settings. Due to the limited HPV vaccination intervention literature in rural U.S. communities, we are somewhat limited in our ability to draw conclusions. Yet the impetus for increasing HPV vaccination in rural settings is well documented and recognized on a national level.
HIGHLIGHTS.
This narrative review examined HPV vaccination interventions in rural settings.
Fifteen HPV vaccination intervention research studies in rural U.S. communities met the inclusion criteria from 2011–2019.
Interventions with clinics and/or healthcare providers had HPV vaccination improvements more often than other types.
Interventions in rural setting were limited when compared to the broader HPV vaccination intervention literature.
More HPV intervention research is needed in rural communities to address low HPV vaccination uptake.
ACKNOWLEDGMENTS:
We wish to thank Amy Edwards, health sciences librarian in University Libraries at the University of South Carolina, for her guidance and assistance.
FUNDING:
This research is the result of work conducted by two of the Cancer Prevention and Control Research Network (CPCRN) collaborating centers funded by the Centers for Disease Control and Prevention (CDC) from 20142019. The research was supported by the following cooperative agreements from the CDC’s Prevention Research Centers Program. The CDC was not involved in the research process or manuscript development and submission; the findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC.
University of Kentucky: Appalachian Center for Cancer Education, Screening and Support [U48DP00501401S2; PI: Vanderpool, 20142019]
University of South Carolina: Multi-Level, Community-Clinical Cancer Prevention and Control Interventions [U48 DP00500001S2; PI: Friedman, 20142019]
Dr. Robin Vanderpool was formerly with the University of Kentucky and principal investigator of the CPCRN collaborating center noted above; she is now employed by the National Cancer Institute. The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health or the National Cancer Institute.
Footnotes
CRediT AUTHOR STATEMENT:
Heather M. Brandt, PhD: Conceptualization, methodology, validation, formal analysis, writing – original draft, writing – review & editing, supervision. Robin C. Vanderpool, DrPH: Conceptualization, methodology, validation, formal analysis, writing – original draft, writing – review & editing, supervision. Meagan Pilar, MPH: Formal analysis, writing – original draft, writing – review & editing. Maria Zubizarreta, MSPH: Formal analysis, writing – original draft, writing – review & editing. Lindsay R. Stradtman, MPH: Formal analysis, writing – original draft, writing – review & editing.
The authors have no conflicts of interest to report.
REFERENCES
- American Hospital Association, 2019. Challenges facing rural communities and the roadmap to ensure local access to high-quality, affordable care. Chicago (IL). [Google Scholar]
- Appalachian Regional Commission, 2018. Exploring bright spots in Appalachian health: Case studies. Washington (DC). [Google Scholar]
- Barefoot KN, Warren JC, Smalley KB, 2017. Women’s health care: the experiences and behaviors of rural and urban lesbians in the USA. Rural Remote Health 17, 3875. 10.22605/rrh3875 [DOI] [PubMed] [Google Scholar]
- Bednarczyk RA, Whitehead JL, Stephenson R, 2017. Moving beyond sex: Assessing the impact of gender identity on human papillomavirus vaccine recommendations and uptake among a national sample of rural-residing LGBT young adults. Papillomavirus Res. 3, 121–125. 10.1016/j.pvr.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta MP, Phillips L, 2015. Human papillomavirus vaccine awareness, uptake, and parental and health care provider communication among 11- to 18-year-old adolescents in a rural Appalachian Ohio county in the United States. J. Rural Heal 31, 67–75. 10.1111/jrh.12079 [DOI] [PubMed] [Google Scholar]
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT, 2017. Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol. Biomarkers Prev 26, 992–997. 10.1158/1055-9965.EPI-17-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake KD, Ottenbacher AJ, Finney Rutten LJ, Grady MA, Kobrin SC, Jacobson RM, Hesse BW, 2015. Predictors of human papillomavirus awareness and knowledge in 2013: gaps and opportunities for targeted communication strategies. Am. J. Prev. Med 48, 402–410. https://do.org/10.1016/j.amepre.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd ED, Phillips JM, Schoenberger Y-MM, Simpson T, 2018. Barriers and facilitators to HPV vaccination among rural Alabama adolescents and their caregivers. Vaccine 36, 4126–4133. 10.1016/j.vaccine.2018.04.085 [DOI] [PubMed] [Google Scholar]
- Bragge P, Grimshaw JM, Lokker C, Colquhoun H, The AIMD Writing/Working Group, 2017. AIMD – a validated, simplified framework of interventions to promote and integrate evidence into health practices, systems, and policies. BMC Med. Res. Methodol 17. 10.1186/s12874-017-0314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, Shen AK, 2020. Decline in child vaccination coverage during the COVID-19 pandemic - Michigan care improvement registry, May 2016-May 2020. MMWR. Morb. Mortal. Wkly. Rep 69, 630–631. 10.15585/mmwr.mm6920e1 [DOI] [PubMed] [Google Scholar]
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C, 2017. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics 139. 10.1542/peds.2016-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman AL, McGladrey ML, Goodman Hoover A, Crosby RA, 2015. Organizational variation in implementation of an evidence-based human papillomavirus intervention. Am. J. Prev. Med 49, 301–308. 10.1016/j.amepre.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Cartmell KB, Young-Pierce J, McGue S, Alberg AJ, Luque JS, Zubizarreta M, Brandt HM, 2018. Barriers, facilitators, and potential strategies for increasing HPV vaccination: A statewide assessment to inform action. Papillomavirus Res. 5, 21–31. 10.1016/j.pvr.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BR, Crosby RA, Vanderpool RC, Dignan M, Bates W, 2013. Predictors of initial uptake of human papillomavirus vaccine uptake among rural Appalachian young women. J. Prim. Prev 34, 71–80. 10.1007/s10935-013-0295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates JR, Brewer NT, Fazekas KI, Mitchell CE, Smith JS, 2009. Racial differences in HPV knowledge, HPV vaccine acceptability, and related beliefs among rural, southern women. J. Rural Heal 25, 93–97. 10.1111/j.1748-0361.2009.00204.x [DOI] [PubMed] [Google Scholar]
- Cates JR, Crandell JL, Diehl SJ, Coyne-Beasley T, 2018. Immunization effects of a communication intervention to promote preteen HPV vaccination in primary care practices. Vaccine 36, 122–127. 10.1016/j.vaccine.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates JR, Ortiz R, Shafer A, Romocki LS, Coyne-Beasley T, 2012. Designing messages to motivate parents to get their preteenage sons vaccinated against human papillomavirus. Perspect. Sex. Reprod. Health 44, 39–47. 10.1363/4403912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates JR, Ortiz RR, North S, Martin A, Smith R, Coyne-Beasley T, 2015. Partnering with middle school students to design text messages about HPV vaccination. Health Promot. Pract 16, 244–255. 10.1177/1524839914551365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates JR, Shafer A, Diehl SJ, Deal AM, 2011. Evaluating a county-sponsored social marketing campaign to increase mothers’ initiation of HPV vaccine for their pre-teen daughters in a primarily rural area. Soc. Mar. Q 17, 4–26. 10.1080/15245004.2010.546943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2017b. About Rural Health https://www.cdc.gov/ruralhealth/about.html, Accessed date: 30 May 2020.
- Centers for Disease Control and Prevention, 2020. Cancers associated with human papillomavirus, United States – 2013 – 2017 https://www.cdc.gov/cancer/uscs/pdf/USCS-DataBrief-No18-September2020-h.pdf, Accessed date: 16 October 2020.
- Centers for Disease Control and Prevention, 2017a. Genital HPV Infection – CDC Fact Sheet https://www.cdc.gov/std/hpv/HPV-FS-print.pdf, Accessed date: 30 May 2020.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE, 2014. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis 41, 660–664. 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RJ, Walter EB, Kemper AR, Dayton A, 2015. Keen on teen vaccines: improvement of adolescent vaccine coverage in rural North Carolina. J. Adolesc. Heal 56, S14–6. 10.1016/j.jadohealth.2014.10.272 [DOI] [PubMed] [Google Scholar]
- Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR, 2011. Uptake of free HPV vaccination among young women: a comparison of rural versus urban rates. J. Rural Heal 27, 380–384. 10.1111/j.1748-0361.2010.00354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do EK, Rossi B, Miller CA, Ksinan AJ, Wheeler DC, Chukmaitov A, Cyrus JW, Fuemmeler BF, 2020. Area-level variation and human papillomavirus vaccination among adolescents and young adults in the United States: a systematic review. Cancer Epidemiol. Biomarkers Prev In Press. 10.1158/1055-9965.EPI-20-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Silva I, 1999. Chapter 5, Overview of study designs in: Cancer Epidemiology: Principles and Methods. International Agency for Research on Cancer, Lyon (Fr). [Google Scholar]
- Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, Fredua B, McNamara L, Stokley S, 2020. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2019. MMWR. Morb. Mortal. Wkly. Rep 69, 1109–1116. 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas KI, Brewer NT, Smith JS, 2008. HPV vaccine acceptability in a rural Southern area. J. Womens. Health 17, 539–548. 10.1089/jwh.2007.0489 [DOI] [PubMed] [Google Scholar]
- Francis DB, Cates JR, Wagner KPG, Zola T, Fitter JE, Coyne-Beasley T, 2017. Communication technologies to improve HPV vaccination initiation and completion: A systematic review. Patient Educ. Couns 100, 1280–1286. 10.1016/j.pec.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Fu LY, Bonhomme L-A, Cooper SC, Joseph JG, Zimet GD, 2014. Educational interventions to increase HPV vaccination acceptance: A systematic review. Vaccine 32, 1901–1920. 10.1016/j.vaccine.2014.01.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerend MA, Stephens YP, Kazmer MM, Slate EH, Reyes E, 2019. Predictors of human papillomavirus vaccine completion among low-income Latina/o adolescents. J. Adolesc. Heal 64, 753–762. 10.1016/j.jadohealth.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessert C, Waring S, Bailey-Davis L, Conway P, Roberts M, VanWormer J, 2015. Rural definition of health: A systematic literature review. BMC Public Health 15, 378. 10.1186/s12889-015-1658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BN, Johnson CD, Adams A, 2006. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J. Chiropr. Med 5, 101–117. 10.1016/S0899-3467(07)60142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AT, Denniss AR, 2018. An introduction to writing narrative and systematic reviews - tasks, tips and traps for aspiring authors. Heart. Lung Circ 27, 893–898. 10.1016/j.hlc.2018.03.027 [DOI] [PubMed] [Google Scholar]
- Harvard TH Chan School of Public Health, 2018. The health and economic concerns of rural Americans https://theforum.sph.harvard.edu/events/the-health-and-economic-concerns-of-rural-americans/, Accessed date: 31 May 2020.
- Head KJ, Vanderpool RC, Mills LA, 2013. Health care providers’ perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs. 30, 351–360. 10.1111/phn.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KA, Swiecki-Sikora AL, Stroup AM, Warner EL, Kepka D, 2017. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health 18, 19. 10.1186/s12889-017-4567-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y, 2018. Vaccination coverage among children aged 19–35 months - United States, 2017. MMWR. Morb. Mortal. Wkly. Rep 67, 1123–1128. 10.15585/mmwr.mm6740a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J, 2020. Vaccine rates drop dangerously as parents avoid doctor’s visits. New York Times. https://www.nytimes.com/2020/04/23/health/coronavirus-measles-vaccines.html, Accessed date: 1 June 2020. [Google Scholar]
- Hoffman T, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt J, Chan A, Michie S, 2014. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348, g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- Hughes MC, Baker TA, Kim H, Valdes EG, 2019. Health behaviors and related disparities of insured adults with a health care provider in the United States, 2015–2016. Prev. Med 120, 42–49. 10.1016/j.ypmed.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Implementation Science Working Group, n.d. Accelerating implementation of evidence-based cancer prevention and screening strategies. Bethesda (MD). [Google Scholar]
- Inguva S, Barnard M, Ward LM, Yang Y, Pittman E, Banahan BF 3rd, Kirby TR, Noble SL, 2020. Factors influencing human papillomavirus (HPV) vaccination series completion in Mississippi medicaid. Vaccine 8, 2051–2057. 10.1016/j.vaccine.2019.12.030 [DOI] [PubMed] [Google Scholar]
- Jacobson RM, Agunwamba AA, St Sauver JL, Finney Rutten LJ, 2016. The most effective and promising population health strategies to advance human papillomavirus vaccination. Expert Rev. Vaccines 15, 257–269. 10.1586/14760584.2016.1116947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Parker TS, Ahearn M, Mishra AK, Variyam JN, 2009. Health status and health care access of farm and rural populations, Rural America: Issues and Developments. Washington (DC). [Google Scholar]
- Kaul S, Do TQN, Hsu E, Schmeler KM, Montealegre JR, Rodriguez AM, 2019. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 8, 100189. 10.1016/j.pvr.2019.100189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AE, Vanderpool RC, Croyle RT, Srinivasan S, 2018. An overview of the National Cancer Institute’s initiatives to accelerate rural cancer control research. Cancer Epidemiol. Biomarkers Prev 27, 1240–1244. 10.1158/1055-9965.epi-18-0934 [DOI] [PubMed] [Google Scholar]
- Kepka D, Coronado GD, Rodriguez HP, Thompson B, 2011. Evaluation of a radionovela to promote HPV vaccine awareness and knowledge among Hispanic parents. J. Community Health 36, 957–965. 10.1007/s10900-011-9395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskan AM, Dominick LN, Helitzer DL, 2019. Rural caregivers’ willingness to community pharmacists to adminster the HPV vaccine to their age-eligible children. J. Cancer Educ. In press 10.1007/s13187-019-01617-z [DOI] [PubMed] [Google Scholar]
- Lai D, Ding Q, Bodson J, Warner EL, Kepka D, 2016. Factors associated with increased HPV vaccine use in rural-frontier U.S. states. Public Health Nurs. 33, 283–294. 10.1111/phn.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque JS, Mason M, Reyes-Garcia C, Hinojosa A, Meade CD, 2011. Salud es vida: development of a cervical cancer education curriculum for promotora outreach with Latina farmworkers in rural Southern Georgia. Am. J. Public Health 101, 2233–2235. 10.2105/AJPH.2011.300324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JM, Swerdlow DL, Khan F, Will O, Curry A, Snow V, Isturiz RE, Jodar L, 2019. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum. Vaccin. Immunother 15, 841–849. 10.1080/21645515.2018.1564434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites E, Kempe A, Markowitz LE, 2016. Use of a 2-dose schedule for human papillomavirus vaccination - Updated recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep 65, 1405–1408. 10.15585/mmwr.mm6549a5 [DOI] [PubMed] [Google Scholar]
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE, 2019. Human papillomavirus vaccination for adults: Updated recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep 68, 698–702. 10.15585/mmwr.mm6832a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzouk MD, Courtney P, Garrett-Albaugh S, Janoo J, Hobbs G, Vernon M, 2011. Knowledge of HPV in West Virginia high school health students and the effects of an educational tool. J. Pediatr. Adolesc. Gynecol 24, 278–281. 10.1016/j.jpag.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Mohammed KA, Subramaniam DS, Geneus CJ, Henderson ER, Dean CA, Subramaniam DP, Burroughs TE, 2018. Rural-urban differences in human papillomavirus knowledge and awareness among US adults. Prev. Med 109, 39–43. 10.1016/j.ypmed.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Moss JL, Gilkey MB, Rimer BK, Brewer NT, 2016. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum. Vaccin. Immunother 12, 1476–1483. 10.1080/21645515.2015.1128601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, 2019. HPV vaccine uptake in cancer centers https://healthcaredelivery.cancer.gov/hpvuptake/, Accessed date: 31 May 2020.
- National Cancer Institute, 2018. Rural cancer control https://cancercontrol.cancer.gov/research-emphasis/rural.html, Accessed date: 31 May 2020.
- National Cancer Institute, 2016. Cancer moonshot blue ribbon panel report 2016. Bethesda (MD). [Google Scholar]
- National Cancer Institute, n.d. Evidence-based cancer control programs (EBCCP): HPV vaccination evidence-based programs listing https://ebccp.cancercontrol.cancer.gov/topicPrograms.do?topicId=22626661&choice=default, Accessed date: 16 October 2020.
- National HPV Vaccination Roundtable, 2019. HPV vaccination: Improving clinical systems to increase HPV vaccine uptake http://hpvroundtable.org/wp-content/uploads/2019/08/Evidence-Summary-Clinical-System_Final.pdf, Accessed date: 31 May 2020.
- National Quality Forum, 2018. A core set of rural-relevant measures and measuring and improving access to care: 2018 recommendations from the MAP Rural Health Workgroup. Washington (DC). [Google Scholar]
- Newcomer SR, Caringi J, Jones B, Coyle E, Schehl T, Daley MF, 2020. A mixed-methods analysis of barriers to and facilitators of human papillomavirus vaccination among adolescents in Montana. Public Health Rep. In press. https://doi.org/10.1177%2F0033354920954512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolai LM, Hansen CE, 2015. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: A systematic review. JAMA Pediatr. 169, 686–692. 10.1001/jamapediatrics.2015.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskett ED, Krok-Schoen JL, Pennell ML, Tatum CM, Reiter PL, Peng J, Bernardo BM, Weier RC, Richardson MS, Katz ML, 2016. Results of a multilevel intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiol. Biomarkers Prev 10.1158/1055-9965.EPI-15-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, Fabio A, 2014. Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program. Vaccine 32, 320–326. 10.1016/j.vaccine.2013.11.070 [DOI] [PubMed] [Google Scholar]
- Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS, 2015. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 33, 1223–1229. 10.1016/j.vaccine.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Peterson CE, Silva A, Holt HK, Balanean A, Goben AH, Dykens A, 2020. Barriers and facilitators to HPV vaccine uptake among US rural populations: a scoping review. Cancer Causes Control 31, 801–814. 10.1007/s10552-020-01323-y [DOI] [PubMed] [Google Scholar]
- Pinnock H, Barwick M, Carpenter C, Eldridge S, Grandes G, Griffiths CJ, Rycoft-Malone J, Meissner P, Murray E, Patel A, Sheikh A, Taylor JC, StaRI Group, 2017. Standards for reporting implementation studies (StaRI) statement. BMJ 356, i6795. 10.1136/bmj.i6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter PL, Katz ML, Paskett ED, 2013. Correlates of HPV vaccination among adolescent females from Appalachia and reasons why their parents do not intend to vaccinate. Vaccine 31, 3121–3125. 10.1016/j.vaccine.2013.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter PL, Oldach BR, Randle KE, Katz ML, 2014. Acceptability of HPV vaccine for males and preferences for future education programs among Appalachian residents. Am. J. Mens. Health 8, 167–174. 10.1177/1557988313505319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman AR, Maddy L, Torres E, Goldberg EJ, 2016. A randomized intervention study to evaluate whether electronic messaging can increase human papillomavirus vaccine completion and knowledge among college students. J Am. Coll. Health 64, 269–278. 10.1080/07448481.2015.1117466 [DOI] [PubMed] [Google Scholar]
- Richman AR, Torres E, Wu Q, Carlston L, O’Rorke S, Moreno C, Olsson J, 2019. Text and email messaging for increasing human papillomavirus vaccine completion among uninsured or medicaid-insured adolescents in rural eastern North Carolina. J. Health Care Poor Underserved 30, 1499–1517. 10.1353/hpu.2019.0090 [DOI] [PubMed] [Google Scholar]
- Rodriguez SA, Mullen PD, Lopez DM, Savas LS, Fernández ME, 2020. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev. Med 131, 105968. 10.1016/j.ypmed.2019.105968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, Kang Y, Lucas P, Noblit C, Tropper J, Vogt T, Weintraub E, 2020. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR. Morb. Mortal. Wkly. Rep 69, 591–593. 10.15585/mmwr.mm6919e2 [DOI] [PubMed] [Google Scholar]
- Shafer A, Cates JR, Diehl SJ, Hartmann M, 2011. Asking mom: formative research for an HPV vaccine campaign targeting mothers of adolescent girls. J. Health Commun 16, 988–1005. 10.1080/10810730.2011.571343 [DOI] [PubMed] [Google Scholar]
- Shipman SA, Lan J, Chang C-H, Goodman DC, 2011. Geographic maldistribution of primary care for children. Pediatrics 127, 19–27. 10.1542/peds.2010-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PG, Morrow RH, Ross DA, (Ed.), 2015. Field trials of health interventions: A toolbox, in: Field Trials of Health Interventions: A Toolbox. Oxford OUP, Oxford (UK). [PubMed] [Google Scholar]
- Smulian EA, Mitchell KR, Stokley S, 2016. Interventions to increase HPV vaccination coverage: A systematic review. Hum. Vaccin. Immunother 12, 1566–1588. 10.1080/21645515.2015.1125055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber NR, Brewer NT, Smith JS, 2008. Influence of parent characteristics and disease outcome framing on HPV vaccine acceptability among rural, Southern women. Cancer Causes Control 19, 115–118. 10.1007/s10552-007-9074-9 [DOI] [PubMed] [Google Scholar]
- Spleen AM, Kluhsman BC, Clark AD, Dignan MB, Lengerich EJ, 2012. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, age 9–17 years, in Appalachian Pennsylvania. J. Cancer Educ 27, 312–319. 10.1007/s13187-011-0294-z [DOI] [PubMed] [Google Scholar]
- Szilagyi PG, Serwint JR, Humiston SG, Rand CM, Schaffer S, Vincelli P, Dhepyasuwan N, Blumkin A, Albertin C, Curtis CR, 2015. Effect of provider prompts on adolescent immunization rates: A randomized trial. Acad. Pediatr 15, 149–157. 10.1016/j.acap.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiggelaar SM, Rafalski M, Davidson MA, Hu Y, Burnett L, 2014. HPV knowledge and vaccine acceptability in Appalachian Tennessee and Kentucky, USA. J. Fam. Plan. Reprod. Heal. care 10.1136/jfprhc-2013-100697 [DOI] [PubMed] [Google Scholar]
- Underwood NL, Gargano LM, Jacobs S, Seib K, Morfaw C, Murray D, Hughes JM, Sales JM, 2016. Influence of sources of information and parental attitudes on human papillomavirus vaccine uptake among adolescents. J. Pediatr. Adolesc. Gynecol 29, 617–622. 10.1016/j.jpag.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Underwood NL, Weiss P, Gargano LM, Seib K, Rask KJ, Morfaw C, Murray D, DiClemente RJ, Hughes JM, Sales JM, 2015. Human papillomavirus vaccination among adolescents in Georgia. Hum. Vaccin. Immunother 11, 1703–1708. 10.1080/21645515.2015.1035848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2020. Rural action plan https://www.hhs.gov/sites/default/files/hhs-rural-action-plan.pdf, Accessed date: 25 September 2020.
- US Department of Health and Human Services, n.d. Social and behavioral intervention research to address modifiable risk factors for cancer in rural populations https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-20-051.html, Accessed date: 06 October 2020.
- Vanderpool RC, Cohen E, Crosby RA, Jones MG, Bates W, Casey BR, Collins T, 2013. “1–2-3 Pap” intervention improves HPV vaccine series completion among Appalachian women. J. Commun 63, 95–115. 10.1111/jcom.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool RC, Breheny PJ, Tiller PA, Huckelby CA, Edwards AD, Upchurch KD, Phillips CA, Weyman CF, 2015a. Implementation and evaluation of a school-based human papillomavirus vaccination program in rural Kentucky. Am. J. Prev. Med 49, 317–323. 10.1016/j.amepre.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Vanderpool RC, Dressler EVM, Stradtman LR, Crosby RA, 2015b. Fatalistic beliefs and completion of the HPV vaccination series among a sample of young Appalachian Kentucky women. J. Rural Heal 31, 199–205. 10.1111/jrh.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool RC, Stradtman LR, Brandt HM, 2019. Policy opportunities to increase HPV vaccination in rural communities. Hum. Vaccin. Immunother 15, 1527–1532. 10.1080/21645515.2018.1553475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielot NA, Butler AM, Brookhart MA, Becker-Dreps S, Smith JS, 2017. Patterns of use of human papillomavirus and other adolescent vaccines in the United States. J. Adolesc. Heal 61, 281–287. 10.1016/j.jadohealth.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M, Green CL, Lee HY, Pierce JY, Daniel CL, 2019. Factors associated with HPV vaccination uptake and HPV-associated cancers: a county-level analysis in the state of Alabama. J. Community Health 44, 1214–1223. 10.1007/s10900-019-00690-1 [DOI] [PubMed] [Google Scholar]
- Vogel NP, Appel SJ, Winker G, 2018. Improving HPV vaccination rates among young males in rural areas of the United States. Nurse Pract. 43, 1–6. 10.1097/01.NPR.0000527572.74477.a5 [DOI] [PubMed] [Google Scholar]
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S, 2019. National, regional, state, and selected local area vaccination coverage among adolescents Aged 13–17 Years - United States, 2018. MMWR. Morb. Mortal. Wkly. Rep 68, 718–723. 10.15585/mmwr.mm6833a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Mbaeyi SA, Fredua B, Stokley S, 2018. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years - United States, 2017. MMWR. Morb. Mortal. Wkly. Rep 67, 909–917. 10.15585/mmwr.mm6733a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling EB, Benzoni N, Dornfeld J, Bhandari R, Sisk BA, Garbutt J, Colditz G, 2016. Interventions to improve HPV vaccine uptake: A systematic review. Pediatrics 138, e20153863. 10.1542/peds.2015-3863 [DOI] [PubMed] [Google Scholar]
- Weaver SJ, Blake KD, Vanderpool RC, Gardner B, Croyle RT, Srinivasan S, 2020. Advancing rural cancer control research: National Cancer Institute efforts to identify gaps and opportunities. Cancer Epidemiol. Biomarkers Prev 29, 1515–1518. 10.1158/1055-9965.epi-20-0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch VA, Norheim OF, Jull J, Cookson R, Sommerfelt H, Tugwell P, CONSORT-Equity and Boston Equity Symposium, 2017. CONSORT-Equity 2017 extension and elaboration for better reporting of health equity in randomised trials. BMJ. 359, j5085. 10.1136/bmj.j5085 [DOI] [PubMed] [Google Scholar]
- Williams CL, Walker TY, Elam-Evans LD, Yankey D, Fredua B, Saraiya M, Stokley S, 2020. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey-Teen, 2016–2017. Hum. Vaccin. Immunother 16, 562–572. 10.1080/21645515.2019.1670036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnd WE, Fogleman AJ, Jenkins WD, 2018. Rural-urban disparities in stage of diagnosis among cancers with preventive opportunities. Am. J. Prev. Med 54, 688–698. 10.1016/j.amepre.2018.01.021 [DOI] [PubMed] [Google Scholar]
- Zahnd WE, Mueller-Luckey GS, Fogleman AJ, Jenkins WD, 2019a. Rurality and health in the United States: Do our measures and methods capture our intent? J. Health Care Poor Underserved 30, 70–79. 10.1353/hpu.2019.0008 [DOI] [PubMed] [Google Scholar]
- Zahnd WE, Rodriguez C, Jenkins WD, 2019b. Rural-urban differences in human papillomavirus-associated cancer trends and rates. J. Rural Heal 35, 208–215. 10.1111/jrh.12305 [DOI] [PubMed] [Google Scholar]


