Abstract
Background:
Earlier studies have described the neural markers of apathy in Alzheimer’s disease (AD) and mild cognitive impairment (MCI), but few focused on the motivation circuits. Here, we targeted hypothalamus, a hub of the motivation circuit.
Objective:
To examine hypothalamic resting state functional connectivity (rsFC) in relation to apathy.
Methods:
We performed whole-brain regression of hypothalamic rsFC against Apathy Evaluation Scale (AES) total score and behavioral, cognitive, and emotional subscores in 29 patients with AD/MCI and 28 healthy controls (HC), controlling for age, sex, education, cognitive status, and depression. We evaluated the results at a corrected threshold and employed path analyses to assess possible interaction between hypothalamic rsFCs, apathy and depression/memory. Finally, we re-examined the findings in a subsample of amyloid-β-verified AD.
Results:
AES total score correlated negatively with hypothalamic precuneus (PCu)/posterior cingulate cortex (PCC) and positively with left middle temporal gyrus (MTG) and supramarginal gyrus rsFCs. Behavioral subscore correlated negatively with hypothalamic PCu/PCC and positively with middle frontal gyrus rsFC. Cognitive subscore correlated positively with hypothalamic MTGrsFC. Emotional subscore correlated negatively with hypothalamic calcarine cortex rsFC. Inpathanalyses, hypothalamic-PCu/PCC rsFC negatively modulated apathy and, in turn, depression. The model where hypothalamic MTG rsFC and memory independently modulated apathy also showed a good fit. The findings of diminished hypothalamic-PCu/PCC rsFC in relation to apathy and, in turn, depression were confirmed in amyloid-verified AD.
Conclusion:
The findings together support a role of altered hypothalamic connectivity in relation to apathy and depression, and modulation of apathy by memory dysfunction.
Keywords: Alzheimer’s disease, apathy, depression, hypothalamus, memory, resting state functional connectivity
INTRODUCTION
Apathy or reduced goal-directed behavior is a common neuropsychiatric symptom with prevalence ranging from 11–45% and 19–88% in mild cognitive impairment (MCI) and Alzheimer’s disease (AD), respectively [1]. Apathy elevates dementia risk in individuals with MCI and predicts more severe cognitive decline, reduced quality of life, and higher morbidity in those with AD [2]. Earlier studies implicated meso-corticolimbic and nigrostriatal dopaminergic dysfunction in AD apathy [3–6]. However, mounting evidence from anatomical, physiological, and behavioral studies has identified key roles of parallel, prefrontal cortical-basal ganglia circuits in motivated behavior [3, 7, 8]. Other studies characterized metabolic and structural changes in the anterior cingulate cortex, other prefrontal cortical structures, basal ganglia, lateral parietal, and temporal cortex [1, 9] as well as functional brain changes during emotional processing [10, 11] in association with apathy.
Resting state functional connectivity (rsFC) describes low-frequency blood-oxygenation level dependent signals that are correlated between functionally related brain regions in the absence of task demand or external stimulation [12]. Such patterns of brain activity are instrumental to understanding brain function and dysfunction in health and illness. In AD research, for instance, rsFCs could predict corresponding regional tau protein levels as revealed by positron emission tomography imaging [13]. Another study reported that the rsFCs of the default mode network (DMN) moderated the association of amyloid-β(Aβ) pathology with cognitive dysfunction in AD [14]. Further, studies have identified rsFC correlates of apathy in AD in the default mode, executive, and salience networks with varying patterns of findings across studies [15–17].
Importantly, manifested as a reduction of goal-directed behavior, apathy represents a dys-motivational syndrome [18]. The hypothalamus is best known for its role in supporting survival-related goal behavior [19]. However, imaging studies in humans have demonstrated hypothalamic function more broadly to meet cognitive, affective, and motivational challenges [20–29], in accord with its widespread brain connectivity [19, 30, 31]. The hypothalamus-pituitary axis provides afferent inputs to the thalamic mediodorsal nucleus, a hub of the cortical-striatal-thalamic-cortical circuit that mediates a wide array of cognitive and affective processes [32]. Injection of the neurotoxin, 6-hydroxydopamine, in the medial forebrain bundles of the lateral hypothalamus induced symptoms of akinetic mutism, a form of profound cognitive-motor apathy [33]. Further, global dysfunction of the hypothalamic-pituitary-gonadal/adrenal (HPA) axes has often been described in the pathophysiology of AD [34, 35]. Studies of AD have noted a 10–12% decrease in the volume [34] along with the accumulation of amyloid plaques and neurofibrillary tangles [36], in accord with postmortem reports of loss of neurons [37, 38], in the hypothalamus. In another study, hypothalamic suprachiasmatic nucleus showed a sharp decrease in volume and total cell count in AD compared to non-AD controls [39]. Further, in an acute rat (oAβ25-35) model of AD, investigators have noted long-lasting activation of HPA axis and associated changes in glucocorticoid and mineralocorticoid receptors in the hypothalamus [40]. These structural, ultrastructural changes of the hypothalamus may disrupt its communication with rest of the brain and its physiological functions. For instance, an earlier study reported reduced hypothalamus-temporal cortical functional connectivity in AD patients with versus without depression, a comorbidity of apathy [41]. Hypothalamus rsFC with the subgenual cingulate cortex was reduced in patients with major depressive disorder, as compared to healthy subjects [42]. While HPA-axis dysregulation and related structural/functional changes are well studied in AD and other neurodegenerative disorders [43], no studies have examined how hypothalamic circuits may be involved in the manifestation of apathy in AD, in contrast with healthy aging.
Here, in a cross-sectional study, we investigated hypothalamic rsFCs in relation to apathy in individuals with AD/MCI and healthy controls (HC). We hypothesized greater severity of apathy in AD/MCI as well as differential hypothalamic rsFC correlates of apathy in AD/MCI and HC. As it remains unclear whether the neural processes of apathy would differ categorically or in a spectrum across AD/MCI cases and controls, we performed two sets of analyses. First, we performed seed-based voxel-wise analyses in multiple regression, accounting for the influences of age, sex, education, depression, and cognitive ability, and examined the rsFC correlates of apathy across the entire sample of AD/MCI cases and controls. Next, we assessed whether the strength of these rsFCs correlated with apathy score/subscore differently between AD/MCI and HC. Second, we performed whole-brain regressions in a subsample of Aβ-verified AD and matched HC to verify the findings. Finally, as apathy and depression overlap considerably in the pathophysiology of AD [44] and in cognitively normal individuals [45], we investigated the inter-relationship between hypothalamic rsFCs, apathy, and depression with path analyses.
METHODS
Participants and assessments
Twenty-nine participants with AD dementia (n = 25)/amnestic MCI (aMCI) (n = 4) and 28 HC participants, matched in age, sex, and years of education (Table 1), were recruited from Yale Alzheimer’s Disease Research Center and the greater New Haven area. Participants with AD met the criteria for probable dementia because of AD according to the National Institute of Aging-Alzheimer’s Association [46]: Clinical Dementia Rating (CDR) score of 0.5 to 1.0; Mini-Mental State Examination (MMSE) score of 16 to 26 (inclusive); impaired episodic memory as evidenced by a Logical Memory II (LMII, Delayed Paragraph Recall) score of 1.5 SDs less than an education-adjusted norm:≤8 for 16 or more years of education;≤4 for 8–15 years of education;≤2 for 0–7 years of education. Amyloid pathology was confirmed by positron emission tomography imaging in 23 of the 25 AD participants [47]. Participants with aMCI met the criteria for amnestic MCI [48]: CDR score of 0.5; MMSE score of 24 to 30 (inclusive); impaired episodic memory as evidenced by LMII score of 1.5 SDs less than an education-adjusted norm. HC participants had a CDR score of 0, MMSE score > 26, and normal education adjusted LMII score. Note that all participants were also assessed for LMI (immediate paragraph recall). The exclusion criteria for all participants included current or history of diagnosis of psychotic or substance (except nicotine) use disorders; current diagnosis of major depressive disorder according to DSM-5 criteria or as assessed by the Beck Depression Inventory-II (BDI, score ≥ 29); current use of any psychoactive drugs; significant current medical condition including other neurological, cardiovascular, endocrine, renal, hepatic or thyroid pathology; history of brain injury with loss of consciousness or of a cerebrovascular event, including transient ischemic attack; MRI contraindications; pregnant or nursing women. Written informed consent was obtained prior to the study in accordance with institutional guidelines and a protocol approved by the Yale Human Investigation Committee.
Table 1.
Demographic and clinical characteristics of HC and AD/MCI participants
| HC (n = 28) | AD/MCI (n = 29) | t/χ2/z-, p | |
|---|---|---|---|
|
| |||
| Age (y)a | 70.6 ± 4.9 | 73.4 ± 6.7 | 1.81, 0.075 |
| Sex (male/female)b | 13/15 | 14/15 | 0.16, 0.689 |
| Education (y)a | 17.1 ± 2.0 | 16.2 ± 2.3 | 1.61, 0.113 |
| CDR | 0 | 0.5 (n = 4) | – |
| 1.0 (n = 22) | |||
| 2.0 (n = 3) | |||
| MMSEa | 29.8 ± 0.4 | 22.4 ± 6.3 | 6.15,<0.001* |
| LMI immediate)c | 22.3 ± 4.9 | 8.6 ± 5.6 | 5.97,<0.001* |
| LMII (delayed)c | 19.6 ± 5.8 | 2.0 ± 4.5 | 6.28,<0.001* |
| BDI-IIc | 4.1 ± 4.6 | 8.2 ± 6.1 | 2.77, 0.006* |
| AES totalc | 24.9 ± 5.8 | 31.0 ± 12.9 | 1.97, 0.049* |
| AES cognitivec | 11.1 ± 2.8 | 13.5 ± 6.0 | 1.54, 0.126 |
| AES behavioralc | 6.7 ± 1.6 | 8.9 ± 3.6 | 2.51, 0.011* |
| AES emotionalc | 3.0 ± 1.1 | 3.2 ± 1.7 | 0.27, 0.816 |
p < 0.05
two-sample t-test
χ2-test
two-sample Wilcoxon rank-sum (Mann-Whitney)test
MMSE, Mini-Mental State Examination; LMI, logical memory immediate; LMII, logical memory delayed; BDI, Beck Depression Inventory; AES, Apathy Evaluation Scale.
All participants completed self-rated Apathy Evaluation Scale (AES-S) [49], a questionnaire of 18 items to assess behavioral (five items), cognitive (eight items), emotional (two items), and the other (three items) aspects of apathy. The items are scored on 4-point Likert scale and the score ranges from 18 to 72, with a higher score indicating more severe apathy [50, 51]. Participants were also assessed with BDI-II [52], a self-reported measure of depression that consists of 21 items with each item referencing a symptom (e.g., sadness) and related responses (generally four to six evaluative statements in the order of increasing severity) to reflect the severity of the symptom over the past 2 weeks. The total BDI-II score ranges from 0 to 63, with a higher score indicating greater severity of depression [53].
Imaging protocol, data preprocessing, and statistical analyses
Participants were scanned on a 3-Tesla Siemens Trio TIM with a 32-channel head coil. Conventional T1-weighted spin echo sagittal anatomical images were collected for slice localization. Anatomical images for functional slice localization were acquired using spin echo imaging in the axial plane parallel to the AC–PC line with TR = 2530 ms, TE = 3.66 ms, bandwidth = 181 Hz/pixel, flip angle = 7°, field of view = 250×250 mm, matrix = 256×256, 176 slices with slice thickness = 1 mm in isotropic voxels and no gap. Resting state blood oxygen level-dependent (BOLD) signals with participants in eyes-closed condition, were then acquired with a single-shot gradient echo echoplanar imaging sequence in 51 axial slices parallel to the AC–PC line covering the whole brain using TR = 1,000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210×210 mm, matrix = 84×84, slice thickness = 2.5 mm without gap, and total acquisition time = 10 m.
Data were analyzed with Statistical Parametric Mapping (SPM12). We followed and applied published preprocessing pipeline to functional images after first discarding the images of the initial five TRs so only BOLD signals in steady state equilibrium were included in analyses [54, 55]. Next, the functional images of each subject were slice time corrected for the temporal offset and then were motion corrected (realigned). Realignment produced a mean functional image which was co-registered with high resolution structural image and segmented for normalization with affine registration followed by nonlinear transformation. The estimated normalization parameters were subsequently applied to the corresponding functional image volumes for each subject. Next, the functional images were normalized to Montreal Neurological Institute (MNI) space with resampled voxel size of 2.5×2.5×2.5 mm3 and smoothed with a Gaussian kernel of 6-mm FWHM.
Nuisance signals unlikely to reflect neural activity were removed using linear regression by including the six motion parameters from realignment, signal from whole brain, ventricular system, white matter, and their first-order derivatives [56–60]. Next, functional images were checked for micro-head motion (> 0.1 mm) as this may lead to spurious correlations in rsFC analysis, followed by “scrubbing” to remove time points affected by head motions, as successfully applied in previous studies using the thresholds of FD(t) > 0.5 mm [56, 61] or DVARS(t) > 75 [62]. Before computing the correlation maps to estimate rsFC, we applied a temporal band-pass filter (0.009 Hz<f<0.08 Hz) to the time course to obtain low-frequency fluctuations [58–60, 63].
We employed the hypothalamus mask from the WFU Pick-Atlas [64] as the seed, according to a previous study [65]. The correlation coefficients r’s between the averaged time course of the hypothalamus seed and time courses of all other brain voxels were computed for each participant. Next, the correlation maps were converted into z-score maps by Fisher’s Z transform: z = 0.5loge [1 + r/1 − r]. In group analyses we assessed the correlations between hypothalamus rsFC and apathy across participants, using multiple regression of hypothalamus rsFCs each against AES total score and individual subscores with age, sex, education, MMSE, and BDI-II scores as covariates. We evaluated the results at cluster p < 0.05 family-wise error (FWE)-corrected with a cluster-forming voxel p < 0.001, uncorrected [56, 65]. Clusters were identified in MNI coordinates and mean hypothalamus-cluster rsFCs were extracted for further statistical analyses and graphical presentation. In particular, as these rsFC correlates were identified from the entire sample, to assess how these correlates might differ between AD/MCI and HC, we compared AD/MCI and HC in the regression of hypothalamus-regional rsFCs (mean values across all voxels in individual clusters) on apathy scores with slope tests [66].
In a separate set of analyses, we examined the hypothalamus rsFC correlates in a subsample of 23 AD cases verified with amyloid scans and 22 matched HCs separately. As the smaller sample would come with reduced statistical power, we evaluated the voxel-wise findings at the statistical threshold of cluster p < 0.05 FWE-corrected with a cluster-forming voxel p < 0.005, uncorrected [67]. We described the main findings and showed the detailed results in the Supplementary Material.
Statistical analysis of clinical data
Clinical data were analyzed using two-sample t-test, two-sample Wilcoxon rank-sum (Mann-Whitney) test, or χ2 -test, depending upon the nature and distribution of the variable, to estimate group differences between HC and AD/MCI. We performed Pearson’s partial correlation (i.e., with covariates) to assess the correlation between variables and evaluated the findings at two-sided p < 0.05. Likewise, in separate analyses we examined the differences between amyloid-verified AD and HC and presented these findings in the Supplementary Material.
Path analysis
With path analysis based on structural equation modelling, we assessed inter-relationship between hypothalamus connectivity, apathy (AES-total), depression (BDI-II), and cognition (LMI score; see Results). Path analysis is a hypothesis-driven multivariate tool based on a structural model representing hypothesized “causal” relationships among variables [68]. It accounts for measurement error and simultaneously estimates the linear causal relationships among variables. The strength of each connection/path (e.g., A → B) is specified by a path coefficient or the β weight, which is estimated while the influences of other variables are kept constant. Standardized path coefficients convey assumptions about the directionality of interactions between variables. A good model fit is typically assessed with indices including χ2 p > 0.05, root mean square error of approximation (RMSEA)≤0.08, standardized root mean squared residual (SRMR)≤0.08, and comparative fit index (CFI)≥0.90 [69, 70].
RESULTS
Clinical characteristics
AD/MCI and HC participants were comparable in age, sex composition, and education. AD/MCI relative to HC showed lower MMSE and logical memory test scores and higher BDI-II score, AES total score, and AES behavioral but not cognitive and emotional subscores (Table 1).
Correlation between AES scores and clinical variables
Across all (HC+AD/MCI) participants, AES total score and cognitive and behavioral subscores correlated negatively with MMSE and LMI as well as positively with BDI-II scores (Table 2). AES emotional subscore correlated with BDI-II but not with MMSE or LMI/LMII score. LMII correlated negatively with behavioral but not with other apathy subscores (Table 2).
Table 2.
Correlation of AES apathy scores with clinical variables across all participants
| MMSE | LMI | LMII | BDI-II | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| r | p | r | p | r | p | r | P | |
|
| ||||||||
| AES Total | −0.52 | <0.001* | −0.33 | 0.016* | −0.19 | 0.166 | 0.64 | <0.001* |
| Cognitive | −0.50 | <0.001* | −0.33 | 0.013* | −0.17 | 0.218 | 0.61 | <0.001* |
| Behavioral | −0.53 | <0.001* | −0.37 | 0.006* | −0.29 | 0.035* | 0.61 | <0.001* |
| Emotional | −0.21 | 0.129 | 0.02 | 0.895 | 0.08 | 0.556 | 0.54 | <0.001* |
r, Pearson’s partial correlation coefficient adjusted for age, sex, and education
p < 0.05
MMSE, Mini-Mental State Examination; LMI, logical memory immediate; LMII, logical memory delayed; BDI: Beck Depression Inventory; AES: Apathy Evaluation Scale.
In the analyses separately for AD/MCI, and HC, apathy total score and behavioral and cognitive subscores correlated negatively with MMSE and all apathy scores correlated positively with BDI II in AD/MCI but not in HC. The correlations between apathy (except emotional subscore) and LMI scores were significant in HC alone. The correlations between apathy and LMII scores were mostly not significant for either group. These results are shown in Supplementary Table 1.
Apathy and hypothalamus resting state functional connectivity (hypothalamus rsFC)
Whole-brain multiple regressions in all participants (HC+AD/MCI) showed a negative correlation between AES total score and hypothalamus rsFC with bilateral precuneus (PCu)/posterior cingulate cortex (PCC). AES total score also correlated positively with hypothalamus rsFC with the left middle temporal gyrus (MTG) and left supramarginal gyrus (SMG) (Fig. 1A). We extracted the mean rsFC for all three regions of interest (ROIs), and post-hoc regression analyses in HC and AD/MCI separately showed significant correlations for all three regions in AD/MCI (rPCu/PCC = −0.64; rMTG = 0.63, rSMG = 0.69; ps ≤ 0.001). In HC, the correlation for HT-PCu/PCC rsFC (rPCu/PCC = −0.61, p = 0.002) but not MTG or SMG rsFCs (rMTG = 0.38, rSMG = 0.20; ps > 0.076) was significant. Slope tests showed significant differences in the regression slope between HC and AD/MCI for HT-SMG rsFC but not for HT rsFC with the other ROIs (Fig. 1B).
Fig. 1.
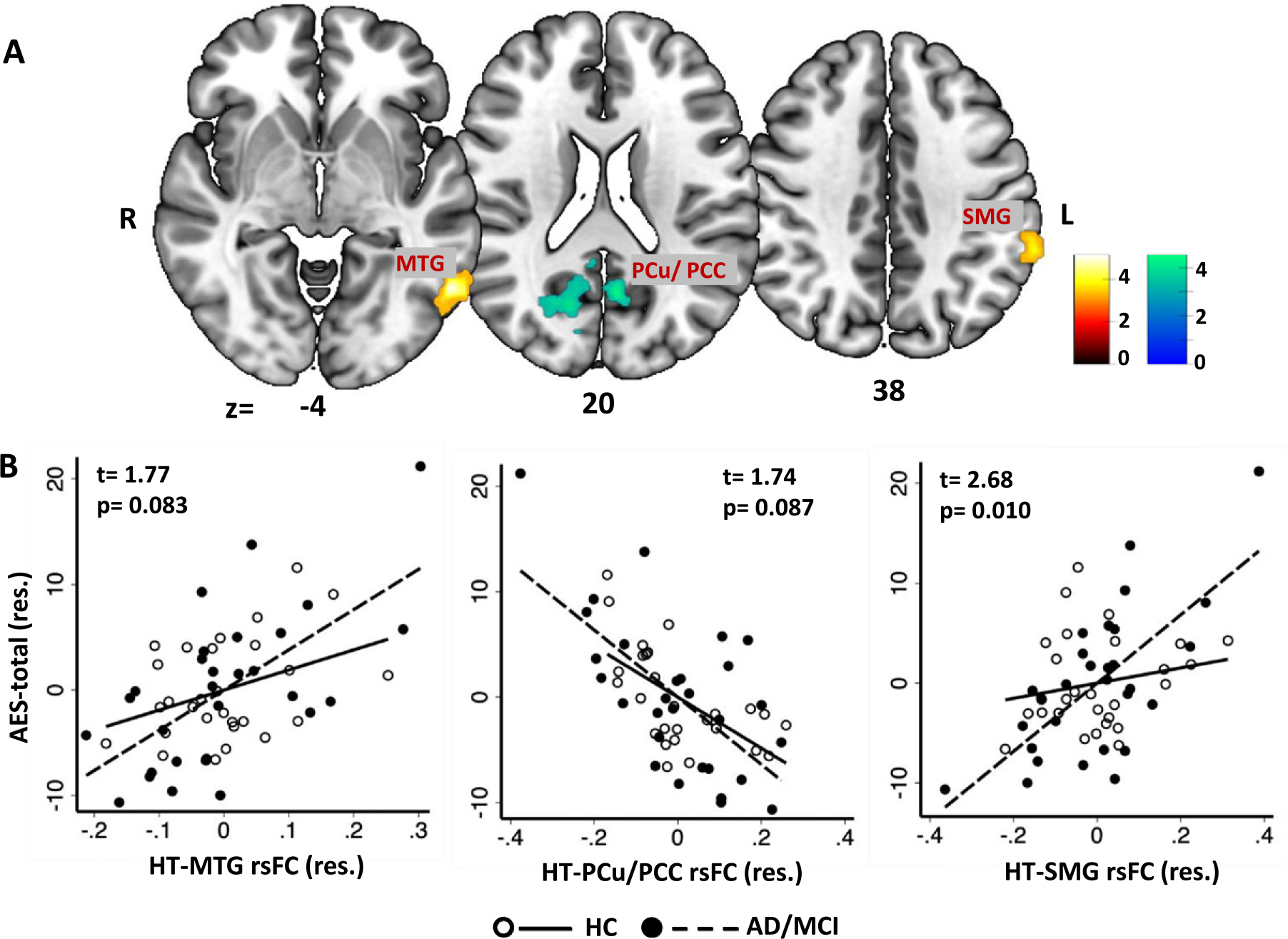
Whole-brain multiple regression of hypothalamus (HT) resting state functional connectivity (rsFC) with (A) AES-total score in all (HC and AD/MCI) sample. Warm and winter color bars represent T-values of positive and negative correlations, respectively. B) Scatterplot of AES-total and HT rsFCs (β) with regression line shown separately for HC and AD/MCI. Note that the scatterplots show values in residuals (res.) and the t- and p-values show slope test between HC and AD-MCI for the corresponding correlations. For all regressions, we controlled for age, sex, education, MMSE, and BDI-II. MTG, middle temporal gyrus; PCu, precuneus; PCC, posterior cingulate cortex; SMG, supramarginal gyrus.
We also examined hypothalamic rsFC correlates of AES subscores (Fig. 2A). AES behavioral subscore correlated positively with HT-right middle frontal gyrus (MFG) and negatively with HT-bilateral PCu/PCC rsFC. These correlations were significant in both HC and AD/MCI in post-hoc regressions (AD/MCI: rPCu/PCC = −0.68, rMFG = 0.68, ps < 0.001; HC: rPCu/PCC = −0.45, rMFG = 0.50, ps < 0.05). However, slope test showed significant difference in the regressions, with stronger correlations in AD/MCI versus HC (Fig. 2B). The cognitive subscore correlated positively with HT-left MTG rsFC. In post-hoc regressions, the correlation was significant in AD/MCI (r = 0.56, p = 0.005) but not in HC (r = 0.38, p = 0.073). However, the regression slopes did not significantly differ between HC and AD/MCI (Fig. 2C). AES emotional subscore correlated negatively with HT-left calcarine cortex rsFC. This correlation was significant both in AD/MCI (r = −0.61, p = 0.001) and in HC (r = −0.41, p = 0.049); the slopes did not differ significantly between AD/MCI and HC (Fig. 2D).
Fig. 2.
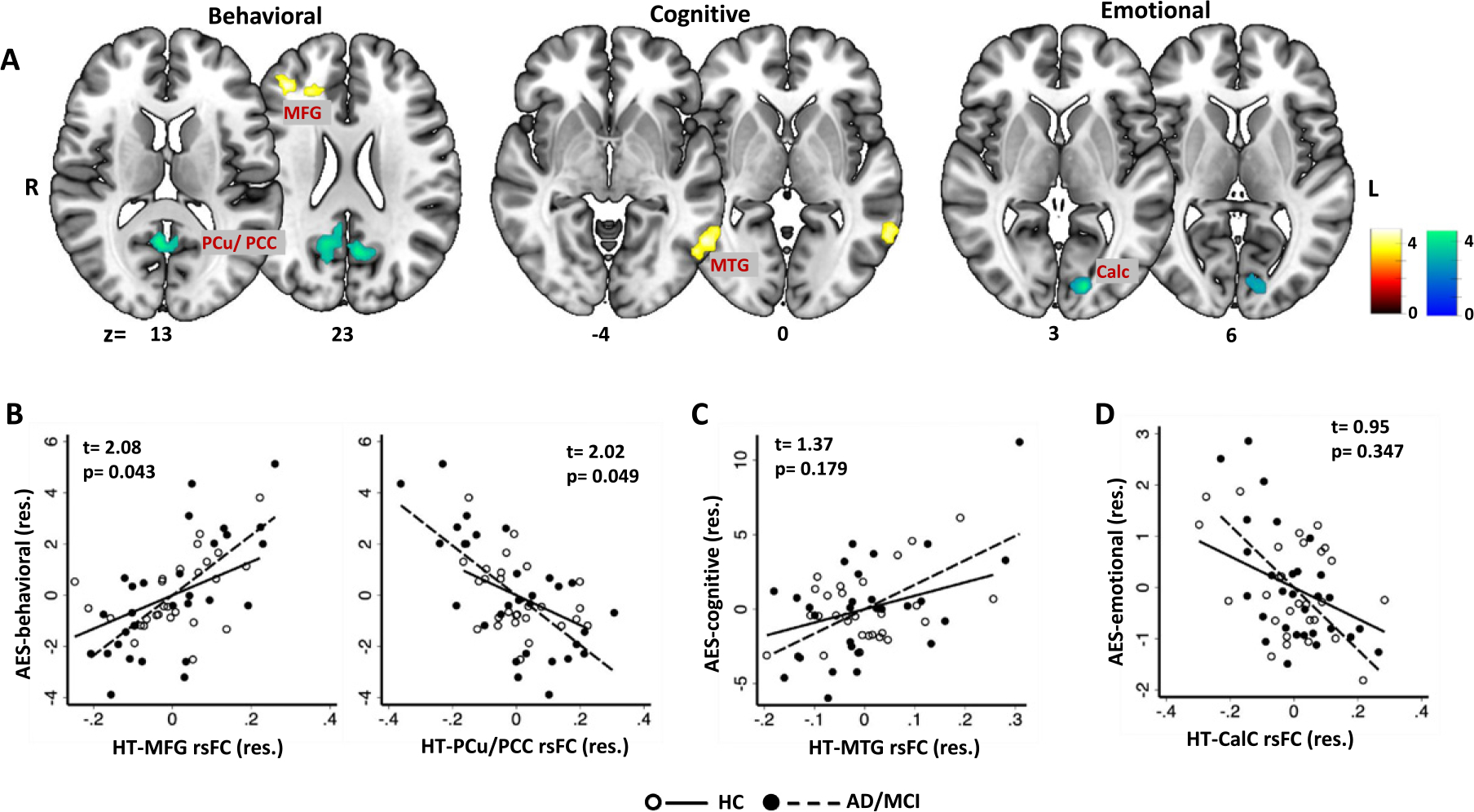
Whole-brain multiple regression of hypothalamus (HT) resting state functional connectivity (rsFC) with (A) AES behavioral, cognitive, and emotional subscores across all subjects. Color bars indicate voxel T values (warm/cool: positive/negative correlation). MFG, middle frontal gyrus; MTG, middle temporal gyrus; PCu, Precuneus; PCC, posterior cingulate cortex; Calc, calcarine cortex. The bottom row shows the scatterplots of AES (B) behavioral, (C) cognitive, and (D) emotional sub-scores versus extracted HT rsFCs (β) with regression lines shown separately for HC and AD/MCI. Note that the scatterplot represents values in residuals (res.) and the t- and p-values are of the slope tests of HC versus AD/MCI for the corresponding correlations. For all analyses, we controlled for age, sex, education, MMSE, and BDI-II.
For all of these regional hypothalamic rsFCs, we compared the mean HT rsFCs between AD/MCI and HC, with age, sex, education, MMSE, and BDI-II scores as covariates. The results showed that HT-left MTG rsFC was significantly reduced (t = 3.75, p < 0.001) in AD/MCI versus HC. No significant group differences were noted for other HT rsFCs (Supplementary Figure 1).
Path analysis
As shown in Table 2, apathy (AES total score) correlated significantly with depression (BDI-II score) and with memory (LMI score). Thus, we conducted path analyses to assess their inter-relationships, each in six models: A1-6 and B1-6 (Supplementary Figure 2). As both MFG and MTG connect with PCu/PCC [71, 72], we hypothesized a link between hypothalamic (HT)-MFG/MTG rsFCs and HT-PCu/PCC rsFC, with paths HT-MTG rsFC→HT-PCu/PCC rsFC←HT-MFG rsFC as well as HT-MTG rsFC←HT-PCu/PCC rsFC→HT-MFG rsFC considered in different models. The model fit indices are shown in Supplementary Tables 2 and 3.
Of models A1-6, model A1 and A5, where individual HT rsFCs served as exogenous variables, showed a good fit. Both HT-MTG and HT-MFG rsFCs negatively modulated HT-PCu/PCC rsFC, which in turn negatively modulated AES total score (Fig. 3A). In addition, HT-MTG but not HT-MFG rsFC showed a significant direct positive modulation of AES total score. The modulation by HT-SMG and CalC rsFCs were not significant. Model A5, with path from BDI-II to AES total score, also showed a good fit (Fig.3B). Thus, across models A1 and A5, AES and BDI-II scores showed mutual, positive modulation.
Fig. 3.
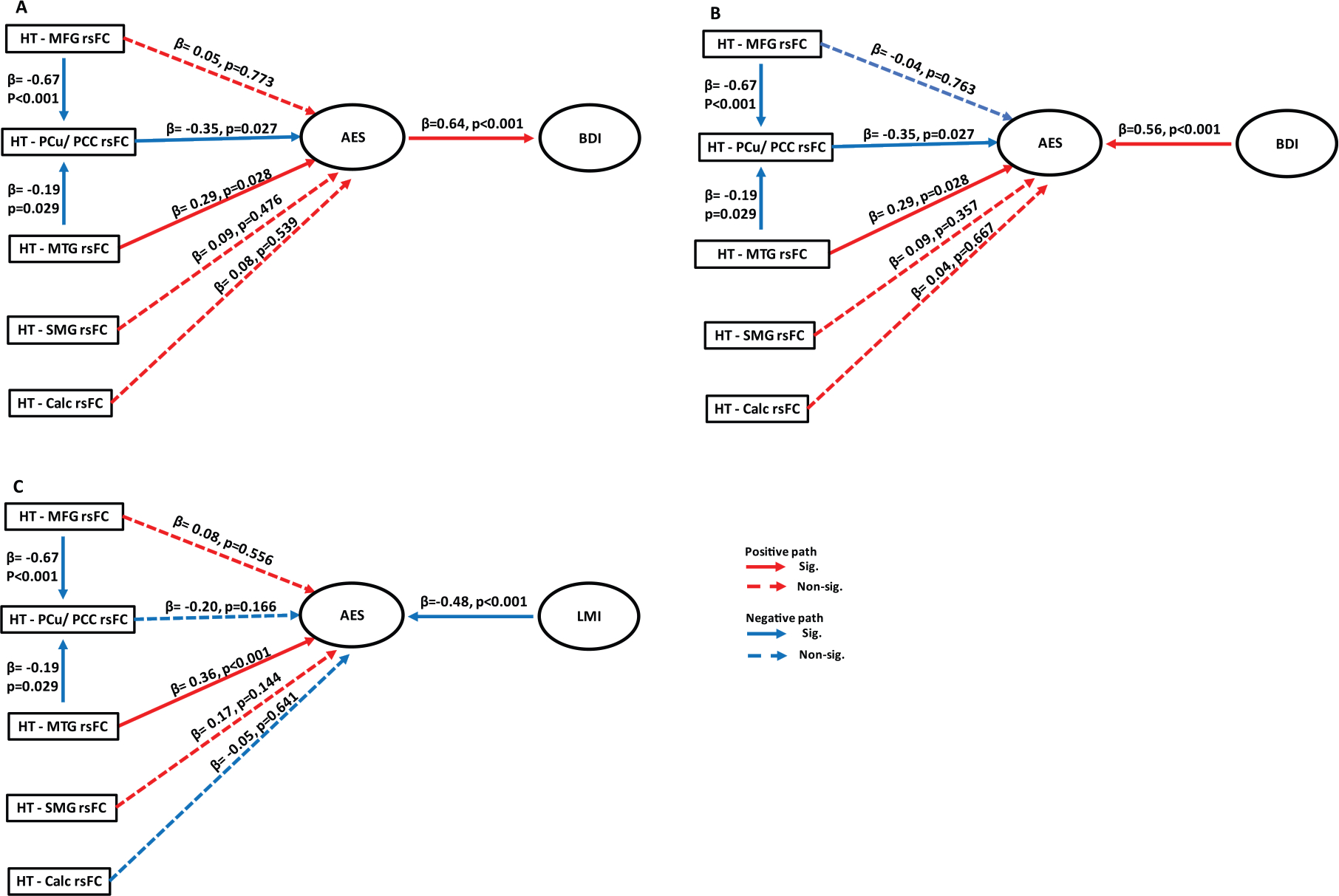
Models showing a good fit in path analyses. Hypothalamus (HT)-middle frontal gyrus (MFG) rsFC and HT-middle temporal gyrus (MTG) rsFC both negatively modulated HT-precuneus (PCu)/posterior cingulate cortex (PCC) rsFC which (A) negatively affected AES score and, in turn, BDI-II score; and (B) negatively modulated AES score, with BDI-II independently facilitating apathy. In both models, HT-MTG rsFC also significantly and positively modulated AES score. C) In the model of HT rsFC, apathy and memory, HT-PCu/PCC did not significantly affect AES score. HT-MTG rsFC positively and LMI score negatively modulated apathy. The β (path) coefficients and p-values were obtained from regression analyses using maximum likelihood method. The solid and dashed line each represents a significant and non-significant path coefficient.
Models B1-6 were analogous to A1-6 with BDI-II replaced with LMI score. Here, only model B5 showed a good fit, with negative and positive modulation of AES total by HT-MTG rsFC and LMI score (Fig. 3C). The model where AES total score modulated LMI score was not significant.
Findings in Aβ-verified AD and matched HC
We repeated the analyses in a reduced sample of 23 amyloid positive AD and 22 HCs with comparable age, sex, and education. The demographic and clinical characteristics (Supplementary Table 4) as well as clinical correlates of apathy (Supplementary Table 5) appeared similar across the original and reduced samples, except that the AES emotional score did not correlate significantly with LMII score in amyloid positive AD.
In multiple regression against apathy score, HT rsFC with a cluster in the left PCu/PCC showed a significant, negative correlation with the AES total score in AD. In post-hoc analyses, the negative correlation was significantly stronger in AD (r = −0.76, p < 0.001) than in HC (r = −0.53, p = 0.030) in a slope test (t = 2.43, p = 0.020). In HC, AES total showed a significant, positive correlation with HT rsFC with the rostral anterior cingulate cortex (rACC). In post-hoc analysis, this correlation was significant in HC, as expected (r = 0.82,p < 0.001), but not in AD(r = 0.42, p = 0.082), but the two regressions did not significantly differ in slope (t = 0.81, p = 0.424). These results are shown in Supplementary Figure 3.
Of the subscores, AES-behavioral subscore showed a negative correlation with HT-PCu/PCC rsFC in AD. In post-hoc analysis, HT-PCu/PCC rsFC correlation with the subscore was significant in AD (r = −0.81, p < 0.001), as expected, but not in HC (r = −0.30, p = 0.240). AES cognitive subscore correlated positively with HT-left SFG and left SPL rsFC in AD. post-hoc analyses showed significant association in AD (rSFG = 0.82, rSPL = 0.74, ps < 0.001), but not in HC (rSFG = −0.37, p = 0.138; rSPL = −0.40, p = 0.108). AES emotional subscore showed a negative correlation with HT-right CalC/Cuneus (Cun) rsFC in both groups. Thus, in post-hoc analysis, significant association was noted both in AD (r = − 0.84, p < 0.001) and HC (r = −0.91, p < 0.001). In HC, cognitive subscore correlated positively with HT-rACC rsFC and negatively with HT-left angular gyrus (AG)/PCC. post-hoc, the correlation was significant in HC (rrACC = 0.93, p < 0.001, rAG/PCC = −0.92, p < 0.001), but not in AD (rrACC = 0.44, p = 0.066, rAG/PCC = −0.25, p = 0.322). These results as well as the corresponding slope tests are shown in Supplementary Figure 4. Overall, the correlations of AES-total score and AES-behavioral subscore with HT-PCu/PCC rsFC shown for the original sample of AD/MCI and HC was replicated in amyloid-verified AD.
We thus focused on HT-PCu/PCC rsFC and tested the inter-relationships between HT-PCu/PCC rsFC, apathy, and depression in amyloid-verified AD with mediation analyses. The model HT-PCu/PCC rsFC → AES-total score → BDI-II score showed a significant and complete mediation, again replicating the findings of the original sample. The results of mediation analyses are shown in Supplementary Figure 5.
DISCUSSION
Apathy was more severe in AD/MCI relative to HC, with the group differences most prominently demonstrated in behavioral apathy. Across all subjects, the overall cognitive status and memory correlated negatively with the severity of cognitive and behavioral, but not emotional apathy, in accord with previous reports [73, 74]. Apathy was associated with distinct hypothalamic (HT) rsFCs, with HT rsFC with the SMG and MFG showing a significantly stronger positive correlation with the AES total score and behavioral subscore, respectively, and HT rsFC with the PCu/PCC showing a significantly stronger negative correlation with the behavioral subscore in AD/MCI as compared to HC. Path analyses showed that these rsFCs contributed to apathy and, in turn, depression, although the model where HT rsFCs and depression independently contributed to apathy was also significant. In contrast, in the analyses to assess the inter-relationships between HT rsFCs, apathy, and memory, only the model where HT rsFCs and memory contributed to apathy showed a significant fit. In the subsample, the correlations of HT-PCu/PCC rsFCs with AES-total/behavioral score were also significant and stronger in amyloid-positive AD than in matched HC. Mediation analyses likewise supported significant mediation by apathy of the relationship between HT-PCu/PCC rsFC and depression. Together, these findings highlighted HT rsFCs in association with apathy and depression across the spectrum of elderly adults and those with MCI and AD, as well as more specifically in Aβ-verified AD. We highlighted the main findings in the discussion below.
Apathy and hypothalamic rsFCs
The severity of overall and subdomains of apathy was associated with both distinct and shared HT rsFCs. HT-PCu/PCC rsFC correlated negatively with AES-total/behavioral scores across AD/MCI and HC, as well as independently in amyloid-positive AD and HC. However, the correlation was more significant in AD relative to HC. PCu/PCC is a hub of the DMN [75], a network of brain regions implicated in self-awareness and reflection [76]. Impaired ability to imagine and project one’s future mirror a reduction in motivation towards goal-directed behavior, as evidenced in behavioral apathy [77]. Precuneus pathology, including gray matter atrophy, cortical thinning, hypometabolism, decreased white matter integrity, and increased Aβ deposition, were noted in AD [78] as well as in specific association with AD depression and anxiety, a common comorbidity of apathy [79].
Emotional apathy correlated negatively with HT-CalC connectivity across MCI/AD (left hemisphere) and HC as well as in Aβ-verified AD alone (right hemisphere). The calcarine cortex responds to visual attention and imagery [80]. It processes salient and behaviorally relevant visual stimuli. For instance, the visual cortex partakes in processing visual emotional images of both positive and negative valence in social contexts [81, 82]. Consistent with present findings, apathy correlated with occipital cortical hypoperfusion in patients with MCI [83]. Further, atrophy [84] and excessive tau accumulation [85] in the occipital cortex were noted in AD pathology and in association with the severity of neuropsychiatric symptoms in AD patients [79].
We also observed positive correlation between HT-MFG/SMG rsFC with AES-total and HT-MTG rsFC with AES-cognitive subscore across MCI/AD and HC. The SMG and MFG is each part of the ventral attention and fronto-parietal executive control network central to attentional monitoring and effort-based decision making [8]. Previous studies have associated apathy with disruption of MFG circuit activities in various neuropsychiatric conditions [86] and MFG rsFC in individuals with MCI [17] as well as in cognitively normal older adults [87], broadly consistent with the current findings. Along with the hippocampal complex, the MTG is involved in episodic memory formation [88] and cued attention and working memory [41]. The MTG also responds to anticipation and receipt of reward [89–91]. Notably, the association between HT-MTG rsFC and AES cognitive subscore was significant in AD/MCI but not in HC, with lower HT-MTG rsFC in AD/MCI as compared to HC, consistent with an earlier report of reduction of HT-MTG rsFC in AD patients with depression [41]. Thus, despite an overall reduced connectivity in AD/MCI, cognitive apathy relates to higher HT-MTG connectivity. A post-hoc explanation is that higher HT-MTG connectivity may reflect functionally compensatory processes in link with apathy [92]. Notably, the correlation of HT-MTG rsFC with AES-cognitive subscore was not observed in the subsample of Aβ-verified AD, suggesting that individuals with more severe dementia may no longer be able to sustain the compensatory process.
The inter-relationship of hypothalamic rsFC, apathy, and depression/memory
Although apathy and depression represent separate diagnostic entities [93], studies have reported apathy as a risk factor for depression in AD. In a study of 150 AD patients, apathy was present in 19% of the sample, of which 62% also showed depression, whereas only 28% of depressed patients had comorbid apathy [94]. In a 4-year longitudinal study, baseline apathy predicted risk for depression in AD [95]. Here, depression correlated with all subdomains of apathy in AD/MCI and with cognitive/behavioral apathy in HC. In path analysis to assess the inter-relationships between HT connectivity, apathy, and depression, we observed negative modulation of HT-MFG/MTG rsFC on HT-PCu/PCC rsFC, which in turn negatively modulated apathy. PCu/PCC is a hub of the DMN [75] and MFG and MTG both project to PCu/PCC [71, 72]. In accord, the modulation of HT-MFG/MTG on HT-PCu/PCC rsFC was significant. However, the modulation was negative and a higher HT-MFG/MTG connectivity was associated with reduced HT-PCu/PCC connectivity, consistent with the PCu/PCC and MFG/MTG each belonging to the functionally antagonistic DMN and executive control network [96,97]. Apathy and depression were frequently comorbid, and, in accord, path analyses supported mutual modulation of apathy and depression. Mediation analysis in Aβ-verified AD alone showed similar interaction between HT-PCu/PCC rsFC, apathy and depression.
In path models that included memory deficits, HT-MTG rsFC and memory performance each positively and negatively modulated apathy, whereas the model where apathy modulated memory was not significant. Of note, other HT-regional rsFCs did not significantly modulated apathy, suggesting a unique role of the MTG in manifesting memory dysfunction and its impact on apathy across the spectrum of healthy aging, MCI, and AD. As discussed earlier, higher HT-MTG connectivity may reflect functionally compensatory processes in link with apathy. Thus, one is tempted to speculate that, as AD and apathy escalates in severity, memory dysfunction may no longer contributes significantly to apathy. These findings together suggest reciprocal influences of apathy and depression and a unidirectional effect of memory on apathy across elderly, MCI, and AD. As dementia grows in severity and takes hold, the decreases in HT-PCu/PCC rsFC becomes a dominant neural feature of apathy.
Limitations and conclusions
The study is limited by small sample size and a cross-sectional design. The findings would thus need to be replicated and it remains to be seen whether hypothalamic rsFCs may predict changes in apathy. Secondly, we did not evaluate dysfunction in executive control and decision making, which may be disproportionally impacted by apathy [98, 99]. More studies should assess the impact of hypothalamic rsFCs on apathy and, in turn, these specific domains of cognitive deficits. Finally, the findings of path and mediation analyses suggest but do not indicate causal relationship between the connectivity markers and clinical variables. Longitudinal and/or treatment studies to investigate whether and how apathy and hypothalamic rsFC are inter-related temporally would provide stronger evidence for causal links. In addition, a good model fit may indicate over-fitting of the data and thus limit generalizability of the findings. Due to the small sample, we could not perform cross validation of the findings.
In conclusion, focusing on the hypothalamus, a hub of the motivation circuit, we demonstrated altered hypothalamic functional connectivity with the precuneus/ posterior cingulate cortex in association with apathy, which may contribute to depression across cognitively normal older adults and those with AD/MCI as well as in amyloid-verified AD alone.
Supplementary Material
ACKNOWLEDGMENTS
The current study is supported by NIH grants R21AG067024 (Li), R01AG072893 (Li), R01CA218502 (Chao), and P30AG066508 (van Dyck) as well as a VA Merit Award CX001301 (Chao). The NIH and VA are otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish these findings.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0708r2).
SUPPLEMENTARYMATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220708.
REFERENCES
- [1].Mortby ME, Adler L, Agüera-Ortiz L, Bateman DR, Brodaty H, Cantillon M, Geda YE, Ismail Z, Lanctôt KL, Marshall GA, Padala PR, Politis A, Rosenberg PB, Siarkos K, Sultzer DL, Theleritis C, ISTAART NPS PIA (2022) Apathy as a treatment target in Alzheimer’s disease: Implications for clinical trials. Am J Geriatr Psychiatry 30, 119–147. [DOI] [PubMed] [Google Scholar]
- [2].Nobis L, Husain M (2018) Apathy in Alzheimer’s disease. Curr Opin Behav Sci 22, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Levy R, Dubois B (2006) Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 16, 916–28. [DOI] [PubMed] [Google Scholar]
- [4].David R, Koulibaly M, Benoit M, Garcia R, Caci H, Darcourt J, Robert P (2008) Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases A SPECT study with partial volume effect correction. Clin Neurol Neurosurg 110, 19–24. [DOI] [PubMed] [Google Scholar]
- [5].Udo N, Hashimoto N, Toyonaga T, Isoyama T, Oyanagi Y, Narita H, Shiga T, Nakagawa S, Kusumi I (2020) Apathy in Alzheimer’s disease correlates with the dopamine transporter level in the caudate nuclei. Dement Geriatr Cogn Dis Extra 10, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Starkstein SE, Merello M, Brockman S, Bruce D, Petracca G, Power BD (2009) Apathy predicts more severe parkinsonism in Alzheimer’s disease. Am J Geriatr Psychiatry 17, 291–298. [DOI] [PubMed] [Google Scholar]
- [7].van Dyck CH, Arnsten AFT, Padala PR, Brawman-Mintzer O, Lerner AJ, Porsteinsson AP, Scherer RW, Levey AI, Herrmann N, Jamil N, Mintzer JE, Lanctôt KL, Rosenberg PB (2021) Neurobiologic rationale for treatment of apathy in Alzheimer’s disease with methylphenidate. Am J Geriatr Psychiatry 29, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Walton ME, Bannerman DM, Rushworth MFS (2002) The role of rat medial frontal cortex in effort-based decision making. J Neurosci 22, 10996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaudhary S, Zhornitsky S, Chao HH, van Dyck CH, Li C-SR (2022) Cerebral volumetric correlates of apathy in Alzheimer’s disease and cognitively normal older adults: Meta-analysis, label-based review, and study of an independent cohort. J Alzheimers Dis 85, 1251–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D (2007) A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biol Psychiatry 62, 1388–95. [DOI] [PubMed] [Google Scholar]
- [11].Zhao H, Tang W, Xu X, Zhao Z, Huang L (2014) Functional magnetic resonance imaging study of apathy in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 26, 134–141. [DOI] [PubMed] [Google Scholar]
- [12].Biswal BB (2015) Resting-state functional connectivity. In Brain Mapping, Toga AW, ed. Academic Press, Waltham, pp. 581–585. [Google Scholar]
- [13].Franzmeier N, Rubinski A, Neitzel J, Kim Y, Damm A, Na DL, Kim HJ, Lyoo CH, Cho H, Finsterwalder S, Duering M, Seo SW, Ewers M (2019) Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain 142, 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koch K, Myers NE, Göttler J, Pasquini L, Grimmer T, Förster S, Manoliu A, Neitzel J, Kurz A, Förstl H, Riedl V, Wohlschläger AM, Drzezga A, Sorg C (2015) Disrupted intrinsic networks link amyloid-β pathology and impaired cognition in prodromal Alzheimer’s disease. Cereb Cortex 25, 4678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Büyükgök D, Bayraktaroǧlu Z, Buker HS, Kulaksızoǧlu MIB, Gurvit İH (2020) Resting-state fMRI analysis in apathetic Alzheimer’s disease. Diagn Interv Radiol 26, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Altunkaya S, Huang S-M, Hsu Y-H, Yang J-J, Lin C-Y, Kuo L-W, Tu M-C (2022) Dissociable functional brain networks associated with apathy in subcortical ischemic vascular disease and Alzheimer’s disease. Front Aging Neurosci 13, 717037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Joo SH, Lee CU, Lim HK (2017) Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr Dis Treat 13, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marin RS (1991) Apathy: A neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 3, 243–254. [DOI] [PubMed] [Google Scholar]
- [19].Sternson SM (2013) Hypothalamic survival circuits: Blueprints for purposive behaviors. Neuron 77, 810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murayama K, Matsumoto M, Izuma K, Sugiura A, Ryan RM, Deci EL, Matsumoto K (2015) How self-determined choice facilitates performance: A key role of the ventromedial prefrontal cortex. Cereb Cortex 25, 1241–1251. [DOI] [PubMed] [Google Scholar]
- [21].Murayama K, Matsumoto M, Izuma K, Matsumoto K (2010) Neural basis of the undermining effect of monetary reward on intrinsic motivation. Proc Natl Acad Sci U S A 107, 20911–20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J (2014) Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology 47, 31–42. [DOI] [PubMed] [Google Scholar]
- [23].Pfabigan DM, Seidel E-M, Sladky R, Hahn A, Paul K, Grahl A, Küblböck M, Kraus C, Hummer A, Kranz GS, Windischberger C, Lanzenberger R, Lamm C (2014) P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: An EEG and fMRI experiment. Neuroimage 96, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE (2006) Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron 50, 507–517. [DOI] [PubMed] [Google Scholar]
- [25].Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS (2009) fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 118, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vorobyev V, Kwon MS, Moe D, Parkkola R, Hämäläinen H (2015) Risk-taking behavior in a computerized driving task: Brain activation correlates of decision-making, outcome, and peer influence in male adolescents. PLoS One 10, e0129516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farrell MJ, Trevaks D, Taylor NAS, McAllen RM (2015) Regional brain responses associated with thermogenic and psychogenic sweating events in humans. J Neurophysiol 114, 2578–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peters AC, Blechert J, Sämann PG, Eidner I, Czisch M, Spoormaker VI (2014) One night of partial sleep deprivation affects habituation of hypothalamus and skin conductance responses. J Neurophysiol 112, 1267–1276. [DOI] [PubMed] [Google Scholar]
- [29].Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R (2008) Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. Neuroimage 42, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73, 759–768. [DOI] [PubMed] [Google Scholar]
- [31].Lecea L de, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R (2006) Addiction and arousal: Alternative roles of hypothalamic peptides. J Neurosci 26, 10372–10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weitzner MA, Kanfer S, Booth-Jones M (2005) Apathy and pituitary disease: It has nothing to do with depression. J Neuropsychiatry Clin Neurosci 17, 159–166. [DOI] [PubMed] [Google Scholar]
- [33].Moretti R, Signori R (2016) Neural correlates for apathy: Frontal-prefrontal and parietal cortical- subcortical circuits. Front Aging Neurosci 8, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vercruysse P,Vieau D,Blum D,Petersén Å, Dupuis L(2018) Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front Mol Neurosci 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Canet G, Hernandez C, Zussy C, Chevallier N, Desrumaux C, Givalois L (2019) Is AD a stress-related disorder? Focus on the HPA axis and its promising therapeutic targets. Front Aging Neurosci 11, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishii M, Iadecola C (2015) Metabolic and non-cognitive manifestations of Alzheimer’s disease: The hypothalamus as both culprit and target of pathology. Cell Metab 22, 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG (2014) The hypothalamus in Alzheimer’s disease: A Golgi and electron microscope study. Am J Alzheimers Dis Other Demen 30, 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A (2008) Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain 131, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goudsmit E, Hofman MA, Fliers E, Swaab DF (1990) The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer’s disease. Neurobiol Aging 11, 529–536. [DOI] [PubMed] [Google Scholar]
- [40].Brureau A, Zussy C, Delair B, Ogier C, Ixart G, Maurice T, Givalois L (2013) Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiol Aging 34, 1426–1439. [DOI] [PubMed] [Google Scholar]
- [41].Liu X, Chen W, Tu Y, Hou H, Huang X, Chen X, Guo Z, Bai G, Chen W (2018) The abnormal functional connectivity between the hypothalamus and the temporal gyrus underlying depression in Alzheimer’s disease patients. Front Aging Neurosci 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sudheimer K, Keller J, Gomez R, Tennakoon L, Reiss A, Garrett A, Kenna H, O’Hara R, Schatzberg AF (2015) Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology 40, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Du X, Pang TY (2015) Is dysregulation of the HPA-axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases? Front Psychiatry 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Benoit M, Berrut G, Doussaint J, Bakchine S, Bonin-Guillaume S, Frémont P, Gallarda T, Krolak-Salmon P, Marquet T, Mékiès C, Sellal F, Schuck S, David R, Robert P (2012) Apathy and depression in mild Alzheimer’s disease: A cross-sectional study using diagnostic criteria. J Alzheimers Dis 31, 325–334. [DOI] [PubMed] [Google Scholar]
- [45].Batail JM, Palaric J, Guillery M, Gadoullet J, Sauleau P, Le Jeune F, Vérin M, Robert G, Drapier D (2018) Apathy and depression: Which clinical specificities? Pers Med Psychiatry 7-8, 21–26. [Google Scholar]
- [46].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Dell RS, Mecca AP, Chen M-K, Naganawa M, Toyonaga T, Lu Y, Godek TA, Harris JE, Bartlett HH, Banks ER, Kominek VL, Zhao W, Nabulsi NB, Ropchan J, Ye Y, Vander Wyk BC, Huang Y, Arnsten AFT, Carson RE, van Dyck CH (2021) Association of Aβ deposition and regional synaptic density in early Alzheimer’s disease: A PET imaging study with [(11)C]UCB-J. Alzheimers Res Ther 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38, 143–162. [DOI] [PubMed] [Google Scholar]
- [50].Raimo S, Trojano L, Spitaleri D, Petretta V, Grossi D, Santangelo G (2014) Apathy in multiple sclerosis: A validation study of the apathy evaluation scale. J Neurol Sci 347, 295–300. [DOI] [PubMed] [Google Scholar]
- [51].Umucu E, Wyman M, Lee B, Zuelsdorff M, Benton SF, Nystrom N, Johnson SC, Carlsson CM, Asthana S, Gleason CE (2019) Apathy in preclinical Alzheimer’s disease: Psychometric validation of the Apathy Evaluation Scale. Am J Alzheimers Dis Other Demen 34, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- [53].Hubley AM (2014) Beck Depression Inventory. In Encyclopedia of Quality of Life and Well-Being Research, Michalos AC, ed. Springer Netherlands, Dordrecht, pp. 338–345. [Google Scholar]
- [54].Chaudhary S, Zhornitsky S, Roy A, Summers C, Ahles T, Li C-SR, Chao HH (2022) The effects of androgen deprivation on working memory and quality of life in prostate cancer patients: The roles of hypothalamic connectivity. Cancer Med 11, 3425–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang S, Zhornitsky S, Wang W, Le TM, Dhingra I, Chen Y, Li C-SR (2021) Resting state hypothalamic and dorsomedial prefrontal cortical connectivity of the periaqueductal gray in cocaine addiction. Addict Biol 26, e12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang S, Wang W, Zhornitsky S, Li C-SR (2018) Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: An exploratory study. Front Psychiatry 9, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rombouts SARB, Stam CJ, Kuijer JPA, Scheltens P, Barkhof F (2003) Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage 20, 1236–1245. [DOI] [PubMed] [Google Scholar]
- [58].Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE (2007) A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 35, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700–711. [DOI] [PubMed] [Google Scholar]
- [61].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li J, Kong R, Liégeois R, Orban C, Tan Y, Sun N, Holmes AJ, Sabuncu MR, Ge T, Yeo BTT (2019) Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage 196,126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001) Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22, 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- [64].Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- [65].Le TM, Liao D-L, Ide J, Zhang S, Zhornitsky S, Wang W, Li C-SR (2020) The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int J Obes 44, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zar JH (1999) Biostatistical analysis, Prentice Hall, Upper Saddle River. [Google Scholar]
- [67].Lieberman MD, Cunningham WA (2009) Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci 4, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Beran TN, Violato C (2010) Structural equation modeling in medical research: A primer. BMC Res Notes 3, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fan Y, Chen J, Shirkey G, John R, Wu SR, Park H, Shao C (2016) Applications of structural equation modeling (SEM) in ecological studies: An updated review. Ecol Process 5, 19. [Google Scholar]
- [70].Li G, Le TM, Wang W, Zhornitsky S, Chen Y, Chaudhary S, Zhu T, Zhang S, Bi J, Tang X, Li C-SR (2021) Perceived stress, self-efficacy, and the cerebral morphometric markers in binge-drinking young adults. Neuroimage Clin 32, 102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- [72].Andrews-Hanna JR (2012) The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Perri R, Turchetta CS, Caruso G, Fadda L, Caltagirone C, Carlesimo GA (2018) Neuropsychological correlates of cognitive, emotional-affective and auto-activation apathy in Alzheimer’s disease. Neuropsychologia 118, 12–21. [DOI] [PubMed] [Google Scholar]
- [74].Yu S-Y, Lian T-H, Guo P, Li L-X, Ding D-Y, Li D-N, Liu L, Zhao H, Hu Y, Zuo L-J, Gao J-H, Yu Q-J, Jin Z, Wang R-D, Zhu R-Y, Wang X-M, Zhang W (2020) Correlations of apathy with clinical symptoms of Alzheimer’s disease and olfactory dysfunctions: A cross-sectional study. BMC Neurol 20, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schacter DL, Addis DR, Buckner RL (2007) Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci 8, 657–661. [DOI] [PubMed] [Google Scholar]
- [77].Forlim CG, Klock L, Bächle J, Stoll L, Giemsa P, Fuchs M, Schoofs N, Montag C, Gallinat J, Kühn S (2020) Reduced resting-state connectivity in the precuneus is correlated with apathy in patients with schizophrenia. Sci Rep 10, 2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen Y, Dang M, Zhang Z (2021) Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener 16, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Winlove CIP, Milton F, Ranson J, Fulford J, MacKisack M, Macpherson F, Zeman A (2018) The neural correlates of visual imagery: A co-ordinate-based meta-analysis. Cortex 105, 4–25. [DOI] [PubMed] [Google Scholar]
- [81].Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V (1998) Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 35, 199–210. [PubMed] [Google Scholar]
- [82].Camacho MC, Karim HT, Perlman SB (2019) Neural architecture supporting active emotion processing in children: A multivariate approach. Neuroimage 188, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kazui H, Takahashi R, Yamamoto Y, Yoshiyama K, Kanemoto H, Suzuki Y, Sato S, Azuma S, Suehiro T, Shimosegawa E, Ishii K, Tanaka T (2017) Neural basis of apathy in patients with amnestic mild cognitive impairment. J Alzheimers Dis 55, 1403–1416. [DOI] [PubMed] [Google Scholar]
- [84].Harper L, Bouwman F, Burton EJ, Barkhof F, Scheltens P, O’Brien JT, Fox NC, Ridgway GR, Schott JM (2017) Patterns of atrophy in pathologically confirmed dementias: A voxelwise analysis. J Neurol Neurosurg Psychiatry 88, 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cho H, Choi JY, Lee SH, Lee JH, Choi Y-C, Ryu YH, Lee MS, Lyoo CH (2017) Excessive tau accumulation in the parieto-occipital cortex characterizes early-onset Alzheimer’s disease. Neurobiol Aging 53, 103–111. [DOI] [PubMed] [Google Scholar]
- [86].Le Heron C, Holroyd CB, Salamone J, Husain M (2019) Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry 90, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jang JY, Han SD, Yew B, Blanken AE, Dutt S, Li Y, Ho JK, Gaubert A, Nation DA (2021) Resting-state functional connectivity signatures of apathy in community-living older adults. Front Aging Neurosci 13, 691710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Squire LR, Wixted JT, Clark RE (2007) Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci 8, 872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B (2002) The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci U S A 99, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cao Z, Bennett M, Orr C, Icke I, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, Quinlan EB, Desrivières S, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot J-L, Nees F, Orfanos DP, Paus T, Poustka L, Hohmann S, Fröhner JH, Smolka MN, Walter H, Schumann G, Whelan R (2019) Mapping adolescent reward anticipation, receipt, and prediction error during the monetary incentive delay task. Hum Brain Mapp 40, 262–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Murty VP, LaBar KS, Adcock RA (2016) Distinct medial temporal networks encode surprise during motivation by reward versus punishment. Neurobiol Learn Mem 134 Pt A, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sheline YI, Raichle ME (2013) Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry 74, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Agüera-Ortiz L,García-Ramos R, Grandas Perez FJ, López- Álvarez J, Montes Rodríguez JM, Olazarán Rodríguez FJ, Olivera Pueyo J, Pelegrin Valero C, Porta-Etessam J (2021) Depression in Alzheimer’s disease: A Delphi consensus on etiology, risk factors, and clinical management. Front Psychiatry 12, 638651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Starkstein SE, Ingram L, Garau ML, Mizrahi R (2005) On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry 76, 1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Starkstein SE, Jorge R, Mizrahi R, Robinson RG (2006) A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77, 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Peterson AC, Li C-SR (2018) Noradrenergic dysfunction in Alzheimer’s and Parkinson’s diseases-an overview of imaging studies. Front Aging Neurosci 10, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Manza P, Zhang S, Hu S, Chao HH, Leung H-C, Li C-SR (2015) The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. Neuroimage 107, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rouch I, Padovan C, Boublay N, Pongan E, Laurent B, Trombert-Paviot B, Krolak-Salmon P, Dorey J-M (2020) Association between executive function and the evolution of behavioral disorders in Alzheimer’s disease. Int J Geriatr Psychiatry 35, 1043–1050. [DOI] [PubMed] [Google Scholar]
- [99].Bayard S, Jacus J-P, Raffard S, Gely-Nargeot M-C (2014) Apathy and emotion-based decision-making in amnesic mild cognitive impairment and Alzheimer’s disease. Behav Neurol 2014, 231469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


