Abstract
Background
Due to the lack of effective treatment, metastasis is the main cause of cancer related deaths. TGF-β pathway has been reported related to cervical cancer metastasis. However, mechanism is still unclear.
Methods
After agonist of TGF-β treatment, RNA sequencing revealed the expression profiles of circRNA in cervical cancer. In situ hybridization was used to analysis relationship between CDR1as and prognosis. Real-time PCR, Western blot, RNA interference, Transwell assay, Wound healing assay, RNA pulldown assay and RIP assays were performed in vitro. And in vivo cervical cancer model (including foot pad model and subcutaneous tumor formation) was also performed.
Results
CDR1as was found upregulated obviously following TGF-β activation. In situ hybridization showed CDR1as was positively correlated with lymph node metastasis and shortened survival length. Simultaneously, overexpression of CDR1as promoted cervical cancer metastasis in vitro and in vivo. It was also found that CDR1as could facilitate the orchestration of IGF2BP1 on the mRNA of SLUG and stabilize it from degradation. Silencing IGF2BP1 hampers CDR1as related metastasis in cervical cancer. Additionally, effective CDR1as has been proven to activate TGF-β signaling factors known to promote EMT, including P-Smad2 and P-Smad3.
Conclusions
Our study proved TGF-β signaling may promote cervical cancer metastasis via CDR1as.
Keywords: Cervical cancer, Metastasis, TGF-β, CDR1as, Slug, IGF2BP1
Introduction
Although cervical cancer screening and administration of HPV vaccines have been widely used, cervical cancer still remains the fourth greatest global burden of female cancer on incidence and mortality because of metastasis [1–3]. The overall survival rate for metastasis is still poor. Hence, effective predictive biomarkers are vital to alleviate the progression.
More and more evidence show that metastasis is a multi-step process involving complex interaction of transcriptome changes. TGF-β is a multifunctional cytokine that is associated with cancer metastasis in various cancer cells [4]. While it is well known that TGF-β-induced metastasis is very complex, the underlying mechanisms involved in cervical cancer are not fully understood.
N6-methyladenosine (m6A) is the most common modification in messenger RNA (mRNA). The insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) have been considered as m6A readers, which could target thousands of mRNA transcripts through recognizing the consensus GG(m6A) C sequence [5]. IGF2BPs belong to a conserved family of RNA-binding oncofetal proteins. Several studies have shown that IGF2BPs could influence polarization, migration, morphology, metabolism, proliferation and differentiation [6–8].
CDR1as is a conventional circular RNA with a length of 1485 bp. It is located in chromosome X and directly transcribed from LINC000632 [9, 10]. There are 73 binding sites between CDR1as and let-7. It was found to promote brain maturation [11] and accelerate lung [12–14], esophagus [15, 16], and bladder cancer progression [17, 18]. Additionally, it has also been proven to be involved in cancer drug resistance [19]. The effects were suggested on a multi-post-transcriptional level [10, 20]. So, it seems that CDR1as may function as a potential biomarker and therapeutic target. However, the role of CDR1as in cervical cancer has not been clearly identified.
In this study, we firstly induced endothelial-mesenchymal transition (EMT) in cervical cancer via TGF-β induction; then, the expression of different circular RNAs was analyzed. It was found that CDR1as was significantly elevated when EMT was induced and stabilized Slug expression via binding with IGF2BP1. Our research firstly addressed that TGF-β played a major part in the regulation of CDR1as and required m6A modification on multiple metastatic-related genes in cervical cancer.
Material and methods
Patient information
65 patients diagnosed with cervical cancer from July 2010 to February 2014 in Sun Yat-sen University were recruited. All patients signed the contract for permission to use their tissue for experimental purposes. All tissues that were used for pathological classification were assessed by at least three pathologists. Chips were purchased from Shanghai Outdo Biotech Co., Ltd., including 111 cervical cancer tissue samples.
Cell culture and reagents
Hela, Siha, and C33a were all cultured in DMEM (HyClone, USA) culture medium, with 10% FBS (Gibco, USA), 1% penicillin streptomycin (Gibco, USA), 1% Mycoplasma Removal Agent (Yeasen Biotechnology, Shanghai), and were stored at 37 °C and a humidified atmosphere with 5% CO2 (Thermo Fisher, Germany). The TGF-beta activator SRI-011381 hydrochloride and the TGF-beta inhibitor LY2109761 were purchased from Selleck Chemicals.
RNase R digestion
RNA (1 μg) was digested with 3 μL 10X RNase R reaction buffer and 3 U RNase R for 2 h at 37 °C. 500 μL pre-cooled isopropanol, 1 μL glycogen, and 5 μL 30% NaAc were added into each sample. RNA was precipitated at− 80 °C for 24 h and extracted.
Agarose electrophoresis
Cell genomic DNA was extracted using human genomic DNA extraction kit (Kangwei, China). Cell cDNA was reverse transcribed using reverse transcription kit (TaKaRa, Japan). Primers for CDR1, CDR1as, and linc were concluded in Table 1. 2% agarose was diluted with boiled 0.5% TAE and cooled for gel making. 9 μL of cDNA and gDNA, along with 1 μL of loading buffer were added to each hole for electrophoresis. Gels were luminated by Invitrogen iBright CL1000.
Table 1.
Primers
| Primer | sense (5′-3′) | Anti-sense(5′-3′) |
|---|---|---|
| CDR1as | TCTGCTCGTCTTCCAACATC | CGGAAACCCTGGATATTGCA |
| CDR1 | CGGATTTCCTGGAAGACCTGGA | TCCGTGTCTTCCAGCAAGTCCA |
| β-actin | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| MALAT1 | TTCCGGGTGTTGTAGGTTTC | AAAAAACCCACAAACTTGCC |
| E-cadherin | GCCTCCTGAAAAGAGAGTGGAAG | TGGCAGTGTCTCTCCAAATCCG |
| N-cadherin | CCTCCAGAGTTTACTGCCATGAC | GTAGGATCTCCGCCACTGATTC |
| SLUG | ATCTGCGGCAAGGCGTTTTCCA | GAGCCCTCAGATTTGACCTGTC |
| SNAIL | TGCCCTCAAGATGCACATCCGA | GGGACAGGAGAAGGGCTTCTC |
| SMAD2 | GGGTTTTGAAGCCGTCTATCAGC | CCAACCACTGTAGAGGTCCATTC |
| SMAD3 | TGAGGCTGTCTACCAGTTGACC | GTGAGGACCTTGTCAAGCCACT |
| TWIST | GCCAGGTACATCGACTTCCTCT | TCCATCCTCCAGACCGAGAAGG |
| VIM | AGGCAAAGCAGGAGTCCACTGA | ATCTGGCGTTCCAGGGACTCAT |
| ZEB1 | GGCATACACCTACTCAACTACGG | TGGGCGGTGTAGAATCAGAGTC |
| ZEB2 | AATGCACAGAGTGTGGCAAGGC | CTGCTGATGTGCGAACTGTAGG |
| IGF2BP1 | CTTTGTAGGGCGTCTCATTGGC | CCTTCACAGTGATGGTCCTCTC |
| IGF2BP2 | GTTGGTGCCATCATCGGAAAGG | TGGATGGTGACAGGCTTCTCTG |
| IGF2BP3 | TCGTGACCAGACACCTGATGAG | GGTGCTGCTTTACCTGAGTCAG |
| SLUG site1 | TAAAGGAGCCGGGTGACTTC | TGTATGTGTGTCCAGTTCGC |
| SLUG site2 | AAGCCAAACTACAGCGAACT | AACAGTTGAATCTTTGGCTCTTT |
| SLUG site3 | TGCCTGTCATACCACAACCA | GAGGTGTCAGATGGAGGAGG |
| SLUG site4 | ACTACCGCTGCTCCATTCC | GGGTCTGAAAGCTTGGACTG |
| SLUG site5 | GCGCCCTGAAGATGCATATT | TCATGCAAATCCAACAGCCA |
| SLUG site6 | CAGACCCTGGTTGCTTCAAG | AGCAAGAAATGGAGCACTTTGT |
| SLUG site7 | GTGCTTTTAATGATGGACAGTCA | ACAGAACACACATTCAAGCACA |
CircRNA expression profiles in Cervical cancer cell
After treatment of SRI-011381 hydrochloride for three days, Human circRNA Arrays (8 × 15 K, Arraystar, Rockville, MD, USA) was used to detect the different circRNAs with ≥ 2 folds. The data were analyzed by using R software limma and Arraystar program (Arraystar).
Transient transfections and RNA interference
Cells with 30–50% density in 6 well plates were plated 24 h before transfection. Lipofectamine iMAX mixed with siRNAs in opti-MEM was added to each plate. RNA, protein, and cells were harvested for later research after 48 h transfection. siRNA sequences for each target were concluded in Table 1.
Transwell assay
Eight thousand cells were counted and suspended in 200 μL non-serum culture medium (for metastasis assay) or 200 μL non-serum matrix gel (for invasive assay) in upper-channel, while the lower channel was added with 600 μL complete medium. After 24 h, cells were fixed with 500 μL paraformaldehyde and stained with crystal violet. Cells that penetrated through the membrane were observed and photographed with an optical microscope. The relative rate for metastasis and invasion was analyzed and compared with the control group.
Wound-healing assay
Cells with 100% density were plated in 24-well plates. After 24 h, 1 mL pipette was used to create the indicated cellular wound. To observe the healing of wounds, cells were photographed microscopically after 0 h, 12 h, 24 h and 48h from the time of wound creation.
In situ hybridization in tissue (ISH)
Tissues that were embedded in paraffin were individually sliced. Detailed procedures were strictly followed using the enhanced sensitive ISH Detection Kit I (MK1030). Probes of CDR1as were purchased from GeneBio, Shanghai, China. The sequence was 5′ > T + GC + CATCCGGAAAC+C + CTGGATATTGCAGACACTGGAAGACC+TGA + AT< 3′. The expression strength was scored from 0 to 9, and the strength was calculated by multiplication between staining area and intensity. The staining area was defined as such: grade 0: ≤25% positive cells; grade 1: ≤50% positive cells; grade 2: ≤75% positive cells; grade 3: ≤100% positive cells. The staining intensity was defined as such: grade 0: no staining color; grade 1: yellow stain; grade 2: pale brown stain; grade 3: deep brown stain.
In situ florescence hybridization
Ten thousand cells were plated in confocal dishes for 24 h, then fixed with 4% paraformaldehyde. The exact procedures were strictly adhered to using the Fluorescence In Situ Hybridization Kit (RIBOBIO, Guangzhou, China). Positive cells were photographed with a Laser Scanning Confocal Microscope, which was magnified 400-fold. Each sample was randomly photographed more than 10 times. Probes of CDR1as were purchased from Synbio Technologies, and the sequence is GGT + GCCAT+CGGAAACCCT+ GGAT+ ATT+ GCAGACA.
In vivo tumor model
Nude mice (4–6-week-old female mice) were purchased from the animal center of Sun Yat-sen University. Procedures strictly followed the ethical regulations of animal experimentation in Sun Yat-sen University. Subcutaneous tumor formation: 1,000,000 Siha cells were subcutaneously injected at the axillary region of nude mice. Foot pad model: 2,000,000 Siha cells were subcutaneously injected at the foot pad of nude mice. Tumors were observed and measured once a week. Volume was calculated by the function of (V = (L*W2)/2) (V: tumor volume; L: tumor length; W: tumor width). Tumors were harvested from the mice when volume reached 1000 mm3.
RNA-pulldown assay
To pulldown proteins with CDR1as, biotin-labeled probes were designed and synthesized (GenePharma, Suzhou, China). 50 μL streptomycin magnetic beads were incubated with CDR1as probe and oligo probe at room temperature for 2 h. At the same time, 107 cells were fixed and lysed. Cell lysates were mixed with probe-coated beads and rotated overnight at 4 °C. Samples were separated by a magnetic separator and washed with pre-coated PBS for 3 times. 50 μL loading buffer was added and beads were boiled for 5 min. Then, the exact expression of indicated protein was examined by Western blot.
RNA binding protein immunoprecipitation assay (RIP)
Detailed processes during this section were performed according to the recommendation of Magna RIP Kit (MILLIPORE, Germany. Catalog No. 17–700). Antibody for m6A was purchased from ABCAM; IGF2BPs from SANTA.
Protein extraction and Western blot
Harvested cells were washed with PBS and lysed with RIPA (with 1% protease inhibitor and 1% phosphatase inhibitor). Protein content was measured by BCA assay (Thermo Fisher, Germany). Antibody for SMAD2 (Cat.5339), SMAD3 (Cat.9523), p-SMAD2 (Cat.5339), p-SMAD3 (Cat.9520), Vimentin (Cat.46173), E-Cadherin (Cat.14472), N-Cadherin (Cat.13116), and β-actin (Cat.3700) were purchased from CST (U.S.A). Slug (Cat.166476), IGF2BP1 (Cat.166344), IGF2BP2 (Cat.377014), and IGF2BP3 (Cat.365640) were purchased from SANTA(U.S.A).
RNA extraction and real-time PCR
Harvested cells were washed with PBS and lysed with Trizol (TaKaRa, Japan). Detailed procedures were followed form the instruction of TaKaRa. RNA was reverse-transcribed by PrimeScriptTM RT Master Mix Kit (TaKaRa, Japan). TB Green Premix Ex Taq II Kit (TakaRa, Japan) was used for quantitative real-time PCR and analyzed by Light Cycler 480 (Roche, Germany).
Statistical analysis
Statistical analysis was performed by using SPSS 20.0 and GraphPad 8.0. T test was used between two individual groups. Chi-squared test was used for correlation analysis. One-way ANOVA analysis were used among multiple groups. Survival rate of patients was evaluated by Kaplan-Meier curves and log-rank tests. P < 0.05 was considered as statistically significant.
Results
CDR1as was significantly upregulated after induction of TGF-β in cervical cancer cells
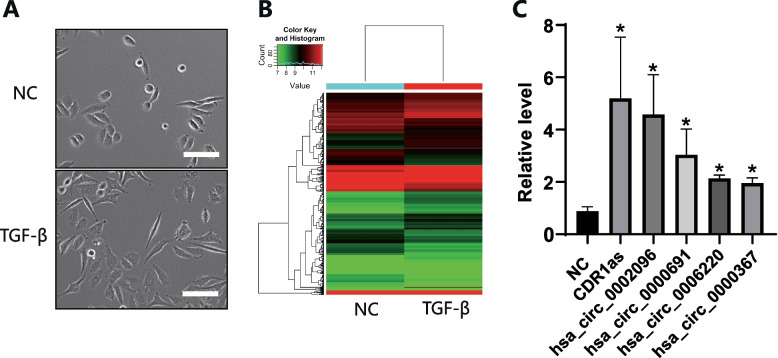
EMT is one of the hallmarks in the early stage of tumor progression, and its signal maintenance depends heavily on TGF- β activation. EMT phenotype was induced in Siha cells after treatment with SRI-011381 for 3 days (Fig. 1A). RNA Sequencing showed expression of 6964 circRNAs was up-regulated and 6596 down- regulated (Fig. 1B). We chose 50 circRNAs with the most obvious changes for RT-PCR test. The results showed that the fold change of CDR1as was the highest (Fig. 1C). Therefore, we boldly speculated that CDR1as was involved in cervical cancer metastasis induced by the TGF-β pathway.
Fig. 1.
Induction of EMT by TGF- β in cervical cancer cells. A Morphological changes of EMT in cervical cancer cells were induced by SRI-011381 treatment. B The cluster heat map demonstrated the differentially expressed circRNAs. C Real time PCR was used to verify the differential circRNAs expression, and the top five circRNAs with the most obvious upregulation were shown in the figure
CDR1as predicted lymph node metastasis and worse overall survival
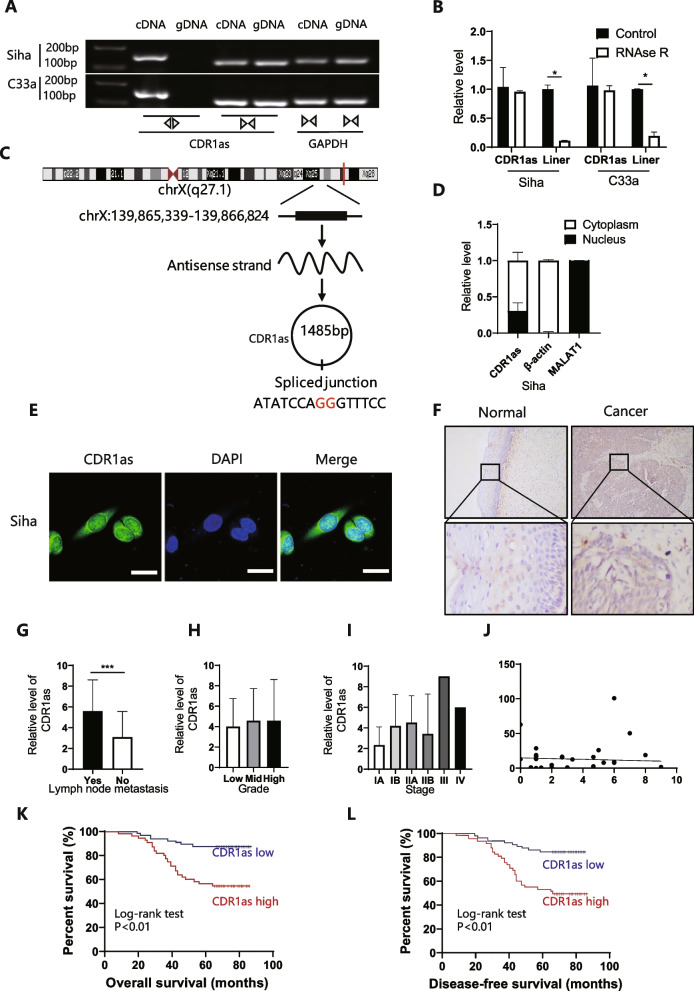
RNase R digestion assay revealed that the circular form of CDR1as could only be amplified in cDNA sample, while with CDR1, the line form existed in both gDNA and cDNA (Fig. 2A); after being digested by RNase R, the circular form remained significantly more stable compared with its line form (Fig. 2B). Sanger sequencing also indicated that the sequence of amplified oligo was similar to the sequence in www.circbase.com (Fig. 2C). These results demonstrated that CDR1as was a circular RNA.
Fig. 2.
Higher CDR1as level indicated lymph node metastasis and worse overall survival of patients with cervical cancer. A To detect if CDR1as is a circular RNA, divergent and convergent primers were designed, agarose gel electrophoresis showed that CDR1as was amplified by divergent primer in cDNA but not in gDNA. GAPDH was taken as the negative control; B RNase R treatment assay was performed, and only CDR1as showed strong stability but not its linear origin; C Chromosome location and transcripts of CDR1as; D, E Nuclear-plasma extraction assay and FISH showed that CDR1as was mainly expressed in cytoplasm; F ISH was used to evaluate the expression level of CDR1as in cervical cancer tissue; G, H, I, J CDR1as level among cervical cancer tissue was analyzed by lymph node metastasis, tumor FIGO stage, tumor grade, and tumor size; *P < 0.05, **P < 0.01; ns, not significant; unpaired Student’s t test K, L K-M analysis of CDR1as in 111 cervical cancer tissue samples
FISH and nuclear-plasma extraction assay indicated that CDR1as was mainly expressed in the cytoplasm (Fig. 2D, E), which suggested that CDR1as may work post-transcriptionally. In situ hybridization was used to evaluate the relationship between CDR1as and clinical parameters (Fig. 2F, Table 2), which demonstrated a higher expression of CDR1as among lymph node metastasis (Fig. 2G) and correlation with shorter overall survival and worse prognosis (Fig. 2K, L). However, there was no significant relationship between CDR1as and tumor grade, stage and primary tumor volume (Fig. 2H-J).
Table 2.
CDR1as with clinicopathological factors
| Low | High | χ2 | P-value | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤ 45 | 13 | 15 | 0.371 | 0.543 |
| >45 | 20 | 17 | ||
| Figo stage | ||||
| I | 32 | 29 | 1.132 | 0.355 |
| ≥ II | 1 | 3 | ||
| Lymph node metastasis | ||||
| No | 26 | 11 | 13.069 | < 0.001 |
| Yes | 7 | 21 | ||
| Grade | ||||
| Low | 14 | 12 | 0.167 | 0.92 |
| Mid | 17 | 18 | ||
| High | 2 | 2 | ||
| Metastasis | ||||
| No | 32 | 29 | 1.132 | 0.287 |
| Yes | 1 | 3 | ||
CDR1as may promote metastasis of cervical cancer cells
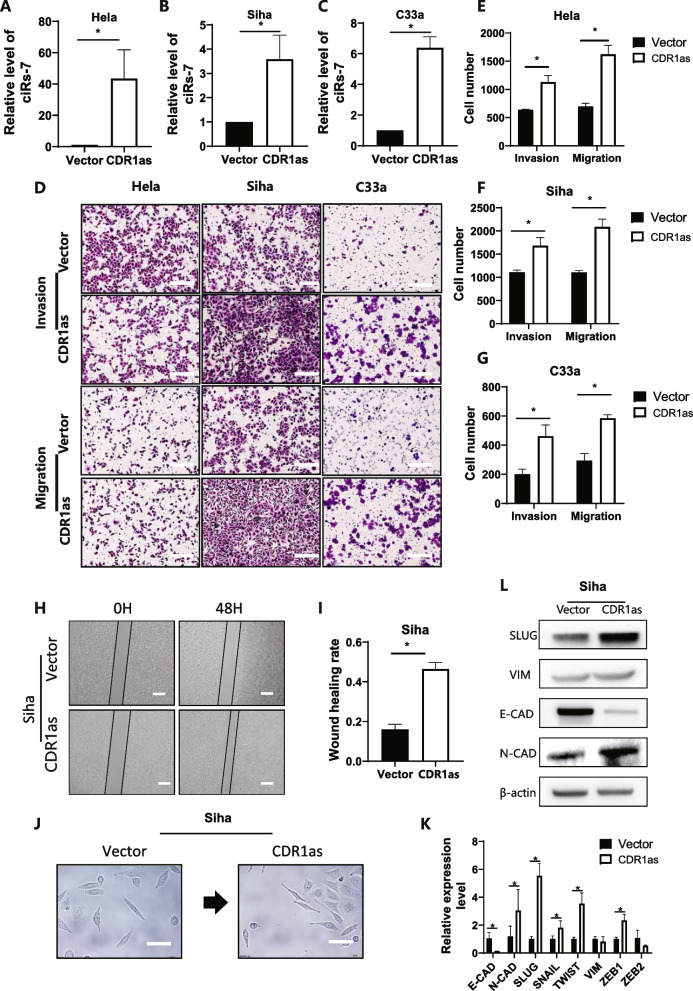
After overexpression of CDR1as in cancer cells (Fig. 3A-C), metastasis of cervical cancer was significantly expedited, according to more penetrated cells in the membrane from the Transwell assay (Fig. 3D), and shorter wound healing time (Fig. 3H). As illustrated, the motility of cancer cells was enhanced by increase of the invasive and metastatic rate by 1.86-fold and 2.07-fold, respectively (Fig. 3E-G). These results suggested that CDR1as promoted the metastasis of cervical cancer.
Fig. 3.
CDR1as promoted metastasis of cervical cancer cells. A, B, C CDR1as level was detected by qPCR after overexpression of CDR1as; D, E, F, G Transwell assay was performed to evaluate the migration and invasion ability after overexpression of CDR1as; H, I Wound healing assay was performed after overexpression of CDR1as; J Morphology change after CDR1as was overexpressed in cervical cancer cells; K, L qPCR and Western blot demonstrated an obvious change of metastasis-related genes and proteins
CDR1as promoted EMT in cervical cancer cells via binding with IGF2BP1
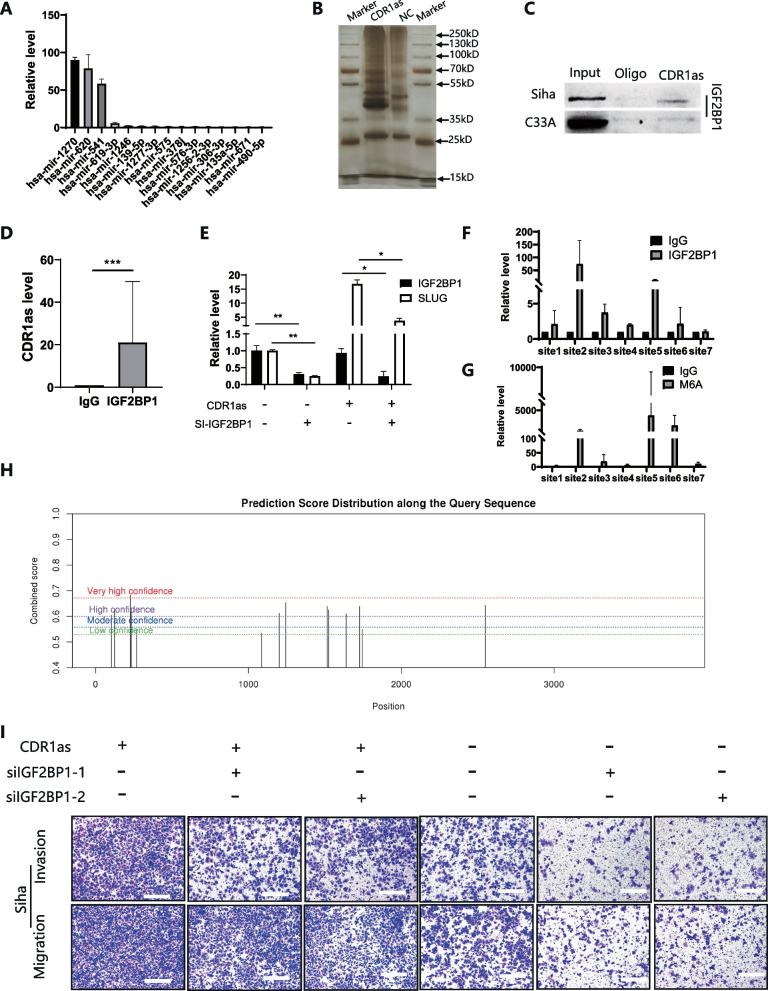
As illustrated in Fig. 3J, after elevated CDR1as, cells adopted a spindle-shaped morphology while cells transfected with vector still exhibited a sharp margin on the vector; qPCR and Western blot suggested that the increase of CDR1as reduced E-cadherin expression and increased the expression of mesenchymal marker, including N-cadherin and vimentin (Fig. 3K, L), while Slug was upregulated the most. According to bioinformatic analysis, we overlapped the search results from www.circinteractome.irp.nia.nih.gov, www.mirdb.org, www.targetscan.org, and www.circbank.cn to identify potential binding between CDR1as and miRNAs. Twenty-five miRNAs were uncovered to bind with CDR1as. Among these miRNAs, miR-203 could regulate Slug expression, upon bio-informative prediction. However, CDR1as probe pull-down assay did not reveal Slug pathway-related miRNA (Fig. 4A), indicating that Slug activation was probably not being regulated by miRNA sponge.
Fig. 4.
CDR1as promoted EMT in cervical cancer cells via binding with IGF2BP1. A qPCR was performed after pull-down using CDR1as probe; B Silver staining was performed after pull-down using CDR1as probe, a distinct band was discovered at 70 kD; C Western blot assay was performed after pull-down using CDR1as probe; D RIP assay using anti-IGF2BP1 shows an obvious enrichment of CDR1as; E qPCR was performed after IGF2BP1 was silenced; F, G, H Primers were designed for Slug’s M6A modification site, RIP assay using anti-m6A and anti- IGF2BP1 showed evident enrichment of Slug; I inhibition of IGF2BP1 reduced CDR1as-promoted metastatic and invasive potential
Apart from competing endogenous RNAs (ceRNAs), several reports suggested that CDR1as was able to affect protein interaction. For example, it was found that CDR1as disrupted the p53/MDM2 complex to inhibit gliomagenesis [20]. In melanoma, CDR1as has been reported to unleash pro-metastatic functions of IGF2BP3, which shed new light onto the proteomics of CDR1as [10]. Hence, we performed RNA-pulldown to identify the potential proteins that could bind with CDR1as. It was found that there were several individual bands between 35kD to 70kD (Fig. 4B). Through bio-informative prediction from www.interactive.irp.nia.nih.gov, IGF2BPs were detected to interact with CDR1as, which was consistent with the previous mentioned research. Moreover, blots were discovered around 70 kD, which just coincided with the molecular weight of IGF2BPs. Then, we performed RIP and pull-down assays and found that IGF2BP 1/2/3 could bind with CDR1as. Specifically, only IGF2BP1 could be detected by CDR1as probe (Fig. 4C, D), indicating that IGF2BP1 could be a specific potential downstream target.
IGF2BP1 is well known for its implication in m6A regulation, and its major role was as an m6A reader. Especially, IGF2BP1 could recognize the modified site of m6A on mRNA, prevent the decay and maintain the stability of its targeted mRNA [21]. To explore whether IGF2BP1 affects CDR1as elevated Slug, we silenced IGF2BP1 in CDR1as overexpressed cells. It showed that reduced CDR1as induced Slug upregulation (Fig. 4E). Besides, RIP assays suggested that IGF2BP1 preferentially bound with m6A modified sites on the mRNA of Slug (Fig. 4F&H). Silencing IGF2BP1 also inhibited the metastatic and invasive potential promoted by CDR1as (Fig. 4I). Taken together, CDR1as bound specifically with IGF2BP1 to stabilize and elevate Slug expression.
TGF-β signaling may activate EMT via CDR1as and m6A in cervical cancer cell
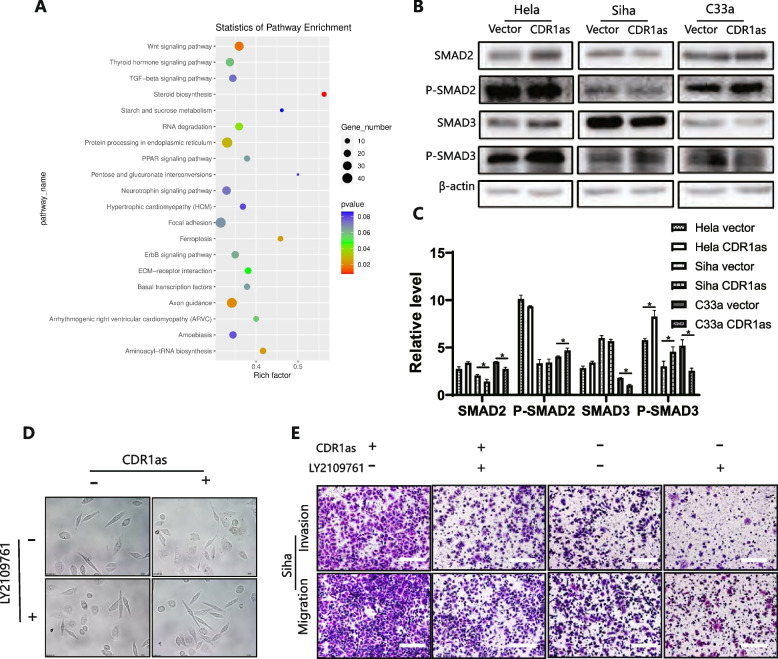
N6-methyladenosine is involved in almost all steps of RNA metabolism, which is an important part of transcriptional modification [22]. In conjunction with the preliminary verification of CDR1as functions and mechanisms, we then analyzed the levels of different mRNAs and m6A levels after the upregulation of CDR1as. KEGG and GO analysis indicated that CDR1as significantly activated the signal of TGF-β (Fig. 5A). Additionally, overexpressed CDR1as dramatically activated p-smad2 and p-smad3 by western blot (Fig. 5B-C). As expected, these EMT phenotypes were all reversed to the vector level when treating overexpressed CDR1as cells with LY2109761, which implied that CDR1as promoted EMT via activating TGF-β signaling (Fig. 5D). It was found that the number of penetrated cells were significantly decreased compared with the overexpressed CDR1as cells (Fig. 5E). These results suggested that CDR1as activated TGF-β signaling and EMT to promote metastasis in cervical cancer cells.
Fig. 5.
CDR1as activates TGF-β signaling and promotes EMT in cervical cancer cells; A Sanger sequencing shows that overexpressed CDR1as activated TGF-β signaling pathway; B C Western blot shows that P-SMAD2 and P-SMAD3 were upregulated after CDR1as was overexpressed; D Inhibition of TGF-β in CDR1as overexpressed cells reversed the EMT change by Wound healing; E Inhibition of TGF-β in CDR1as overexpressed cells reversed the EMT change by Transwell test
CDR1as promoted progression of cervical cancer cells in vivo
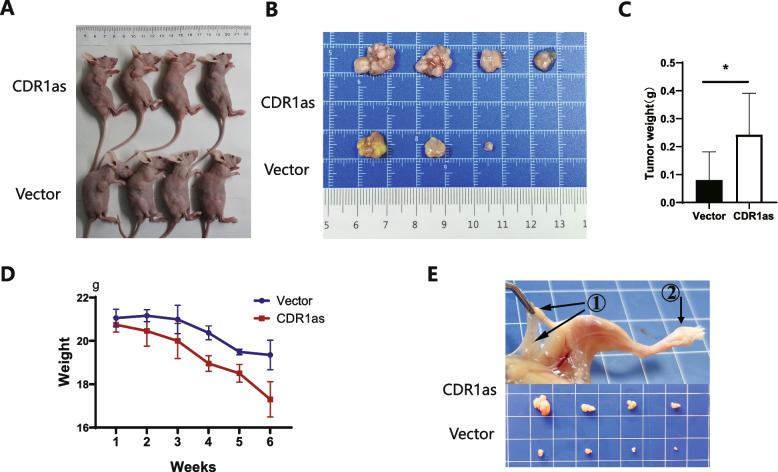
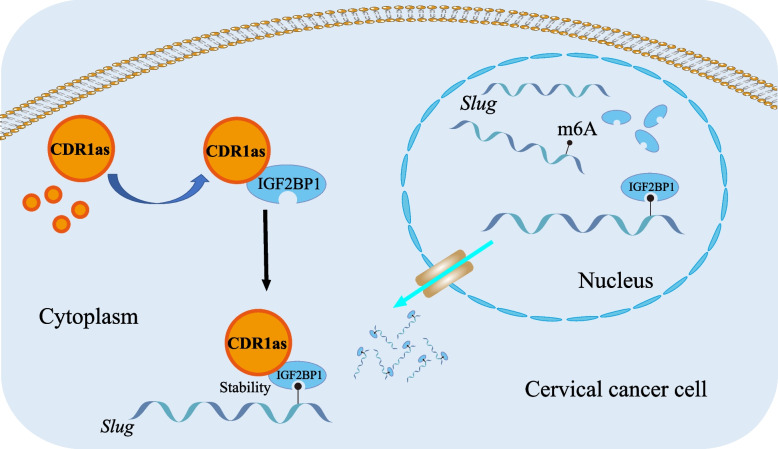
We then evaluated the in vivo role of CDR1as on cervical cancer metastasis by injection of cervical cancer cells into the subcutaneous tissue and foot pad of mice. After 6 weeks of injection, the growth of primary tumors showed a difference between control group and CDR1as group. As for injected subcutaneously into anterior flank of nude mouse, the volume of tumor was increased compared to control group (Fig. 6A-B), as well as a bigger tumor masses and reduced body weight when CDR1as was over-expressed (Fig. 6C-D). As for injection of foot pad of mice, there was obvious swelling of Inguinal lymph nodes in CDR1as group (Fig. 6E). Cartoon for ‘TGF-β signaling may promote cervical cancer metastasis via CDR1as’ has been shown Fig. 7.
Fig. 6.
Over-expressed CDR1as promotes cervical cancer progression in vivo. A, B, C, D Overexpressed CDR1as of Siha was subcutaneously injected into mice, the mice were weighed once a week, tumors were harvested and weighted after 6 weeks; E Overexpressed CDR1as of Siha was injected into the foot pad of mice and popliteal lymph nodes were harvested after 6 weeks
Fig. 7.
Cartoon for ‘TGF-β signaling may promote cervical cancer metastasis via CDR1as’ has been shown
Discussion
Most cervical cancer deaths are attributable to metastasis. Mechanisms responsible for metastasis are incompletely penetrated. TGF-β signaling has been reported to orchestrated an intricate signaling network to modulate tumorigenesis and cancer progression. Among multi-steps of TGF-β related metastasis in other cancers, R-SMAD/coSMAD complex was accumulated in the nucleus as transcription factors to promote EMT after TGF-β activation [23, 24]. Target genes including slug, snail, zeb1, zeb2, and twist were all known for promoting EMT [25]. To reveal the mechanism of TGF-β in metastatic spread would yield new insights of cervical cancer.
Epigenetic and transcriptional changes could drive metastatic processes. In our study, after treating cells with TGF-β molecular, metastasis was obviously promoted and the whole gene regulation and expression was reorganized. Among these changes, we focused on the circular RNA with regard to its extra-cellular stability and potential use as biomarker. Besides metastasis [15], CDR1as was also found to promote carcinogenesis [18] and cancer chemoresistance [26, 27]. In previous studies, CDR1as has been reported as a sponge for specific miRNAs, leading to regulation of downstream target mRNA expression and modulation of EMT processes. This mechanism has been commonly referred as a ceRNA network. However, we failed to identify a miRNA that could form a ceRNA network involved in the activation of Slug. Therefore, we speculate that other biomolecules may be involved in the regulation of Slug expression.
CircRNAs have been reported interacted with RNA binding proteins. CDR1as has been proved to interact with proteins such as IGF2BP3 and p53 [10, 20]. IGF2BPs belong to oncofetal proteins to regulate target mRNA transcripts in numerous cancers. In our study, after overexpression of CDR1as, the mRNA levels of IGF2BP1 did not demonstrate any change, while the protein levels of IGF2BP1 were significantly increased our data identify several IGF2BP3 targets that may contribute to this phenotype. Taken together, we hypothesize that IGF2BP1 is a key downstream effector of CDR1as.
After overexpression of CDR1as, we also found one of TGF-β signaling target gene, Slug, was increased. Besides its role as an m6A reader, activated IGF2BP1 could bind with the promoter of multiple genes to promote translation. Silencing bp1 efficiently reduced the expression of TGF-β signaling-related factors. To the m6A modified site of Slug mRNA, this binding specifically improved the stability of Slug and prevented the decay of Slug.
Conclusion
Our research addressed that effective CDR1as and m6A activate TGF-β signaling and promotes cervical cancer metastasis, which suggests potential therapeutic targets for cervical cancer.
Acknowledgements
None.
Authors’ contributions
GuangLei Zhong conceived the study. Qian Zhao acquired patient samples and performed the experiments and data analyses. Zhiliao Chen analyzed and interpreted the data. Tingting Yao and GuangLei Zhong wrote and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Guangdong Province Natural Scientific Grant (2021A1515010267), and Csco-Pilot Cancer Research Fund (Y-2019AZMS-0393).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Association of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (2020000820). All enrolled patients provided written informed consent before participation.
Consent for publication
Manuscript is approved by all authors for publication.
Competing interests
No potential conflict of interest was reported by the author(s).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gadducci A, Guerrieri ME, Cosio S. Adenocarcinoma of the uterine cervix: pathologic features, treatment options, clinical outcome and prognostic variables. Crit Rev Oncol Hematol. 2019;135:103–114. doi: 10.1016/j.critrevonc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez Villalba S, Díaz-Caneja Planell C, Cervera Grau JM. Current opinion in cervix carcinoma. Clin Transl Oncol. 2011;13(6):378–384. doi: 10.1007/s12094-011-0671-4. [DOI] [PubMed] [Google Scholar]
- 4.Nishizuka M, Komada R, Imagawa M. Knockdown of RhoE expression enhances TGF-β-induced EMT (epithelial-to-mesenchymal transition) in cervical Cancer HeLa cells. Int J Mol Sci. 2019;20(19). [DOI] [PMC free article] [PubMed]
- 5.Tong J, Flavell RA, Li HB. RNA m (6) a modification and its function in diseases. Front Med. 2018;12(4):481–489. doi: 10.1007/s11684-018-0654-8. [DOI] [PubMed] [Google Scholar]
- 6.Jiang T, et al. RNA m6A reader IGF2BP3 promotes metastasis of triple-negative breast cancer via SLIT2 repression. FASEB J. 2022;36(11):e22618. doi: 10.1096/fj.202200751RR. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, et al. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J Hematol Oncol. 2018;11(1):88. doi: 10.1186/s13045-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell JL, et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70(15):2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen TB, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 10.Hanniford D, et al. Epigenetic silencing of CDR1as drives IGF2BP3-mediated melanoma invasion and metastasis. Cancer Cell. 2020;37(1):55–70.e15. doi: 10.1016/j.ccell.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memczak S, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 12.Su C, et al. CiRS-7 targeting miR-7 modulates the progression of non-small cell lung cancer in a manner dependent on NF-κB signalling. J Cell Mol Med. 2018;22(6):3097–3107. doi: 10.1111/jcmm.13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan B, et al. Circular RNA ciRS-7 correlates with advance disease and poor prognosis, and its down-regulation inhibits cells proliferation while induces cells apoptosis in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2018;22(24):8712–8721. doi: 10.26355/eurrev_201812_16636. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco Targets Ther. 2018;11:3979–3987. doi: 10.2147/OTT.S158316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li RC, et al. CiRS-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of miR-7/HOXB13. Cell Death Dis. 2018;9(8):838. doi: 10.1038/s41419-018-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, et al. Circular RNA ciRS-7 triggers the migration and invasion of esophageal squamous cell carcinoma via miR-7/KLF4 and NF-κB signals. Cancer Biol Ther. 2019;20(1):73–80. doi: 10.1080/15384047.2018.1507254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P, et al. CircRNA-Cdr1as exerts anti-oncogenic functions in bladder Cancer by sponging MicroRNA-135a. Cell Physiol Biochem. 2018;46(4):1606–1616. doi: 10.1159/000489208. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W, et al. Circular RNA Cdr1as sensitizes bladder cancer to cisplatin by upregulating APAF1 expression through miR-1270 inhibition. Mol Oncol. 2019;13(7):1559–1576. doi: 10.1002/1878-0261.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, et al. CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes to cisplatin resistance in non-small cell lung cancer (NSCLC) Cancer Cell Int. 2020;20:289. doi: 10.1186/s12935-020-01390-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou J, et al. Circular RNA CDR1as disrupts the p53/MDM2 complex to inhibit Gliomagenesis. Mol Cancer. 2020;19(1):138. doi: 10.1186/s12943-020-01253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y. Downregulation of METTL14 improves postmenopausal osteoporosis via IGF2BP1 dependent posttranscriptional silencing of SMAD1. Cell Death Dis. 2022;13(11):919. doi: 10.1038/s41419-022-05362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa A, et al. N (6)-methyladenosine (m (6) a) is an endogenous A3 adenosine receptor ligand. Mol Cell. 2021;81(4):659–674.e7. doi: 10.1016/j.molcel.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, et al. TGF-β-activated SMAD3/4 complex transcriptionally upregulates N-cadherin expression in non-small cell lung cancer. Lung Cancer. 2015;87(3):249–257. doi: 10.1016/j.lungcan.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Tong X, et al. MYOCD and SMAD3/SMAD4 form a positive feedback loop and drive TGF-β-induced epithelial-mesenchymal transition in non-small cell lung cancer. Oncogene. 2020;39(14):2890–2904. doi: 10.1038/s41388-020-1189-4. [DOI] [PubMed] [Google Scholar]
- 25.Pavlič A, et al. Long non-coding RNAs as potential regulators of EMT-related transcription factors in colorectal Cancer-a systematic review and bioinformatics analysis. Cancers (Basel). 2022;14(9). [DOI] [PMC free article] [PubMed]
- 26.Yang W, et al. Silencing CDR1as enhances the sensitivity of breast cancer cells to drug resistance by acting as a miR-7 sponge to down-regulate REGγ. J Cell Mol Med. 2019;23(8):4921–4932. doi: 10.1111/jcmm.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, et al. Circular RNA CDR1as alleviates cisplatin-based Chemoresistance by suppressing MiR-1299 in ovarian Cancer. Front Genet. 2021;12:815448. doi: 10.3389/fgene.2021.815448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.