Abstract
Background
Crohn’s disease (CD) is associated with altered body composition, affecting clinical outcomes. We evaluated the impact of biologics on body composition in CD patients.
Methods
This multicenter longitudinal study across four Korean university hospitals conducted from January 2009 to August 2021 retrospectively reviewed data of CD patients with abdominal computed tomography (CT) before and after the biologic treatment. Skeletal muscle area (SMA), visceral fat area (VFA), and subcutaneous fat area (SFA) of the third lumbar vertebra (L3) on CT were measured. Myopenia was defined as L3 skeletal muscle index (SMI) of < 49 and < 31 cm2/m2 for men and women, respectively.
Results
Among 112 participants, 79 (70.5%) had myopenia. In the myopenia group, all body composition parameters were significantly increased after the biologic treatment: SMI (37.68 vs. 39.40 cm2/m2; P < 0.001), VFA (26.12 vs. 54.61 cm2; P < 0.001), SFA (44.29 vs. 82.42 cm2; P < 0.001), while no significant differences were observed in the non-myopenia group. In multivariate analysis, penetrating CD (hazard ratio, 5.40; P = 0.020) was the independent prognostic factor for surgery. Operation-free survival rate tended to decrease in the myopenia group (Log-rank test, P = 0.090).
Conclusions
Biological agents can increase all body composition parameters in CD patients with myopenia. These patients are more likely to experience surgery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-023-02742-2.
Keywords: Crohn's disease, Sarcopenia, Myopenia, Biological products, Body composition
Background
Crohn's disease (CD), a chronic inflammatory disease of the gastrointestinal tract, is frequently associated with malnutrition and weight loss and is also accompanied by sarcopenia in 52% of CD patients. [1, 2] Sarcopenia is defined as a syndrome characterized by progressive and generalized loss of skeletal muscle mass plus either low muscle strength or low physical performance, according to the European Working Group on Sarcopenia in Older Persons 2. [3, 4] Sarcopenia, which was considered to be a physical change due to aging, can also occur in young people, reduces the quality of life, and is closely related to mortality. [5, 6] Systemic diseases, such as cancer or autoimmune diseases, can cause sarcopenia. Sarcopenia affect disease prognosis, [7, 8] which lowers an individual’s quality of life and becomes an obstacle to the improvement of the disease, repeating a vicious cycle. Sarcopenia in CD patients affects the clinical outcome of the disease. Sarcopenia is a prognostic factor for intestinal resection in patients with Crohn's disease [9] and increases the risk of postoperative complications. [10] Sarcopenia is associated with loss of response to anti-TNF therapy in CD. [11]
The introduction of biologics has brought major changes to CD patients' quality of life. [12] Biologic agents including infliximab (IFX), adalimumab (ADA), ustekinumab (UST), vedolizumab (VDZ), and so on improved nutritional status, addressing the malnutrition in inflammatory bowel disease (IBD) patients, which can occur in 85% of patients. [13, 14] Moreover, among IBD patients undergoing surgery due to high-dose steroids and complications, the incidence rate of surgery was reduced by 33%–77% with biologic treatment, and long-term remission was maintained. [15, 16]
Computed tomography (CT) can assess myopenia defined as the presence of clinically relevant muscle wasting due to any disease that is not associated with loss of muscle strength or poor physical performance. [17] The previous study showed that the use of infliximab affected the improvement of sarcopenia in Crohn's disease by using various techniques of body composition analysis. [18, 19] However, to our knowledge, there is no study through CT evaluation on the effect of the use of biological agents on body composition changes in Crohn's disease. Therefore, we analyzed the patients’ body composition through CT imaging studies, such as before and after the use of biologics to determine any changes in the parameters. In addition, we evaluated the risk factors associated with surgery after biologic treatment.
Methods
Study design
This was a multicenter, longitudinal study of CD patients at four university hospitals in South Korea from January 2009 to August 2021. Eligible patients included those who were at least 18 years of age with a diagnosis of CD, based on clinical, endoscopic, and histopathological criteria. We enrolled patients with moderate-to-severe CD with the indication of biological therapy. Moderate-to-severe CD is defined as the Crohn's Disease Activity Index (CDAI) of 220 or greater. If there is no response to two or more drugs such as corticosteroids or immunosuppressants, or the CDAI of 220 or higher, it is an indication for biologics therapy. [20] The patients underwent abdominal CT within 3 months before starting the biological therapy and follow-up abdominal CT while receiving treatment with biological agents. The exclusion criteria were patients with severe comorbidities, such as cardiovascular disease, chronic renal disease, chronic liver disease, and malignancy.
This study was approved by the Institutional Review Board of Busan Paik Hospital (IRB No. 2021–08-028) and was conducted following the ethical guidelines of the Declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective nature of the study and the analysis used de-identified clinical data.
Body composition assessment based on CT
The cross-sectional area at the level of the L3 vertebra on CT was selected for the assessment of body composition, such as skeletal muscle area (SMA), visceral fat area (VFA), subcutaneous fat area (SFA), right and left psoas muscle areas (PSAs), total fat area (TFA), and intramuscular fat area (IMFA). The areas of body composition were measured according to predetermined thresholds for the Hounsfield unit on CT using AsanJ-MorphometryTM software based on ImageJ (NIH, Bethesda, MD, USA; Fig. 1; this open-source software is available at https://datasharing.aim-aicro.com/morphometry). The measurements were performed by a researcher blinded to patient information and outcomes. Also, the measurements of body composition were performed according to the instructions of the radiology doctor.
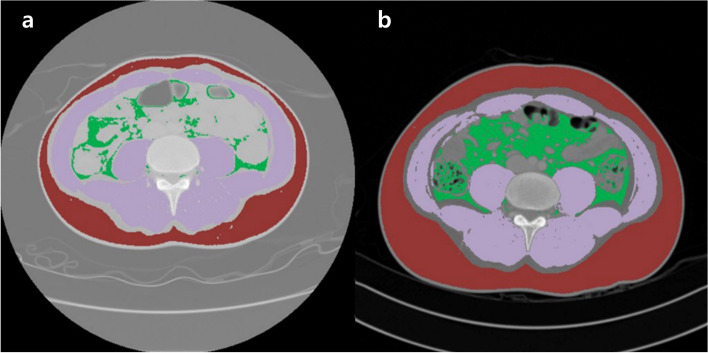
Fig. 1.
Evaluation of the body composition parameters at the L3 vertebral level using computed tomography scans. (a) Baseline (b) After treatment with biologic agents. Purple: skeletal muscle area (SMA), red: subcutaneous fat area (SFA), green: visceral fat area (VFA)
Skeletal muscle index (SMI) was defined as the SMA divided by height in meters squared and used to identify patients with myopenia. The cut-off values of SMI were 49 cm2/m2 and 31 cm2/m.2 for Korean men and women, respectively. [21]
Demographic and clinical parameters
The electronic medical records were used to analyze patient demographics, including age, sex, height, body weight, body mass index (BMI), smoking history, disease characterization, and according to the Montreal classification duration of disease and laboratory parameters, such as serum C-reactive protein (CRP), albumin, and hemoglobin levels. Biological agents included IFX, ADA, UST, and VDZ. More than one biologic was used, as patients may lose their response to some biological agents. Abdominal surgeries associated with CD included colectomy, ileocolonic resection, segmental small bowel resection, and anal fistula operation.
Statistical analysis
Continuous variables were presented as median (interquartile range) because variables were unevenly distributed. Categorical variables were presented as numbers (percentages). The Chi-square (χ2) test was used to compare categorical variables, and Mann–Whitney U test was used for continuous variables. Wilcoxon signed-rank test was used to compare the parameters of the laboratory, including CRP, hemoglobin, albumin, and CT values, including SMA, SMI, PMA, SFA, VFA, TFA, and IMFA before and after the biologic treatment. The correlation between the SMI and clinical variables was analyzed using Spearman's rank correlation. To evaluate the prognostic outcomes of surgery according to myopenia, Kaplan–Meier methods and log-rank test were used. Cox regression analysis was performed to evaluate the risk factors of surgery among CD patients treated with biological agents. A p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using the R statistical software version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
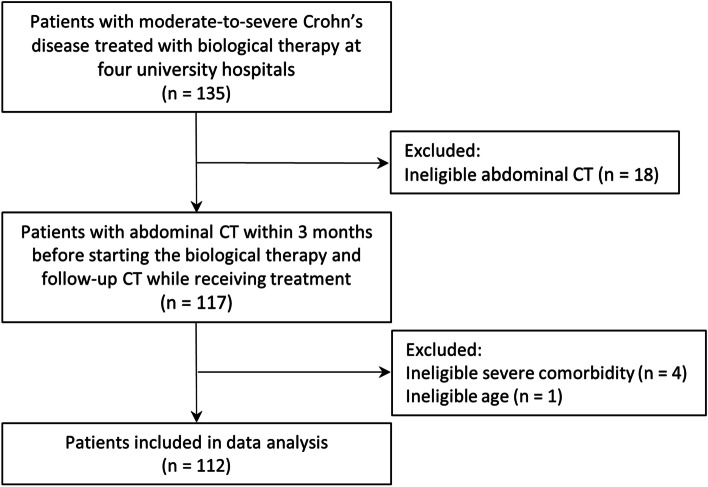
A total of 112 patients were included in this study (Fig. 2). The prevalence of myopenia was 70.5% (79 patients) according to Korean-specific cut-off values. The baseline characteristics of patients stratified according to the presence or absence of myopenia are summarized in Table 1. Myopenia was significantly associated with the male sex (P < 0.001) and BMI (P = 0.010). Altogether, 48 patients (42.9%) had previously undergone abdominal surgery. The location of the disease was the ileocolon in 66 patients (58.9%) and the stricturing type was the most common behavior of the disease in 49 patients (43.8%), followed by the inflammatory type in 42 patients (37.5%). The majority of patients were treated with IFX (67.9%), followed by ADA (40.2%), and the median duration of biological treatment was 64.0 months. There were no significant differences in the characteristics, except for sex and BMI, between the patients with and without myopenia.
Fig. 2.
Flowchart of the study population
Table 1.
Baseline characteristics of patients with Crohn's disease
| Characteristics | Total (n = 112) | Myopenia (n = 79) | Non-myopenia (n = 33) | P-value |
|---|---|---|---|---|
| Age (years) | 35.5 (28.5–41.0) | 36.0 (29.0–40.5) | 32.0 (27.0–41.0) | 0.293 |
| Male sex | 82 (73.2%) | 68 (86.1%) | 14 (42.4%) | < 0.001 |
| BMI (kg/m2) | 21.0 (18.5–23.7) | 20.6 (17.7–22.8) | 22.3 (20.1–24.5) | 0.010 |
| Previous abdominal surgery | 48 (42.9%) | 34 (43.0%) | 14 (42.4%) | 1.000 |
| Smoking habit | 17 (15.2%) | 15 (19.0%) | 2 (6.1%) | 0.147 |
| Montreal classification | ||||
| Age at diagnosis (years) | ||||
| A2(18–40) | 91 (81.2%) | 65 (82.3%) | 26 (78.8%) | 0.868 |
| A3(> 40) | 21 (18.8%) | 14 (17.7%) | 7 (21.2%) | 0.868 |
| Location | ||||
| L1(Ileum) | 20 (17.9%) | 12 (15.2%) | 8 (24.2%) | 0.384 |
| L2(Colon) | 26 (23.2%) | 21 (26.6%) | 5 (15.2%) | 0.289 |
| L3(Ileocolon) | 66 (58.9%) | 46 (58.2%) | 20 (60.6%) | 0.982 |
| L4(Upper GI) | 18 (16.1%) | 12 (15.2%) | 6 (18.2%) | 0.912 |
| Behavior | ||||
| B1(Inflammatory) | 42 (37.5%) | 30 (38.0%) | 12 (36.4%) | 1.000 |
| B2(Stricturing) | 49 (43.8%) | 33 (41.8%) | 16 (48.5%) | 0.657 |
| B3(Penetrating) | 21 (18.8%) | 16 (20.3%) | 5 (15.2%) | 0.715 |
| P(Perianal disease) | 70 (62.5%) | 49 (62.0%) | 21 (63.6%) | 1.000 |
| Extra-intestinal manifestation | 44 (39.3%) | 30 (38.0%) | 14 (42.4%) | 0.820 |
| Disease duration (years) | 10.9 (7.2–14.4) | 11.0 (7.6–14.6) | 9.2 (6.8–13.8) | 0.264 |
| Biologics | ||||
| Infliximab | 76 (67.9%) | 51 (64.6%) | 25 (75.8%) | 0.350 |
| Adalimumab | 45 (40.2%) | 33 (41.8%) | 12 (36.4%) | 0.748 |
| Ustekinumab | 6 (5.4%) | 4 (5.1%) | 2 (6.1%) | 1.000 |
| Vedolizumab | 1 (0.9%) | 0 (0.0%) | 1 (3.0%) | 0.651 |
| Refractory (2 or more biologics) | 13 (11.6%) | 7 (8.9%) | 6 (18.2%) | 0.280 |
| Biologics treatment duration (months) | 64.0 (30.5–93.5) | 66.0 (32.0–97.5) | 58.0 (26.0–82.0) | 0.457 |
| Operation after biologics | 21 (18.8%) | 18 (22.8%) | 3 (9.1%) | 0.154 |
| Concurrent medical treatment | ||||
| steroid | 25 (22.3%) | 16 (20.3%) | 9 (27.3%) | 0.572 |
| 5-ASA | 94 (83.9%) | 65 (82.3%) | 29 (87.9%) | 0.650 |
| Azathioprine | 56 (50.0%) | 40 (50.6%) | 16 (48.5%) | 1.000 |
Values are presented as median (Interquartile range) or number (%)
BMI, body mass index; 5-ASA, 5-aminosalicylic acid
The serum albumin level was significantly associated with myopenia (3.7 vs. 4.0 mg/dL, P = 0.005). The body composition values were measured based on CT before the initiation of the biologic treatment and are presented in Table S1. The median SMI was 37.7 and 44.8 cm2/m2 in the myopenia and non-myopenia groups, respectively (P = 0.001). The SFA (44.3 vs. 90.4 cm2/m2, P < 0.001), VFA (26.1 vs. 33.8 cm2/m2, P = 0.039), and TFA (86.1 c vs. 156.5 cm2/m2, P < 0.001) were significantly lower in the myopenia group than in the non-myopenia group. The median duration between baseline and follow-up CT was 4.1 years. All patients in our study were continuously treated with biologics between the initial assessment and the follow-up CT.
Comparison of the laboratory and body composition values before and after biological therapy
Significant changes in laboratory values were observed after biological therapy, which includes a decrease in CRP levels (1.46 vs. 0.27 mg/dL, P < 0.001) and an increase in albumin (3.80 vs. 4.37 mg/dL, P < 0.001) and hemoglobin (12.15 vs. 13.35 mg/dL, P < 0.001) levels (Table S2).
All body composition parameters were significantly increased after treatment with biological agents. The median SMI was 38.1 cm2/m2 and 39.80 cm2/m2 at baseline and after biologic therapy (P < 0.001), respectively. The SFA (54.61 vs. 93.20 cm2/m2, P < 0.001), VFA (28.98 vs. 53.15 cm2/m2, P < 0.001), and TFA (94.40 vs. 173.32 cm2/m2, P < 0.001) were significantly increased after treatment with biologics.
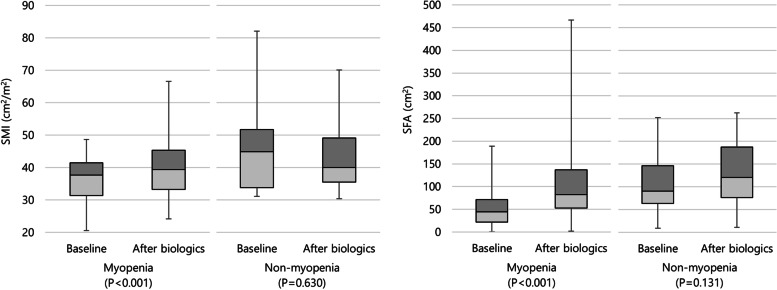
The results of the subgroup analysis by myopenia are shown in Tables 2 and S3. In the myopenia group, all values of laboratory and body composition on CT were significantly different before and after the biologic treatment (Table 2). However, in the patient without myopenia, no significant differences were found in the body composition parameter values at baseline and post-biological therapy (Table S3). Figure 3 shows the comparison of SMI and SFA before and after the use of biological agents in the myopenia and non-myopenia.
Table 2.
Laboratory and body composition parameter values at baseline and after biologic treatment for Crohn's disease patients with myopenia
| Baseline | Post biologics | P-value | |
|---|---|---|---|
| Laboratory parameters | |||
| CRP (mg/dL) | 1.63 (0.58–4.40) | 0.26 (0.06–1.67) | 0.004 |
| Hemoglobin (mg/dL) | 12.20 (10.50–13.68) | 13.60 (11.43–14.78) | 0.002 |
| Albumin (mg/dL) | 3.70 (3.30–4.10) | 4.30 (3.89–4.59) | < 0.001 |
| CT parameter value | |||
| SMA (cm2) | 110.86 (93.45–122.57) | 118.50 (96.18–136.83) | < 0.001 |
| SMI (cm2/m2) | 37.68 (31.40–41.48) | 39.40 (33.29–45.35) | < 0.001 |
| PMA (cm2) | 17.81 (12.35–22.97) | 17.93 (13.51–25.60) | 0.003 |
| SFA (cm2) | 44.29 (21.62–71.61) | 82.42 (51.88–137.10) | < 0.001 |
| VFA (cm2) | 26.12 (13.55–43.35) | 54.61 (32.04–89.87) | < 0.001 |
| TFA (cm2) | 86.11 (41.52–137.24) | 165.36 (103.34–263.28) | < 0.001 |
| IMFA (cm2) | 5.42 (2.52–19.97) | 10.43 (5.48–29.49) | < 0.001 |
Values are presented as median (interquartile range)
CRP C-reactive protein, SMA Skeletal muscle area, SMI Skeletal muscle index, PMA Psoas muscle area, SFA Subcutaneous fat area, VFA Visceral fat area, TFA Total fat area, IMFA Intramuscular fat area
Fig. 3.
The changes in SMI and SFA at baseline and after biologic treatment for Crohn’s disease patients in the myopenia and non-myopenia
Association between SMI and clinical variables
The correlations between SMI and clinical variables in patients with CD are shown in Table 3. The BMI (rho = 0.437), PMA (rho = 0.672), Albumin (rho = 0.285), and hemoglobin (r = 0.282) showed an increasing trend along with increasing SMI. The BMI was significantly correlated with SFA (rho = 0.548) and TFA (rho = 0.539).
Table 3.
Correlation between SMI and clinical parameters
| BMI | Height | Age | SFA | VFA | PMA | TFA | IMFA | Albumin | CRP | Hb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SMI | 0.437b | 0.180 | − 0.076 | 0.128 | 0.137 | 0.672b | 0.111 | − 0.083 | 0.285b | − 0.194a | 0.282b |
| BMI | 0.098 | − 0.159 | 0.548b | 0.447b | 0.318b | 0.539b | 0.288b | 0.180 | − 0.084 | 0.195a | |
| Height | − 0.016 | − 0.186a | − 0.012 | 0.514b | − 0.097 | 0.151 | − 0.151 | 0.152 | 0.201a | ||
| Age | − 0.078 | 0.151 | − 0.110 | − 0.006 | − 0.010 | − 0.071 | − 0.108 | − 0.109 | |||
| SFA | 0.698b | 0.027 | 0.932b | 0.364b | 0.222a | − 0.212a | − 0.103 | ||||
| VFA | 0.081 | 0.857b | 0.466b | − 0.004 | − 0.082 | − 0.141 | |||||
| PMA | 0.101 | 0.260b | 0.095 | − 0.051 | 0.319b | ||||||
| TFA | 0.546b | 0.105 | − 0.163 | − 0.117 | |||||||
| IMFA | − 0.252b | 0.176 | − 0.072 | ||||||||
| Albumin | − 0.309b | 0.487b | |||||||||
| CRP | − 0.114 |
aCorrelation is significant at the 0.05 level
bCorrelation is significant at the 0.01 level
SMI Skeletal muscle index, BMI Body mass index, SFA Subcutaneous fat area, VFA Visceral fat area, PMA Psoas muscle area, TFA Total fat area, IMFA Intramuscular fat area, CRP C-reactive protein, Hb Hemoglobin
Predictors of surgery in patients with myopenia
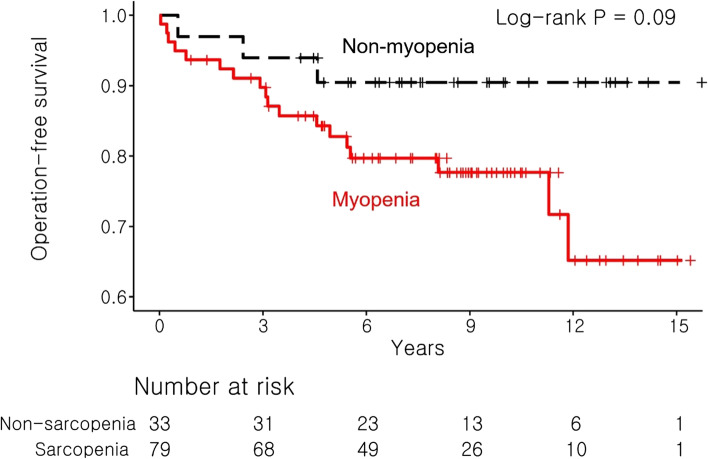
The results of the Kaplan–Meier analysis for CD patients showed that the operation-free survival rate tended to decrease in patients with myopenia, as compared to that of patients without myopenia (Log-rank test, P = 0.090; Fig. 4). The result of the univariable Cox regression analysis showed that sex (hazard ratio [HR], 8.859; P = 0.034) and disease location at the ileocolon (HR, 0.338; P = 0.046) were significantly associated with operation-free survival. Contrarily, myopenia was not significantly related to operation-free survival (HR, 2.759; P = 0.104). In the multivariable analysis, sex (HR, 17.217; P = 0.021) and penetrating CD (HR, 5.399; P = 0.020) were independent predictive factors of surgery (Table 4).
Fig. 4.
Operation-free survival rate by Kaplan–Meier analysis for Crohn's disease patients with or without myopenia
Table 4.
Cox regression hazard ratio in the univariate and multivariate analyses for surgery in patients with Crohn’s disease
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Myopenia | 2.759 (0.812–9.375) | 0.104 | 0.739 (0.185–2.948) | 0.668 |
| Male sex | 8.859 (1.182–66.4) | 0.034 | 17.217 (1.542–192.250) | 0.021 |
| Age | 0.975 (0.927–1.026) | 0.329 | ||
| BMI | 0.997 (0.884–1.124) | 0.961 | ||
| Age at diagnosis (years) | ||||
| A2 (18–40) | Ref | |||
| A3 (> 40) | 0.963 (0.321–2.893) | 0.946 | ||
| Location | ||||
| L1(Ileum) | Ref | Ref | ||
| L2(Colon) | 0.697 (0.233–2.087) | 0.519 | 1.279 (0.390–4.194) | 0.684 |
| L3(Ileocolon) | 0.338 (0.117–0.979) | 0.046 | 0.464 (0.144–1.494) | 0.198 |
| L4(Upper GI) | 0.947 (0.698–1.286) | 0.729 | ||
| Behavior | ||||
| B1(Inflammatory) | Ref | Ref | ||
| B2(Stricturing) | 2.056 (0.797–7.878) | 0.116 | 1.709 (0.483–6.051) | 0.406 |
| B3(Penetrating) | 3.395 (0.956–12.056) | 0.059 | 5.399 (1.311–22.241) | 0.020 |
| P(Perianal disease) | 0.482 (0.205–1.136) | 0.095 | 0.396 (0.150–1.047) | 0.062 |
| Disease duration | 1.039 (0.960–1.125) | 0.344 | ||
| Biologics | ||||
| Biologics one | Ref | |||
| Biologics 2 or more | 1.157 (0.337–3.974) | 0.817 | ||
| Biologics duration | 1.003 (0.993–1.014) | 0.554 | ||
| Laboratory parameters | ||||
| Hemoglobin | 0.950 (0.770–1.173) | 0.636 | ||
| Albumin | 0.736 (0.355–1.527) | 0.411 | ||
| CRP | 0.940 (0.838–1.054) | 0.288 | ||
| CT parameter value | ||||
| SMA | 1.005 (0.992–1.017) | 0.466 | ||
| PMA | 1.027 (0.972–1.085) | 0.342 | ||
| SFA | 0.991 (0.981–1.001) | 0.075 | 0.963 (0.777–1.193) | 0.731 |
| VFA | 0.985 (0.966–1.003) | 0.103 | 0.934 (0.754–1.155) | 0.527 |
| TFA | 0.994 (0.987–1.000) | 0.053 | 1.044 (0.844–1.292) | 0.693 |
| IMFA | 0.973 (0.935–1.012) | 0.166 | 0.952 (0.767–1.182) | 0.656 |
CRP C-reactive protein, SMA Skeletal muscle area, PMA Psoas muscle area, SFA subcutaneous fat area, VFA Visceral fat area, TFA Total fat area, IMFA Intramuscular fat area, HR Hazard ratio
Discussion
The present study evaluated the body composition changes in patients with CD before and after the use of biological agents. Several key observations were noted. First, the skeletal muscle and body fat in CD patients with myopenia were significantly increased after the administration of biologics. In particular, the increase in body fat was notable. Second, the BMI and albumin levels showed tendencies to increase along with an increase in skeletal muscle mass. Third, CD patients with myopenia may be at a higher risk for surgery than those without myopenia.
Biologics brought a paradigm shift in ways to treat CD. Inflammation suppressed through biological agents might contribute to an increase in muscle mass, which again suppress inflammation, thereby forming a virtuous cycle. Considering that in Crohn's disease, muscle loss may continue due to inflammation, [22] it is thought that the slight increase in muscle mass after the biological treatment is meaningful despite of median rather long duration of biological treatment. In this study, most of the patients received anti-TNF, and this increase in body composition may be caused by a direct effect on anti-TNF. Therefore, these results may differ from patients using another biological agents.
On the other hand, the increase in fat mass left us with another problem. In this study, the increase in fat mass was relatively higher than that in muscle mass after administration of the biologic agent. In CD, creeping fat, characterized by localized fat accumulation near the intestinal wall, induces intestinal inflammation. [23] Moreover, improvements in nutritional status may also negatively affect the patients, as this may result in metabolic syndrome. After successful treatment with biological agents in patients with CD, it might be necessary to modify their sedentary lifestyle and diet, such as establishing a healthy diet to prevent overweight. [24, 25]
Recent studies showed that albumin and BMI are associated with body composition parameters. [26, 27] In this study, BMI and albumin showed a possible correlation with skeletal muscle measurement. Therefore, BMI and albumin might be possible alternative indicators for estimating myopenia in patients who had not undergone an imaging study, but more research is needed to confirm this.
Although CD can be treated conservatively, surgery is required for cases with free perforation with peritonitis and massive hemorrhage, acute severe colitis, complete bowel obstruction, or bowel ischemia. [28] Myopenia was an independent predictor of surgery according to a study by Adrienn Erős et al. [29] Myopenia tended to increase the risk of surgery in CD patients in this study. Increasing muscle mass in CD patients can reduce the need for surgical intervention and postoperative complications. [2] Besides myopenia, the only independent predictor of surgery analyzed in this study was the penetrating type CD according to the Montreal classification. [30] The penetrating type also was reported to be associated with an increased risk of recurrence after surgery. [31] This is thought to be due to the nature of fistulas and abscesses which can develop. The predictive factors for surgery in CD patients may help in making treatment decisions, such as the type of drug and the timing of its introduction.
The cutoff values used to define the sarcopenia are applied depending on the study and remain controversial. Considering that there is a difference in muscle mass according to race in previous studies, the threshold should be set differently according to race. Zhang et al. defined sarcopenia as an SMI < 49.9 cm2/m2 for men and < 28.7 cm2/m2 for women, which is a new cut-off value of sarcopenia determined specifically for the Asian population. [32] Because the physical characteristics are different in the case of Asians. CT-based studies in Korean patients also vary from the cutoffs presented. [33, 34] We defined sarcopenia by Korean specific cut-off values for the SMI of 49 cm2/m2 for men and 31 cm2/m2 for women, which was used in several previous studies which included Korean population. [21, 35] Additional research is needed to consensus on the definition of sarcopenia in the future.
This study has a few limitations. First, there was an imbalance between the male and female ratios. It is known that the prevalence of CD in women is high in Europe and the United States, but the opposite result was observed in Asian patients. [36] In this study, the proportion of men was 73.2%. There is a difference in the degree of muscle formation and muscle mass between men and women, and this needs to be considered. Second, the effect of concurrent medications, such as steroids and 5-aminosalicylic acid, on myopenia was not considered. Corticosteroids, used as first-line treatments for CD, are effective in inducing remission and reducing inflammation. [37] Although corticosteroids may reduce skeletal muscle mass and strength with a different mechanism of action from biological agents, it is thought that they may have a role in contributing to sarcopenia in terms of reducing inflammation, and thus additional research is needed. In addition to the effects of medications, it is also necessary to consider the effects of diet and physical activity during the period of therapy. Third, the number of enrolled CD patients without myopenia was too small, limiting the comparison between myopenia and non-myopenia patients. According to a previous study, approximately 50% of CD patients had myopenia, whereas, in this study, 70.5% had myopenia [1, 20]. This is because biologics are used when the first-line treatment is not effective, and the patients in our study are more likely to have advanced diseases. Finally, there is a selection bias because it is difficult to accurately determine the indications for which CT was performed due to a retrospective multicenter design. Due to several confounding factors, the cohort examined may be atypical. Therefore, the results should be clarified later through more prospective studies.
Conclusions
This study revealed that biological agents can increase all body composition parameters among CD patients with myopenia. Anti-TNF may reverse cachexia in CD patients, allowing them to survive without surgery.
Supplementary Information
Additional file 1: Supplementary Table S1. Laboratory and bodycomposition parameter values at baseline in patients with Crohn's disease.Table S2. Laboratory and body composition parameter values at baseline andafter biologic treatment for Crohn'sdisease patients. Table S3. Laboratory and body composition parametervalues at baseline and after biologic treatment for Crohn's disease patientswithout myopenia.
Acknowledgements
This work was supported by a grant from the Research year of Inje University in 20210012.
The authors would like to thank Yu Sub Sung, Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea for his sharing and instructing of AsanJ-Morphometry software.
Busan Ulsan Gyeongnam Intestinal Study Group Society (BIGS)
Eun Jeong Choi1, Dong Hoon Baek2, Hong Sub Lee1, Geun Am Song2, Tae Oh Kim3, Yong Eun Park3, Chang Min Lee4 & Jong Hoon Lee.5
1Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea, 2Department of Internal Medicine, Pusan National University College of Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea, 3Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea, 4Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea, 5Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. A full list of members and their affiliations appears in the Supplementary Information.
Consortia representative: Tae Oh Kim, MD, PhD.
Division of Gastroenterology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea. Tel: + 82–51-797–0220, Fax: + 82–51-797–0298, E-mail: kto0440@paik.ac.kr.
Abbreviations
- BMI
Body mass index
- CD
Crohn’s disease
- CRP
C-reactive protein
- CT
Computed tomography
- HR
Hazard ratio
- IBD
Inflammatory bowel disease
- IMFA
Intramuscular fat area
- IRB
Institutional Review Board
- SFA
Subcutaneous fat area
- SMA
Skeletal muscle area
- SMI
Skeletal muscle index
- TFA
Total fat area
- VFA
Visceral fat area
Authors’ contributions
Conceptualization, Lee HS, Song GA, Kim TO, Lee JH.; Formal analysis: Choi EJ, Baek DH, Lee HS.; Investigation: Choi EJ, Baek DH, Park YE, Lee CM.; Methodology: Park YE, Lee CM.; Project administration: Lee HS.; Writing – original draft: Choi EJ, Baek DH.; Writing – review & editing: Lee HS.; Approval of final manuscript: all authors.
Funding
Not applicable.
Availability of data and materials
The de-identified participant data that support the findings of this study are available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Busan Paik Hospital (IRB No. 2021–08-028) and was conducted following the ethical guidelines of the Declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective nature of the study and the analysis used de-identified clinical data.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eun Jeong Choi and Dong Hoon Baek have equally contributed to this work.
References
- 1.Dhaliwal A, Quinlan JI, Overthrow K, Greig C, Lord JM, Armstrong MJ, et al. Sarcopenia in inflammatory bowel disease: a narrative overview. Nutrients. 2021;13. [DOI] [PMC free article] [PubMed]
- 2.Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflam Bowel Dis. 2019;25:67–73. doi: 10.1093/ibd/izy212. [DOI] [PubMed] [Google Scholar]
- 3.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Par A, Hegyi JP, Vancsa S, Par G. Sarcopenia - 2021: pathophysiology, diagnosis, therapy. Orv Hetil. 2021;162:3–12. doi: 10.1556/650.2021.32015. [DOI] [PubMed] [Google Scholar]
- 6.Tsekoura M, Kastrinis A, Katsoulaki M, Billis E, Gliatis J. Sarcopenia and its impact on quality of life. Adv Exp Med Biol. 2017;987:213–218. doi: 10.1007/978-3-319-57379-3_19. [DOI] [PubMed] [Google Scholar]
- 7.Therakomen V, Petchlorlian A, Lakananurak N. Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: a cross-sectional study. Sci Rep. 2020;10:19551. doi: 10.1038/s41598-020-75250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed]
- 9.Bamba S, Sasaki M, Takaoka A, Takahashi K, Imaeda H, Nishida A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn's disease. PLoS One. 2017;12:e0180036. doi: 10.1371/journal.pone.0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Yu D, Hong L, Zhang T, Liu H, Fan R, et al. Prevalence of sarcopenia and its effect on postoperative complications in patients with Crohn's disease. Gastroenterol Res Pract. 2021;2021:3267201. doi: 10.1155/2021/3267201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding NS, Malietzis G, Lung PFC, Penez L, Yip WM, Gabe S, et al. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther. 2017;46:883–891. doi: 10.1111/apt.14293. [DOI] [PubMed] [Google Scholar]
- 12.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 13.Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare A, Cicala M. Nutritional aspects in inflammatory bowel diseases. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed]
- 14.Wiese D, Lashner B, Seidner D. Measurement of nutrition status in Crohn's disease patients receiving infliximab therapy. Nutr Clin Pract. 2008;23:551–556. doi: 10.1177/0884533608323421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13. doi: 10.1111/apt.13847. [DOI] [PubMed] [Google Scholar]
- 16.Binion DG. Biologic therapies for Crohn’s disease: update from the 2009 ACG meeting. Gastroenterol Hepatol. 2010;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011;2:1–3. doi: 10.1007/s13539-011-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding NS, Tassone D, Al Bakir I, Wu K, Thompson AJ, Connell WR, et al. Systematic review: the impact and importance of body composition in inflammatory bowel disease. J Crohns Colitis. 2022;16:1475–1492. doi: 10.1093/ecco-jcc/jjac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramaniam K, Fallon K, Ruut T, Lane D, McKay R, Shadbolt B, et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn's disease. Aliment Pharmacol Ther. 2015;41:419–428. doi: 10.1111/apt.13058. [DOI] [PubMed] [Google Scholar]
- 20.Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd european evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Yoon H, Oh DJ, Lee JM, Choi YJ, Shin CM, et al. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn's disease. Intest Res. 2020;18:79–84. doi: 10.5217/ir.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuoco L, Vescovo G, Castaman R, Ravara B, Cammarota G, Angelini A, et al. Skeletal muscle wastage in Crohn's disease: a pathway shared with heart failure? Int J Cardiol. 2008;127:219–227. doi: 10.1016/j.ijcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9. [DOI] [PMC free article] [PubMed]
- 24.Chicco F, Magri S, Cingolani A, Paduano D, Pesenti M, Zara F, et al. Multidimensional impact of mediterranean diet on IBD patients. Inflam Bowel Dis. 2021;27:1–9. doi: 10.1093/ibd/izaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Van Ancum JM, Tuttle CSL, Koopman R, Pijnappels M, Meskers CGM, Paul SK, et al. Albumin and C-reactive protein relate to functional and body composition parameters in patients admitted to geriatric rehabilitation after acute hospitalization: findings from the RESORT cohort. Eur Geriatr Med. 2022;13:623–632. doi: 10.1007/s41999-022-00625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn's cisease undergoing bowel resection. JPEN J Parenter Enter Nutr. 2017;41:592–600. doi: 10.1177/0148607115612054. [DOI] [PubMed] [Google Scholar]
- 28.Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, et al. ECCO-ESCP consensus on surgery for Crohn's disease. J Crohns Colitis. 2018;12:1–16. doi: 10.1093/ecco-jcc/jjx061. [DOI] [PubMed] [Google Scholar]
- 29.Eros A, Soos A, Hegyi P, Szakacs Z, Benke M, Szucs A, et al. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: a meta-analysis. Surg Today. 2020;50:1138–1150. doi: 10.1007/s00595-019-01893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechara Cde S, Lacerda Filho A, Ferrari Mde L, Andrade DA, Luz MM, da Silva RG. Montreal classification of patient operated for Crohn's disease and identification of surgical recurrence predictors. Rev Col Bras Cir. 2015;42:97–104. doi: 10.1590/0100-69912015002006. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Ding C, Xie T, Yang J, Dai X, Lv T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr. 2017;36:1586–1592. doi: 10.1016/j.clnu.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67:1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JK, Lee S, Kim KW, Lee JE, Hwang JA, Park T, et al. Reference values for skeletal muscle mass at the third lumbar vertebral level measured by computed tomography in a Healthy Korean Population. Endocrinol Metab (Seoul) 2021;36:672–677. doi: 10.3803/EnM.2021.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, Kim YS, Kim EY, Jin W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE. 2018;13:e0202700. doi: 10.1371/journal.pone.0202700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greuter T, Manser C, Pittet V, Vavricka SR, Biedermann L. Gender differences in inflammatory bowel disease. Digestion. 2020;101:98–104. doi: 10.1159/000504701. [DOI] [PubMed] [Google Scholar]
- 37.Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;2:CD006792. doi: 10.1002/14651858.CD006792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table S1. Laboratory and bodycomposition parameter values at baseline in patients with Crohn's disease.Table S2. Laboratory and body composition parameter values at baseline andafter biologic treatment for Crohn'sdisease patients. Table S3. Laboratory and body composition parametervalues at baseline and after biologic treatment for Crohn's disease patientswithout myopenia.
Data Availability Statement
The de-identified participant data that support the findings of this study are available upon reasonable request to the corresponding author.