Abstract
Purpose
Clinical target volumes (CTVs) and organs at risk (OARs) could be autocontoured to save workload. This study aimed to assess a convolutional neural network for automatic and accurate CTV and OARs in prostate cancer, while comparing possible treatment plans based on autocontouring CTV to clinical treatment plans.
Methods
Computer tomography (CT) scans of 217 patients with locally advanced prostate cancer treated at our hospital were retrospectively collected and analyzed from January 2013 to January 2019. A deep learning-based method, CUNet, was used to delineate CTV and OARs. A training set of 195 CT scans and a test set of 28 CT scans were randomly chosen from the dataset. The mean Dice similarity coefficient (DSC), 95th percentile Hausdorff distance (95HD), and subjective evaluation were used to evaluate the performance of this strategy. Predetermined evaluation criteria were used to grade treatment plans, and percentage errors for clinical doses to the planned target volume (PTV) and OARs were calculated.
Results
The mean DSC and 95HD values of the defined CTVs were (0.84 ± 0.05) and (5.04 ± 2.15) mm, respectively. The average delineation time was < 15 s for each patient's CT scan. The overall positive rates for clinicians A and B were 53.15% versus 46.85%, and 54.05% versus 45.95%, respectively (P > .05) when CTV outlines from CUNet were blindly chosen and compared with the ground truth (GT). Furthermore, 8 test patients were randomly chosen to design the predicted plan based on the autocontouring CTVs and OARs, demonstrating acceptable agreement with the clinical plan: average absolute dose differences in mean value of D2, D50, D98, Dmax, and Dmean for PTV were within 0.74%, and average absolute volume differences in mean value of V45 and V50 for OARs were within 3.4%.
Conclusion
Our results revealed that the CTVs and OARs for prostate cancer defined by CUNet were close to the GT. CUNet could halve the time spent by radiation oncologists in contouring, demonstrating the potential of the novel autocontouring method.
Keywords: prostate cancer radiotherapy, convolutional neural networks, clinical target volume, deep learning, DVH dosimetric index
Note: A preprint has been previously published.1
Introduction
Prostate cancer is the commonest noncutaneous cancer in males, and has the second highest mortality among all cancers.2 Prostate cancer is expected to affect 1 in every 9 males at some point in their lives.3 Intensity-modulated radiation therapy (IMRT) is an important aspect of current cancer care for patients with prostate cancer; and with appropriate treatment, the 5-years survival rate is 98.2%.4 Radiation doses to the clinical target volume (CTV) and organs at risk (OARs) can often be significant and one of the essential steps for successful IMRT delivery owing to the complex dose distribution in IMRT. Laborious manual delineation is conventionally performed by radiotherapy oncologists for such delineation. However, this delineation is time-consuming.5 Therefore, automatic and accurate computational tools that could significantly lower the manual efforts put in by radiotherapy oncologists are warranted.
In recent years, the hoppoint convolutional neural networks (CNNs) have demonstrated their advantages in performing medical segmentation tasks,6,8 having been commonly used in the CTV or OARs delineation of brain cancer,9 head and neck (H&N) cancer,10,13 breast cancer, esophageal cancer,14 rectal cancer,15 and cervical cancer.16 U-Net, a well-known CNN architecture for medical image segmentation, was commonly used among all the CNN-based contour delineation models.17 These studies demonstrate that combining U-Net with an elegant feature extraction architecture would efficiently help obtain an effective CNN architecture for target region segmentation. On the other hand, attention has been paid to how to effectively improve network performance.18–23 The network could focus on important features and suppress unnecessary features via the attention mechanism. Herein, we integrate Convolutional Block Attention Module (CBAM) module that focus on meaningful features along the channel and spatial dimensions into U-Net to facilitate automatic CTV and OARs delineation.24
Intra-observer and interobserver variability is one of the major challenges in radiotherapy planning for prostate cancer.25 We further tried to design the clinical plan on autocontouring CTV and OARs, to compare its performance with clinical treatment plans, for exploring its clinical feasibility and accuracy. The autocontouring computed tomography (CT)-based dose prediction could decrease the time required for the iterative optimization and structural contouring, allowing physicians and dosimetrists to focus their expertise on more challenging cases. To the best of our knowledge, this is the first report to predict the dose distribution for IMRT using autocontouring deep learning CT images.
Materials and Methods
Patients Database
The Peking Union Medical College Hospital's Institutional Review Board ethnically approved the study in December 2021(approval number: K22C2234). The reporting of this study conforms to STROBE guidelines.26 CT data from 217 de-identified patients with locally advanced prostate cancer were retrospectively collected from January 2013 to January 2019. We statistically examined patient characteristics such as age and pathological type. The patients were given a total dose of 70 Gy in 28 fraction, using 6-Mv X ray of IMRT.
All the data were recorded using a Brilliance CT Big Bore with a size of 512 × 512 pixels (pixel spacing: ranging from 0.77 × 0.77 to 1.95 × 1.95 mm) and a 5 mm thickness (Philips Healthcare, Best, the Netherlands). Qualified radiation oncologists manually drew the CTV and OAR contours prior to irradiation in clinical practice as the segmentation ground truth (GT). Widely acknowledged consensus criteria with certain modifications were used to define the CTV.27 The prostate and seminal vesicle were combined to create the CTV. A professional radiation oncologist committee comprising 8 oncologists with > 10 years of experience in radiotherapy for pelvic tumors reviewed and modified (only when necessary) all of the delineated contours together, as is the clinical routine in our department, to ensure the quality of the delineation.
Machine Learning Models Data and Preprocessing
The threshold of the supplied CT slice intensity was set between −1024 and 2048 HU. Zero-mean normalization was used to make the input picture data zero-centred and distributed in the same range for each patient, making training less sensitive to feature size and allowing for well-conditioned optimization. Patients from the dataset were randomly assigned to a training-validation set (195 patients) and a test set (28 patients).
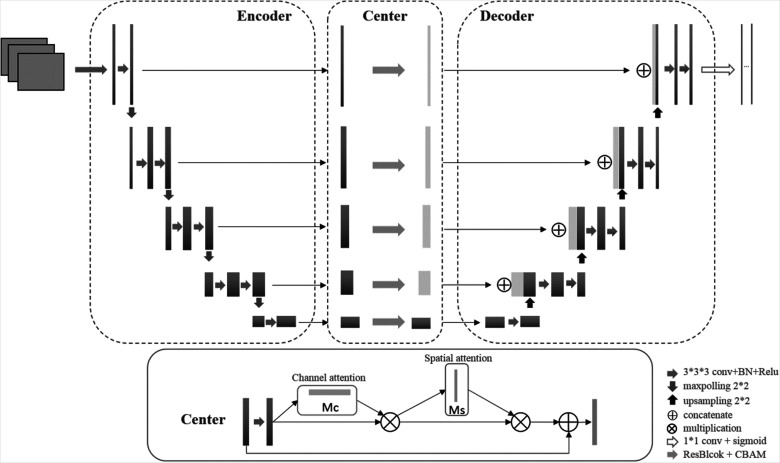
The CUNet architecture is illustrated in Figure 1. Three-dimensional (3D) U-Net backbone architecture comprising encoding and decoding paths was used. The encoding and decoding paths were combined by a skip connection in the typical U-Net to concatenate the multilevel features, thus taking advantage of the low-level and high-level information. Thus, the network learns to use the features equally. An attention center block is proposed to weigh the features before concatenating them to the encoder part instead of combing the features directly in CUNet. Thus, the network will focus on specific features instead of all the features. The attention center block is a CBAM integrated with ResBlock in ResNet.23 CBAM infers attention maps along the channel and spatial dimensions sequentially. For adaptive feature refinement, each attention map is multiplied by the input feature map. A residual block skip connection then connects the final feature map with the initial input feature map. The channel attention map exploits the interchannel relationship of features, showing “what” is meaningful in terms of input image. The interspatial relationship of features generates the spatial attention map. The informative features will be emphasized while the other features will be suppressed.
Figure 1.
The architecture of the proposed CUNet.
The CTV, bladder, rectum, left femoral head, and right femoral head contours were trained using CUNet with 5 output channels.
Model Training
Using a 5-fold cross-validation technique, 195 CT scans were used for training and validation. During training, patches with dimensions (64, 64, 64) were randomly generated and the model was trained from the scratch. Augmentation was not used. The output value of the model ranged from 0 to 1. The foreground of the segmented mask was set to pixels with an output value > 0.5. Following this, a contour extraction was given to the foreground. The network was trained on NVIDIA TITAN Xp (12 GB RAM). The Adam optimizer was used throughout the network, with an initial learning rate of 0.0001 and a decay rate of gamma 0.9 for each epoch. The total number of epochs was 50.
Model Evaluation
Quantitative evaluation metrics
The Dice similarity coefficient (DSC) and 95th percentile Hausdorff distance (95HD) were used to assess the contouring accuracy. The volumetric overlap ratio between segmented masks is calculated using the DSC; a higher value indicates a higher overlap ratio. When 2 masks are totally identical, the value equals 1. The 95HD indicates the agreement between 2 outlines; a higher value suggests a greater divergence.
Oncologist evaluation
Red and green outlines were used to randomly mark the artificial intelligence (AI) and GT outlines respectively, in a clinically blinded assessment of drawing layers from 2237 slice layers of 28 patients, sorting after creating 111 layers. Two clinicians with > 10 years of experience in prostate cancer radiation blindly decided which contour was superior for clinical use. A positive outcome was recorded if the AI group performed better, and the number of AI layers that had better clinical applicability was reported.
Plan Evaluation
Anticipated treatment plans based on autocontouring CTV and clinical plans were compared. The predicted dose was based on a reoptimized plan which was optimized based on the AI contours. The total dose-volume histogram (DVH) curves of planned target volume (PTV) and distinct OARs were first exhibited between the prediction and clinical truth plans in terms of DVH parameters. Second, the clinically relevant dosimetric indices (DI) were determined, including the mean dose (Dmean), D2, D50, and D98 for PTV (where Di represents the dose received by i percent of PTV volume) and Dmean, V45, and V50 for OARs (where Vi represents the volume fraction of OARs irradiated by i Gy). The following formula was used to calculate the homogeneity index and conformation index where Vptv and Vpres are the volumes of the PTV and the prescription dose region, respectively, and Vref is the volume of the irradiated PTV of the prescription dose.
Statistical Analysis
The CTV and OARs contours predicted from using CUNet were transferred to a treatment planning system for designing the radiotherapy plan. During radiotherapy planning, the total amount of time spent refining the CTV and OARs contours was documented in minutes per case. Per cent error relative to the prescribed dose was used to further evaluate the performance of the models, which was calculated as (predicted dose−clinical dose)/prescribed dose ×100%. The dosimetric parameters were indicated with median and interquartile range, and the parameters in clinical and predicted groups were compared by means of the Wilcoxon rank sum test. The significance between the average and maximum doses of the clinical and the predicted and mimicked plans of each models were investigated and analyzed. Statistical significance was set at P < .05 (2-tailed).
Results
Quantitative Analysis of the CTV and OAR Contour
CT data from 217 patients were enrolled, and 28 CT slices from the test dataset were analyzed. The mean DSC and 95HD values for the defined CTVs were (0.84 ± 0.05) and (5.04 ± 2.15) mm, respectively. The mean DSC and 95HD values for the OARs were reported in Table 1. The average delineation time was < 15 s for the CT scan of 1 patient.
Table 1.
The Mean DSC and 95HD Values for OARs.
| Anatomy | DSC ± STD | 95HD ± STD |
|---|---|---|
| Bladder | 0.913 ± 0.078 | 2.462 ± 3.984 |
| Bone marrow | 0.850 ± 0.051 | 1.973 ± 0.924 |
| Femoral head left | 0.899 ± 0.027 | 1.424 ± 0.329 |
| Femoral head right | 0.897 ± 0.023 | 1.547 ± 0.421 |
| Rectum | 0.783 ± 0.032 | 6.278 ± 2.275 |
| Small intestine | 0.822 ± 0.035 | 5.369 ± 2.294 |
| Spinal cord | 0.824 ± 0.056 | 3.345 ± 3.431 |
Abbreviations: 95HD, 95th percentile Hausdorff distance; DSC, Dice similarity coefficient; OAR, organs at risk; STD, sexually transmitted diseases.
The Turing Imitation Test
A total of 10% of the slices with GT masks were chosen at random to mark both AI and GT contours simultaneously in the test dataset. The AI and GT CTV contours were demonstrated for each slice, with the color of these 2 contours randomly assigned as red or green. A total of 112 slices were chosen from the 28 CT images. The results are presented in Table 2.
Table 2.
The Results of the Turing-Like Imitation Test.
| Clinician A | Clinician B | |||
|---|---|---|---|---|
| Slice | Ratio | Slice | Ratio | |
| Positive | 59 | 53.15% | 60 | 54.05% |
| Negative | 53 | 46.85% | 52 | 45.95% |
| Total | 112 | P > .05 | 112 | P > .05 |
Subclass analysis was performed to evaluate the oncologists individually and the CT slice (Table 3). The sample CTV delineations are presented in Figure 2.
Table 3.
The Subclass Analysis Results of the Turing-Like Imitation Test.
| Clinician A | Clinician B | ||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 28 | 25 | 53 |
| Positive | 24 | 35 | 59 |
| Total | 52 | 60 | 112 |
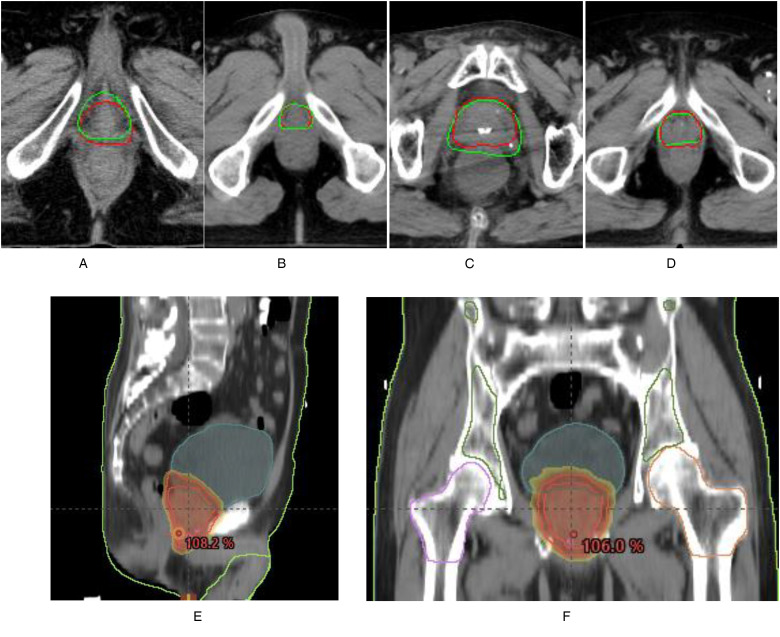
Figure 2.
The distribution map of the positive results. (A) A sample slice wherein both the oncologists approved the GT contour. The GT contours are presented in green. AI contours are presented in red. (B) A sample slice wherein both oncologists approved the AI contours. The AI contours are presented in red. GT contours are presented in green. (C) A sample slice wherein only oncologist A approved the AI contour. AI contours are presented in green line. GT contours are presented in red. (D) A sample slice wherein only oncologist B approved the AI contour. AI contours are presented in green line. GT contours are presented in red. (E) and (F) Three-dimensional display of prostate contouring. Abbreviations: AI, artificial intelligence; GT, ground truth.
Statistics of the DVH DI
Overall DVH comparisons of the PTVs and OARs between the clinical and predicted results for 8 randomly selected test participants, revealed that the clinical and anticipated DVHs of the PTV and OARs had an acceptable agreement for each patient.
The mean and standard deviation of the clinically relevant DI for PTV and OARs in the 8 test patients are shown in Table 4. The average absolute dose differences in mean value of D2, D50, D98, Dmax and Dmean for the PTVs are within 0.74%, and the average absolute volume differences in mean value of V45 and V50 for OARs are within 3.4%. The projected findings are comparable to the clinical truth without any statistical significance (P > .05).
Table 4.
Median and Interquartile Range of DIs for PTV and OARs.
| DI | Clinical | Prediction | P Value |
|---|---|---|---|
| PTV | |||
| D98(Gy) | 68.57 (1.31) | 68.55 (1.63) | .65 |
| D50(Gy) | 71.84 (1.35) | 71.73 (1.06) | .62 |
| D2(Gy) | 73.10 (1.59) | 72.90 (1.24) | .51 |
| Dmean(Gy) | 71.67 (1.12) | 71.53 (0.96) | .50 |
| Dmax(Gy) | 75.02 (2.04) | 74.30 (1.04) | .18 |
| HI | 1.04 (0.03) | 1.04 (0.03) | .96 |
| CI | 0.87 (0.006) | 0.87 (0.009) | .57 |
| Rectum | |||
| V50(%) | 14.47 (9.90) | 13.48 (4.90) | .94 |
| V45(%) | 16.60 (11.39) | 15.43 (3.97) | .95 |
| D25(Gy) | 28.03 (23.64) | 26.51 (15.45) | .88 |
| D50(Gy) | 6.88 (6.38) | 8.02 (5.32) | .63 |
| Dmean(Gy) | 18.93 (8.72) | 18.56 (7.02) | .88 |
| Bladder | |||
| V50(%) | 10.65 (11.95) | 14.02 (14.48) | .64 |
| V45(%) | 12.10 (13.91) | 16.15 (16.44) | .67 |
| D25(Gy) | 19.11 (27.89) | 30.21 (33.00) | .67 |
| D50(Gy) | 5.17 (12.58) | 9.93 (13.68) | .73 |
| Dmean(Gy) | 15.16 (13.89) | 21.66 (16.86) | .70 |
| Left femoral head | |||
| D5(Gy) | 16.42 (3.90) | 16.16 (4.09) | .65 |
| Dmean(Gy) | 11.73 (5.23) | 10.77 (4.50) | .98 |
| Right femoral head | |||
| D5(Gy) | 16.29 (2.37) | 16.99 (5.47) | .63 |
| Dmean(Gy) | 11.88 (3.38) | 11.28 (4.48) | .72 |
Abbreviations: CI, conformation index; DI, dosimetric indices; Dmax, maximum dose; Dmean, mean dose; HI, homogeneity index; OAR, organs at risk; PTV, planned target volume.
Discussion
Radiation oncology for prostate cancer is of significance because it can decrease its associated mortality. CTV and OAR contouring remains fundamental for treatment; however, it is time-consuming and prone to human errors, leading to potentially avoidable delays in treatment initiation. Currently, several CNNs have focused on autosegmenting prostate CTV contouring using a CNN-based method, and despite the satisfactory performance of the models, there is scope for improvement. There is a reasonably long way before these models can be used safely and effectively in clinical practice. Hence, we collected and standardized CT images from 237 patients in our department and proposed a new deep learning network, CUNet, to automatically segment the CTV and OARs in prostate cancer.
The mean DSC and 95HD values were used for comprehensively evaluating the performance of CUNet for prostate CTV contouring. The mean DSC and 95HD values for the delineated CTVs were 0.84 and 5.04 mm, respectively, demonstrating the highly quantitative CTV contours. The results were also acceptable for OARs delineation. Moreover, in the Turing imitation test, 2 clinicians with > 10 years of experience in radiotherapy for prostate tumors blindly selected which contour had better clinical applicability. The overall positive rate for clinicians A and B were 53.15% versus 46.85%, and 54.05% versus 45.95% (P > .05), demonstrating that our deep machine learning model performed equally well or even better than human delineation.
An inherent product of CTV autocontouring is a reduction in the time spent by the radiation oncologist on contouring. The average manual CTV contouring time for 1 patient with prostate cancer was 10 to 20 min,28 while the corresponding time was 15 s when CUNet was employed. Following this, an average time of 2 min was required for each case to refine the contours. Thus, it took approximately 8 min including 5 min for manual data transfer.
The clinical and predicted (autocontouring) CTV treatment plans were further scored and compared. The overall DVH comparisons of the PTV and OARs for the 8 randomly selected test patients revealed that the average absolute dose differences of D2, D50, D98, Dmax, and Dmean for PTV were within 0.74%, and the average absolute volume differences of V45 and V50 for OARs were < 3.4%. The predicted results are comparable to the clinical truth, and no statistical significance was observed (P > .05) (comparison in Figure 3). All these results revealed that the dose distribution predictions using autocontouring CTV are accurate. Differences between the predicted doses for OARs of the models were lesser when compared with the clinical plans, which were not clinically relevant, thus demonstrating the potential of automated treatment planning for prostate cancer. The predicted dose distribution could serve as a quality control tool for treatment planning, wherein the planners can know whether or where the dose distributions can be improved, and the physicians can immediately visualize the 3D dose distributions to adjust the dose constraint requirements of the OARs. Meanwhile, the planners can take advantage of these OARs DVHs to define optimization, which improve the quality and consistency of treatment plans, and lower the planning time. However, this was still idealistic because it would take time for planners to make radiotherapy plans and new DVHs. In the future, this could be even faster if clinicians could get new DVHs automatically after contouring using the deep learning method.
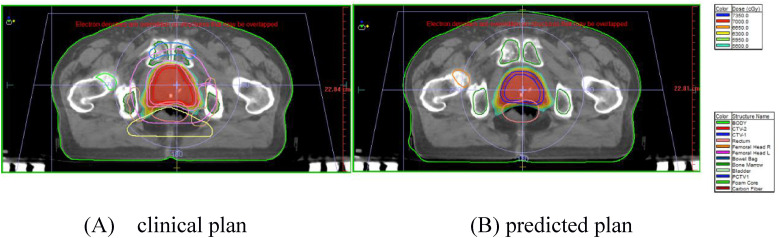
Figure 3.
An example of the clinical and predicted plans. (A)The clinical and (B) predicted plans based on the autocontoured CTV in the same patient. Abbreviation: CTV, clinical target volumes.
Our study has several shortcomings. First of all, sample size was not well justified in the current study, and the number of DVHs included were small. Additionally, the majority of the participants were from Peking Union Medical College Hospital, where medical capacity is high, leading to possible selection bias. Further studies need to be carried out among medical researches in other regions and countries for comparisons of AI technology and manual contouring in different cultural contexts.
The current results for prostate CTV and OAR contouring demonstrate that CUNet can learn high-level semantic features well, making it possible to use this method for volume delineations in other cancers. It would form a sound foundation to further collect more data from multiple manufacturers and institutions for validating our study findings; however, this possibility warrants further research.
Conclusions
Accurate and consistent delineation of the CTV and OARs is of significance in radiotherapy. This study presents a novel CNN (CUNet) for automatic and accurate CTV and OAR segmentation in prostate cancer. The performance of CUNet was quantitatively and subjectively evaluated for 28 patients. CUNet provided accurate delineation results, which had high clinical acceptability. The results demonstrate that CUNet can be used to improve efficiency and reduce the adverse effects associated with random errors in delineating the CTV and OARs in patients with prostate cancer.
Abbreviations
- 3D
three-dimensional
- 95HD
95th percentile Hausdorff distance
- AI
artificial intelligence
- CBAM
convolutional block attention module
- CNN
convolutional neural network
- CT
computed tomography
- CTV
clinical target volumes
- DI
dosimetric index
- Dmean
mean dose
- DSC
Dice similarity coefficient
- DVH
dose-volume histogram
- GT
ground truth
- IMRT
intensity-modulated radiation therapy
- OAR
organs at risk
- PTV
planned target volume.
Appendix 1. OARs’ Dose Constraints.
| Normal Organs | Volume/Dose | Volume/Dose Constraints |
|---|---|---|
| Rectum | 25% | ≤3000 (cGy) |
| 35% | ≤2500 (cGy) | |
| 50% | ≤2000 (cGy) | |
| Bladder | 25% | ≤4000 (cGy) |
| 35% | ≤3000 (cGy) | |
| 50% | ≤3000 (cGy) | |
| Colon | 25% | ≤1000 (cGy) |
| 2cc | ≤3500 (cGy) | |
| Femoral head left | 5% | ≤2500 (cGy) |
| Femoral head right | 5% | ≤2500 (cGy) |
Abbreviation: OAR, organs at risk.
Footnotes
Authors’ Contributions: SJ and TJY were responsible for data collection and drafted the manuscript. SJ and TYJ contributed equally to this manuscript. GQ, ZHN, DTT, and HL participated in the design of the study. SJ performed statistical analysis and data interpretation. SJ and LZK designed the study and revised the manuscript. All authors read and approved the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the 13th Five-Year Research Fund of China (grant no. 2016YFC0105207).
Ethical Approval and Consent to Participate: The Peking Union Medical College Hospital's Institutional Review Board reviewed the study protocol and ethnically approved the study in December 2021(Approval number: K22C2234).
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
ORCID iD: Yinjie Tao https://orcid.org/0000-0003-4042-0379
References
- 1.Shen J, Tao Y, Guan H, et al. Clinical validation and treatment plan evaluation based on auto-delineation of the clinical target volume for prostate cancer radiotherapy. 2022. [DOI] [PMC free article] [PubMed]
- 2.Men K, Dai J, Li Y. Automatic segmentation of the clinical target volume and organs at risk in the planning CT for rectal cancer using deep dilated convolutional neural networks. Med Phys. 2017;44(12):6377-6389. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, Andrews KS, Brooks Det al. et al. Cancer screening in the United States, 2019: a review of current American Cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184-210. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 5.Cao M, Stiehl B, Yu VYet al. et al. Analysis of geometric performance and dosimetric impact of using automatic contour segmentation for radiotherapy planning. Front Oncol. 2020;10:1762. 10.3389/fonc.2020.01762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB. Advances in auto-segmentation. Semin Radiat Oncol. 2019;29(3):185-197. [DOI] [PubMed] [Google Scholar]
- 7.Anwar SM, Majid M, Qayyum A, Awais M, Alnowami M, Khan MK. Medical image analysis using convolutional neural networks. A review . J Med Syst. 2018;42(11):226. [DOI] [PubMed] [Google Scholar]
- 8.Fabijanska A. Segmentation of corneal endothelium images using a U-net-based convolutional neural network. Artif Intell Med. 2018;88:1-13. 10.1016/j.artmed.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 9.Shusharina N, Soderberg J, Edmunds D, Lofman F, Shih H, Bortfeld T. Automated delineation of the clinical target volume using anatomically constrained 3D expansion of the gross tumor volume. Radiother Oncol. 2020;146:37-43. 10.1016/j.radonc.2020.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrtovec T, Mocnik D, Strojan P, Pernus F, Ibragimov B. Auto-segmentation of organs at risk for head and neck radiotherapy planning: from atlas-based to deep learning methods. Med Phys. 2020;47(9):e929-e950. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Li X, Zhang X, Song F, Huang S, Xia Y. Segmentation of organs at risk in nasopharyngeal cancer for radiotherapy using a self-adaptive Unet network. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(11):1579-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Y, Yang Y, Fang Y, Wang J, Hu W. A preliminary experience of implementing deep-learning based auto-segmentation in head and neck cancer: a study on real-world clinical cases. Front Oncol. 2021;11:638197. 10.3389/fonc.2021.638197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Wang H, Tian S, et al. A slice classification model-facilitated 3D encoder-decoder network for segmenting organs at risk in head and neck cancer. J Radiat Res. 2021;62(1):94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Chen X, Yang Bet al. et al. Evaluation of automatic segmentation model with dosimetric metrics for radiotherapy of esophageal cancer. Front Oncol. 2020;10:564737. 10.3389/fonc.2020.564737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Hu J, Wu Qet al. et al. Automatic delineation of the clinical target volume and organs at risk by deep learning for rectal cancer postoperative radiotherapy. Radiother Oncol. 2020;145:186-192. 10.1016/j.radonc.2020.01.020 [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Liu X, Xiao Bet al. et al. Segmentation of organs-at-risk in cervical cancer CT images with a convolutional neural network. Phys Med. 2020;69:184-191. 10.1016/j.ejmp.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 17.Rhee DJ, Cardenas CE, Elhalawani Het al. Automatic detection of contouring errors using convolutional neural networks. Med Phys. 2019;46(11):5086-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mnih V, Heess N, Graves A. Recurrent models of visual attention. Advances in neural information processing systems. 2014, 27. [Google Scholar]
- 19.Ba J, Mnih V, Kavukcuoglu K. Multiple object recognition with visual attention. Computer ence 2014.
- 20.Bahdanau D, Cho K, Bengio Y. Neural machine translation by jointly learning to align and translate. In.: Computer ence; 2014.
- 21.Xu K, Ba JL, Kiros R. et al. et al. Show, attend and tell: neural image caption generation with visual attention. Pr Mach Learn Res. 2015;37:2048–2057. [Google Scholar]
- 22.Jaderberg M, Simonyan K, Zisserman A, Kavukcuoglu K. Spatial transformer networks. Adv Neur In 2015, 28.
- 23.Gregor K, Danihelka I, Graves A, Rezende DJ, Wierstra D. DRAW: a recurrent neural network for image generation. Pr Mach Learn Res. 2015;37:1462–1471. [Google Scholar]
- 24.Woo SH, Park J, Lee JY, Kweon IS. CBAM: convolutional block attention module. Proceedings of the European conference on computer vision (ECCV) 2018:3–19. [Google Scholar]
- 25.Kuisma A, Ranta I, Keyrilainen Jet al. Validation of automated magnetic resonance image segmentation for radiation therapy planning in prostate cancer. Phys Imaging Radiat Oncol. 2020;13:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger Met al. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. [DOI] [PubMed] [Google Scholar]
- 27.Salembier C, Villeirs G, De Bari B, et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127(1):49-61. [DOI] [PubMed] [Google Scholar]
- 28.Patrick HM, Souhami L, Kildea J. Reduction of inter-observer contouring variability in daily clinical practice through a retrospective, evidence-based intervention. Acta Oncol. 2021;60(2):229-236. [DOI] [PubMed] [Google Scholar]