Abstract
Purpose
To assess the efficacy of lung low-dose radiotherapy (LD-RT) in the treatment of patients with COVID-19 pneumonia.
Materials and methods
Ambispective study with two cohorts to compare treatment with standard of care (SoC) plus a single dose of 0.5 Gy to the whole thorax (experimental prospective cohort) with SoC alone (control retrospective cohort) for patients with COVID-19 pneumonia not candidates for admission to the intensive care unit (ICU) for mechanical ventilation.
Results
Fifty patients treated with LD-RT were compared with 50 matched controls. Mean age was 85 years in both groups. An increase in arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (PAFI) in the experimental LD-RT-treated group compared to the control group could not be found at 48 h after LD-RT, which was the primary endpoint of the study. However, PAFI values significantly improved after 1 month (473 vs. 302 mm Hg; p < 0.0001). Pulse oxymetric saturation/fraction of inspired oxygen (SAFI) values were also significantly higher in LD-RT-treated patients than in control patients at 1 week (405 vs. 334 mm Hg; p = 0.0157) and 1 month after LD-RT (462 vs. 326 mm Hg; p < 0.0001). All other timepoint measurements of the respiratory parameters were similar across groups. Patients in the experimental group were discharged from the hospital significantly earlier (23 vs. 31 days; p = 0.047). Fifteen and 26 patients died due to COVID-19 pneumonia in the experimental and control cohorts, respectively (30% vs. 48%; p = 0.1). LD-RT was associated with a decreased odds ratio (OR) for 1‑month COVID-19 mortality (OR = 0.302 [0.106–0.859]; p = 0.025) when adjusted for potentially confounding factors. Overall survival was significantly prolonged in the LD-RT group compared to the control group (log-rank p = 0.027). No adverse events related to radiation treatment were observed.
Conclusion
Treatment of frail patients with COVID-19 pneumonia with SoC plus single-dose LD-RT of 0.5 Gy improved respiratory parameters, reduced the period of hospitalization, decreased the rate of 1‑month mortality, and prolonged actuarial overall survival compared to SoC alone.
Keywords: COVID-19 pneumonia, Low-dose radiation therapy, Standard of care, Anti-inflammatory effects, Frai patients
Introduction
Person-to-person respiratory transmission of a new severe acute respiratory syndrome coronavirus (SARS-Cov-2) led to the global pandemic that emerged in December 2019. At that time, an outbreak of pneumonia cases of unknown origin was reported in China, which were subsequently recognized as the first cases of coronavirus disease 2019 (COVID-19). On March 12, 2020, the World Health Organization (WHO) formally declared that humankind was suffering from a pandemic with highly variable clinical manifestations. While most patients experience asymptomatic or mildly symptomatic infection, a minority of cases present with the severe form, with pneumonia, associated with a remarkable inflammatory response.
SARS-Cov‑2 is an RNA virus with a lipid membrane taken from the host cells where viral proteins are inserted. The spike (S) protein is essential and gives the virus its name due to a corona-like appearance. SARS-Cov‑2 uses the S protein to bind to host cells through a receptor-binding domain to the human angiotensin-converting enzyme 2 (ACE2) receptor [1]. COVID-19 infection is distinguished by a time sequence in which after the initial phase of viral replication, a small percentage of patients with SARS-CoV‑2 infection experience a serious form of pneumonia due to dysregulation of the immune response, which triggers a cytokine-release syndrome [2]. Then, an excessive inflammatory response can lead to adult respiratory distress syndrome with severe respiratory failure due to attraction and accumulation of immune cells in the lung parenchyma [3], leading to acute lung tissue damage [4]. Cytokine dysregulation associated with SARS-Cov‑2 infection has a distinctive profile, with decreases in some cytokines (CD3, CD4, CD8, interleukin [IL]-10, and interferon [IFN]), natural killer cells and B cells, while others are increased (IL‑1, IL‑2, IL‑6, IL‑4, IL‑8, IL-17, tumor necrosis factor-alpha [TNF], G‑CSF, GM-CSF [2, 4]).
Therefore, the medications currently approved for COVID-19 infection with pneumonia belong to two main groups: treatments administered to reduce viral replication and treatments with an anti-inflammatory effect. The most frequently used are remdesivir [5] and dexamethasone [6]. The efficacy of these treatments is controversial, with reports both for and against, and at best, the benefits are limited, which warrants investigation on new treatments for COVID-19. The anti-inflammatory potential of low-dose radiotherapy (LD-RT) has long been known, becoming an option for treating selected cases of patients with non-malignant inflammatory diseases [7, 8], including infectious pneumonia [9]. The anti-inflammatory effect of LD-RT is mediated by a decrease in polymorphonuclear and endothelial cells, decreased production of nitric oxide, increased activation of apoptosis mediators, and increased production of IL-10 [10] and transforming growth factor β1 (TGF-β1) [11].
The ethical standards that mandate that assisted ventilation and admission to intensive care units (ICUs) should be limited to one segment of the population means that patients in the other segments have very limited therapeutic options. The strong anti-inflammatory effects of LD-RT could prevent or reduce the inflammatory cascade caused by COVID-19. Therefore, to test the hypothesis that total-lung LD-RT treatment could benefit these patients by improving their oxygenation and decreasing mortality, we conducted a multicenter, prospective comparative clinical trial (IPACOVID trial, NCT04380818). The preliminary results of this trial have been reported previously [12] and the final results are reported herein.
Methods
Study design
This study was designed as a non-randomized comparison of two cohorts, with an ambispective design. Patients received standard of care (SoC) treatment in the control cohort or SoC plus LD-RT in the experimental cohort. SoC included antiviral or anti-inflammatory pharmacological treatment. We planned to include a prospective series of 50 consecutive patients in the experimental cohort and to compare the results with those of 50 consecutive patients in the control cohort, matched by age, gender, comorbidities, and the rate of pulse oxymetric saturation (SpO2)/fraction of inspired oxygen (FiO2; SAFI) values. The primary endpoint of the study was the difference in the variation of the rate of arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (PAFI) between the experimental and the control cohorts measured at 48 h after LD-RT treatment. If PAFI measurement was not possible, SAFI was determined. Secondary endpoints were SAFI, adverse events, radiological response, overall mortality, and 30-day actuarial overall survival. The baseline reference date had to be the day of the LD-RT treatment in the experimental cohort and the day of the start of SoC treatment for COVID-19 pneumonia in the control cohort. Clinical follow-up and radiological parameters were assessed before LD-RT, at 7 days, and 1 month after treatment. PAFI and SAFI ratios were assessed at 24 h, 1 week, and 1 month after treatment. Radiological CT scan images were classified according to the degree of parenchymal involvement. CURB-65 score (based on age, vital signs, urea level, and presence of confusion) was used to assess the severity of pneumonia and to predict mortality risk (score 1 assigned to low risk and scores 3 or 4 assigned to high risk). In the control group, the diagnosis was made with chest X‑ray.
The study was conducted in accordance with the Declaration of Helsinki in its latest version, Fortaleza 2013, and was approved by the institutional review board (IRB) of the Hospital Universitari Sant Joan de Reus (project code: 089/2020). All patients allocated to the prospective experimental cohort provided written informed consent. Patients allocated to the retrospective control cohort did not provide consent to the IRB agreement (most patients had died, others were discharged and lost to follow-up; data were anonymized and taken from a single, one-time retrospective review without further follow-up).
Patients
Patients eligible for the prospective study were diagnosed with COVID-19 pneumonia, deemed to not be candidates for invasive mechanical ventilation and therefore not admitted to the ICU, who were treated during the acute phase of the viral infection with a single dose of 0.5 Gy to the whole thorax. Patients included in the study were those aged 18 years and over, with fewer than 8 days from onset of symptoms or having PAFI below 300 or SAFI below 315, needing supplemental oxygen, and receiving standard COVID-19 medication at appropriate doses. Also, the lung infiltrates has to affect more than 50% of the parenchyma and present one of the following biochemical changes: dimer-D > 1500 ng/mL, IL-6 > 40 UI, CRP > 100 mg/l.
Treatment
It was planned for all patients to undergo CT for simulation using a simple and repositionable immobilization device (pillow and leg wedge) exclusively for COVID-19 patients. For the preparation and administration of the treatment, all the necessary measures had to be taken to guarantee the safety of patients and staff faced with the risk of SARS-CoV‑2 infection, following the regulations and instructions of the hospital. On the day of treatment, two technicians wearing appropriate personal protective equipment worked with the patient. The treatment of patients with COVID-19 pneumonia was carried out in the same linear accelerator, grouping all patients in the same time slot to avoid the risk of transmission to other patients. According to the established protocol, the room was decontaminated after completing the treatments.
The planning of the irradiation volume was based on the definition of the clinical target volume (CTV) involving the volume of both lungs, which was increased by 5 mm in all directions. Planning was carried out to achieve a homogeneous distribution in the area to be treated that met International Commission on Radiation Units (ICRU) criteria of between 95% and 107% of the prescribed dose [13]. The heart was defined as the organ at risk and the dose received was calculated, although it was not necessary to establish dose limits in critical organs beyond the ALARA principle (as low as reasonably achievable), in accordance with the common procedure with the use of ultra-low doses [14]. Patients had to receive a dose of 0.5 Gy in a single dose.
Statistical analyses
For sample size calculation, to demonstrate that a 50% difference between the medians of the PAFIs is statistically significant from the 20% expected, with an alpha risk of 0.05 and a beta risk of less than 0.2 in a bilateral contrast, accepting a loss of patients of 10%, with a 1:1 allocation, it was necessary to include 100 patients, 50 in the experimental group and 50 in the control group. We used the Mann–Whitney U test to compare continuous clinical variables in the two groups. We used Fisher’s exact test to assess differences between categorical variables. Multiple groups were compared with the Kruskal–Wallis test or the analysis of variance (ANOVA) test. Multivariable logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI) to evaluate the association between clinical risk factors and COVID-19 mortality. Categorical variables were expressed as frequencies and percentages, and continuous variables were presented as means and standard deviation. Kaplan–Meier curves and the log-rank test were used to calculate actuarial survival. Statistical analyses, logistic regressions, and graph representations were performed in SPSS Statistics version 25 (IBM SPSS Statistics, Armonk, NY, USA) and GraphPad Prism 8.01 (GraphPad Software, San Diego, CA, USA).
Results
Baseline demographics
Between June 15, 2020, and February 28, 2021, 50 inpatients treated at a single center were included (LD-RT-treated experimental cohort) and compared with 50 inpatient controls matched blindly by age, gender, comorbidities, and SAFI values (control cohort). Table 1 shows the demographic, clinical, and imaging baseline characteristics of the patients. Mean age was 85 years, and 46% of the patients were female in both groups. Participants presented a similar incidence of comorbidities, including neurological (28% vs. 38%), cardiovascular (82% in both groups), and respiratory diseases (40% vs. 28%) in the LD-RT-treated group and the control group, respectively. SoC treatment for COVID-19 was dexamethasone for all patients in both groups. In addition, one patient had received tocilizumab and four patients remdesivir in the control group. The functional status of the participants according to the Barthel index was not statistically different between the two groups. However, the control group presented a higher number of minimally dependent patients (p = 0.044). Also, when looking at the Geriatric Depression Scale, there was heterogeneity in the cognitive decline status, with only one LD-RT-treated patient in the moderate cognitive decline classification grade, whereas 11 patients were classified as such in the control group (p = 0.004). We believe that these heterogeneities appear as a result of difficulties in recruitment. It is important to bear in mind that incomprehension of the treatment or agitation is, worldwide, a contraindication for radiotherapy, considering patient’s safety.
Table 1.
Baseline characteristics of patients with COVID-19 treated with low-dose radiotherapy (LD-RT) and control patients
| Characteristic | LD-RT cohort (n = 50) |
Control cohort (n = 50) |
p-value | |
|---|---|---|---|---|
| Female sexb | 23 (46) | 23 (46) | > 0.999 | |
| Agea | 84.74 ± 6.583 | 85.04 ± 6.934 | 0.819 | |
| Comorbiditiesb | ||||
| Neurological diseases | 14 (28) | 19 (38) | 0.395 | |
| Cardiovascular diseases | 41 (82) | 41 (82) | > 0.999 | |
| Respiratory diseases | 20 (40) | 14 (28) | 0.291 | |
| Other comorbidities | 44 (88) | 37 (74) | 0.125 | |
| Pharmacological treatmentb | ||||
| Corticosteroids (dexamethasone) | 50 (100) | 50 (100) | – | |
| Tocilizumab | 0 (0) | 1 (2) | − | |
| Remdesivir | 0 (0) | 4 (8) | – | |
| Functional status (Barthel index)b | ||||
| Independent | 9 (18) | 15 (30) | 0.241 | |
| Minimally dependent | 19 (38) | 9 (18) | 0.044* | |
| Partially dependent | 12 (24) | 11 (22) | > 0.999 | |
| Very dependent | 7 (14) | 6 (12) | 0.234 | |
| Totally dependent | 3 (6) | 9 (18) | 0.121 | |
| Geriatric Depression Scale (GDS)b | ||||
| No cognitive decline | 25 (50) | 16 (32) | 0.103 | |
| Very mild cognitive decline | 10 (20) | 10 (20) | > 0.999 | |
| Mild cognitive decline | 9 (18) | 5 (10) | 0.388 | |
| Moderate cognitive decline | 1 (2) | 11 (22) | 0.004* | |
| Moderately severe cognitive decline | 2 (4) | 4 (8) | 0.678 | |
| Severe cognitive decline | 3 (6) | 4 (8) | > 0.999 | |
| Very severe cognitive decline | – | – | – | |
| Basal SpO2a | 93.20 ± 2.969 | 93.32 ± 3.395 | 0.832 | |
| Basal SAFI (SpO2/FIO2)a | 280.86 ± 93.021 | 274.88 ± 88.41 | 0.572 | |
| Mild (310–460) | 29 (58)b | 28 (56) | 0.820 | |
| Moderate (160–310) | 10 (20) | 10 (20) | 0.774 | |
| Severe (< 160) | 11 (22) | 12 (24) | > 0.999 | |
| CURB-65 scoreb | ||||
| Score 1 | – | – | – | |
| Score 2 | 13 (26.5) | 13 (26) | > 0.999 | |
| Score 3 | 26 (53.1) | 15 (30) | 0.025* | |
| Score 4 | 10 (20.4) | 22 (44) | 0.018* | |
| Score 5 | – | – | – | |
| First radiological findingsb | CT | Chest X‑ray | ||
| CT lung involvement < 5% | – | None: 1 (2) | – | |
| CT lung involvement 5–25% | 1 (2) | Unilateral: 4 (8) | – | |
| CT lung involvement 26–49% | 8 (16) | NA | – | |
| CT lung involvement 50–75% | 23 (46) | Bilateral: 45 (90) | – | |
| CT lung involvement > 75% | 17 (34) | NA | – | |
SAFI ratio of pulse oximetry saturation (SpO2) to fractional inspired oxygen (FiO2), CURB-65 clinical criteria validated to guide the treatment of community-acquired pneumonia based on confusion, BUN respiratory rate, systolic blood pressure and age, CT computed tomography, NA not available
aData shown as mean ± standard deviation
bData shown as number of patients and percentages in parenthesis
*Statistically significant p-value
A CURB-65 score of 3 for the severity of pneumonia was reported more frequently in the patients in the LD-RT-treated group (54% vs. 30%; p = 0.025), while a CURB-65 score of 4 was reported more frequently in the control group (21% vs. 44%; p = 0.018). Although higher CURB-65 scores are associated with higher mortality, the differences in CURB-65 scores within the different groups were analyzed and there were no significant differences in the mortality ratio. This could be due to the fact that there is an homogenization between groups and, consequently, the generally reported effect that higher CURB-65 scores are associated with higher mortality was not observed in this sample. To further investigate whether a different distribution of CURB-65 at baseline in the control and the LD-RT-treated groups could have an impact on COVID-19 mortality and hospitalization, the following groups were considered: control group vs. LD-RT-treated group both with CURB-65 scores of 4, control group vs. LD-RT-treated group both with CURB-65 scores of 3, and, finally, control group vs. LD-RT-treated group both with CURB-65 scores of 2. None of the groups showed a significant association with either COVID-19 mortality (using a Fisher’s exact test) or hospitalization length (employing the Mann–Whitney U test) when comparing the same CURB-65 scores in both studied groups before LD-RT treatment. Most patients (46%) in the experimental group had a value higher than 50% of lung parenchyma affected before irradiation, as measured with CT scan, while most patients in the control group (90%) had bilateral pneumonia assessed with chest X‑ray. No differences were observed between the groups in terms of pre-irradiation SpO2 and SAFI values.
Outcomes
Thirty-four patients (68%) in the LD-RT cohort were evaluated 1 week after irradiation (11 patients died within 1 week and five were in too poor a condition for further examination) and 32 patients (64%) were evaluated 1 month after LD-RT. During the first week, 11 and 15 patients died in the LD-RT and control groups, respectively. In the experimental group, 18 (36%) patients died, of whom 15 died due to COVID-19 pneumonia and three due to other causes, whereas in the control group, 26 patients died (56%), 24 were due to COVID-19 pneumonia and two due to other causes (Table 2).
Table 2.
Comparison of outcomes between the case groups treated with low-dose radiotherapy (LD-RT) and the control group
| Outcome | LD-RT cohort (n = 50) |
Control cohort (n = 50) |
p-value |
|---|---|---|---|
| Duration of symptoms (d)a | 5.10 ± 1.644 | 6.88 ± 3.662 | 0.015* |
| Duration of hospitalization (d)a | 18.58 ± 13.902 | 20.04 ± 56.845 | 0.010* |
| Discharge from hospital (d)a | 22.69 ± 14.915 | 31.46 ± 79.821 | 0.047* |
| Final status, 1 month after treatmentb | |||
| Alive | 32 (64) | 24 (48) | 0.158 |
| Death due to COVID-19 | 15 (30) | 24 (48) | 0.100 |
| Death due to other causes | 3 (6) | 2 (4) | > 0.999 |
aData shown as mean ± standard deviation
bData shown as number of patients and percentage in parenthesis
*Statistically significant p-value
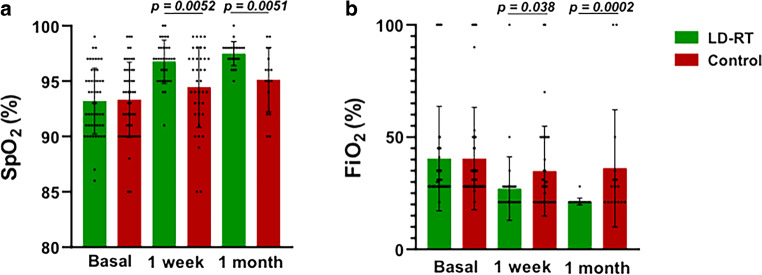
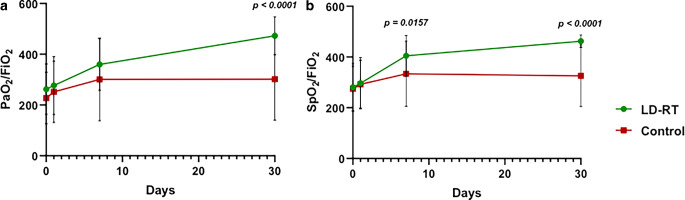
Regarding respiratory parameters, our primary endpoint hypothesis was not fulfilled due to PAFI values not improving by over 20% at 48 h after LD-RT treatment. However, a post-hoc analysis showed significant long-term respiratory parameter improvement. At baseline, respiratory parameters revealed no differences between the experimental and the control cohorts, but a post-hoc analysis showed that significant changes in respiratory parameters appeared 1 week after the intervention. Seven days after treatment, LD-RT-treated patients experienced a significant increase in SpO2 (97% vs. 94%; p = 0.0052) and a significant reduction in the FiO2 they needed (27% vs. 35%; p = 0.038; Fig. 1). Regarding PAFI (277 vs. 252; p = 0.113) and SAFI (297 vs. 292; p = 0.577) parameters, no significant differences were found at 24 h after treatment. At 1 week after treatment, the LD-RT-treated patients’ SAFI values were significantly higher than in control patients (405 vs. 334; p = 0.0157), but no PAFI differences were reported (360 vs. 301; p = 0.117). A significant improvement was observed in all respiratory parameters in the LD-RT group after 1 month of LD-RT compared with the control group: SpO2 (97 vs. 95; p < 0.0051), FiO2 (21% vs. 36; p = 0.0002), SAFI (462 vs. 326; p < 0.0001), and PAFI (473 vs. 302; p < 0.0001; Fig. 2). All other timepoint measurements of the respiratory parameters were similar across the groups (Fig. 2).
Fig. 1.
Comparison of SpO2 and FiO2 parameters between the LD-RT-treated and the control group at different timepoints. a SpO2 values in treated and control patients at baseline (n = 50), at 1 week (LD-RT n = 32, control n = 38), and at 1 month (LD-RT n = 23 and control n = 16). b FiO2 values in treated and control patients at baseline (n = 50), at 1 week (LD-RT n = 34, control n = 38), and at 1 month (LD-RT n = 23, control n = 16). Data are expressed as mean ± standard deviation. P-values are from a Mann–Whitney test
Fig. 2.
Evolution of respiratory parameters PAFi and SAFI in patients treated with LD-RT and control patients. a PAFi (PaO2/FiO2) values in treated and control patients at baseline (LD-RT n = 48, control n = 50), at 24 h (LD-RT n = 47, control n = 46), at 1 week (LD-RT n = 32, control n = 38), and at 1 month (LD-RT n = 23, control n = 16). b SAFI (SpO2/FiO2) values in treated and control patients at baseline (LD-RT and control n = 50), at 24 h (LD-RT and control n = 46), at 1 week (LD-RT n = 33, control n = 38), and at 1 month (LD-RT n = 28, control n = 16). Data are expressed as mean ± standard deviation. P-values are from a Mann–Whitney test
The length of hospitalization was not an endpoint in the IPACOVID trial. However, regarding the global health crisis during the COVID-19 pandemic, these data were extracted from our cohorts. Using Mann–Whitney U, the length of hospitalization was significantly shorter in the LD-RT-treated group than in the control group (19 vs. 20 days; p = 0.01). Patients allocated to the experimental group were discharged from the hospital significantly earlier (23 vs. 31 days; p = 0.047). Fifteen and 26 patients died due to COVID-19 pneumonia in the experimental and control cohorts, respectively, resulting in a non-significant trend towards lower mortality due to COVID-19 (30 vs. 48%; p = 0.1). Three and two patients died of other causes in the LD-RT and control cohorts, respectively. The same trend was observed for all-cause mortality (36% vs. 56%; p = 0.158; Table 2). When adjusted for potentially confounding factors (sex, age, comorbidities, and number of days with symptoms), only age and LD-RT treatment showed a significant association with COVID-19 mortality (Table 3). Specifically, age was associated with an increased odds ratio (OR) for COVID-19 mortality (OR = 1.147; 95% confidence interval [1.033–1.272]; p = 0.010) whereas LD-RT was associated with a decreased OR for COVID-19 mortality (OR = 0.302 [0.106–0.859]; p = 0.025). Therefore, the odds of dying due to COVID-19 were significantly reduced by 70% in patients treated with LD-RT. In line with this, actuarial analysis with Kaplan–Meier curves showed prolonged survival in the LD-RT group compared to the control group (log-rank p = 0.027; Fig. 3). We considered the log-rank test to be the best statistical method to estimate survival over time, rather than Mann–Whitney U, as reported previously, due to the fact that the specific time of patient death was known.
Table 3.
Multivariate logistic regression analysis of factors associated with COVID-19 mortality
| Factors associated with mortality | OR (95% CI) | p-value |
|---|---|---|
| LD-RT treatment | 0.302 (0.106–0.859) | 0.025* |
| Sex (female) | 0.859 (0.331–2.231) | 0.755 |
| Age | 1.147 (1.033–1.272) | 0.010* |
| Neurological disease | 1.592 (0.567–4.484) | 0.377 |
| Respiratory disease | 1.838 (0.654–5.155) | 0.249 |
| Cardiovascular disease | 1.044 (0.250–4.348) | 0.953 |
| Other comorbidities | 3.311 (0.769–14.286) | 0.108 |
| Days with symptoms | 0.927 (0.773–1.111) | 0.410 |
Data were obtained from a multivariate logistic regression model including the different clinically relevant factors able to be associated with the following outcomes: COVID-19 death, death from other causes, and alive. This table shows concretely the odds for COVID-19 mortality compared to alive patients
OR odds ratio, CI confidence interval
Fig. 3.
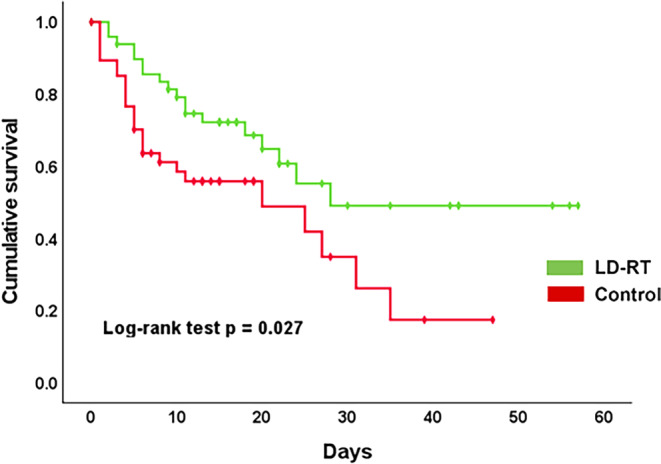
Kaplan–Meier overall survival curves stratified according to LD-RT treatment or control group. ■■■
One week after treatment, 35 patients (70%) underwent a CT scan in the experimental group. According to the comparison with percentage of lung parenchyma involved at baseline CT scan, 66% of patients experienced radiological improvement (no patient had > 75% involvement, 5 [14%] had involvement of 50–75%, 13 [37%] of 25–50%, 13 [37%] of 5–25%, and 4 [11%] involvement < 5%). Only one patient experienced radiological worsening, and another experienced no changes on CT images. One month after LD-RT, 29 patients had their comparative CT, and 2% (1/29) of patients presented 25–50% parenchyma involvement, 10% (5/29) presented 5–25% involvement, and 46% (23/29) presented < 5% involvement. In the control group, the image obtained during the diagnosis was a chest X‑ray. The second control was taken after a mean of 33 days in 28 patients. Forty percent of the patients experienced radiological improvement, and 16% experienced radiological worsening, with an increased area of parenchymal infiltration. In the control group, 15 patients died in the first week, and seven did not get the image control after individual clinical decisions.
No acute adverse events related to radiation treatment were observed.
Discussion
The results of this study show that SoC treatment plus LD-RT significantly improved respiratory parameters compared with SoC alone in patients with COVID-19 pneumonia. This improvement was associated with a reduction in the percentage of lung volume involvement and a statistically nonsignificant but numerically remarkable improvement in 1‑month mortality from half in the SoC cohort to one third in the LD-RT cohort. LD-RT treatment is associated with lower mortality due to COVID-19 pneumonia when adjusted for risk factors and a prolonged actuarial overall survival. Of note, these benefits were achieved with no acute adverse events experienced by patients.
One of the most relevant concerns of radiation treatment for patients with COVID-19 is the safety of healthcare personnel and other patients using the same facilities [15]. The movement of patients with an infectious contagious disease through the hospital is an unavoidable risk that has to be minimized. In our experience with 50 patients, we did not observe any outbreaks as a result of the treatment during this clinical trial. The careful use of personal protective equipment and individual material for the patients, and the disinfection of the surfaces of the facilities, have allowed this successful result, adding to reports in the recently published studies that we will comment on below. Although these procedures may seem time consuming, such treatment is possible if coordinated well [16]. Furthermore, if the patients’ benefit was indisputable, assuming a reasonable level of risk in a controlled manner and making every effort to avoid it should not be an obstacle to the implementation of this treatment for COVID-19 pneumonia in the future.
The results of the present study, the largest of those published to date, run opposite to the results of the other comparative study, which is the only randomized one available to date. Indeed, Papachristofilou et al. reported the results of a randomized trial [17] in which 22 elderly and comorbid patients undergoing mechanical ventilation in the ICU were randomized to LD-RT vs. sham irradiation. No benefit of LD-RT was observed in that study, measured as ventilator-free interval and 28-day mortality. However, 22 patients seem too small a sample size for a randomized trial, and the lack of statistical power may have impacted this negative result. Nevertheless, that study had a positive design feature with a sham irradiation control group, which should be the standard control in randomized trials of radiation in patients with COVID-19 pneumonia. Another two randomized trials, one of them for patients with moderate COVID-19 (seven patients in the LD-RT arm and six in the control group) [18] and the other for patients with moderate to severe COVID-19 (34 patients in the LD-RT and 17 patients in the control group) [19], have shown that LD-RT can be an option for these patients. Another study, a prospective comparative cohort with 58 patients (31 in the treatment group and 29 in the control group), has demonstrated that LD-RT could be an alternative to lessen the mortality of patients with moderate COVID-19 pneumonia [20]. Other previous reports are based on small case series, with two cases [21], nine cases [22], and ten cases [23–25]. A systematic review published recently including four studies with 61 patients does not support mortality benefit, clinical course improvement, or imaging changes with LD-RT [17, 22–26]. The present study shows benefits for respiratory function and mortality at 1 month and radiological improvement, the latter being more difficult to compare between groups due to different evaluation methods (thoracic CT vs. chest X‑ray). The other noncomparative studies evaluated the same parameters and also found improvement in one or more of these endpoints. Thus, an impact on radiological [21], clinical [22], or respiratory parameters [19, 20] has been observed in some studies, while in others, improvement was observed for a combination of clinical and radiological endpoints [18]. We administered a dose of 0.5 Gy, which is in line with that administered to patients with COVID-19 pneumonia in these previous studies, ranging from 0.5 Gy [20] to 1.5 Gy [21]. The mean age of study participants is over 70 in almost all of these studies, including one in which the mean age was 90 years [21]. Our study sample also consisted of elderly and frail patients. Investigating initially in frail patients not admitted to ICUs made sense, as it allowed us to offer these patients the treatment option under investigation. At the same time, if it resulted beneficial, LD-RT could be considered in the future to treat patients during the acute phase, before mechanical ventilation becomes necessary. This could be valuable in other similar scenarios in which the health system could collapse, resulting in a shortage of respiratory devices. We note that a subgroup of these patients rapidly worsened, although that was due to COVID-19 infection, not LD-RT, because this deterioration was also reported in patients in the control cohort who did not receive radiotherapy. This issue raises the need to investigate this strategy in less frail patients using LD-RT associated with SoC, including mechanical ventilation and ICU admission.
This study has several weaknesses. The non-randomized design cannot definitively establish causality. The retrospective nature of the control cohort may be of concern. The comparability of the radiological imaging is not optimal for establishing causality, because in patients treated with LD-RT, thoracic CT was used, and in the patients of the control cohort, chest X‑ray. Furthermore, better homogenization between the control and the cohort groups was limited owing to the fact that this study was conducted during the COVID-19 outbreak and hospital personnel were clearly overwhelmed. In addition, patients fitting our inclusion criteria were limited, and difficulties were also encountered regarding the organization of a study involving irradiation. Although some parameters such as age, gender, comorbidities, and respiratory parameters were homogenous, others such as grade of independence and cognitive decline were more difficult to match between the groups. Several factors hindered a higher homogenization between the two groups, including the reluctance of potential candidates and physicians to participate in a radiotherapy study, the advanced age of patients, and agitation, which is a significant contraindication to radiotherapy. On the other hand, a strength of the study is its sample size, which compares favorably with previous studies, as well as the concordance of improvement in the three endpoints (respiratory function, mortality at 1 month, and radiological assessment), suggesting that LD-RT could be related to better outcomes observed in the experimental group. The next steps in COVID-19 research are difficult to predict, because they will depend on how the pandemic evolves. Let us suppose it behaves as it has so far, with successive waves of greater or lesser intensity. In that case, a multicenter effort is needed to conduct a prospective randomized clinical trial of SoC plus LD-RT versus SoC plus sham irradiation, given that the data from the studies conducted so far indicate a possible benefit of this treatment, even though this has not yet been demonstrated conclusively [15]. Also, the most pressing issues would seem to be the leap to including less frail patients in the sample and combining LD-RT in an experimental regimen in patients admitted to the ICU.
In conclusion, although in this prospective, non-randomized comparative study, SoC plus LD-RT 0.5 Gy single dose to treat frail patients with COVID-19 pneumonia did not meet the primary endpoint, we observed an improvement of respiratory parameters, 1‑month mortality, and actuarial overall survival compared to SoC alone, thus fulfilling the secondary endpoints. No acute radiation-related adverse events were observed. Further multicenter collaboration in a large randomized trial including patients in earlier stages of infection is key to determining the actual value of LD-RT for treating COVID-19 pneumonia.
Acknowledgments
Acknowledgements
To the Spanish Group for Clinical Research in Radiation Oncology—Spanish Society of Radiation Oncology (GICOR-SEOR 2020) for an extraordinary grant for radiation research during COVID-19 to cover the cost of legal insurance for the clinical trial.
To all professionals who make the treatment of patients possible: radiotherapy technicians, nurses, physicists, hospital porters, cleaning staff, and all the doctors, especially M. Arguís, D. Gómez, F.A. Castaño, J. Trilla, M. Murcia, Y. López, and E. Roquer.
Funding
Grant from the Spanish Group for Clinical Research in Radiation Oncology—Spanish Society of Radiation Oncology (GICOR-SEOR 2020) for radiation research during COVID-19 to cover the cost of legal insurance for the clinical trial.
Author Contribution
Conceptualization of the study: M.A.R., M.A.L., and Á.M.; methodology of the study: M.A.R. and M.A.L.; formal analysis: E.R.-T., J.C.A., L.T.-R., G.D.F., G.B.-G., H.C., A.J., C.V., and P.A.; investigation of the study: J.G., B.M., M.Á, D.C., J.M.S., X.G.-B., B.P., and S.S.; writing—original draft preparation: E.R.-T., J.C., J.J., and M.A.R.; writing—review and editing the mansucript: E.R.-T., J.C., J.J., and M.A.R. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
M. Arenas, B. Piqué, L. Torres-Royo, A.J. Acosta, E. Rodríguez-Tomàs, G. De Febrer, C. Vasco, P. Araguas, J.A. Gómez, B. Malave, M. Árquez, M. Algara, A. Montero, M. Montero, J.M. Simó, X. Gabaldó, D. Parada, F. Riu, S. Sabater, J. Camps and J. Joven declare that they have no competing interests.
Contributor Information
M. Arenas, Email: meritxell.arenas@urv.cat.
B. Piqué, Email: berta.pique@estudiants.urv.cat.
L. Torres-Royo, Email: lauratroyo@gmail.com.
J. C. Acosta, Email: johanac.acostaa@gmail.com.
E. Rodríguez-Tomàs, Email: elisabet.rodriguez@urv.cat.
G. De Febrer, Email: gabrielde.febrer@urv.cat.
C. Vasco, Email: carlos.vasco@salutsantjoan.cat.
P. Araguas, Email: pablo.araguasmora@gmail.com.
J. A. Gómez, Email: junioranderson.gomez@gmail.com.
B. Malave, Email: barbaraantonia.malave@salutsantjoan.cat.
M. Árquez, Email: miguelarquez@gmail.com.
M. Algara, Email: 85368@parcdesalutmar.cat.
A. Montero, Email: angel.monteroluis@gmail.com.
M. Montero, Email: manueljesus.montero@salutsantjoan.cat.
J. M. Simó, Email: jmsimo@lrsud.cat.
X. Gabaldó, Email: xgabaldo@lrsud.cat.
D. Parada, Email: david.parada@salutsantjoan.cat.
F. Riu, Email: francesc.riu@salutsantjoan.cat.
S. Sabater, Email: ssabaterm@gmail.com.
J. Camps, Email: jorge.camps@salutsantjoan.cat.
J. Joven, Email: jorge.joven@salutsantjoan.cat.
References
- 1.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenas M, Sabater S, Hernández V, et al. Anti-inflammatory effects of low-dose radiotherapy. Strahlenther Onkol. 2012;188:975–981. doi: 10.1007/s00066-012-0170-8. [DOI] [PubMed] [Google Scholar]
- 8.Torres Royo L, Antelo Redondo G, Árquez Pianetta M, Arenas Prat M. Low-Dose radiation therapy for benign pathologies. Rep Pract Oncol Radiother. 2020;25:250–254. doi: 10.1016/j.rpor.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese EJ, Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013;86:555. [PMC free article] [PubMed] [Google Scholar]
- 10.Rödel F, Frey B, Manda K, et al. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose x-irradiation. Front Oncol. 2012;2:120. doi: 10.3389/fonc.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arenas M, Gil F, Gironella M, Hernández V, et al. Time course of anti-inflammatory effect of low-dose radiotherapy: correlation with TGF-beta(1) expression. Radiother Oncol. 2008;86:399–406. doi: 10.1016/j.radonc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Arenas M, Algara M, De Febrer G, et al. Could pulmonary low-dose radiation therapy be an alternative treatment for patients with COVID-19 pneumonia? Preliminary results of a multicenter SEOR-GICOR nonrandomized prospective trial (IPACOVID trial) Strahlenther Onkol. 2021;197:1010–1020. doi: 10.1007/s00066-021-01803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ICRU . ICRU Report. Bethesda: International Commission on Radiation Units and Measurements; 1999. Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50) [Google Scholar]
- 14.https://www.cdc.gov/nceh/radiation/alara.html. Accessed 19 Jan 2022
- 15.Mortazavi SMJ, Shams S, Mohammadi S, Mortazavi AR, Sihver L. Low-dose radiation therapy for COVID-19: a systematic review. Radiation. 2021;1:234–249. doi: 10.3390/radiation1030020. [DOI] [Google Scholar]
- 16.Bonet M, Vázquez S, García E, et al. Saving time in the radiotherapy procedures for COVID-19 pneumonia treatment. A single-institution experience. Clin Transl Oncol. 2021;23:2344–2349. doi: 10.1007/s12094-021-02634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papachristofilou A, Finazzi T, Blum A, et al. Low dose radiation therapy for severe COVID-19 pneumonia: a randomized double-blind study. Int J Radiat Oncol Biol Phys. 2021;110:1274–1282. doi: 10.1016/j.ijrobp.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sing P, Mandal A, Singh D. Interim analysis of impact of adding low dose pulmonary radiotherapy to moderate COVID-19 pneumonia patients: IMpact-RT study. Front Oncol. 2022;12:822902. doi: 10.3389/fonc.2022.822902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesan G, Ponniah S, Sundaram V, et al. Whole lung irradiation as a novel treatment for COVID-19: Interim results of an ongoing phase 2 trial in India. Radiother Oncol. 2021;163:83–90. doi: 10.1016/j.radonc.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz CS, Hernández D, Trujillo C, et al. The clinical efficacy of low-dose whole-lung irradiation in moderate-to-severe COVID-19 pneumonia: RTMX-20 trial. Radiother Oncol. 2022;166:133–136. doi: 10.1016/j.radonc.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Olmedo E, Suárez-Gironzini V, Pérez M, Filigheddu T, et al. COVID-19 pneumonia treated with ultra-low doses of radiotherapy (ULTRA-COVID study): a single institution report of two cases. Strahlenther Onkol. 2021;197:429–437. doi: 10.1007/s00066-020-01743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanmamed N, Alcantara P, Cerezo E, et al. Low-dose radiation therapy in the management of Coronavirus disease 2019 (COVID-19) pneumonia (LOWRAD-Cov19): preliminary report. Int J Radiat Oncol Biol Phys. 2021;109:880–885. doi: 10.1016/j.ijrobp.2020.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameri A, Ameri P, Rahnama N, et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: final results of a pilot study. Int J Radiat Oncol Biol Phys. 2021;109:859–866. doi: 10.1016/j.ijrobp.2020.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess CB, Buchwald ZS, Stokes W, et al. Low-dose whole-lung radiation for COVID-19 pneumonia: planned day 7 interim analysis of a registered clinical trial. Cancer. 2020;126:5109–5113. doi: 10.1002/cncr.33130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma DN, Guleria R, Wig N, et al. Low-dose radiation therapy for COVID-19 pneumonia: a pilot study. Br J Radiol. 2021;94(1126):20210187. doi: 10.1259/bjr.20210187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey SR, Adhikari Yadav S, Gautam S, et al. Effectiveness of low-dose radiation therapy in COVID-19 patients globally: a systematic review. F1000Res. 2022;11:62. doi: 10.12688/f1000research.74558.1. [DOI] [PMC free article] [PubMed] [Google Scholar]