Abstract
Background
Orsellinic acid (2,4-dihydroxy-6-methylbenzoic acid, OA) and its structural analog o-Orsellinaldehyde, have become widely used intermediates in clinical drugs synthesis. Although the research on the biosynthesis of such compounds has made significant progress, due to the lack of suitable hosts, there is still far from the industrial production of such compounds based on synthetic biology.
Results
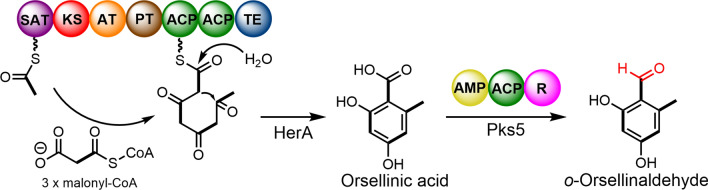
With the help of genome mining, we found a polyketide synthase (PKS, HerA) in the genome of the Hericium erinaceus, which shares 60% amino acid sequence homology with ArmB from Armillaria mellea, an identified PKS capable of synthesizing OA. To characterize the function of HerA, we cloned herA and heterologously expressed it in Aspergillus oryzae, and successfully detected the production of OA. Subsequently, the introduction of an incomplete PKS (Pks5) from Ustilago maydis containing only three domains (AMP-ACP-R), which was into herA-containing A. oryzae, the resulted in the production of o-Orsellinaldehyde. Considering the economic value of OA and o-Orsellinaldehyde, we then optimized the yield of these compounds in A. oryzae. The screening showed that when maltose was used as carbon source, the yields of OA and o-Orsellinaldehyde were 57.68 mg/L and 15.71 mg/L respectively, while the yields were 340.41 mg/Kg and 84.79 mg/Kg respectively in rice medium for 10 days.
Conclusions
Herein, we successfully expressed the genes of basidiomycetes using A. oryzae heterologous host. As a fungus of ascomycetes, which not only correctly splices genes of basidiomycetes containing multiple introns, but also efficiently produces their metabolites. This study highlights that A. oryzae is an excellent host for the heterologous production of fungal natural products, and has the potential to become an efficient chassis for the production of basidiomycete secondary metabolites in synthetic biology.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12934-023-02071-9.
Keywords: Orsellinic acid, o-Orsellinaldehyde, Polyketide synthase, Basidiomycetes, Aspergillus oryzae
Background
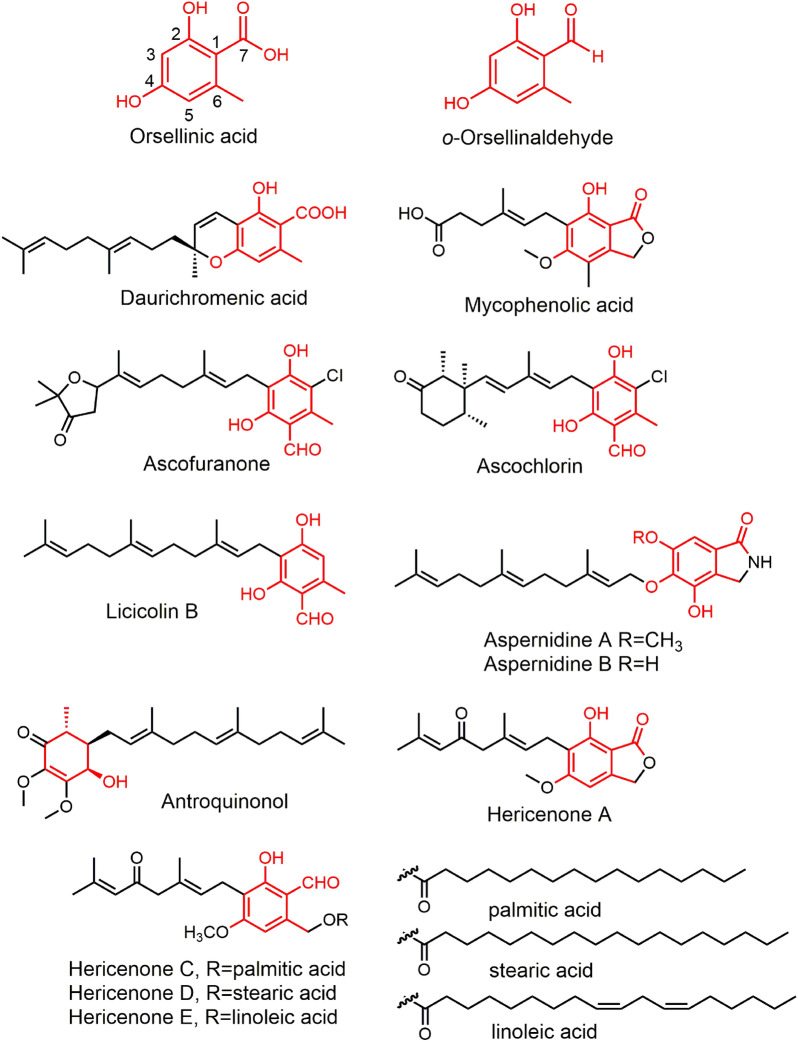
Orsellinic acid (OA) is a dihydroxybenzoic acid derivative with an extra methyl group, and its structural analogue o-Orsellinaldehyde is a benzene carbaldehydes compound. Their natural derivatives are typically contained isopentenyl units. More than 200 OA derivatives compounds have been isolated and identified in plants, lichens, fungi, and bacteria [1], which exhibit essential biological activities. For example, Daurichromenic acid (DCA), isolated from the leaves of the plant Rhododendron dauricum, has potent anti-HIV activity [2]. Mycophenolic acid (MPA), derived from the filamentous fungi Penicillium brevicompactum, has been a first-line immunosuppressive drug for organ transplantations and autoimmune diseases [3]. Ascofuranone (AF) and ascochlorin (AC) are produced by Acremonium egyptiacum, of which AF is a promising drug candidate against African trypanosomiasis and a potential anticancer lead compound [4]. Aspernidine A and B two phthalaldehydes, containing an O-farnesyl moiety, metabolites of Aspergillus nidulans, exhibited moderate antiproliferative activities [5]. Llicicolin B (LL-Z1272β), a prenylated aryl aldehyde produced by various fungi, such as Stachybotrys bisbyi, it not only inhibits pathogenic microorganisms and African trypanosomes, but is also less harmful to human cells [6, 7]. Antroquinonol, from the basidiomycete fungus Antrodia camphorata, has non-small cell cancer inhibitory activity and is currently a Phase II clinical lead drug [8]. Hericenone A, C, D, and E, isolated from the basidiomycete H. erinaceus, they were the first compounds discovered to have Nerve Growth Factor promoting activity and were pioneering drugs against Alzheimer's disease [9–11] (Fig. 1).
Fig. 1.
Representative natural products containing the OA scaffold
OA is a structurally simple aromatic polyketide formed by the stepwise condensation of acetyl coenzyme A with three malonyl coenzymes A. In fungi and bacteria, OA is mediated by repetitive type I PKS enzymes [12–14], while it is produced in plants by type III PKS [15]. In 2012, Ishiuchi et al. identified a PKS (CC1G_05377) from the model basidiomycete Coprinopsis cinerea, whose heterologous expression this gene in Saccharomyces cerevisiae produced OA [16]. In 2013, Lackner et al. identified armB in Armillaria mellea through genome mining. In vitro catalytic realization indicated that ArmB catalyzed acetyl-CoA and malonyl-CoA to produce OA [17]. PKS1 and 2 were identified from Stereum sp. when expressed in A. niger the OA was detected [18]. PKS63787 from A. cinnamomea and the metabolites of Δpks63787 transformants were deficient in several aromatic compounds, including OA [19]. Although OA is the structural backbone of numerous secondary metabolites, only a few of the OA synthases described above have been characterized from basidiomycetes.o-Orsellinaldehyde is a natural product of reducing the C1-position carboxyl group of OA to an aldehyde group. There are two forms of enzymatic reactions that catalyze the conversion of OA to o-Orsellinaldehyde, one is the catalytic reaction responsible for the Non-ribosomal peptide synthase-like (NRPS-like) enzymes, such as StbB, AscB, ATEG_03630 [4, 7, 20], and the other is the catalytic reaction responsible for the R domain of PKS, such as PkfA, TropA [21, 22] (Additional file 1: Fig. S1). In 2019, Reyes-Fernández et al. identified Pks5 from the U. maydis genome, and based on gene knockout experiments, proved that the function of the enzyme is to convert OA into o-Orsellinaldehyde [23].
Currently, most OA derivatives are of plant origin [24], but they are present in relatively small amounts and difficult to isolate and extract. In recent years, the emergence of synthetic biology has promoted the development of related technologies. Through the concept and technology of synthetic biology, the construction of microbial cell factories not only dramatically reduces the production cost, but also provides a new way to protect rare plant resources and drugs development. E. coli and several streptomyces species are frequently utilized for bacterial genes. The most popular plant heterologous host for plant genes is the tobacco “Nicotiana benthamiana”, and the microbial chassis for these organisms is Saccharomyces cerevisiae. For fungal genes, there are several strains available, including S. cerevisiae and well-characterized Aspergillus sp. [25]. To achieves efficient biopreparation of OA and its derivatives, the filamentous fungus A. oryzae was selected as the heterologous expression host, which can synthesize polyketides, terpenoids, non-ribosomal peptides, and their post-modification products [26–28].
Through sequence similarity networks (SNNs) analysis, we found a gene herA in the genome of the basidiomycete H. erinaceus, that prediction involved in the synthesis of OA. Using A. oryzae as host, construction of herA heterologous expression strain (AO-herA), we not only verified the function of HerA, but also obtained an engineering strain of A. oryzae with high OA production. Next, transformed pks5 from U. maydis into AO-herA transformants, and successfully obtained a strain-producing o-Orsellinaldehyde. Through the optimization of carbon sources and the comparison of fermentation methods, we finally determined the best production conditions for high-yielding OA and o-Orsellinaldehyde. This study provides a new strategy for the construction and optimization of the biosynthetic pathway of OA derivatives. In addition, our study also provides an effective method for the efficient expression of genes in basidiomycetes.
Results and discussion
Genome mining polyketide synthase in H. erinaceus
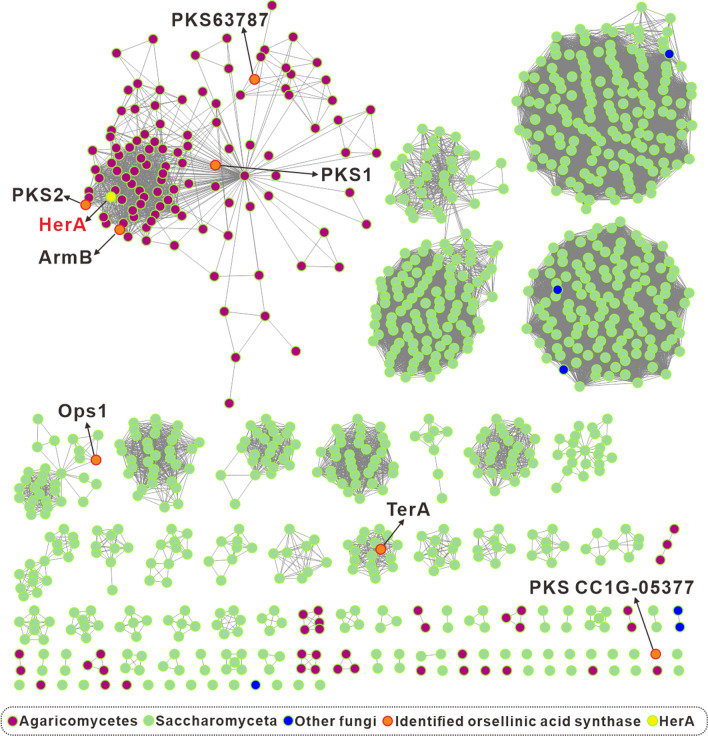
OA is biosynthesized by non-reducing polyketide synthase (NR-PKS) [29]. ArmB, an orsellinic acid synthase (OAS) from A. mellea, has the canonical nonreducing architecture (SAT–KS–AT–PT–ACP–TE) [17]. The OAS gene from the basidiomycetes fungi has rarely been studied or identified. To obtain insights into the OAS homolog in the basidiomycetes fungi, a BLAST search of the fungi genomic database using ArmB as the target gene. For better visualization and classification, the SSNs were built for the 1000 curated NR-PKS using the Enzyme Function Initiative-Enzyme Similarity Tool [30]. The results showed that most OAS are derived from ascomycetes, and only a minority originates from basidiomycetes (Fig. 2). The reason for is that the number of ascomycetes in the reported genome database is much higher than that of basidiomycetes. Based on the data analyzed, all the reported genomes of basidiomycetes contain sequences with high homology for OAS, and this result indicates that it is ubiquitous in basidiomycetes. Therefore, identifying the functions of OAS in basidiomycetes, for the biosynthetic study of OA derivatives was essential.
Fig. 2.
The SSNs network analysis based on ArmB and its homologous sequences. All homologous sequences were from Uniport and other databases. The purple group is from Basidiomycetes. The green group is from ascomycetes. The blue group is from other fungi. The orange dots represent identified OAS. The yellow dots represent HerA
Hericium erinaceus, also known as lion’s mane mushroom, is a widely distributed edible and medicinal fungus in Asian countries. The H. erinaceus mushroom contains a class of compounds “hericenones”, that promote the synthesis of Nerve Growth Factors [31]. This class of compounds is based on the OA backbone, the biosynthetic of hericenones processes remain unknown. Elucidation of the function of OAS in H. erinaceus will provide an insight into the biosynthesis of hericenones. Through SSNs analysis, we found an NR-PKS in the basidiomycete H. erinaceus and named it HerA. Through further analysis, HerA contained 2147 amino acid sequence, which was distributed in the same classic as the previously reported ArmB [17], CC1G-05377 [16], PKS1, 2 [18], and PKS63787 [19] from basidiomycetes, while Osp1 and TerA of ascomycete origin were in a different classic. According to bioinformatic analysis, except for PKS CC1G-05377, these enzymes share the structural SAT, KS, AT, PT, ACP, and TE domains for OA biosynthesis (Additional file 1: Table S1). Noteworthy, HerA contains two ACP domains. We speculated that it can synthesize OA.
Heterologous expression of herA in A. oryzae to form OA
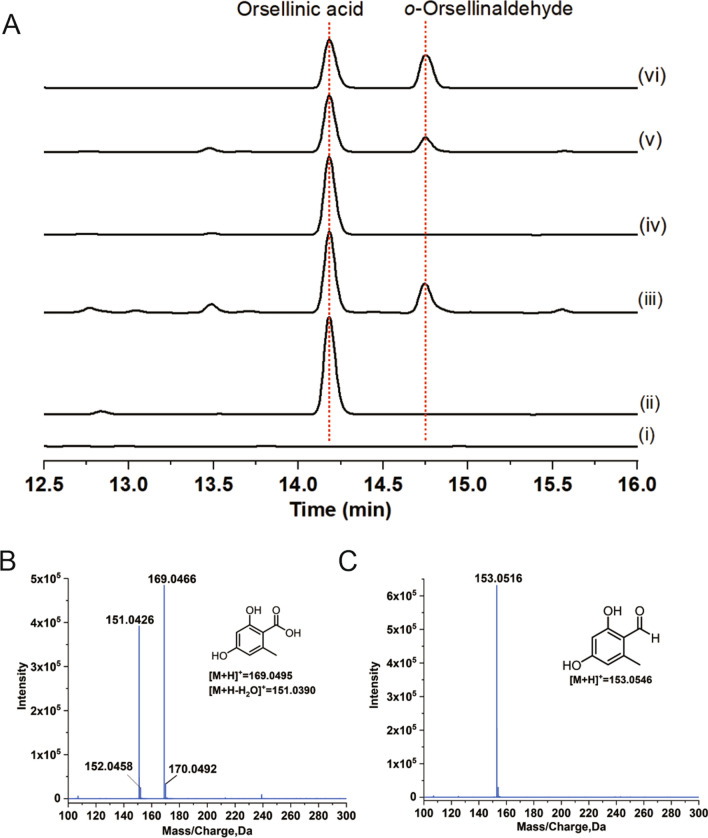
To investigate the function of HerA, the herA gene was amplified from the H. erinaceus CS4 genomic DNA (gDNA) and cloned into the pUARA2 plasmid. The pUARA2 containing herA was subsequently transformed into A. oryzae NSAR1 to obtain AO-herA transformants. HPLC analysis of the AO-herA mycelial extract showed a new peak with maximum UV absorption of 207, 262, and 300 nm (Additional file 1: Fig. S2), while this peak was not found in the control of the wild-type strain (Fig. 3A). To determine the structure of this compound, the AO-herA transformants were cultured on rice medium on a large scale. The crude ethyl acetate extract of the ferment was isolated by silica gel column chromatography and HPLC purification to obtain the pure monomeric compound. High-resolution electrospray mass spectrometry (HR-ESI–MS) analysis determined the molecular weight [M + H]+ 169.0466 of the compound with the presumed molecular formula of C8H8O4 (calculated as [M + H]+169.0495) (Fig. 3B). Further NMR examination characterized the structure, and its NMR data (Additional file 1: Figs. S3, S4, and Table S2) were consistent with the reported NMR data of OA [32].
Fig. 3.
Heterologous expression and characterization of metabolites. A HPLC profiles of OA and o-Orsellinaldehyde produced by the transformants. (i) A. oryzae NSAR1, (ii) AO-herA, (iii) AO-herA-pks5, (iv) Bioconversion of OA by A. oryzae NSAR1, (v) Bioconversion of OA by AO-pks5, (vi) Standard of Orsellinic acid and o-Orsellinaldehyde. B MS spectrum of OA. C MS spectrum of o-Orsellinaldehyde
The heterologous expression of herA in A. oryzae, that indicates HerA is an OAS from the H. erinaceus and is responsible for OA production. The ability of A. oryzae to correctly recognize the seven introns of herA (Additional file 1: Table S3), as a filamentous fungus, it can efficiently express the gDNA gene of basidiomycetes.
Functional analysis of Pks5
Ustilago maydis is a typical plant pathogenic fungus, and there are five PKS genes in its genome sequence. A recent study showed that pks5 is a polyketide synthase-encoding gene from U. maydis associated with the biosynthesis of melanin. Inactivation of pks5 results in the loss of o-Orsellinaldehyde, and the accumulation of OA in U. maydis. This result suggests that the function of Pks5 is responsible for the conversion of OA to o-Orsellinaldehyde [23]. Bioinformatics analysis revealed that Pks5 contains two introns, and three structural domains, AMP, ACP, and R (Additional file 1: Table S1), with 30% identity to the NRPS-like enzyme ATEG_03630. To construct a cell factory to produce o-Orsellinaldehyde, we cloned the pks5 gene from U. maydis and transferred it into the AO-herA strain to obtain an AO-herA-pks5 transformant. HPLC analysis revealed a new peak in the metabolites of the AO-herA-pks5 transformants relative to the AO-herA strain, whose retention time was consistent with that of the standard o-Orsellinaldehyde (Fig. 3A). To further confirm the function of pks5, an A. oryzae transformant containing pks5 was constructed. AO-pks5 was fed with OA as substrate, and HPLC analysis showed that the AO-pks5 strain could convert OA into a new compound with a retention time consistent with that of o-Orsellinaldehyde (Fig. 3A). HR-ESI–MS analysis showed that the compound had a [M + H]+ of 153.0516, with the presumed molecular formula C8H7O3 (calculated as [M + H]+153.0546) (Fig. 3C). The 1H and 13C NMR data of this compound (Additional file 1: Figs. S5, S6, and Table S2) agree with the known o-Orsellinaldehyde data [30]. Our results indicated that A. oryzae can correctly splice the two introns of pks5 and that the incomplete structural domain with Pks5 has the function of converting carboxyl groups to aldehyde groups.
Optimization of OA and o-Orsellinaldehyde yields
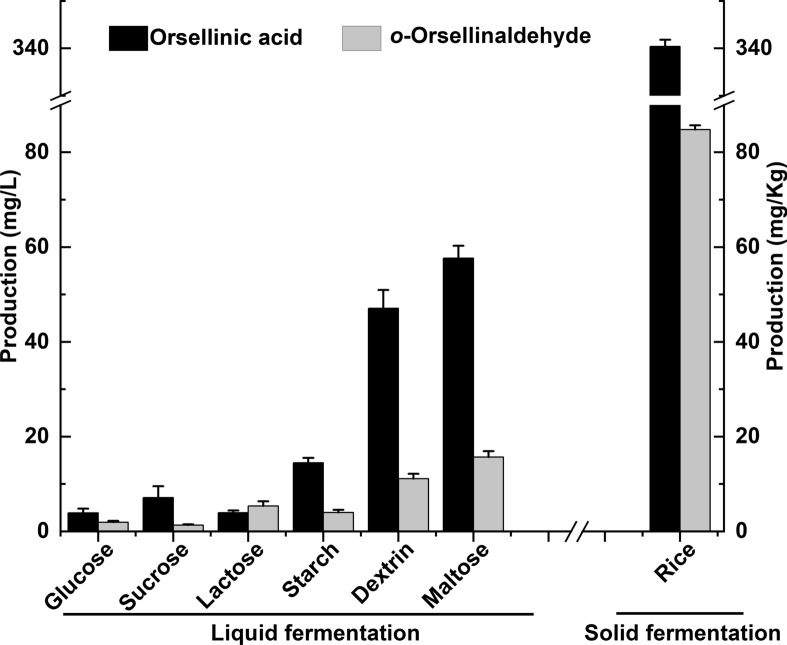
Since both OA and o-Orsellinaldehyde are important pharmaceutical intermediates and chemical raw materials, we proceeded to optimize the yields of the two compound-producing. Carbon sources provide the energy for microbial growth, and the carbon skeleton for metabolites. Considering the cost of large-scale production, we needed to screen for carbon sources that were less expensive and high yields of metabolites. The six different carbon sources (glucose, sucrose, lactose, dextrin, maltose, and starch) were selected for carbon source screening to compare the effects on the production of OA and o-Orsellinaldehyde. The results showed that the addition of different carbon sources had different effects on OA and o-Orsellinaldehyde production, where the highest OA and o-Orsellinaldehyde yields of 57.68 mg/L and 15.71 mg/L, respectively, were obtained when maltose was used as the carbon source (Fig. 4). In addition, OA and o-Orsellinaldehyde production was higher than that of glucose, sucrose, and lactose when starch and dextrin were used as carbon sources. In the present experiment, the α-amylase promoter was used for the expression of exogenous genes, and maltose was able to induce the expression of amylases, including α-amylase in A. oryzae [33]. Therefore, the yield of OA and o-Orsellinaldehyde was highest when maltose was used as the carbon source.
Fig. 4.
Effects of different carbon sources and fermentation conditions on the yield of Orsellinic acid and o-Orsellinaldehyde
Compared to liquid cultures, solid cultures with rice medium showed a 5.90- and 5.40-fold increase in OA and o-Orsellinaldehyde production, with yields of 340.41 mg/kg and 84.79 mg/kg, respectively (Fig. 4). Tagami K et al. reconstructed the biosynthesis of the indole diterpenoid in A. oryzae, when using maltose as a carbon source for liquid fermentation, the yield of paxilline was 35 mg/L [34], however the yield of aflatrem was 54 mg/Kg when rice was used as a solid medium [35]. This suggests that during solid culture, A. oryzae has support attached to it during growth. It can form a differentiation between trophic and aerial mycelium, this form facilitates the production of a large number of enzymes, that able to increase the efficiency of heterologous expression of proteins, thus increasing the yield of metabolites. In contrast, under liquid culture conditions, which are relatively unstable, A. oryzae tends to form balls of varying sizes, which may have affected the production of metabolites. Furthermore, in the later stages of liquid culture, the pH of the medium tends to be acidic. Aldehyde groups are unstable under acid conditions. This may be one of the reasons why o-Orsellinaldehyde is more productive in solid medium. So far, A. oryzae is suitable for heterologous expression of basidiomycetes PKS products.
Here, we have demonstrated the successful expression of PKS genes herA and pks5 from basidiomycetes in A. oryzae, resulting in the construction of a cell factory capable of producing OA and o-Orsellinaldehyde. Our findings highlight the potential of A. oryzae as a promising platform to produce mushroom polyketide compounds, which offering several advantages such as low cost, high efficiency, environmental friendliness, and sustainability. These results not only provide valuable insights into the biosynthesis of important pharmaceutical intermediates, but also offer an important contribution to the elucidation of gene function.
Conclusion
In this work, we identified the functions of HerA and Pks5 by heterologous expression in A. oryzae as hosts, and simultaneously obtained OA and o-Orsellinaldehyde producer strains. Carbon source optimization and screening of fermentation methods pointed to the optimal production of OA and o-Orsellinaldehyde. This result lays the foundation for the possible future industrial production of both compounds.
It is well known that basidiomycetes are a demanding species with different growth cycles. In some cases, the correct cDNA sequence cannot be obtained. This limits the research on the biosynthesis of basidiomycetes natural products. A. oryzae is an excellent host for heterologous expression. It is competent to directly express intron-containing genes, with the ability to correctly splice mRNA and translate. This strategy saves time in basidiomycetes cultivation and provides a new approach to the biosynthesis of basidiomycetes natural products. We believe that A. oryzae heterologous expression tools will have more widespread applications.
Material and methods
General experimental procedures
All reagents commercially supplied were used as received. HPLC analysis was performed using an Agilent 1260 Series with a DAD detector (California, CA, USA). 1H- and 13C-NMR spectra were recorded on Bruker AVAN CEIII HD 500. Chemical shifts were reported as δ scale in ppm as an internal reference (CD3OD; 1H NMR = 3.31 ppm, 13C NMR = 49.0 ppm). Mass spectra were obtained with an AB SCEIX Triple TOF 6600. Column chromatography was carried out on C18 silica gel (Agilent Technologies. USA). Oligonucleotides for polymerase chain reaction (PCR) were purchased from RuiBiotech Biotechnology Co., Ltd.
Strains and culture conditions
H. erinaceus CS-4 (CCTCC AF 2018025) [36] was cultivated at 25 °C, 170 rpm in potato dextrose broth (PDB) liquid medium for one week and used as a source for the cloning of herA gene. The U. maydis (CGMCC 5.208) was grown at 28 °C, 200 rpm in YEPS (yeast extract-pep-tone-sucrose: 1% yeast extract, 2% peptone, 2% sucrose, 100 mL) for one week and used as a source for the cloning of Pks5 gene. Standard DNA engineering was performed with Escherichia coli DH5α and culture at 37 ℃ for grown. A. oryzae NSAR1 (niaD-, sC-, ΔargB, adeA-) was used as the fungal heterologous expression host in this study, and growth at 30 °C, 200 rpm in DPY (dextrin-polypeptone-yeast extract: 2% dextrin, 1% polypeptone, 0.5% yeast extract, 100 mL) medium supplemented with appropriate nutrients.
Extraction of the genomes and construction of plasmids
Extraction of the genomes of H. erinaceus and U. maydis was carried out according to the literature procedure [34]. The herA was divided into two fragments for amplification from the genome of H. erinaceus with the primers described in Additional file 1: Table S4, and then introduced into pUARA2 vector using ClonExpress MultiS One Step Cloning Kit (Vazymebiotech Laboratories) to construct expression plasmids, pUARA2-herA (Additional file 1: Table S5). The pks5 was amplified from the genome of U. maydis with the primers described in Additional file 1: Table S4 and then introduced into pUSA2 vector, using a ClonExpress Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd) to construct expression plasmids, pUSA2-pks5 (Additional file 1: Table S5).
Transformation of Aspergillus oryzae
Transformation of A. oryzae NSAR1 [37] was carried out by the previously reported protoplast–polyethylene glycol method [38]. The plasmid pUARA2-herA was used for the first transformation to construct AO-herA. This transformant was further transformed with pUSA2-pks5 to construct AO-herA-pks5. pUSA2-pks5 was used for the transformation to construct AO-pks5.
Biotransformation of OA using AO-pks5
Mycelia of the transformant with pks5 were inoculated into MPY (maltose-peptone-yeast extract: 3% maltose, 1% polypeptone, 0.5% yeast extract) medium (2 mL) containing appropriate nutrients in 10 mL test tube. OA (20 μg, methanol solution) was then administered to the culture medium. After an additional 3 days of incubation at 30 °C, 200 rpm. The mycelium was removed by filtration, and the broth was extracted with ethyl acetate, then the organic layers were concentrated in vacuo. The crude extracts were directly analyzed by HPLC and LC–MS.
Extraction and analysis of metabolites
Mycelia of AO-herA and AO-herA-pks5 transformants were inoculated into a solid medium containing polished rice (1 g) and appropriate adenine at 30 °C for 10 days. After extraction with ethyl acetate, the extract was concentrated in vacuo to afford crude extracts. The crude extracts were analyzed by HPLC equipped with an Agilent TC-C18 (250 mm × 4.6 mm) at the following conditions: 0–5 min, 10% B; 5–20 min, a linear gradient 10–100% B; 20–30 min, 100% B (A: H2O + 0.1% of formic acid, B: CH3OH + 0.1% of formic acid) at a flow rate of 1 mL/min. Samples were analyzed using a TripleTOF 6600 mass spectrometer (AB/SCIEX, Milford, MA) and an HPLC system (AB/SCIEX). Chromato- graphic separation was achieved using a 150 mm × 4.6 mm, 2.6 μm Kinetex C18 100A column (Phenomenex) at the following conditions: 0-10 min, 5–100% B;10–15 min, 100% B (A: H2O + 0.1% of formic acid, B: CH3CN + 0.1% of formic acid) at a flow rate of 0.6 mL/min.
Effect of different carbon sources on the production of OA and o-Orsellinaldehyde
The spore suspension (1 × 108 Cell/mL) of AO-herA-pks5 transformant was inoculated into 100 mL of PY (1% polypeptone, 0.5% yeast extract) liquid medium supplemented with 2% with different carbon sources (glucose, sucrose, lactose, dextrin, starch, and maltose) in 500 mL Erlenmeyer flasks. After 3 days of incubation at 30 ℃ and 200 rpm. The mycelium was removed by filtration, and the broth was extracted with ethyl acetate three times. Then, the organic layers were concentrated in vacuo. The crude extracts were obtained and analyzed by the above method. All experiments were replicated three times.
Isolation and purification of each metabolite
Mycelia of AO-herA-pks5 transformants were inoculated into a solid medium containing polished rice (1 kg) and appropriate adenine at 30 °C for 10 days. The mycelia were extracted with ethyl acetate at room temperature overnight. The ethyl acetate layer was washed with brine and concentrated in vacuo. The crude extracts were isolated using silica gel column chromatography (Petroleum ether: ethyl acetate, 6:1 to 2:1). The isolated compounds were further purified by semi-preparative HPLC.
Supplementary Information
Additional file 1: Figure S1. NR-PKS and NRPS-like enzyme has been reported to be responsible for the formation of synthetic aldehyde groups. Figure S2. UV spectroscopy of OA. Figure S3. 1H-NMR spectrum of OA (CD3OD-d4, 500 MHz). Figure S4. 13C-NMR spectrum of OA (CD3OD-d4, 125 MHz). Figure S5. 1H-NMR spectrum of o-Orsellinaldehyde (CD3OD-d4, 500 MHz). Figure S6. 13C-NMR spectrum of o-Orsellinaldehyde (CD3OD-d4, 125 MHz). Table S1. Domain organizations of PKS in this study. Table S2. NMR data for OA and o-Orsellinaldehyde in CD3OD-d4 (500 MHz for 1H NMR,125 MHz for 13C NMR). Table S3. DNA and protein sequences. Table S4. Primers used in this study. Table S5. Plasmids constructed in this study.
Acknowledgements
We are grateful to Prof. Hideaki Oikawa, Hokkaido University for providing A. oryzae NSAR1 and the expression vectors pUSA2 and pUARA2, we are also grateful to Dr. Shengnan Tan and Dan Sui, Instrumental analysis center in Northeast Forestry University for supplying HR-ESI-MS and NMR analysis.
Abbreviations
- OA
Orsellinic acid
- PKS
Polyketide synthase
- NRPS-like
Non-ribosomal peptide synthase-like
- SSNs
Sequence similarity networks
- AO
Aspergillus oryzae
- NR-PKS
Non-reducing polyketide synthase
- OAS
Orsellinic acid synthase
- HPLC
High performance liquid chromatography
- HR-ESI–MS
High resolution electrospray ionization mass spectrum
- NMR
Nuclear Magnetic Resonance
- gDNA
Genomic DNA
Author contributions
HH, JQ, and CL designed the study and wrote the manuscript. CX, XX, and CL critically revised the manuscript. HH, CY, JQ, PW, PZ, and GW performed the experiments and analyzed the results. XX, and CL designed and supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by the Innovation & Development Joint Fund of Natural Science Foundation from Shandong Province (No. ZR2021LSW022), the National Natural Science Foundation of China (No. U22A20369 and 31800031), the Key innovation Project of the Qilu University of Technology (Shandong Academy of Sciences, No. 2022JBZ01-06) and Natural Science Foundation from Shandong Province (No. ZR2022QC186).
Availability of data and materials
All data for this study are included in this published article and its additional file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xuekui Xia, Email: xiaxk@sdas.org.
Chengwei Liu, Email: liuchw@nefu.edu.cn.
References:
- 1.Chen GD, Hu D, Huang MJ, Tang J, Wang XX, Zou J, Xie J, Zhang WG, Guo LD, Yao XS, et al. Sporormielones A-E, bioactive novel C-C coupled orsellinic acid derivative dimers, and their biosynthetic origin. Chem Commun (Camb) 2020;56:4607–4610. doi: 10.1039/D0CC00855A. [DOI] [PubMed] [Google Scholar]
- 2.Saeki H, Hara R, Takahashi H, Iijima M, Munakata R, Kenmoku H, Fuku K, Sekihara A, Yasuno Y, Shinada T, et al. An aromatic farnesyltransferase functions in biosynthesis of the anti-HIV meroterpenoid daurichromenic acid. Plant Physiol. 2018;178:535–551. doi: 10.1104/pp.18.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Du L, Qu Z, Zhang X, Li F, Li Z, Qi F, Wang X, Jiang Y, Men P, et al. Compartmentalized biosynthesis of mycophenolic acid. Proc Natl Acad Sci USA. 2019;116:13305–13310. doi: 10.1073/pnas.1821932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki Y, Awakawa T, Matsuzaki M, Cho R, Matsuda Y, Hoshino S, Shinohara Y, Yamamoto M, Kido Y, Inaoka DK, et al. Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. Proc Natl Acad Sci USA. 2019;116:8269–8274. doi: 10.1073/pnas.1819254116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherlach K, Schuemann J, Dahse HM, Hertweck C. Aspernidine A and B, prenylated isoindolinone alkaloids from the model fungus Aspergillus nidulans. J Antibiot (Tokyo) 2010;63:375–377. doi: 10.1038/ja.2010.46. [DOI] [PubMed] [Google Scholar]
- 6.Mogi T, Ui H, Shiomi K, Omura S, Miyoshi H, Kita K. Antibiotics LL-Z1272 identified as novel inhibitors discriminating bacterial and mitochondrial quinol oxidases. Biochim Biophys Acta. 2009;1787:129–133. doi: 10.1016/j.bbabio.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Matsuda Y, Gao H, Hu D, Yao XS, Abe I. Biosynthesis of LL-Z1272beta: discovery of a new member of NRPS-like enzymes for aryl-aldehyde formation. ChemBioChem. 2016;17:904–907. doi: 10.1002/cbic.201600087. [DOI] [PubMed] [Google Scholar]
- 8.Kuang Y, Li B, Wang Z, Qiao X, Ye M. Terpenoids from the medicinal mushroom Antrodia camphorata: chemistry and medicinal potential. Nat Prod Rep. 2021;38:83–102. doi: 10.1039/D0NP00023J. [DOI] [PubMed] [Google Scholar]
- 9.Kawagishi H, Ando M, Sakamoto H, Yoshida S, Ojima F, Ishiguro Y, Ukai N, Furukawa S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991;32:4561–4564. doi: 10.1016/0040-4039(91)80039-9. [DOI] [Google Scholar]
- 10.Ueda K, Tsujimori M, Kodani S, Chiba A, Kubo M, Masuno K, Sekiya A, Nagai K, Kawagishi H. An endoplasmic reticulum (ER) stress-suppressive compound and its analogues from the mushroom Hericium erinaceum. Bioorg Med Chem. 2008;16:9467–9470. doi: 10.1016/j.bmc.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Kawagishi H, Ando M, Mizuno T. Hericenone A and B as cytotoxic principles from the mushroom Hericium erinaceum. Tetrahedron Lett. 1990;31:373–376. doi: 10.1016/S0040-4039(00)94558-1. [DOI] [Google Scholar]
- 12.Weitnauer G, Muhlenweg A, Trefzer A, Hoffmeister D, Sussmuth RD, Jung G, Welzel K, Vente A, Girreser U, Bechthold A. Biosynthesis of the orthosomycin antibiotic avilamycin A: deductions from the molecular analysis of the avi biosynthetic gene cluster of Streptomyces viridochromogenes Tu57 and production of new antibiotics. Chem Biol. 2001;8:569–581. doi: 10.1016/S1074-5521(01)00040-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahlert J, Shepard E, Lomovskaya N, Zazopoulos E, Staffa A, Bachmann BO, Huang K, Fonstein L, Czisny A, Whitwam RE, et al. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science. 2002;297:1173–1176. doi: 10.1126/science.1072105. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez JF, Chiang YM, Szewczyk E, Davidson AD, Ahuja M, Elizabeth Oakley C, Woo Bok J, Keller N, Oakley BR, Wang CC. Molecular genetic analysis of the orsellinic acid/F9775 gene cluster of Aspergillus nidulans. Mol Biosyst. 2010;6:587–593. doi: 10.1039/B904541D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taura F, Iijima M, Yamanaka E, Takahashi H, Kenmoku H, Saeki H, Morimoto S, Asakawa Y, Kurosaki F, Morita H. A novel class of plant type III polyketide synthase involved in orsellinic acid biosynthesis from Rhododendron dauricum. Front Plant Sci. 2016;7:1452. doi: 10.3389/fpls.2016.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishiuchi K, Nakazawa T, Ookuma T, Sugimoto S, Sato M, Tsunematsu Y, Ishikawa N, Noguchi H, Hotta K, Moriya H, Watanabe K. Establishing a new methodology for genome mining and biosynthesis of polyketides and peptides through yeast molecular genetics. ChemBioChem. 2012;13:846–854. doi: 10.1002/cbic.201100798. [DOI] [PubMed] [Google Scholar]
- 17.Lackner G, Bohnert M, Wick J, Hoffmeister D. Assembly of melleolide antibiotics involves a polyketide synthase with cross-coupling activity. Chem Biol. 2013;20:1101–1106. doi: 10.1016/j.chembiol.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Braesel J, Fricke J, Schwenk D, Hoffmeister D. Biochemical and genetic basis of orsellinic acid biosynthesis and prenylation in a stereaceous basidiomycete. Fungal Genet Biol. 2017;98:12–19. doi: 10.1016/j.fgb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Yu PW, Chang YC, Liou RF, Lee TH, Tzean SS. pks63787, a polyketide synthase gene responsible for the biosynthesis of benzenoids in the medicinal mushroom Antrodia cinnamomea. J Nat Prod. 2016;79:1485–1491. doi: 10.1021/acs.jnatprod.5b00798. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Beissner M, Zhao H. Aryl-aldehyde formation in fungal polyketides: discovery and characterization of a distinct biosynthetic mechanism. Chem Biol. 2014;21:257–263. doi: 10.1016/j.chembiol.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaegashi J, Praseuth MB, Tyan SW, Sanchez JF, Entwistle R, Chiang YM, Oakley BR, Wang CC. Molecular genetic characterization of the biosynthesis cluster of a prenylated isoindolinone alkaloid aspernidine A in Aspergillus nidulans. Org Lett. 2013;15:2862–2865. doi: 10.1021/ol401187b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison J, Al Fahad A, Cai M, Song Z, Yehia SY, Lazarus CM, Bailey AM, Simpson TJ, Cox RJ. Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis. Proc Natl Acad Sci USA. 2012;109:7642–7647. doi: 10.1073/pnas.1201469109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Fernandez EZ, Shi YM, Grun P, Bode HB, Bolker M. An unconventional melanin biosynthesis pathway in Ustilago maydis. Appl Environ Microbiol. 2021;87:e01510–01520. doi: 10.1128/AEM.01510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulck T, Moller BL. Phytocannabinoids: origins and biosynthesis. Trends Plant Sci. 2020;25:985–1004. doi: 10.1016/j.tplants.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Chiang C-Y, Ohashi M, Tang Y. Deciphering chemical logic of fungal natural product biosynthesis through heterologous expression and genome mining. Nat Prod Rep. 2022;40:89–127. doi: 10.1039/D2NP00050D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oikawa H. Reconstitution of biosynthetic machinery of fungal natural products in heterologous hosts. Biosci Biotechnol Biochem. 2020;84:433–444. doi: 10.1080/09168451.2019.1690976. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Minami A, Ozaki T, Wu J, Kawagishi H, Maruyama JI, Oikawa H. Efficient reconstitution of Basidiomycota Diterpene Erinacine gene cluster in ascomycota host Aspergillus oryzae based on genomic DNA sequences. J Am Chem Soc. 2019;141:15519–15523. doi: 10.1021/jacs.9b08935. [DOI] [PubMed] [Google Scholar]
- 28.Qi J, Han H, Sui D, Tan S, Liu C, Wang P, Xie C, Xia X, Gao JM, Liu C. Efficient production of a cyclic dipeptide (cyclo-TA) using heterologous expression system of filamentous fungus Aspergillus oryzae. Microb Cell Fact. 2022;21:146. doi: 10.1186/s12934-022-01872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lackner G, Misiek M, Braesel J, Hoffmeister D. Genome mining reveals the evolutionary origin and biosynthetic potential of basidiomycete polyketide synthases. Fungal Genet Biol. 2012;49:996–1003. doi: 10.1016/j.fgb.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, Whalen KL. Enzyme function initiative-enzyme similarity tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim Biophys Acta. 2015;1854:1019–1037. doi: 10.1016/j.bbapap.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion's Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J Agric Food Chem. 2015;63:7108–7123. doi: 10.1021/acs.jafc.5b02914. [DOI] [PubMed] [Google Scholar]
- 32.Tian YQ, Lin XP, Liu J, Kaliyaperumal K, Ai W, Ju ZR, Yang B, Wang J, Yang XW, Liu Y. Ascomycotin A, a new citromycetin analogue produced by Ascomycota sp. Ind19F07 isolated from deep sea sediment. Nat Prod Res. 2015;29:820–826. doi: 10.1080/14786419.2014.988620. [DOI] [PubMed] [Google Scholar]
- 33.Tada S, Gomi K, Kitamoto K, Takahashi K, Tamura G, Hara S. Construction of a fusion gene comprising the Taka-amylase A promoter and the Escherichia coli beta-glucuronidase gene and analysis of its expression in Aspergillus oryzae. Mol Gen Genet. 1991;229:301–306. doi: 10.1007/BF00272170. [DOI] [PubMed] [Google Scholar]
- 34.Tagami K, Liu C, Minami A, Noike M, Isaka T, Fueki S, Shichijo Y, Toshima H, Gomi K, Dairi T, Oikawa H. Reconstitution of biosynthetic machinery for indole-diterpene paxilline in Aspergillus oryzae. J Am Chem Soc. 2013;135:1260–1263. doi: 10.1021/ja3116636. [DOI] [PubMed] [Google Scholar]
- 35.Tagami K, Minami A, Fujii R, Liu C, Tanaka M, Gomi K, Dairi T, Oikawa H. Rapid reconstitution of biosynthetic machinery for fungal metabolites in Aspergillus oryzae: total biosynthesis of aflatrem. ChemBioChem. 2014;15:2076–2080. doi: 10.1002/cbic.201402195. [DOI] [PubMed] [Google Scholar]
- 36.Gong W, Wang Y, Xie C, Zhou Y, Zhu Z, Peng Y. Whole genome sequence of an edible and medicinal mushroom, Hericium erinaceus (Basidiomycota, Fungi) Genomics. 2020;112:2393–2399. doi: 10.1016/j.ygeno.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Jin FJ, Maruyama J, Juvvadi PR, Arioka M, Kitamoto K. Development of a novel quadruple auxotrophic host transformation system by argB gene disruption using adeA gene and exploiting adenine auxotrophy in Aspergillus oryzae. FEMS Microbiol Lett. 2004;239:79–85. doi: 10.1016/j.femsle.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Tagami K, Minami A, Matsumoto T, Frisvad JC, Suzuki H, Ishikawa J, Gomi K, Oikawa H. Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole Diterpene Penitrem. Angew Chem Int Ed. 2015;54:5748–5752. doi: 10.1002/anie.201501072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. NR-PKS and NRPS-like enzyme has been reported to be responsible for the formation of synthetic aldehyde groups. Figure S2. UV spectroscopy of OA. Figure S3. 1H-NMR spectrum of OA (CD3OD-d4, 500 MHz). Figure S4. 13C-NMR spectrum of OA (CD3OD-d4, 125 MHz). Figure S5. 1H-NMR spectrum of o-Orsellinaldehyde (CD3OD-d4, 500 MHz). Figure S6. 13C-NMR spectrum of o-Orsellinaldehyde (CD3OD-d4, 125 MHz). Table S1. Domain organizations of PKS in this study. Table S2. NMR data for OA and o-Orsellinaldehyde in CD3OD-d4 (500 MHz for 1H NMR,125 MHz for 13C NMR). Table S3. DNA and protein sequences. Table S4. Primers used in this study. Table S5. Plasmids constructed in this study.
Data Availability Statement
All data for this study are included in this published article and its additional file.