Abstract
Purpose
Transarterial chemoembolization (TACE) with tyrosine kinase inhibitors (TKIs) has been increasingly used to treat unresectable hepatocellular carcinoma (uHCC). However, the superiority of combination therapy to TACE monotherapy remains controversial. Therefore, here we performed a meta-analysis to evaluate the efficacy and safety of TACE plus TKIs in patients with uHCC.
Methods
We searched four databases for eligible studies. The primary outcome was time to progression (TTP), while the secondary outcomes were overall survival (OS), tumor response rates, and adverse events (AEs). Pooled hazard ratios (HRs) with 95% confidence intervals (95% CIs) were collected for TTP and OS, and the data were analyzed using random-effects meta-analysis models in STATA software. OR and 95% CIs were used to estimate dichotomous variables (complete remission[CR], partial remission[PR], stable disease[SD], progressive disease[PD], objective response rate[ORR], disease control rate[DCR], and AEs) using RStudio’s random-effects model. Quality assessments were performed using the Newcastle–Ottawa scale (NOS) for observational studies and the Cochrane risk of bias tool for randomized controlled trials (RCTs).
Results
The meta-analysis included 30 studies (9 RCTs, 21 observational studies) with 8246 patients. We judged the risk of bias as low in 44.4% (4/9) of the RCTs and high in 55.6% (5/9) of the RCTs. All observational studies were considered of high quality, with a NOS score of at least 6. Compared with TACE alone or TACE plus placebo, TACE combined with TKIs was superior in prolonging TTP (combined HR 0.72, 95% CI 0.65–0.80), OS (combined HR 0.57, 95% CI 0.49–0.67), and objective response rate (OR 2.13, 95% CI 1.23–3.67) in patients with uHCC. However, TACE plus TKIs caused a higher incidence of AEs, especially hand-foot skin reactions (OR 87.17%, 95%CI 42.88–177.23), diarrhea (OR 18.13%, 95%CI 9.32–35.27), and hypertension (OR 12.24%, 95%CI 5.89–25.42).
Conclusions
Our meta-analysis found that TACE plus TKIs may be beneficial for patients with uHCC in terms of TTP, OS, and tumor response rates. However, combination therapy is also associated with a significantly increased risk of adverse reactions. Therefore, we must evaluate the clinical benefits and risks of combination therapy. Further well-designed RCTs are needed to confirm our findings.
Trial registration
PROSPERO registration number: CRD42022298003.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-02961-7.
Keywords: Tyrosine kinase inhibitors, Hepatocellular carcinoma, TACE, Systematic review, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third leading cause of cancer-related death. Primary liver cancers include HCC (75–85% of cases) and intrahepatic cholangiocarcinoma (10–15%). The incidence of HCC is increasing annually. More than 700,000 people are diagnosed with HCC each year, and more than half of these cases occur in developing countries, especially Asian countries [1]. The main risk factors for HCC are chronic infection with hepatitis B or C virus, exposure to aflatoxin-contaminated food, alcohol consumption, overweight, type 2 diabetes, and smoking [2].
Due to the high incidence of advanced HCC, palliative care aimed at prolonging life after diagnosis is an important component of its management [3]. Many therapies have been used to treat advanced HCC, including transarterial chemoembolization (TACE), radiation, immunotherapy, systemic chemotherapy, portal vein stenting, percutaneous ethanol injection, and conservative therapy [4–10].
TACE is a local treatment strategy for the palliative treatment or management of most unresectable HCC (uHCC) patients that aims to prevent and alleviate patient suffering and improve their quality of life. A reported 55% of patients achieved partial remission (PR) after TACE treatment, significantly delaying tumor progression and macrovascular invasion [11]. However, TACE induces hepatocyte hypoxia by blocking the blood vessels and upregulating vascular endothelial growth factor (VEGF) in normal and tumor cells. High expression of hypoxia-inducible factor-1α can alter VEGF stability and enhance its mRNA expression. Hypoxia can also induce high VEGF receptors expression in endothelial cells. Binding of VEGF to VEGF receptors leads to angiogenesis, promotes vascular remodeling, and can lead to residual tumor cell growth, which plays an important role in HCC local recurrence or metastasis [12]. Therefore, inhibitors targeting the VEGF signaling pathway have become the main approach for tumor therapy. Tyrosine kinase inhibitors (TKIs) inhibit the activation of downstream signaling pathways (RAS MAPK, PI3K AKT, and JAK STAT) and prevent the proliferation and migration of HCC cells by binding to the corresponding kinases phosphorylated by their substrate tyrosine residues, invasion, and angiogenesis [13]. The survival benefit of a VEGF TKI in a variety of solid tumors was observed in a series of randomized clinical trials (RCTs). Therefore, the combination of antiangiogenic drugs and TACE therapy may improve the therapeutic effect [14–16].
With the development and application of TKIs in the treatment of cancer, TACE combined with TKIs for the treatment of liver cancer has become a hot topic in clinical research. Preliminary clinical research results are represented by TACE plus sorafenib, and many studies have shown that the combined treatment effect is better [17]. TACE plus TKI combination therapy provides additional options, such as combining apatinib, lenvatinib, brivanib, orantinib, and sorafenib. However, some clinical trial results showed that orantinib combined with TACE does not improve overall survival in patients with uHCC [18]; the clinical trial results showed that TACE plus lenvatinib significantly improved clinical outcomes versus TACE monotherapy [19]. Therefore, it remains controversial whether TACE plus TKIs is superior to TACE monotherapy.
This systematic review and meta-analysis aimed to analyze the safety and efficacy of TACE plus TKIs for the treatment of uHCC.
Materials and methods
Search strategy
This meta-analysis was conducted according to the PRISMA guidelines [20]. We searched the PubMed, Embase, Cochrane Library, and Web of Science databases from inception to March 15, 2022. We established search strategies that combined database-specific subject headings (such as MeSH terms) and free text terms (such as hepatocellular carcinoma/liver cancer/hepatoma, TACE/transcatheter arterial chemoembolization/transarterial chemoembolization, orantinib or TSU-68/sorafenib/lenvatinib/apatinib/brivanib, randomized clinical trials/clinical trials) to identify potentially eligible studies. Studies not published in English were also excluded. Letters, commentaries, editorials, and case reports were also excluded. Potential studies were reviewed by two independent reviewers. If there was any uncertainty about eligibility, a third reviewer was consulted.
Study inclusion and exclusion criteria
The inclusion criteria were as follows: (1) study design: RCTs, retrospective or prospective cohort studies, and case control studies; (2) study population: patients with uHCC; (3) intervention: TACE plus sorafenib/lenvatinib/apatinib/brivanib/orantinib versus TACE plus placebo or TACE alone (including conventional TACE and TACE with drug-eluting beads); and (4) the study was limited to English language articles and required adult patient information including overall survival (OS) and time to progression (TTP) (HR and corresponding 95% confidence interval [CI]), tumor response rates, and adverse events (AEs).
Studies were excluded if they met the following criteria: (1) comments, editorials, systematic reviews, meta-analyses, and studies unrelated to our topics were excluded from the final analysis, as were those unrelated to our topic or lacking useful information; (2) the same study was published by the same authors or based on the same database; (3) cases treated with TACE combined with other anti-tumor drugs were excluded; and (4) cases treated with TACE combined with TKIs and immunotherapy were excluded.
Data extraction
The following information was extracted from studies that met the following inclusion criteria: study characteristics (author name, year of publication, study design, sample size), population characteristics (mean age, sex, country, Barcelona Clinical Liver Cancer [BCLC] stage, Child–Pugh score, Eastern Oncology Collaboration Group [ECOG], hepatitis), intervention characteristics (median drug treatment period, dose, tumor response (objective response rate [ORR], disease control rate [DCR], complete remission [CR], partial response[PR], stable disease [SD], progressive disease [PD], and outcomes [OS, TTP, and safety]). Two independent reviewers extracted the data, and a third reviewer resolved any discrepancies.
Quality assessment
Two reviewers independently assessed the quality of non-randomized controlled studies (RCTs) using the Newcastle–Ottawa scale (NOS) [21], which contains three domains: (1) selection, (2) comparability, and (3) outcome. The maximum NOS score was 9 points, and a score ≥ 6 indicated a high-quality study (Additional file 1: Table 1). The Cochrane risk of bias tool 5.1.0 was applied to examine RCTs [22] using a grading scheme for each of its six main aspects: (1) selection bias, (2) performance bias, (3) detection bias, (4) attrition bias, (5) reporting bias, and (6) other bias. These six were further graded, and each part was evaluated as one of the following levels: “low risk of bias, unclear risk of bias,” and “high risk of bias.” A study was assessed as high quality if four or more parts were assessed as having a low risk of bias (Fig. 1).
Fig. 1.
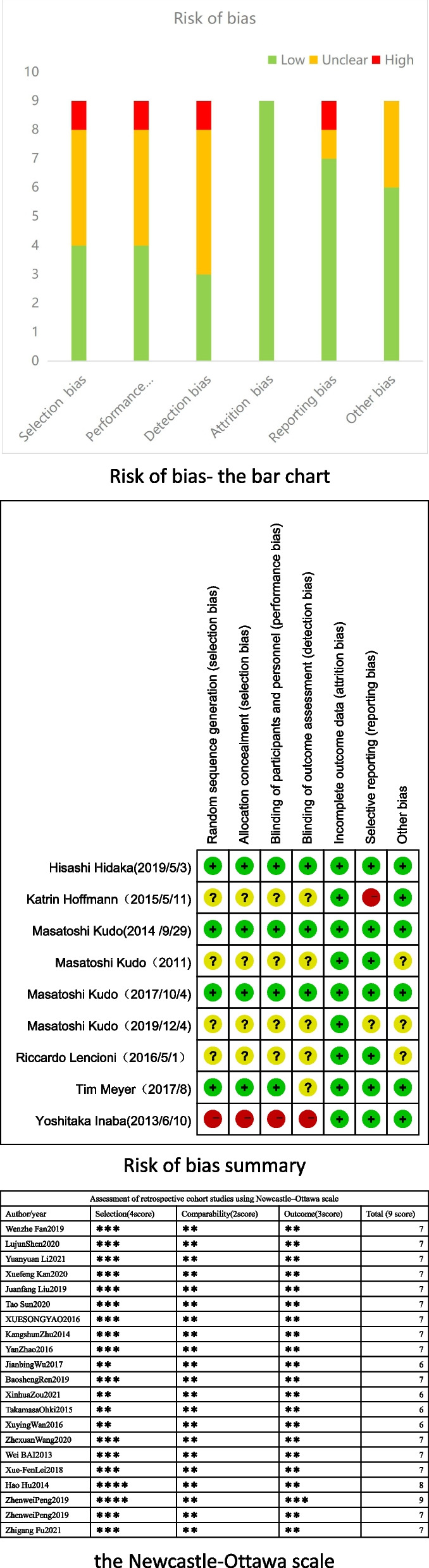
Risk-of-bias graph for randomized controlled trials and observational studies
Statistical analysis
We assessed the overall efficacy of TACE plus TKIs in the treatment of patients with HCC based on data from the included studies. For the time-to-event variables including overall survival (OS), TTP, HRs with 95% CI were directly extracted, and HR values were combined. Pooled HR estimates were calculated using a fixed effects model. However, when heterogeneity was relatively large, the random-effects model was used to summarize the pooled data. Odds ratios (OR) were used to estimate dichotomous variables (CR, PR, SD, PD, ORR, DCR, and AEs), both with corresponding 95% CI.
A test for heterogeneity, defined as the variation between individual trials for a given treatment rather than that expected from chance, was used to assess whether the magnitude of a given treatment effect varied between the trials. The I2 statistic describes the percentage of total variation across studies owing to heterogeneity rather than chance. Studies with an I2 value of < 25%, 50%, 75%, and 100% were considered to have no, low, moderate, and high heterogeneity, respectively.
Publication bias was evaluated using Begg’s and Egger’s tests [19, 20]. Values of p < 0.05 were considered statistically significant. Funnel plots were used to assess publication bias. Statistical analyses were performed using STATA version 14.0 (Stata Corporation, College Station, TX, USA), Review Manager (Revman, version 5.3.0, The Cochrane Collaboration, 2012), and R (version 4.1.2) within the RStudio (2021.09.1) platform.
Evidence certainty
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool was used to assess the overall quality and strength of available evidence [23]. Details of the GRADE evidence profile are shown in Additional file 2: Table 2.
Results
Study selection and quality assessment
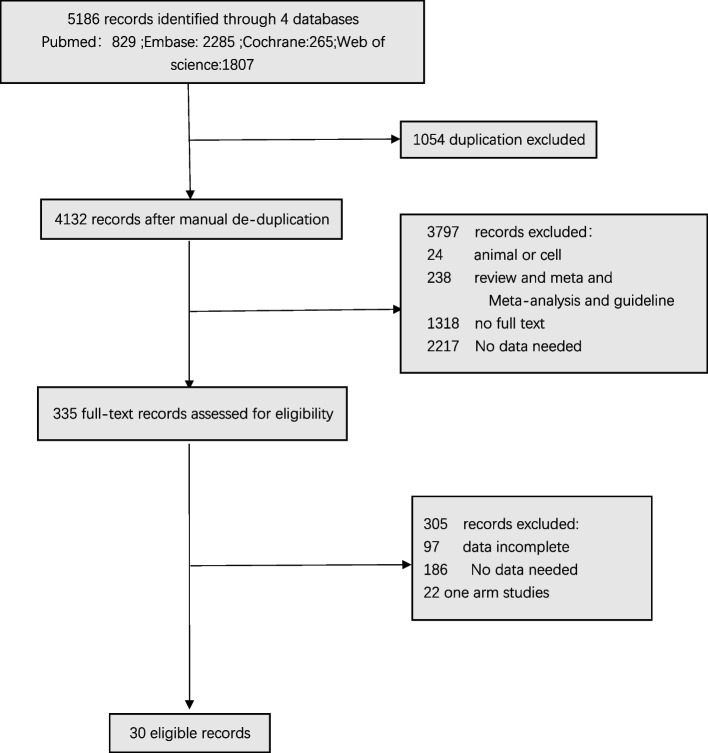
The literature search yielded a total of 5186 studies for screening. The preliminary review excluded 1054 articles, while the title and abstract screening excluded 3797 studies. Thus, 335 articles were subjected to full-text review. Our analysis ultimately included 30 studies: nine (30%) RCTs and 21 (70%) observational studies. A screening flowchart for this study is shown in Fig. 2 [18, 19, 24–51].
Fig. 2.
Study selection
Study characteristics
The baseline characteristics of the 30 studies included in the meta-analysis are presented in Table 1. These 30 studies were published between 2011 and 2021, including 20 in China [19, 26–32, 35, 38, 39, 41, 43–49, 51], 1 in South Korea [40], 7 in Japan [18, 24, 25, 33, 37, 40, 50], 1 in Germany [42], 1 in the USA [36], and 1 in the UK [34], The studies included a total of 8246 patients with uHCC, including 4423 in the combination group and 3823 in the TACE alone or TACE plus placebo, with a total of 48 to 1719 patients each study. The mean age of the studies was 40–73 years, and the majority of patients were men (Table 1). The majority of patients selected had (BCLC) stage A or B, an ECOG physical fitness status (PS) score of 0 or 1, and Child–Pugh grade A or B. The TTP for TACE plus TKIs and TACE alone groups was 71–801 days and 51–492 days, respectively. The OS of the TACE plus TKIs and TACE alone groups was 210–1086 days and 147–990 days, respectively.
Table 1.
Characteristics of included studies in the meta-analysis and systematic review
| Author | Country | Treatment | Mean age (years) | Male/female | Study design | Number of cases | Median treatment period (days) | BCLC stage (%) | Child–Pugh (%) | ECOG (%) | Viral hepatitis (%) (HBV, HCV) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hisashi Hidaka 2019 | Japan | TACE + orantinib VS TACE + placebo | 71 VS 71 |
Male: 178 VS 176 Female: 41 VS 37 |
RCT | 219 VS 213 | 298 |
0: 2.7% VS 4.2% A: 29.7% VS 25.4% B: 55.3% VS 55.9% C: 12.3% VS 14.1% |
A: 100% VS 100% |
0: 94.5% VS 91.5% 1: 5.5% VS 8.5% |
HbsAg positive:17.8% VS 14.1% HCVAb positive:59.8% VS 57.3% |
| Masatoshi Kudo 2017 | Japan | TACE + orantinib VS TACE + placebo | 66·2 VS 65·4 |
Male: 363 VS 364 Female:81 VS 80 |
RCT | 445 VS 444 | 327 |
0: 2% VS 3% A: 33% VS 27% B: 47% VS 52% C: 17% VS 16% |
A: 100% VS 100% |
0: 90% VS 91% 1: 10% VS 9% |
HbsAg positive: 38% VS 45% HbsAb positive: 24% VS 20% HbcAb positive: 70% VS 68% HCV positive:43% VS 37% |
| Yoshitaka Inaba 2013 | Japan | TACE + orantinib VS TACE-alone | NA | Male: 39 VS 43 Female: 11 VS 8 | RCT | 50 VS 51 | 122 |
0: 6.0% VS 17.6% A: 36.0% VS 25.5% B: 48.0% VS 52.9% C: 10.0% VS 10.0% |
A: 80.0% VS 88.2% B: 18.0% VS 11.8% unknown: 2.0% VS 0.0% |
0: 90.0% VS 96.1% 1: 10.0% VS 3.9% |
HbsAg positive: 4.0% VS 7.8% HbcAb positive:80.0% VS 70.6% |
| Tao Sun 2020 | China | TACE + apatinib VS TACE-alone | 55.56 ± 5.2 VS 58.65 ± 6.6 |
Male: 24 VS 21 Female:3 VS 10 |
Retrospective controlled study | 27 VS 31 | NA | C: 100% VS 100% |
A:77.8% VS 74.2% B: 22.2% VS 25.8% |
1: 77.8% VS 80.6% 2: 22.2% VS 19.4% |
B: 92.6% VS 90.3% |
| Wenzhe Fan 2019 | China | TACE + apatinib VS TACE-alone | 49 VS 50 |
Male: 68 VS 71 Female:17 VS 32 |
Retrospective controlled study | 85 VS 103 | NA | B or C: 100% VS 100% |
A: 85.9% VS 84.5% B: 14.1% VS 15.5% |
0: 78.8% VS 87.4% 1–2: 21.2% VS 12.6% |
B: 81.9% VS 75.7% |
| Xuefeng Kan 2020 | China | TACE + apatinib VS TACE-alone | 52.7 ± 9.7 VS 53.1 ± 10.1 |
Male: 77 VS 78 Female:13 VS 12 |
Retrospective controlled study | 90 VS 90 | NA | B: 88.9% VS 87.8% |
A: 87.8% VS 85.6% B: 12.2% VS 14.4% |
1: 82.2% VS 83.3% 2: 17.8% VS 16.7% |
B: 88.9% VS 87.8% |
| Juanfang Liu 2019 | China | TACE + apatinib VS TACE-alone | 53.3 ± 9.4 VS 56.5 ± 9.7 |
Male: 29 VS 39 Female:5 VS 9 |
Retrospective controlled study | 34 VS 48 | NA |
B:52.9% VS 58.3% C:47.1% VS 41.7% |
A: 58.8% VS 60.4% B: 41.2% VS 39.6% |
0–1: 47.1% VS 45.8% 2: 52.9% VS 54.2% |
B: 64.7% VS 77.1% C:14.7% VS 10.4% |
| Yuanyuan Li 2021 | China | TACE‑apatinib VS TACE‑125I | 56.62 ± 10.1 VS 51.63 ± 9.9 |
Male: 19 VS 25 Female:2 VS 2 |
Retrospective controlled study | 21 VS 27 | NA | B or C: 100% VS 100% |
A: 81.0% VS 66.7% B: 19.0% VS 33.3% |
0:71.4% VS 74.1% 1:29.0% VS 25.9% |
NA |
| Zhiyu Qiu 2019 | China | TACE + apatinib VS TACE-alone | NA |
Male: 41 VS 73 Female:1 VS 10 |
A Propensity Score Matching Analysis | 42 VS 83 | NA |
B:21.4% VS 34.9% C:78.6% VS 65.1% |
A:85.7% VS 90.4% B:14.3% VS 9.6% |
NA | B: 92.9% VS 88.0% |
| Lujun Shen 2020 | China | TACE + apatinib VS TACE-alone | NA |
Male: 38 VS 74 Female:2 VS 6 |
Retrospective controlled study | 40 VS 80 | 111 | NA |
A: 82.5% VS 80.0% B: 17.5% VS 20.0% |
NA | B: 90.0% VS 93.8% |
| Masatoshi Kudo 2014 | Japan | TACE + Brivanib VS TACE + placebo | 57 VS 59 | Male: 206 VS 216, female: 43 VS 37 | RCT | 249 VS 253 | NA |
A: 26% VS 23% B: 52% VS 59% C: 22% VS 17% |
A: 96% VS 91% B: 4% VS 8% C: < 1% VS 1% |
0: 80% VS 84% 1: 20% VS 16% |
B: 63% VS 66% C: 20% VS 17% |
| Zhigang Fu 2021 | China | TACE + lenvatinib VS TACE-alone | 60 VS 60 | Male: 50 VS 55, Female: 10 VS 5 | Retrospective controlled study | 60 VS 60 | 246.9 |
A:3.3% VS 5.0% B:55.0% VS 43.3% C:41.7% VS 51.7% |
A:93.3% VS 95.0% B:6.7% VS 5.0% |
NA |
B:80.0% VS 80.0% C:3.3% VS 3.3% |
| Tim Meyer 2017 | UK | DEB-TACE + sorafenib VS DEB-TACE + placebo | 65 VS 68 | Male: 139 VS 138, female: 18 VS 18 | RCT | 157 VS 156 | 120 | NA | A: 100% VS 100%, (5) 68% vs 73% (6) 25% VS 22% (7) 3% VS 1% unknown: 4% VS 3% | 0: 62%: 62% 1: 37%: 37% unknown: 1%: 1% | B: 5% VS 6%, C: 12% VS 6% B + C: 2% VS 2% |
| Xuesong Yao 2016 | China | TACE + sorafenib VS TACE-alone | 56.5 VS 55.9 | Male: 44 VS 87, female: 6VS 13 | Prospective nonrandomized controlled study | 50 VS 100 | NA | B: 42% VS 40%, C: 58% VS 60% | A: 84% VS 86%, B: 16% VS 14% | 0: 42% VS 34% 1: 58% VS 66% | B: 84% VS 83% C: 4% VS 4% B + C: 4% VS 3% |
| Riccardo Lencioni 2016 | USA | DEB-TACE + sorafenib VS DEB-TACE + placebo | 64.5 VS 63.0 | Male: 135 VS 126, female: 19 VS 27 | RCT | 154 VS 153 | 147 | B: 100% VS 100% | A: (5) 63.6% VS 68.6%(6) 35.7% VS 30.7%(7) 0.6% VS 0, unknown: 0: 0.7% | 0: 100% VS 100% | B:35.7% VS 32.7% C: 25.3% VS 26.8% B + C: 1.3% VS 0 |
| Masatoshi Kudo 2019 | Japan | TACE + sorafenib VS TACE-alone | 72.0 VS 73.0 | Male: 63 VS 55, female: 17 VS 21 | RCT | 80 VS 76 | 270.9 | A:33.8% VS 43.4% B:55.0% VS 44.7% C:11.3% VS 11.8% | A: 98.8% VS 93.5% B: 1.3% VS 5.6% | 0: 88.8% VS 88.2%, 1: 11.3% VS 11.8% | B: 12.5% VS 2.6% C: 47.5% VS 69.7% |
| Zhexuan Wang 2020 | China | TACE + sorafenib VS TACE-alone | 53.7 ± 12.0 VS 56.7 ± 12.1 | Male: 267 VS 1183, female: 46 VS 223 | Retrospective controlled study | 1,406 VS 313 | 309 | A:11.5% VS 13.7% B:53.3% VS 53.8% C:35.1% VS 32.6% | A: 95.6% VS 93.8% B: 4.5% VS 6.2% | 0: 64.9% VS 67.4%, 1: 35.1% VS 32.6% | B: 83.1% VS 83.0% C: 5.1% VS 2.6% |
| Kangshun Zhu 2014 | China | TACE + sorafenib VS TACE-alone | 48.4 ± 8.1 VS 51.9 ± 12.2 | Male: 39 VS 38, female: 7 VS 7 | Retrospective controlled study | 46 VS 45 | 330 | NA | A: 84.7% VS 86.7% B: 15.2% VS 13.3% | 0: 47.8% VS 44.4%, 1–2: 52.1% VS 55.6% | B: 82.3% VS 88.9% C: 10.9% VS 2.2% |
| Masatoshi Kudo 2011 | Japan and Korean | TACE + sorafenib VS TACE + placebo | 69 VS 70 | Male: 174VS 168, female: 55 VS 61 | RCT | 229 VS 229 | 513 | NA | NA | 0: 87.8% VS 87.8% 1: 12.2% VS 12.2% | B: 20.5% VS 22.7% C: 60.7% VS 64.6% |
| Yan Zhao 2016 | China | TACE + sorafenib VS TACE-alone | 53 VS 54 | Male: 159 VS 159, female: 24 VS 24 | Multicenter retrospective controlled study | 183 VS 183 | 489 | NA | A: 97.3% VS 3.8% B: 97.3% VS 2.7% | 0: 85.8% VS 14.2%, 1: 88.5% VS 11.5% | B/C: 88.0% VS 88.0% |
| Katrin Hoffmann 2015 | Germany | TACE + sorafenib VS TACE + placebo | 58.5 VS 58.0 | 45\5 | RCT | 24 VS 26 | 125 | NA | A: 58.3% VS 83.3% B: 37.5% VS 23.1% C: 4.2% vs 0% | NA |
B: 12.5% VS 11.5% C: 45.8% VS 26.9% |
| Jianbing Wu 2017 | China | TACE + sorafenib VS TACE-alone | NA | Male: 25 VS 28, female: 2 VS 3 | Retrospective controlled study | 30 VS 31 | NA | C: 100% VS 100% | A: 93.3% VS 80.6% B: 6.6% VS 6.5% | 0: 80% VS 77.4%, 1: 20% VS 22.6% | B/C: 90% VS 96.8% |
| Hao Hu 2014 | china | TACE + sorafenib VS TACE-alone | 61 ± 11 VS 60 ± 11 | Male: 69 VS 140, female: 13 VS 24 | retrospective cohort study | 82 VS 164 | NA | NA | A: 70.7% VS 62.8% B: 29.3% VS 37.2% | NA | B: 82.9% VS 84.8% C: 7.3% VS 6.1% |
| Wei Bai 2013 | China | TACE + sorafenib vs TACE-alone | 54 ± 13 VS 52 ± 12 | Male: 73 VS 146 female: 9 VS 18 | Prospective nonrandomized controlled study | 82 VS 222 | NA |
B: 23.2% VS 27.4% C: 76.8% VS 72.6% |
A:76.8% VS 70.1% B:23.2% VS 29.9% |
0: 36.6% VS 29.3% 1: 46.4% VS 61.6% 2: 14.6% VS 9.1% 3: 1.2% VS 0% 4: 1.2% VS 0% |
B: 87.8% VS 89.6% C: 4.9% VS 4.3% |
| Zhenwei Peng 2019 | China | TACE + sorafenib VS TACE-alone | 55 ± 7.6 VS 56 ± 8.3 | Male: 107 VS 110 female: 21VS 22 | Retrospective cohort study | 128 VS 132 | NA | A: 80.4% VS 72.0%, B: 19.5% VS 28.0% | NA | NA | B: 82.0% VS 85.6% C: 4.7% VS 5.3% |
| Baosheng Ren 2019 | China | TACE + sorafenib VS TACE-alone | NA | Male: 48 VS 102 female: 13 VS 20 | Retrospective controlled study | 61 VS 122 | 351 | B: 49.2% VS 59.0%, C: 50.8% VS 41.0% | A: 90.1% VS 91.0%, B: 9.8% VS 9.0% | 0: 59.0% VS 56.6%, 1–2: 41.0% VS 43.4% | B: 82.0% VS 76.2% C: 8.2% VS 7.3% |
| Xinhua Zou 2021 | China | TACE + sorafenib VS TACE-alone | 58.31 ± 7.83 VS 58.53 ± 8.11 | Male: 32 VS 31 female: 10 VS 12 | Retrospective controlled study | 42 VS 43 | NA | B: 54,8% VS 58.1%, C: 45.2% VS 41.9% | A: 69.0% VS 67.4%, B: 26.2% VS 30.2%, C: 4.8% VS 2.3% | 0: 21.4% VS 23.3% 1: 69.0% VS 69.8, 2: 9.5% VS 7.0% | B: 54.8% VS 58.1% C: 45.2% VS 41.9% |
| Xue-Fen Lei 2018 | China | TACE + sorafenib vs TACE-alone | 52 ± 5 VS 51 ± 6 | Male: 24 VS 18 female: 14 VS 11 | Retrospective controlled study | 38 VS 29 | NA | B: 100% VS 100% |
A:65.8% VS 65.5% B:34.2% VS 34.5% |
0: 100% VS 100% | NA |
| Takamasa Ohki 2015 | Japan | TACE + sorafenib vs TACE-alone | 70.0 VS 72.9 | Male: 20 VS 54 female: 4 VS 17 | Retrospective controlled study | 24 VS 71 | 412 | NA |
A:70.8% VS 29.2% B:56.3% VS 43.7% |
NA | C: 75.0% VS 67.6% |
| Xuying Wan 2016 | China | TACE + sorafenib vs TACE-alone | NA | Male: 218 VS 218 female: 27 VS 27 | Retrospective controlled study | 245 VS 245 | 324 ± 315.3 | NA |
A:86.6% VS 93.7% B:13.4% VS 6.3% |
0/1: 90.6% VS 82.7% 2: 9.4% VS 17.3% |
NA |
| Author | Alcohol hepatitis(%) | Viral hepatitis + alcohol hepatitis | Dose(mg) | ORR | DCR | CR | PR | SD | PD | TTP (days) | OS (days) |
| Hisashi Hidaka 2019 | NA | NA | 200, twice daily | NA | NA | NA | NA | NA | NA | 141 VS 93, HR 0.76 (0.619, 0.940) | 975 VS 990, HR 0.981 (0.717, 1.343) |
| Masatoshi Kudo 2017 | NA | NA | 200, twice daily | NA | NA | NA | NA | NA | NA | 87 VS 75, HR 0.858 (0.744, 0.990) | 933 VS 969, HR 1.09 ( 0.878, 1.352) |
| Yoshitaka Inaba 2013 | NA | NA | 200, twice daily | NA | NA | NA | NA | NA | NA | 157 VS 122, HR 0.699 (0.450, 1.088) | 780: unknown, HR 1.06 (0.578, 1.492) |
| Tao Sun 2020 | NA | NA | 500, twice daily | mRECIST: 37.0% VS 16.1% | 62.9% VS 29.0% | 0% VS 0% | 37.0% VS 16.1% | 25.9% VS 12.9% | 37.0% VS 71.0% | 270 VS 150, HR 0.56 (0.310, 1.022) | 360 VS 270, HR 0.343 ( 0.185, 0.636) |
| Wenzhe Fan 2019 | NA | NA | 500, twice daily | mRECIST:24% VS 4% | 59% VS 14% | 0% VS 0% | 24% VS 4% | 26% VS 10% | 35% VS 89% | 183 VS 111, HR 0.61( 0.48, 0.77) | 360 VS 210, HR 0.443 (0.306, 0.641) |
| Xuefeng Kan 2020 | NA | NA | 500, twice daily | mRECIST:51% VS 10% | 59% VS 33% | 4% VS 0% | 47% VS 10% | 8% VS 23% | 41% VS 67% | 210 VS 90 | 390 VS 240, HR 0.35 ( 0.26, 0.49) |
| Juanfang Liu 2019 | 11.8% VS 8.3% | NA | 500, twice daily | mRECIST: 55.9% vs 31.3% | 70.6% vs 43.8% | 0% VS 0% | 55.9% VS 31.2% | 14.7% VS 12.5% | 29.4% VS 56.3% | NA | 210 VS 167, HR 0.346 (0.203, 0.591) |
| Yuanyuan Li 2021 | NA | NA | 500, twice daily | mRECIST:4.76% VS 40.74% | 23.81% VS 77.78% | 0%VS 0% | 4.8% VS 40.7% | 19% VS 37.0% | 76.2% VS 22,2% | NA | 324 VS 399, HR 0.455 (0.245, 0.848) |
| Zhiyu Qiu 2019 | NA | NA | 500, twice daily | RECIST: 16.7% VS 8.4% | 81.0% VS 53.0% | 4.8% VS 3.6% | 11.9% VS 4.8% | 64.3% VS 44.6% | 19.0% VS 47.0% | NA | 510 VS 321, HR 0.28 (0.158, 0.499) |
| Lujun Shen 2020 | NA | NA | 500, twice daily | NA | NA | NA | NA | NA | NA | NA | 546 VS 255, HR 0.38 ( 0.22, 0.66) |
| Masatoshi Kudo 2014 | 16% VS 15% | NA | 800, once-daily | mRECIST:48% VS 42% | 79% VS 79% | 22% VS 11% | 26% VS 31% | 31% VS 37% | 9% VS 18% | NA | 792 VS 783, HR 0.9 (0.66, 1.23) |
| Zhigang Fu 2021 | NA | NA | 12 mg (≥ 60 kg) or 8 mg (< 60 kg) once daily based on body weight/0, once-daily | mRECIST: 68.3% VS 31.7% | 93.3% VS 86.7% | 10.0% VS 5.0% | 58.3% VS 26.7% | 25.0% VS 55.0% | 6.7% VS 13.3% | NA | NA, HR 0.466 (0.226, 0.886) |
| Tim Meyer 2017 | 34% VS 33% | B + C + alcohol: 2% VS 2% B + alcohol: 2% VS 2% | 400, twice daily | mRECIST: 54% VS 52% | mRECIST: 75% VS 77% | mRECIST: 29% VS 23% | mRECIST: 25% VS 29% | mRECIST: 21% VS 25% | mRECIST: 8% VS 10% | 326 VS 320, HR 0.88 (0.67,1.17) | 631 VS 598, HR 0.91 (0.67, 1.24) |
| Xuesong Yao 2016 | NA | NA | 400, twice daily | mRECIST: 8% VS 1% | 32% VS 24% | 0% VS 0% | 8% VS 1% | 24% VS 23% | 68% VS 76% | 306 VS 201 | 651 VS 345, HR 0.481 (0.297, 0.778) |
| Riccardo Lencioni 2016 | 17.5% VS 19.6% | B + alcohol: 1.9% VS 0.7% C + alcohol: 1.9% VS 2% | 400, twice daily | mRECIST: 42.9% VS 34.6% | 80.5% VS 71.9% | 13.6% VS 13.1% | 29.2% VS 21.6% | 37.7% VS 37.3% | 10.4% VS 19.6% | 169 VS 166, HR 0.797 (0.588, 1.08) | 270 VS 272, HR 0.898 (0.606, 1.330) |
| Masatoshi Kudo 2019 | NA | NA | 400, twice daily | RECICL: 71.3% VS 61.8% | 83.8% VS 77.6% | 28.8% VS 27.6% | 42.5% VS 34.2% | 12.5% VS 15.8% | 2.5% VS 3.9% | 801 VS 492, HR 0.54 (0.35, 0.83) | NA |
| Zhexuan Wang 2020 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | 219 VS 189, HR 0.75 (0.60, 0.93) | 672 VS 666, HR 0.87 (0.74,1.02) |
| Kangshun Zhu 2014 | NA | NA | 400, twice daily | mRECIST: 28.3% VS 4.4% | 57% VS 13% | 0% VS 0% | 28.3% VS 4.4% | 28.3% VS 8.9% | 43.5% VS 86.7% | 180 VS 90 | 330 VS 180, HR 0.429 (0.268, 0.690) |
| Masatoshi Kudo 2011 | 8.2% VS 5.2% | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | 162 VS 111, HR 0.87( 0.7, 1.09) | NA, HR 1.06 (0.69, 1.64) |
| Yan Zhao 2016 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | 393 VS 150 | 669 VS 537, HR 0.4(0.4, 0.83) |
| Katrin Hoffmann 2015 | 29.1% VS 42.3% | NA | 400, twice daily | mRECIST: 20.8%: 26.9% | 66.7% VS 73.1% | 4.3% VS 0% | 17.4% VS 26.9% | 47.8% VS 46.2% | 30.4% VS 26.9% | 71 VS 85, HR 1.106 (0.387, 3.162) | NA |
| Jianbing Wu 2017 | NA | NA | 400, twice daily | mRECIST:NA | 73.4% VS 51.6% | NA | 16.7% VS 6.5% | 56.7% VS 45.1% | 26.6% VS 48.4% | 279 VS 102, | 537 VS 213, HR 0.151 (0.071, 0.322) |
| Hao Hu 2014 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | 78 VS 57 HR 0.62 (0.47,0.82) | 210 VS147, HR 0.63 (0.48, 0.84) |
| Wei Bai 2013 | NA | NA | 400, twice daily | RECIST: 9.7% VS 3.4% | 58.5% VS 44.5% | 0% VS 0% | 9.7% VS 3.4% | 48.8% VS 41.1% | 41.5% VS 55.5% | 189 vs 129, HR 0.6 (0.422, 0.853) | 225 vs 153, HR 0.61(0.42, 0.884) |
| Zhenwei Peng 2019 | 3.9% VS 3.8% | NA | 400, twice daily | mRECIST: 72.3% VS 50.0% | 87.3% VS 80.6% | 34.5% VS 20.8% | 38.1% VS 29.2% | 14.5% VS 30.6% | NA | NA | 516 VS 363, HR 0.62(0.44, 0.89) |
| Baosheng Ren 2019 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | NA | 870 ± 216 VS 447 ± 45, HR 0.684 (0.470,0.997) |
| Xinhua Zou 2021 | NA | NA | 400, twice daily | mRECIST: 23.81% VS 16.28% | 80.95% VS 55.81% | 4.76% VS 0.00% | 19.05% VS 16.28% | 57.14% VS 39.53% | 19.05% VS 44.19% | NA | 960 VS 630, HR 0.6155 (0.3978, 0.9524) |
| Xue-Fen Lei 2018 | NA | NA | 400, twice daily | mRECIST: 60.5% VS 41.4% | 86.8% VS 65.5% | 31.6% VS 13.8% | 28.9% VS 27.6% | 26.3% VS 24.1% | 13.2% VS 34.5% | NA | 1056 VS 660, HR 0.113 (0.036, 0.350) |
| Takamasa Ohki 2015 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | NA | 861 VS 467, HR 0.43 (0.24, 0.76) |
| Xuying Wan 2016 | NA | NA | 400, twice daily | NA | NA | NA | NA | NA | NA | NA | 607 VS 419, HR 0.76 (0.61, 0.94) |
RECIST Response Evaluation Criteria In Solid Tumors, mRECIST modified RECIST, TACE transarterial chemoembolization, DEB-TACE drug-eluting bead transarterial chemoembolization, BCLC The Barcelona Clinic Liver Cancer, ECOG Eastern Cooperative Oncology Group, NA not available, RCT randomized controlled trial, Child–Pugh Child–Turcotte–Pugh, ORR objective response rate, DCR disease control rate, CR complete response, PR partial response, SD stable disease, PD progressive disease
Meta-analysis
Time to progression
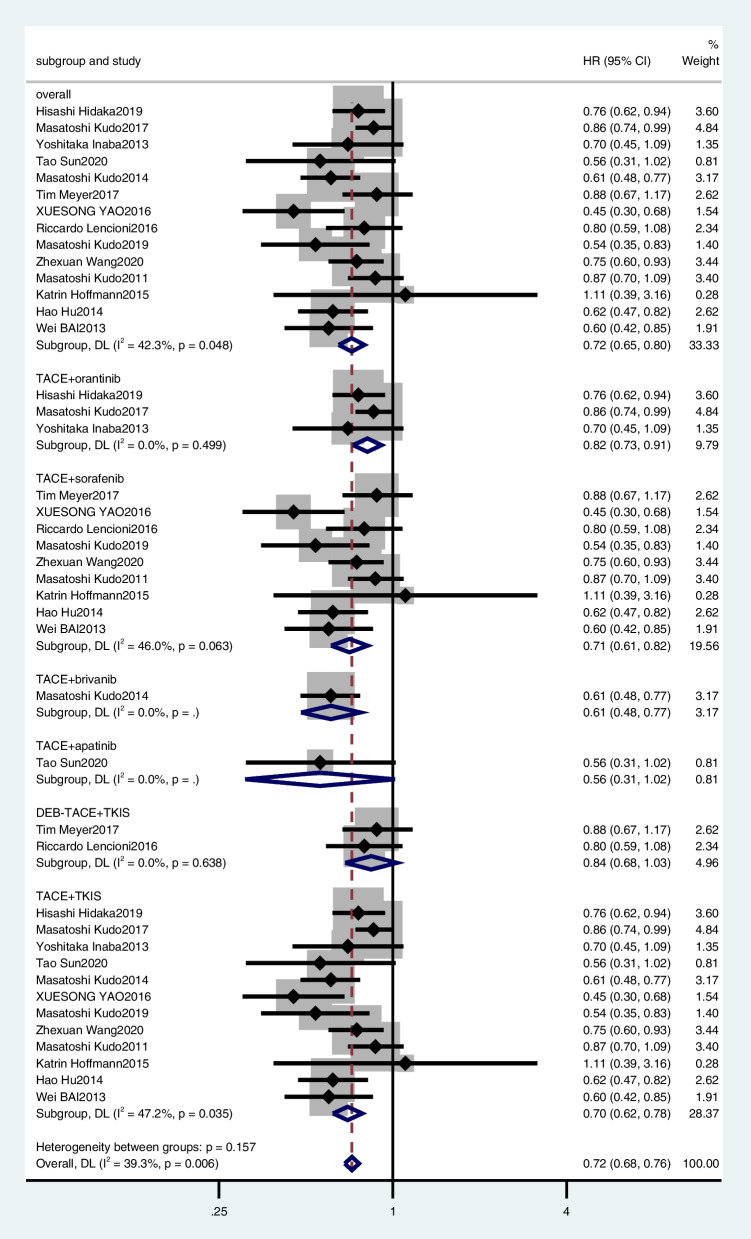
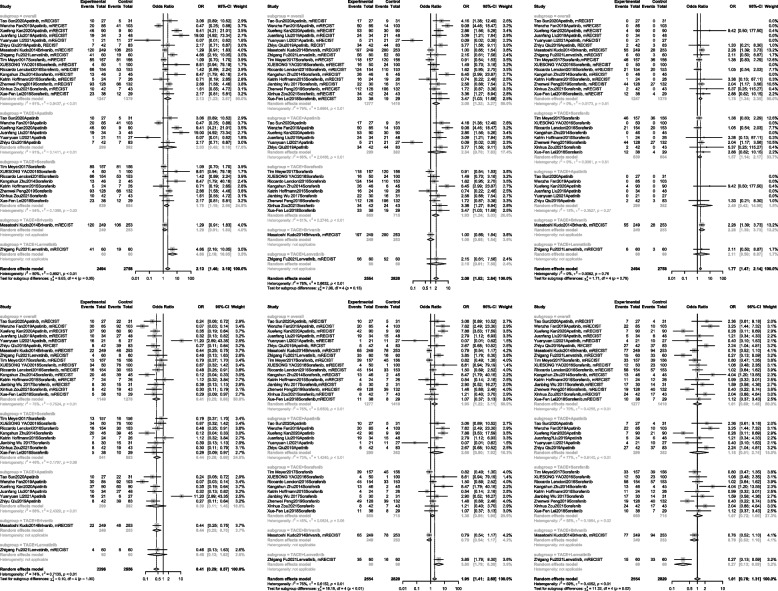
Sixteen [19, 27–32, 39, 41, 43, 46–51] studies were excluded from the TTP meta-analysis because it did not provide HR and 95% CI for TTP. Thus, 14 studies were included in the meta-analysis [18, 24–26, 33–38, 40, 42, 44, 45]. The random-effects model analysis results were as follows: TACE plus TKIs had a better outcome with TTP than TACE plus placebo or TACE alone, with a combined HR of 0.72 (95% CI, 0.65–0.80) (Fig. 3).
Fig. 3.
Meta-analysis for treatment effects of TKIS in combination with TACE on time to progression (TTP) in patients with unresectable hepatocellular carcinoma
In the subgroup analyses, TACE plus orantinib (combined HR, 0.82; 95% CI, 0.73–0.91) (Fig. 3), TACE plus sorafenib (combined HR, 0.71; 95% CI, 0.61–0.82) (Fig. 3), only one article was included in TACE plus apatinib (HR, 0.560; 95% CI, 0.310–1.022), and TACE plus brivanib (HR, 0.61; 95% CI, 0.48–0.77), so the combined HR was no longer used in the analysis. DEB-TACE plus TKIS (combined HR, 0.84, 95%CI, 0.68–1.03), TACE plus TKIS (combined HR, 0.70, 95%CI, 0.62–0.78).
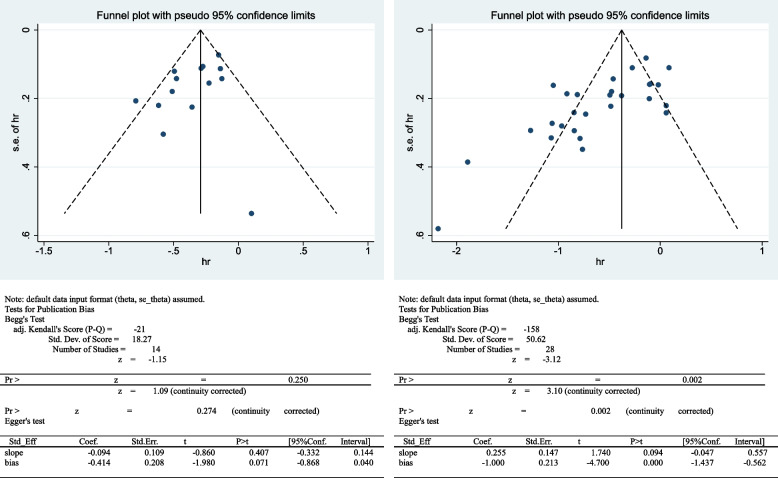
A sensitivity analysis was performed to investigate whether the results were stable. We recalculated the summary HR by excluding each individual study. The range of the combined HR was from 0.70 (95% CI, 0.63–0.78) to 0.73 (95% CI, 0.66–0.81) when the Yao 2016 et al. study was excluded. The heterogeneity was also significantly reduced from 42.3 to 27.4%. The results showed that no individual study significantly affected the pooled effect size. Funnel plots and Egger’s test (t = − 1.98, p = 0.071) showed no evidence of publication bias (Fig. 4).
Fig. 4.
Funnel plot for random effects meta analysis of mean difference in TTP and OS
Overall survival
Of the 30 studies, 28 [18, 19, 24–41, 43–51] provided HR and 95% CI for OS. The pooled results showed that TACE plus TKIs was significantly associated with better OS (combined HR, 0.57; 95% CI, 0.49–0.67) (Fig. 5).
Fig. 5.
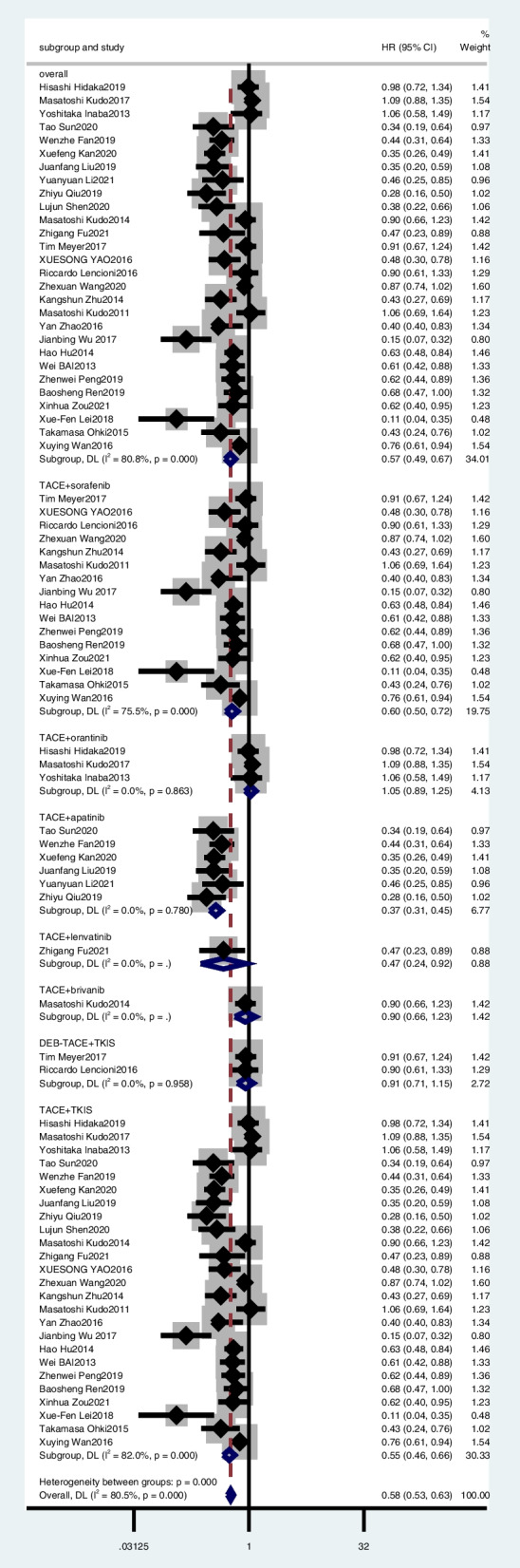
Meta-analysis for treatment effects of TKIS in combination with TACE on overall survival (OS) in patients with unresectable hepatocellular carcinoma
In the subgroup analysis, TACE plus orantinib (combined HR, 1.05; 95% CI, 0.89–1.25) (Fig. 5), TACE plus apatinib (combined HR, 0.37; 95% CI, 0.3–0.44) (Fig. 5), and TACE plus sorafenib (combined HR, 0.60; 95% CI, 0.50–0.72) (Fig. 5). However, TACE plus brivanib (HR, 0.90; 95% CI, 0.66–1.23) and TACE plus lenvatinib (HR, 0.466; 95% CI, 0.226–0.886) were reported by only one article, so the HR values were not combined separately. DEB-TACE plus TKIS (combined HR, 0.91, 95%CI, 0.71–1.15), TACE plus TKIS (combined HR, 0.55, 95%CI, 0.46–0.66).
We performed a sensitivity analysis to explore the robustness of our analysis and recalculated the pooled HR by excluding each individual study. The range of the combined HR was from 0.56 (95% CI, 0.48–0.66) to 0.59 (95% CI, 0.50–0.69). The results showed that no individual study significantly affected the pooled effect size. Funnel plots and Egger’s test (t = − 4.700, p < 0.05) showed publication bias (Fig. 4).
Adverse effects
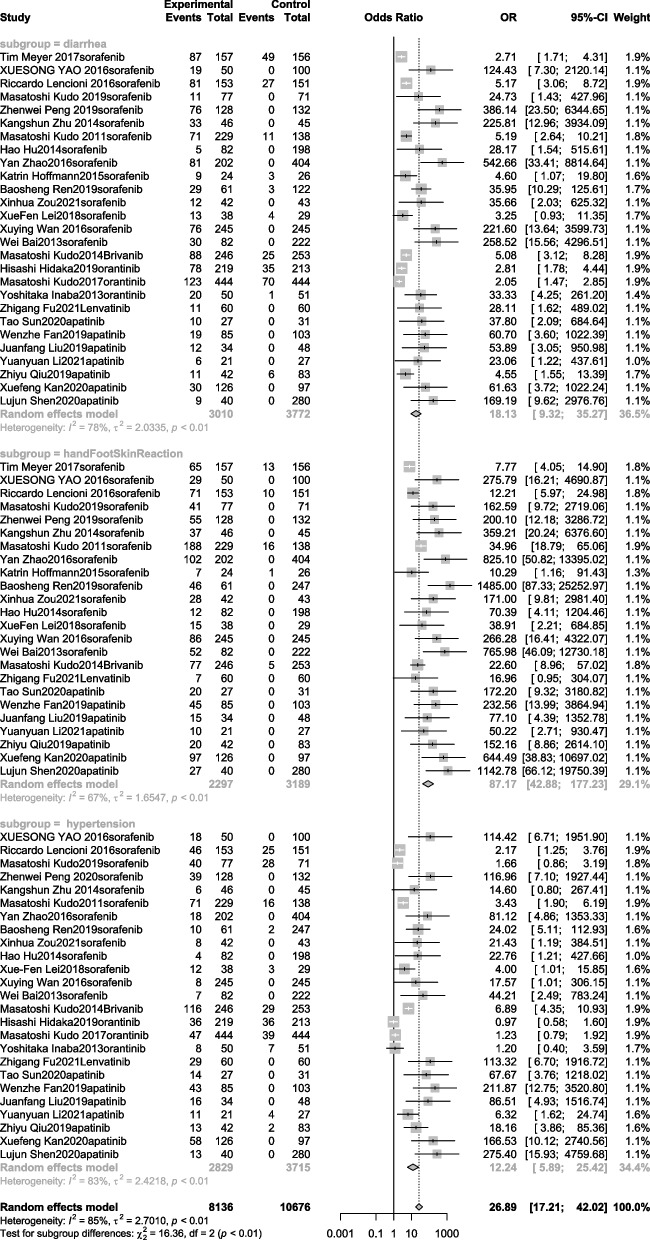
The AEs are summarized in Additional file 3: Table 4. AEs were classified according to the Common Terminology Standard for Adverse Events (version 4.03) [52]. In the random-effects model for adverse reactions of hand-foot skin reaction, diarrhea, and hypertension, the most common AEs in studies related to TACE plus TKIs treatment were hand and foot skin reactions (OR, 87.17; 95% CI, 42.88–177.23), diarrhea (OR, 18.13; 95% CI, 9.32–35.27), and hypertension (OR, 12.24; 95% CI, 5.89–25.42). The forest plot results are shown in Fig. 6.
Fig. 6.
Meta-analysis for treatment effects of TKIS in combination with TACE on adverse events (hand-foot skin reaction, hypertension, diarrhea) in patients with unresectable hepatocellular carcinoma
The common adverse reactions to TACE plus sorafenib were hand-foot skin reactions, diarrhea, hypertension, hair loss, and bleeding. Common adverse reactions to TACE plus brivanib included hand-foot skin reactions, hypertension, rash/desquamation, nausea, and fever. Common adverse reactions to TACE plus orantinib included diarrhea, gastrointestinal disease, abdominal pain, elevated alanine transaminase (ALT) levels, and fever. Common adverse reactions to TACE plus lenvatinib included diarrhea, nausea, hypertension, gastric ulcers, and bleeding. Common adverse reactions to TACE plus apatinib included diarrhea, gastric ulcers, hemorrhage, erythema multiforme, and hypoalbuminemia.
Tumor response rates
Seventeen of the 30 studies [19, 26–31, 33–36, 39, 42, 43, 46, 48, 49] were used to analyze the tumor response rates. The ORR, DCR, CR, PR, SD, and PD were evaluated and described according to modified Response Evaluation Criteria in Solid Tumors [53, 54], ORR (OR, 2.13; 95% CI, 1.23–3.67), DCR (OR, 2.08; 95% CI, 1.32–3.67), CR (OR, 1.78; 95% CI, 1.34–2.35), PR (OR, 1.95; 95% CI, 1.22–3.11), PD (OR, 0.41; 95% CI, 0.25–0.66) were better in the combined therapy versus TACE alone group. However, no significant difference was observed in SD (OR, 1.01; 95% CI, 0.69–1.48) (Fig. 7).
Fig. 7.
Meta-analysis for treatment effects of TKIS in combination with TACE on tumor response rates in patients with unresectable hepatocellular carcinoma
In the subgroup analysis, TACE plus apatinib treatment showed no significant difference between the experimental and control groups in ORR (OR, 2.03; 95% CI, 0.45–9.16), DCR (OR, 2.34; 95% CI, 0.70–7.83), CR (OR, 2.49; 95% CI, 0.42–14.9), PR (OR, 2.68; 95% CI, 0.90–7.92), PD (OR, 0.39; 95% CI, 0.11–1.46), SD (OR, 1.18; 95% CI, 0.51–2.74). For TACE plus sorafenib, TACE plus TKIs was better than TACE alone or TACE plus placebo in terms of ORR (OR, 1.78; 95% CI, 1.19–2.66), DCR (OR, 1.93; 95% CI, 1.24–3.03), CR (OR, 1.57; 95% CI, 1.14–2.17), and PD (OR, 0.44; 95% CI, 0.28–0.69). However, no significant differences were observed in PR (OR, 1.36; 95% CI, 0.95–1.95) and SD (OR, 1.07; 95% CI, 0.72–1.60) (Fig. 7). Moreover, TACE plus brivanib and TACE plus lenvatinib were reported in only one article; therefore, the OR values were not combined separately.
Sensitivity analysis
Sensitivity analyses were performed for TTP and OS. In terms of TTP, according to article type, we divided the included studies into RCTs and non-RCTs and combined their HR values. Nine RCTs [18, 24, 25, 33, 34, 36, 37, 40, 42] had a combined HR of 0.78, 95% CI of 0.70–0.86, I2 = 27.2% and moderate certainty evidence, while 5 non-RCTs [26, 35, 38, 44, 45] had a combined HR of 0.63, 95% CI of 0.53–0.74, and I2 = 22.6%, and heterogeneity was also significantly reduced. Similarly, in terms of OS, 7 RCTs [18, 24, 25, 33, 34, 36, 40] had a combined HR of 0.99, 95% CI of 0.88–1.12, I2 = 0.0% and moderate certainty evidence, while 21 non-RCTs [19, 26–32, 35, 37–39, 41, 43–51] had a combined HR of 0.41, 95% CI of 0.39–0.56, and I2 = 76.9%. Details of the forest plots are shown in Fig. 8.
Fig. 8.
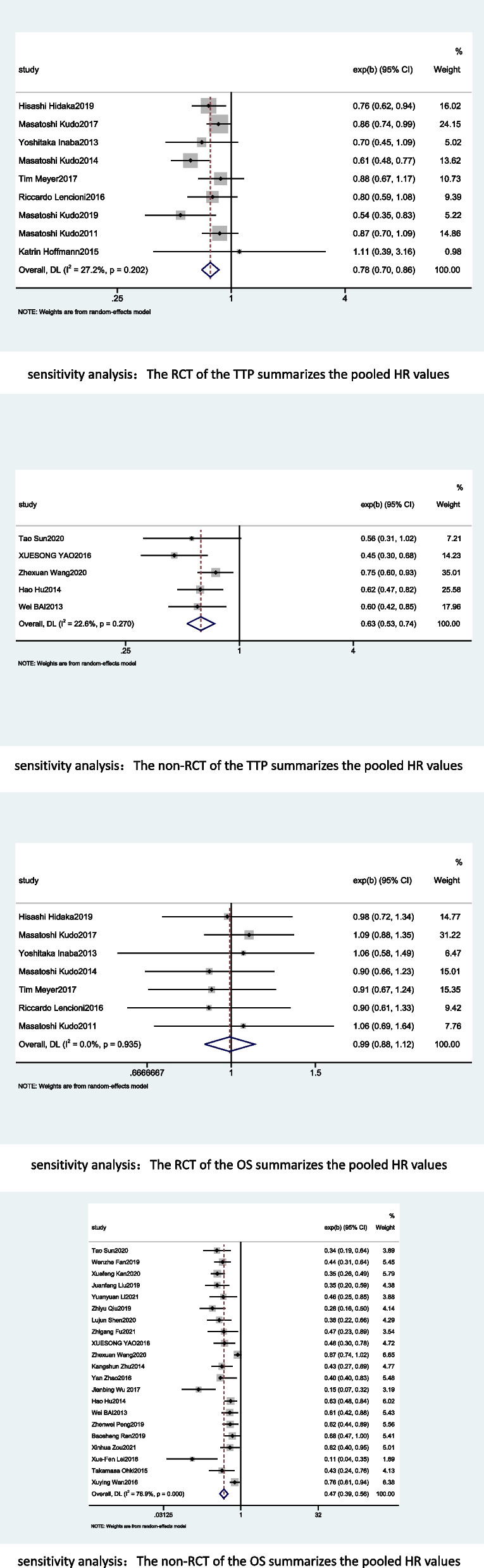
Sensitivity analysis for treatment effects of TKIS in combination with TACE on TTP and OS
Discussion
This meta-analysis enrolled nine RCTs and 21 observational studies with a total of 8246 patients. The results indicated that TACE plus TKIs has an advantage over TACE alone or TACE plus placebo in terms of OS and TTP with an acceptable AE rate. Common AEs associated with TACE plus TKIs therapy mainly include hand-foot skin reaction, diarrhea, hypertension, fatigue, nausea, abdominal pain, vomiting, elevated ALT, fever, and voice change.
Clinically, TACE has a high cost performance because its mini-invasion and excision characteristics are similar to those of surgery. It also has the limitation that a single-pass treatment rarely removes all of the live tumor, and the remaining part can regenerate blood vessels and acquire stronger invasion and metastasis ability in an anaerobic environment. TKIs, a common treatment for advanced uHCC, are usually administered orally, with good patient compliance and slight AEs. Moreover, TKIs can also suppress angiogenesis. To some extent, this seems to compensate for the shortcomings of TACE, a local treatment that can reduce tumor burden, while TKIs are a systemic treatment that can control the disease as a whole. Evidently, the combination of topical and systemic remedies plays a complementary role and provides patients with more benefits.
Several previous meta-analyses evaluated TACE plus sorafenib in patients with uHCC [55–60]. The results of TACE plus sorafenib prolonged TPP in uHCC patients, consistent with our conclusion. This further proves that TACE plus TKIs has a synergistic effect in the treatment of uHCC. However, the results of the five [25, 34, 36, 40, 42] studies we included showed that TACE plus TKIs did not prolong TTP in uHCC. Among them, two [25, 40] studies in Asia indicated that TACE plus TKIs tended to prolong TTP, but this result was not statistically significant. The three [34, 36, 42] studies included more Europeans, and the results showed that combination therapy did not improve TTP in uHCC patients. Interestingly, most of the patients from Asia [25, 40] had hepatitis B or C and most had a Child–Pugh grade of A, while most of the patients included in Europe had alcoholic hepatitis and had a Child–Pugh grade of A. This phenomenon deserves attention since, compared with Europeans, Asians have better results with combination therapy. Most Asian liver cancers are caused by hepatitis B virus infection and are more likely to be treated with TACE combined with TKIs therapy; this may require further research.
Moreover, the Hoffmann 2015 [42] study showed that TACE combined with sorafenib is not suitable for the treatment of HCC patients before liver transplantation. In terms of OS, our study results showed that TACE plus TKIs may prolong OS in patients with uHCC. However, the included studies of TACE plus orantinib, TACE plus brivanib, and TACE plus sorafenib could not prolong OS in uHCC patients, indicating that TACE plus orantinib and TACE plus brivanib after TACE treatment may be a coincidence, but there is a chance that the order of the therapy contributes to the final efficacy. At the same time, the poor treatment effect of TACE plus sorafenib may be caused by the smaller dosage and shorter administration time in the experimental group than in the control group. In terms of tumor response rates, our meta-analysis also demonstrated that TACE plus TKIs had significantly better ORR and DCR. This may be due to the cytotoxic effect of TACE as adjuvant therapy with TKIs. In terms of AEs, our study also showed that the morbidity rate was much higher in the combination treatment group, and the complications were mostly TKI-related. These results are consistent with those of a previous study [61], which showed that hand-foot skin reactions, diarrhea, and hypertension were the most common, indicating that compared with TKIs alone or TACE plus placebo, although the incidence of AEs increased with TACE plus TKIs, there were no unbearable AEs, which were all within the acceptable range.
In terms of TTP, subgroup analysis of included studies according to the type of TACE combination drug showed that, compared to TACE alone, TACE plus orantinib, TACE plus sorafenib, TACE plus brivanib, and TACE plus apatinib all support the idea that TACE plus TKIs are more likely to improve TTP in patients with uHCC. In addition, a sensitivity analysis of the included studies according to study type, the combined HR of nine RCTs [18, 24, 25, 33, 34, 36, 37, 40, 42] and five non-RCTs [26, 35, 38, 44, 45], all illustrate that TACE plus TKIs are more able to prolong TTP in uHCC, demonstrating the stability of our results. In terms of tumor response rates, a subgroup analysis of the included studies according to TACE combination drug, the results demonstrate that, compared to TACE alone, TACE plus apatinib and TACE plus brivanib did not improve ORR and DCR in uHCC patients. However, TACE plus sorafenib and TACE plus lenvatinib resulted in significantly better ORR and DCR. Subgroup analyses according to conventional and drug-eluting beads showed that DEB-TACE did not show superiority over conventional TACE in terms of TTP and OS, but it minimizes systemic toxicity and provides a standardized embolic effect. So it still provides another option for clinicians. Moreover, few studies of DEB-TACE were included in our study, and we hope that more and more comprehensive studies will be conducted in the future.
In terms of OS, a subgroup analysis was performed according to TACE combination drug, and the results indicated that TACE plus orantinib combined HR showed that combination therapy did not improve OS compared to TACE alone. However, TACE plus apatinib, TACE plus sorafenib, TACE plus brivanib, and TACE plus lenvatinib showed that the combination therapy significantly improved the OS of uHCC compared with TACE alone. A sensitivity analysis of the included studies according to study type and the results between RCT and non-RCT-combined HR showed opposite results. The results of seven RCTs [18, 24, 25, 33, 34, 36, 40] combined with HR showed that TACE plus TKIs treatment did not prolong the OS of uHCC patients, and with the I2 = 0, the heterogeneity was low. The seven RCTs, including three of TACE plus orantinib, one of TACE plus brivanib, and three of TACE plus sorafenib with a total sample size of 3002 (37%), were conducted in Japan and Korea. Interestingly, the combined HR of 21 non-RCT studies [19, 26–32, 35, 37–39, 41, 43–51] showed that the combination therapy could prolong OS in patients with uHCC better than TACE alone. Among the 21 RCTs, 20 studies were conducted in China and one was conducted in Japan, with a sample size of 5244 (63%). Perhaps this is a coincidence, but it cannot be ruled out that TACE plus TKIs may be more effective in Chinese patients with prolonged OS. None of the included studies of TACE plus orantinib and TACE plus brivanib support the advantage of combination therapy in prolonging OS in patients with uHCC over TACE alone. Orantinib and brivanib are very good clinical drugs, and their combined treatment with TACE requires additional studies.
Our study has the following strengths. Firstly, it is the first to systematically evaluate the clinical benefits and risks of TACE plus TKIs. Second, it provides more options and evidence to support the clinical treatment of uHCC with TACE plus TKIs. Third, we used rigorous methodological criteria and conducted systematic searches of large sample sizes and in-depth analyses of different subgroups. It also has the following limitations. First, the population characteristics of the included trials (age, etiology of liver disease, vascular invasion, and previous treatment), TKI regimen (treatment lag, treatment duration, treatment sequence, number of prior TACE courses, and dose administered), and study designs vary widely, which may increase heterogeneity and affect the results. Secondly, there are differences chemotherapy agents in TACE with different embolic and drug-eluting bead (e.g., size or type) in different stuies, and these factors may affect the pooled result. Thirdly, the small sample size of some of the included studies may lead to overestimation of the treatment effect.
In conclusion, the current meta-analysis showed that TACE plus TKIs can significantly improve TTP and OS in patients with uHCC with tolerable toxicity. Based on patient specificity, TKIs is a more flexible option for the treatment of uHCC. Owing to the accumulation of new evidence, making the overall situation closer to the real situation, TACE plus TKIs may be a better choice for treating uHCC. We hope that more high-quality studies will be conducted to further support our conclusions.
Supplementary Information
Additional file 1: Table 1 Assessment of retrospective cohort studies using Newcastle–Ottawa scale.
Additional file 2: Table 2 Grade evidence profile of TTP, OS, adverse event and tumor response rates. Certainty of evidence and summary effect estimates assessed byGRADE (grading of recommendations, assessment, development, and evaluation) of randomized controlled trials.
Additional file 3: Table 4 Summary of adverse events.
Authors’ contributions
Manuscript writing, RHD; data collection and analysis, FG,YW and RHD; manuscript revision, RHD, LHH, JMW, ML and CXH; study design, critical manuscript revision, LML , SJQ and YSL; study conception and design, fund collection, corresponding author, YSL; and final manuscript approval, all authors.
Funding
This study was supported by the Guangdong Basic and Applied Research Foundation and Natural Science Foundation of Guangdong Province (2019A1515012118).
Availability of data and materials
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the corresponding author is the sole contact for the editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the corresponding author and which has been configured to accept email from 104,220,421@qq.com.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shijun Qiu, Email: qiu-sj@163.com.
Liming Lu, Email: lulimingleon@126.com.
Yisheng Lin, Email: 104220421@qq.com.
References
- 1.Tan CK, Law NM, Ng HS, Machin D. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:2294–2298. doi: 10.1200/JCO.2003.03.151. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 4.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuan-Xing L, Xu H, Bao-Shan H, Yong L, Pei-Jian S, Xian-Yi Y, et al. Efficacy of therapy for hepatocellular carcinoma with portal vein tumor thrombus: chemoembolization and stent combined with iodine-125 seed. Cancer Biol Ther. 2011;12:865–871. doi: 10.4161/cbt.12.10.17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasai K, Ushio A, Kasai Y, Sawara K, Miyamoto Y, Oikawa K, et al. Combination therapy of intra-arterial 5-fluorouracil and systemic pegylated interferon alpha-2b for advanced hepatocellular carcinoma. Int J Clin Oncol. 2011;16:221–229. doi: 10.1007/s10147-010-0151-9. [DOI] [PubMed] [Google Scholar]
- 7.Katamura Y, Aikata H, Takaki S, Azakami T, Kawaoka T, Waki K, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol. 2009;44:492–502. doi: 10.1007/s00535-009-0033-y. [DOI] [PubMed] [Google Scholar]
- 8.Uka K, Aikata H, Takaki S, Miki D, Jeong SC, Hiramatsu A, et al. Similar effects of recombinant interferon-alpha-2b and natural interferon-alpha when combined with intra-arterial 5-fluorouracil for the treatment of advanced hepatocellular carcinoma. Liver Int. 2007;27:1209–1216. doi: 10.1111/j.1478-3231.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–1252. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 10.Lin CS, Jen YM, Chiu SY, Hwang JM, Chao HL, Lin HY, et al. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212–217. doi: 10.1093/jjco/hyl006. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2007;2003(37):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 12.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 13.Mou L, Tian X, Zhou B, et al. Improving outcomes of tyrosine kinase inhibitors in hepatocellular Carcinoma: new data and ongoing trials. Front Oncol. 2021;11:752725. doi: 10.3389/fonc.2021.752725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 15.Wykosky J, Fenton T, Furnari F, et al. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin J Cancer. 2011;30:5–12. doi: 10.5732/cjc.010.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Meng Q, Tan H, et al. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121:416–424. doi: 10.1002/ijc.22655. [DOI] [PubMed] [Google Scholar]
- 17.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomized and non-randomized clinical trials. BMJ. 1998;317:1185–1190. doi: 10.1136/bmj.317.7167.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Cheng AL, Park JW, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46. doi: 10.1016/S2468-1253(17)30290-X. [DOI] [PubMed] [Google Scholar]
- 19.Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17(8):841–856. doi: 10.1002/(SICI)1097-0258(19980430)17:8<841::AID-SIM781>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- 23.Schunemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 22. The GRADE approach for tests and strategies-from test accuracy to patient-important outcomes and recommendations. J Clin Epidemiol. 2019;111:69–82. doi: 10.1016/j.jclinepi.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka H, Izumi N, Aramaki T, et al. Subgroup analysis of efficacy and safety of orantinib in combination with TACE in Japanese HCC patients in a randomized phase III trial (ORIENTAL) Med Oncol. 2019;36(6):52. doi: 10.1007/s12032-019-1272-2. [DOI] [PubMed] [Google Scholar]
- 25.Inaba Y, Kanai F, Aramaki T, et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer. 2013;49(13):2832–2840. doi: 10.1016/j.ejca.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Ren Y, Kan X, et al. Advanced hepatocellular carcinoma with hepatic arterioportal shunts: combination treatment of transarterial chemoembolization with apatinib. Front Mol Biosci. 2020;7:607520. doi: 10.3389/fmolb.2020.607520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan W, Yuan G, Fan H, et al. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther. 2019;41(8):1463–1476. doi: 10.1016/j.clinthera.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Kan X, Liang B, Zhou G, et al. Transarterial Chemoembolization Combined With Apatinib for Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front Oncol. 2020;10:970. doi: 10.3389/fonc.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Xie S, Duan X, et al. Assessment of efficacy and safety of the transcatheter arterial chemoembolization with or without apatinib in the treatment of large hepatocellular carcinoma. Cancer Chemother Pharmacol. 2020;85(1):69–76. doi: 10.1007/s00280-019-04004-z. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Li H, Hu H, Yuan H, Zhao Y. Efficacy and safety of transcatheter arterial chemoembolization combined with either 125I seed implantation or apatinib in hepatocellular carcinoma with portal vein tumor thrombosis: A retrospective comparative study. J Cancer Res Ther. 2020;16(7):1691–1697. doi: 10.4103/jcrt.JCRT_1587_20. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Z, Shen L, Chen S, et al. Efficacy of apatinib in transcatheter arterial chemoembolization (TACE) refractory intermediate and advanced-stage hepatocellular carcinoma: a propensity score matching analysis. Cancer Manag Res. 2019;11:9321–9330. doi: 10.2147/CMAR.S223271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L, Chen S, Qiu Z, et al. Transarterial chemoembolization combined with apatinib versus transarterial chemoembolization alone for hepatocellular carcinoma with macroscopic vascular invasion: a propensity score matching analysis. J Cancer Res Ther. 2020;16(5):1063–1068. doi: 10.4103/jcrt.JCRT_801_19. [DOI] [PubMed] [Google Scholar]
- 33.Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 34.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial [published correction appears in Lancet Gastroenterol Hepatol. 2017 Sep;2(9):e6] Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Yan D, Zeng H, Liu D, Li H. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J Surg Oncol. 2016;113(6):672–677. doi: 10.1002/jso.24215. [DOI] [PubMed] [Google Scholar]
- 36.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Wang E, Bai W, et al. Exploratory analysis to identify candidates benefitting from combination therapy of transarterial chemoembolization and sorafenib for first-line treatment of unresectable hepatocellular carcinoma: a multicenter retrospective observational study. Liver Cancer. 2020;9(3):308–325. doi: 10.1159/000505692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu K, Chen J, Lai L, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib–a retrospective controlled study. Radiology. 2014;272(1):284–293. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 40.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Li H, Bai W, et al. Early sorafenib-related adverse events predict therapy response of TACE plus sorafenib: a multicenter clinical study of 606 HCC patients. Int J Cancer. 2016;139(4):928–937. doi: 10.1002/ijc.30124. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann K, Ganten T, Gotthardtp D, et al. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in HCC patients - a prospective, randomized, double-blind, phase III trial. BMC Cancer. 2015;15:392. doi: 10.1186/s12885-015-1373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Li A, Yang J, Lu Y, Li J. Efficacy and safety of TACE in combination with sorafenib for the treatment of TACE-refractory advanced hepatocellular carcinoma in Chinese patients: a retrospective study. Onco Targets Ther. 2017;10:2761–2768. doi: 10.2147/OTT.S131022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu H, Duan Z, Long X, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS ONE. 2014;9(5):e96620. doi: 10.1371/journal.pone.0096620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14(4):181–190. doi: 10.1111/1751-2980.12038. [DOI] [PubMed] [Google Scholar]
- 46.Peng Z, Chen S, Xiao H, et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology. 2019;292(1):237–247. doi: 10.1148/radiol.2019181818. [DOI] [PubMed] [Google Scholar]
- 47.Ren B, Wang W, Shen J, Li W, Ni C, Zhu X. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for unresectable hepatocellular carcinoma: a propensity score matching study. J Cancer. 2019;10(5):1189–1196. doi: 10.7150/jca.28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou X, Fan W, Xue M, Li J. Evaluation of the benefits of TACE combined with sorafenib for hepatocellular carcinoma based on untreatable TACE (unTACEable) progression. Cancer Manag Res. 2021;13:4013–4029. doi: 10.2147/CMAR.S304591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei XF, Ke Y, Bao TH, et al. Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: a retrospective comparative study. World J Clin Cases. 2018;6(5):74–83. doi: 10.12998/wjcc.v6.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohki T, Sato K, Yamagami M, et al. Efficacy of transcatheter arterial chemoembolization followed by sorafenib for intermediate/advanced hepatocellular carcinoma in patients in Japan: a retrospective analysis [published correction appears in Clin Drug Investig. 2016 Jan;36(1):93–6] Clin Drug Investig. 2015;35(11):751–759. doi: 10.1007/s40261-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan X, Zhai X, Yan Z, et al. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7(50):83806–83816. doi: 10.18632/oncotarget.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Common terminology criteria for adverse events. Available from:http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 53.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Chen H, Wang M, et al. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS ONE. 2014;9(3):e91124. doi: 10.1371/journal.pone.0091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang G, Liu Y, Zhou SF, et al. Sorafenib combined with transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis and systematic review. Hepatol Int. 2016;10(3):501–510. doi: 10.1007/s12072-015-9700-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS ONE. 2014;9(6):e100305. doi: 10.1371/journal.pone.0100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu MD, Jia LH, Liu HB, Zhang KH, Guo GH. Sorafenib in combination with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20(1):64–74. [PubMed] [Google Scholar]
- 59.Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41(10):6575–6582. doi: 10.1007/s11033-014-3541-7. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):138. doi: 10.1186/s12876-018-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi: 10.1016/j.ctrv.2019.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1 Assessment of retrospective cohort studies using Newcastle–Ottawa scale.
Additional file 2: Table 2 Grade evidence profile of TTP, OS, adverse event and tumor response rates. Certainty of evidence and summary effect estimates assessed byGRADE (grading of recommendations, assessment, development, and evaluation) of randomized controlled trials.
Additional file 3: Table 4 Summary of adverse events.
Data Availability Statement
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the corresponding author is the sole contact for the editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the corresponding author and which has been configured to accept email from 104,220,421@qq.com.