Abstract
Purpose
Study the impact of impaired sleep quality on symptom change and future exacerbation of chronic obstructive pulmonary disease (COPD) patients.
Methods
This was a prospective study. Patients with COPD were recruited into the study and followed up for one year. Pittsburgh sleep quality index (PSQI) was collected at baseline. Symptom change was assessed with Minimum clinically important difference (MCID) in COPD Assessment Test (CAT) at 6-month visit, which is an indicator to assess symptom improvement. Exacerbation was recorded during the one-year visit. PSQI score > 5 was defined as poor sleep quality, whereas PSQI score ≤ 5 was defined as good sleep quality. MCID was defined as attaining a CAT decrease ≥ 2.
Results
A total of 461 patients were enrolled for final analysis. Two hundred twenty-eight (49.4%) patients had poor sleep quality. Overall, 224 (48.6%) patients attained MCID at 6-month visit and the incidence of exacerbation during the one-year visit was 39.3%. Fewer patients with impaired sleep quality achieved MCID than patients with good sleep quality. Good sleepers were significantly more likely to attain MCID (OR: 3.112, p < 0.001) than poor sleepers. Fewer poor sleepers in GOLD A and D groups attained MCID with ICS/LABA, and fewer poor sleepers in the GOLD D group attained MCID with ICS/LABA/LAMA than good sleepers. Poor sleep quality was a greater risk factor of future exacerbation in Cox regression analysis. The ROC curves showed that PSQI score had a predictive capacity for future exacerbation. More patients with poor sleep quality experienced future exacerbation in GOLD B and D group with treatment of ICS/LABA/LAMA compared to good sleepers.
Conclusions
COPD patients with impaired sleep quality were less likely to achieve symptom improvement and were at increased risk of future exacerbation compared to patients with good sleep quality. Besides, sleep disturbance may affect the symptom improvement and future exacerbation of patients with different inhaled medication or in different GOLD groups.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02405-6.
Keywords: COPD, Sleep quality, Symptom change, Exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease with persistent airflow limitation caused by toxic particles or gases [1]. Globally, 174.5 million (2.4%) people suffer from COPD [2], and the prevalence in patients aged 40 years and older in China is 13.7% [3]. COPD is the one of third leading causes of death worldwide [4].
The symptoms of COPD patients include, but are not limited to, dyspnoea, cough, and wheezing, as well as poor sleep quality, depression, and fatigue [5, 6]. Compared with healthy control subjects, poor sleep quality is more frequent in COPD patients. The prevalence of sleep-disordered breathing is reported to be between 50% and 60% in patients with COPD [7, 8]. The most common symptoms include sleep onset insomnia, nighttime awakenings, and sleep deprivation [9, 10]. Factors reported to affect sleep quality in COPD patients include respiratory symptoms [11], depression and anxiety [12], and obstructive sleep apnoea syndrome (OSAS) [13].
The Pittsburgh Sleep Quality Index (PSQI) is a questionnaire mainly used to assess the subjective sleep quality of subjects [14]. A higher PSQI score represents poorer sleep quality. A study demonstrated that PSQI scores of COPD patients were higher compared with individuals without COPD, and sleep quality worsens with disease severity [11, 15]. It has been indicated that patients with a COPD Assessment Test (CAT) ≥ 10 points, or a Modified Medical Research Council Dyspnea Scale (mMRC) ≥ 2 points, had impaired sleep quality [11]. What’s more, at least one daytime COPD symptom, such as dyspnoea or cough, had a negative influence on sleep quality [16–18]. Current studies show that poor sleep quality is one of the important predictors of adverse disease outcomes and mortality [19, 20]. Shorofsky et al. [21] showed that higher PSQI scores were associated with increased risk of future exacerbation and earlier deterioration [21]. Even though bad sleep quality in COPD patients has been shown to be associated with severity of disease and risk of exacerbation, no current study has reported whether poor sleep quality would affect the symptom change in COPD. Therefore, the purpose of this study is to explore the impact of impaired sleep quality on symptoms change and future exacerbation in COPD patients.
Methods
Study design and subjects
This was a prospective study. All subjects were obtained from the outpatient COPD database of the Second Xiangya Hospital of Central South University (ChiCTR-POC-17,010,431) from October 2020 to June 2021. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 report, COPD is diagnosed when a ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) < 0.70 after inhaling a bronchodilator [22]. The study excluded patients with a history of bronchiectasis, asthma, lung cancer, or pneumonia, or severe heart, liver, or kidney disease.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. All patients provided written informed consent.
Data collection
All patients accepted three visits. Clinical characteristics collected at baseline visit included age, sex, education degree, body mass index (BMI), smoking history, biofuel and occupational exposure history, pulmonary function data, CAT, mMRC, Clinical COPD Questionnaire (CCQ), pulmonary function, inhalation therapy drugs (including long-acting muscarinic antagonist (LAMA), inhaled corticosteroids (ICS)/long-acting β2-agonists (LABA), LABA/LAMA, and ICS/LABA/LAMA, exacerbation (moderate-to-severe) in the previous year, PSQI, Hospital Anxiety and Depression Scale (HADS), and Berlin Questionnaire. All patients were followed up for 12 months. Patients were followed up at outpatient centre at 6-month visit, we evaluated the symptom by CAT score, as well as inquired and recorded exacerbations the patients within 6 months. Then, data on exacerbation within latter 6 months were collected at the 12-month follow-up by phone calls.
Definition and measure
The PSQI is a questionnaire mainly used to assess the subjective sleep quality of subjects and consists of 19 questions. Questions include subjective sleep quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Total scores range from 0 to 21. A score > 5 defines poor sleep quality [23], thus the patients were divided into two groups: good sleeper and poor sleeper. A validated three-factor analysis was also used, which is statistically more reliable than a single score [24]. Factor 1, Sleep Efficiency, includes sleep duration and efficiency components (score 0–6). Factor 2, Sleep Quality, includes the perceived sleep quality, sleep latency, and sleep medication use components (score 0–9). Factor 3, Daily Disturbances, includes sleep disturbances (bathroom use, breathing issues, pain) and sleep-related daytime dysfunction (sleepiness, enthusiasm) components (score 0–6).
The HADS is mainly used to assess the degree of anxiety and depression. The total score of both anxiety and depression is 21, and a score > 7 is regarded as the cut-off point for determining anxiety and depression.
The Berlin Questionnaire is a screening scale to assess risk for OSAS. Patients with positive screening and at risk of OSAS were advised to see a specialist outpatient clinic, though how many people used non-invasive positive pressure ventilation wasn’t tracked.
According to the GOLD 2020 report [22], patients were assigned to four categories. Briefly, Group A: 0 to 1 exacerbation per year, no hospitalization, CAT score < 10 or mMRC score of 0 to 1; Group B: 0 to 1 exacerbation per year, no hospitalization, CAT score ≥ 10 or mMRC score ≥ 2; Group C: exacerbation ≥ 2 or hospitalization ≥ 1 per year, CAT score < 10 or mMRC score of 0 to 1; Group D: exacerbation ≥ 2 or hospitalization ≥ 1 per year, CAT score ≥ 10 or mMRC score ≥ 2.
Assessment for symptom change
CAT score was used to assessed the symptom of COPD. Change in CAT was defined as CAT score changing between baseline and the 6-month visit. The study evaluated the symptoms change with minimum clinically important difference (MCID) during the 6-month follow-up. Minimum clinically important difference was defined as attaining a CAT score decrease ≥ 2 from baseline at the 6-month visit, which is an indicator to assess symptom improvement [25]. The MCID response rate was calculated based on the proportions of individual patients with a ≥ 2 improvement from baseline in CAT score.
Exacerbations
The study also recorded the incidence of moderate-to-severe exacerbation during the one-year follow-up period. Moderate exacerbation was defined as exacerbation of respiratory symptoms requiring antibiotics and/or oral corticosteroids; severe exacerbation was defined as exacerbation requiring hospitalization or emergency room admission for more than 2 days during the follow-up period. Frequent exacerbation was defined as at least two exacerbations during the follow-up period.
Statistical analysis
SPSS 26.0 (IBM, Armonk, NY, USA) was used for statistical analysis of the data. Continuous variables were expressed as mean ± standard deviation or median and interquartile range as appropriate. Continuous variables were tested using Student’s t-test; otherwise, non-parametric tests were used. The chi-square test was used for categorical variables. The multivariate stepwise logistic regression analysis was used to analyze the clinical features including sleep quality associated with MCID. A multivariate Cox regression analysis was performed for identifying factors predicting exacerbation during one-year follow up, by including variables that were significant (p < 0.05) on univariate analysis. The receiver operating characteristic (ROC) curve was calculated and the area under the curve (AUC) was compared using the Z-test. For all analyses, a p-value of < 0.05 was considered statistically significant.
Results
Participant characteristics
A total of 502 patients with COPD were initially enrolled. At the 6-month visit, 28 patients were excluded from the study due to loss of contact. At the 12-month follow-up, 13 patients dropped out due to loss of contact. Subsequently, 461 patients were recruited for the final analysis (Fig. 1).
Fig. 1.
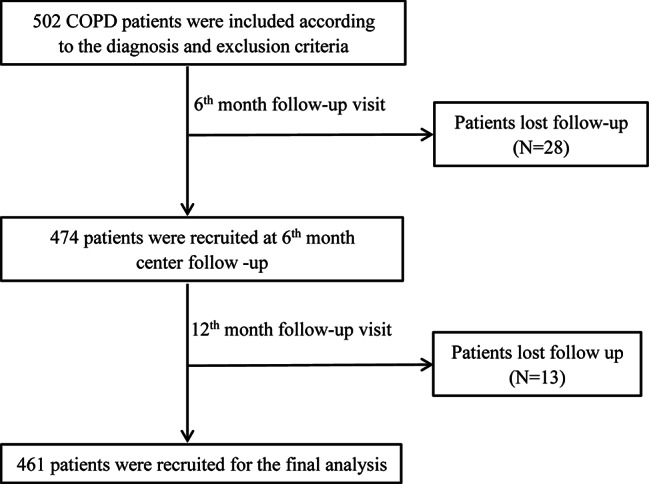
Flow diagram of the inclusion of study
Abbreviations: COPD, Chronic Obstructive Pulmonary Diseases.
As shown in Table 1, the mean age of patients was 63.1 ± 8.2 years and 88.9% of patients were male. The mean CAT score was 12.4 ± 6.8 and the median (IQR) FEV1% was 54.4 (31.2). Most patients were classified as GOLD B (42.3%) and D (34.7%). The mean PSQI score was 5.8 ± 3.4, 228 (49.4%) patients had poor quality sleep. The proportion of patients at high risk for OSAS is 20.1%. Thirty-one (6.8%) patients had anxiety and 12 (2.4%) had depressive tendencies.
Table 1.
The distribution of baseline demographic and clinical characteristics according to sleep quality
| Variables | Total | Bad sleeper | Good sleeper |
P- value |
|---|---|---|---|---|
|
Number of patients, n (%) |
461(100.0) | 228(49.5) | 233(50.5) | |
| Age (years)a | 63.1 ± 8.2 | 64.3 ± 8.5 | 62.1 ± 9.0 | 0.016 |
| Sexb | 0.287 | |||
| Male | 410(88.9) | 199(87.3) | 211(90.6) | |
| Female | 52(11.1) | 29(12.7) | 22(9.4) | |
| Educationb | 0.116 | |||
| Primary school | 158(34.3) | 84(36.8) | 74(31.8) | |
| Junior high school | 189(41.0) | 97(42.5) | 92(39.5) | |
| High school | 77 (16.7) | 35(15.4) | 42(18.0) | |
| University | 37(8.0) | 12 (5.3) | 25 (10.7) | |
| BMI (kg/m2)a | 22.6 ± 3.1 | 22.3 ± 3.2 | 22.9 ± 3.4 | 0.096 |
| Marry statusb | 0.354 | |||
| married | 351 (95.4) | 219 (96.1) | 222(95.2) | |
| unmarried | 17(4.6) | 9(3.9) | 11 (4.8) | |
| Smoking stateb | 0.614 | |||
| Current smoker | 178(38.8) | 84(36.9) | 95(40.7) | |
| Ex-smoker | 210(45.6) | 107(46.9) | 103(44.2) | |
| Non-smoker | 72(16.6) | 37(16.2) | 35(16.0) | |
| Biofuel exposureb | 0.292 | |||
| Yes | 169(36.7) | 90(39.3) | 79(34.1) | |
| No | 292(63.3) | 138(60.7)) | 154 (65.9) | |
| Occupationalb | 0.608 | |||
| Yes | 182(39.4) | 90(39.3) | 79(39.5) | |
| No | 279(60.6) | 138 (60.7)) | 154 (60.5) | |
| CATa | 12.4 ± 6.8 | 14.6 ± 6.3 | 9.8 ± 6.0 | <0.001 |
| mMRCb | 2 (1) | 2(2) | 2(1) | <0.001 |
| 0–1 | 179(38.8) | 71(31.1) | 108(46.4) | 0.001 |
| 2–4 | 282(61.2) | 157(68.9) | 125(53.6) | |
| CCQa | 18.4 ± 8.2 | 20.3 ± 7.7 | 17.7 ± 7.4 | <0.001 |
| FEV1(L)c | 1.4(0.90) | 1.33(0.73) | 1.49(0.99) | 0.031 |
| FEV1 (% predicted)c | 54.40(31.2) | 52.5(28.7) | 56.0(31.7) | 0.178 |
| FEV1/FVCc | 50.9(19.9) | 47.1(19.7) | 51.3(23.9) | 0.030 |
|
Exacerbations in the past yearc |
0(1) | 0(1) | 0(1) | 0.258 |
|
Exacerbations in the past yearb |
0.404 | |||
| 0 | 230(49.9) | 109(47.8) | 121 (51.9) | |
| 1 | 122(26.4) | 59 (25.9) | 63 (27.0) | |
| ≥ 2 | 109(23.7) | 60 (26.3) | 49(21.1) | |
| GOLD groupb | <0.001 | |||
| A | 81(17.6) | 23(10.1) | 58(24.9) | |
| B | 195(42.3) | 110(48.2) | 85(36.5) | |
| C | 25(5.4) | 5(2.2) | 20(8.6) | |
| D | 160(34.7) | 90(39.5) | 70(30.0) | |
| Treatmentb | 0.012 | |||
| LAMA | 91(19.7) | 33(14.5) | 58(24.9) | |
| LABA + ICS | 62(13.4) | 31(13.6) | 31(13.3) | |
| LABA + LAMA | 61(13.2) | 37(16.2) | 24(10.3) | |
| LABA + LAMA + ICS | 217(47.1) | 106(46.5) | 111(47.6) | |
| Others | 30(6.5) | 21(9.2) | 9(3.9) | |
| PSQI scorea | 5.8 ± 3.4 | 8.5 ± 2.7 | 3.2 ± 1.4 | <0.001 |
| Sleep efficiencya | 1.6 ± 1.1 | 2.3 ± 0.9 | 0.8 ± 0.6 | <0.001 |
| Sleep qualitya | 2 ± 1.4 | 3.2 ± 1.2 | 1.3 ± 0.6 | <0.001 |
| Daily disturbancea | 2.0 ± 1.0 | 2.9 ± 1.1 | 1.1 ± 0.7 | <0.001 |
| Risk of OSASb | 0.892 | |||
| positive | 93 (20.1) | 46 (20.2) | 47 (20.0) | 0.890 |
| negative | 368 (79.9) | 182 (79.8) | 186 (80.0) | |
| Anxietyb | 31 (6.8) | 22 (9.8) | 9 (3.8) | 0.030 |
| Depresionb | 11 (2.4) | 8 (3.3) | 3 (1.1) | 0.053 |
aMean ± SD; bCounts with percentage are indicated; cMedian (IQR)
Abbreviations: BMI, Body Mass Index; COPD, Chronic Obstructive Pulmonary Diseas; CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease. ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting β-2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified medical research council dyspnea scale; OSAS, obstructive sleep apnea syndrome ; PSQI, Pittsburgh sleep quality index.
Compared with good sleepers, poor sleepers were older. Poor sleepers had lower absolute FEV1 value and lower FEV1/FVC, but higher baseline CAT and CCQ scores. In addition, more poor sleepers had an mMRC > 1. Furthermore, poor sleepers had a higher proportion of patients in GOLD B and D groups. There was no difference in risk of OSAS between the two groups. However, more poor sleepers had anxiety (Table 1). There was no difference in baseline clinical features in patients with risk of OSAS and without risk of OSAS (Supplement Table 1).
Sleep quality and symptom change
As shown in Table 2, the median change in CAT of all study population was1(8), 224 (48.6%) patients attained MCID in CAT at 6 months. Change in CAT between poor sleepers and good sleepers was different [0(9) vs. 2(6), p = 0.021]. Compared with patients with good sleep quality, fewer patients with poor sleep quality obtained MCID [131 (56.2%) vs. 93 (40.8%), p = 0.001]. In addition, the mean total PSQI score in patients without MCID was higher than patients with MCID (6.9 ± 3.3 vs. 4.5 ± 2.4, p = 0.019), as well as the mean Sleep Quality score (2.4 ± 1.4 vs. 2.0 ± 1.3, p = 0.013) and Daily Disturbance score [2.3 ± 1.3 vs. 1.7 ± 1.2, p < 0.001] (Fig. 2). Furthermore, there was a greater proportion of PSQI > 5 in patients without MCID than patients with MCID (Fig. 2). After adjusting for sex, age, biofuel exposure, CAT score, mMRC, CCQ score, GOLD group, inhalation drug, sleep quality, risk of OSAS, and anxiety and depression, the multivariate logistic regression showed that a PSQI ≤ 5 (OR: 3.112, 95% CI: 1.873–4.711, p < 0.001), higher baseline CAT score (OR: 1.372, 95% CI: 1.274–1.477, p < 0.001), and patients with biofuel exposure (OR: 1.102, 95% CI: 1.009–1.214, p = 0.036) were more likely to attain MCID at the 6-month visit (Table 3).
Table 2.
MCID response rate and exacerbation of patient during the one-year visit according to sleep quality
| Variables | Total N = 461 |
Bad sleeper N = 228 |
Good sleeper N = 233 |
P-value |
|---|---|---|---|---|
| CAT at 6th montha | 10.7 ± 6.1 | 13.6 ± 5.6 | 7.9 ± 4.7 | <0.001 |
| Change in CATc | 1(8) | 0(9) | 2(6) | 0.021 |
| MCID of CAT b | ||||
| Yes | 224(48.6) | 93(40.8) | 131(56.2) | 0.001 |
| No | 237(51.4) | 135(59.2) | 102(43.6) | |
|
Exacerbations in the one yearc |
0(1) | 0(1) | 0(0) | <0.001 |
|
Exacerbation in the one year b |
<0.001 | |||
| Yes | 181(39.3) | 108(47.4) | 73(31.3) | |
| No | 280(60.7) | 120(52.6) | 160(68.7) | |
| Severe exacerbation in the one year b | 0.017 | |||
| Yes | 117(25.4) | 69(30.3) | 48(20.6) | |
| No | 344(74.6) | 159(69.7) | 185(79.4) | |
| Frequent exacerbation in the one year b | 0.008 | |||
| Yes | 65(14.1) | 42(18.4) | 23(9.9) | |
| No | 396(85.9) | 186(81.6) | 210(90.1) |
aMean ± SD; bCounts with percentage are indicated; cMedian (IQR)
Abbreviations: CAT, COPD Assessment Test; MCID, minimum clinically important difference.
Fig. 2.
Baseline PSQI score in those with MCID and without MCID.
Note: (A) Baseline total PSQI score in COPD patients with MICD and without MICD; (B) Percentage of patients with PSQI > 5 in COPD patients with MICD and without MICD; (C) Sleep quality score in COPD patients with MICD and without MICD; (D) Sleep quality score in COPD patients with MICD and without MICD; (E) Daily disturbance score in COPD patients with MICD and without MICD; ns indicates P-values > 0.05;*indicates P-values < 0.05, ** indicates P-values < 0.01, *** indicates P-values < 0.001
Abbreviations: MCID, minimum clinically important difference; PSQI, Pittsburgh sleep quality index.
Table 3.
Univariate and Multivariate logistic regression analysis of factors associated with MCID in CAT.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Factors | OR | 95%CI |
P
-value |
OR | 95%CI |
P
-value |
| Age (years) | 1.013 | 0.991–1.036 | 0.245 | |||
|
Sex (Male versus Female) |
0.890 | 0.502–1.607 | 0.717 | |||
| BMI (kg/m2) | 0.958 | 0.895–1.025 | 0.216 | |||
| Smoking state | ||||||
| Current smoker | Reference | |||||
| Ex-smoker | 1.386 | 0.774–2.477 | 0.271 | |||
| Non-smoker | 0.995 | 0.582–1.703 | 0.986 | |||
|
Biofuel exposure, (Yes versus No) |
1.364 | 1.126–1.579 | 0.000 | 1.102 | 1.009–1.214 | 0.036 |
|
Occupational exposure (Yes versus No) |
1.055 | 0.717–1.522) | 0.786 | |||
| CAT score | 1.179 | 1.136–1.225 | <0.001 | 1.372 | 1.274–1.477 | <0.001 |
|
mMRC (Median, IQR) |
||||||
| 0–1 | Reference | |||||
| 2–4 | 1.021 | 1.002–1.075 | 0.008 | |||
| CCQ score | 1.077 | 1.049–1.106 | <0.001 | |||
| GOLD group | ||||||
| A | Reference | |||||
| B | 2.413 | 1.616–2.907 | 0.000 | |||
| C | 1.043 | 0.583–1.965 | 0.934 | |||
| D | 1.921 | 1.423–2.214 | 0.001 | |||
|
Exacerbations in the past year, (Yes versus No) |
1.321 | 0.914–1.908 | 0.139 | |||
| Treatment | ||||||
| LAMA | Reference | |||||
| LABA + ICS | 1.011 | 0.521–1.961 | 0.975 | |||
| LABA + LAMA | 1.988 | 1.216–3.496 | 0.011 | |||
| LABA + LAMA + ICS | 1.894 | 1.148–3.125 | 0.012 | |||
| PSQI ≤ 5 | 2.364 | 1.388–3.268 | 0.001 | 3.112 | 1.873–4.711 | <0.001 |
| Risk of OSAS | 1.002 | 0.789–1.314 | 0.852 | |||
| Anxiety | 0.957 | 0.568–1.127 | 0.431 | |||
| Depresion | 1.018 | 0.498–1.637 | 0.612 | |||
Notes: Age, sex, Biofuel exposure, CAT score ,mMRC score, CCQ score, GOLD group, OSAS, anxiety, depression, sleep quality and inhalation drug were included as the variables in the multivariate logistic regression model.
Abbreviations: BMI, Body Mass Index; COPD, Chronic Obstructive Pulmonary Disease;GOLD, Global Initiative for Chronic Obstructive Lung Disease. ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting β-2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified medical research council ; dyspnea scale; MCID, minimum clinically important difference; OSAS,obstructive sleep apnea syndrome ; PSQI, Pittsburgh sleep quality index.
Symptom improvement of different GOLD groups or different inhalation therapies in COPD patients with different sleep quality
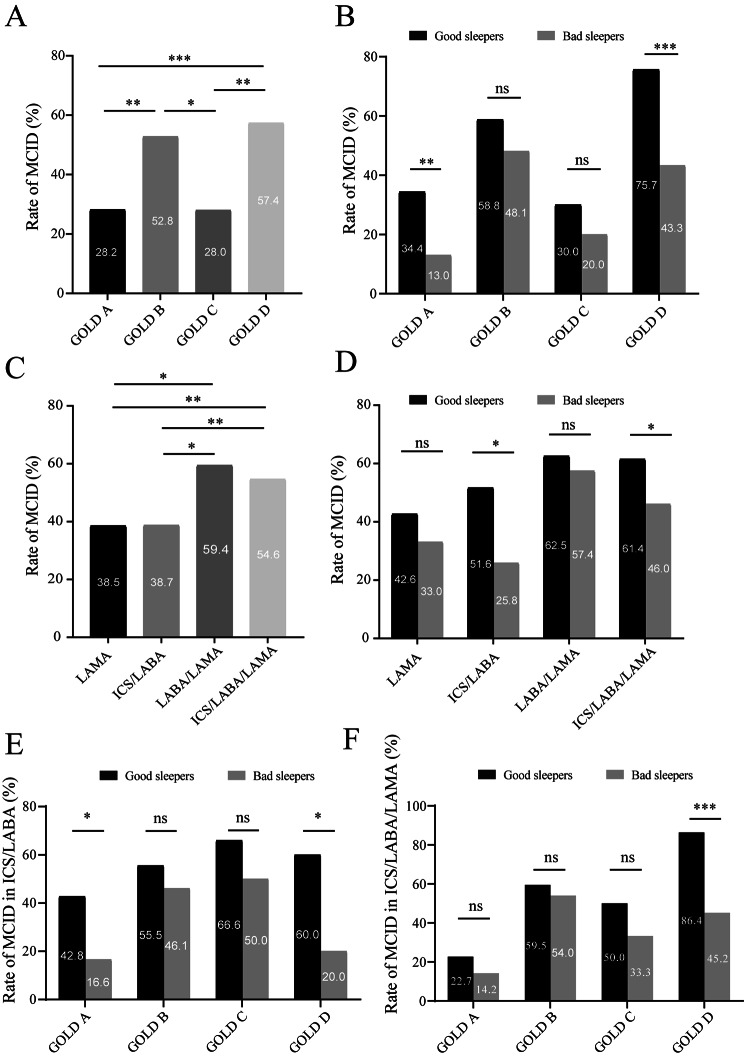
Of all patients, COPD patients in GOLD B and D groups attained a greater proportion of MCID than COPD patients in GOLD A and C groups. The study compared response rates of MCID of different GOLD groups due to sleep quality. Fewer patients with sleep disturbance attained MCID in GOLD A (p = 0.008) and GOLD D (p < 0.001) groups compared with good sleepers (Fig. 3A and B).
Fig. 3.
Comparison of the symptoms improvement between different GOLD group or different main inhalation therapy in COPD patients with different sleep quality
MCID response rate of different GOLD group in COPD patients. (B) MCID response rate of different GOLD group in COPD patients with different sleep quality. (C) MCID response rate of different inhaled medication in COPD patients. (D) MCID response rate of different inhaled medication in COPD patients with different sleep quality. (E) MCID response rate of different GOLD group in treatment of ICS/LABA. (F) MCID response rate of different GOLD group in treatment of ICS/LABA/LAMA. ns indicates P-values > 0.05, *indicates P-values < 0.05, **indicates P-values < 0.01,*** indicates P-values < 0.001
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting β-2-agonist; LAMA, long-acting muscarinic antagonist; MCID, minimum clinically important difference.
In all participants, COPD patients with LAMA/LABA (38/64, 59.3%) or ICS/LABA/LAMA (116/214, 54.6%) were more likely to get symptom improvement than LAMA (35/91, 38.4%) or ICS/LABA (24/62, 38.7%). Whereas sleep quality—good or poor—had no difference in MCID response rate of LAMA and LABA/LAMA. However, fewer patients with impaired sleep quality achieved MCID with ICS/LABA (p = 0.030) and ICS/LABA/LAMA (p = 0.024) compared with good sleepers (Fig. 3C and D).
The study further analysed MCID response rates of different GOLD groups with ICS/LABA and ICS/LABA/LAMA. Fewer patients with impaired sleep quality in GOLD A (p = 0.031) and D (p = 0.042) groups attained MCID with ICS/LABA therapy, and fewer poor sleepers in GOLD D (p < 0.001) group obtained MCID with ICS/LABA/LAMA therapy compared to good sleepers (Fig. 3E-F).
Sleep quality and risk of future exacerbation
Of 461 patients, the median (IQR) number of exacerbations during the one-year visit was 0 (1); 39.3% of patients had at least one moderate-to-severe exacerbation, 25.4% of patients experienced severe exacerbation, and 14.1% of patients experienced frequent exacerbation. In addition, the median (IQR) exacerbation during the one-year visit in poor sleepers and good sleepers were (0, 1) and (0, 0), respectively (p < 0.001) (Table 2). More patients with sleep disturbance had higher incidence of moderate-to-severe exacerbation (p < 0.001), severe exacerbation (p = 0.017), and frequent exacerbation (p = 0.008) than good sleepers (Table 2). Patients with moderate-to-severe exacerbation and severe exacerbation during the 12-month visit had higher total PSQI scores, Sleep Efficiency scores, Sleep Quality scores, and Daily Disturbance scores, and greater proportions of PSQI > 5. Patients with frequent exacerbation had higher total PSQI scores, Sleep Efficiency scores, and Daily Disturbance scores, and greater proportions of PSQI > 5, but not higher Sleep Quality scores (Supplement Fig. 1).
After adjusting for age, sex, CAT score, mMRC score, CCQ score, GOLD group, exacerbation in the past year, inhalation drug, risk of OSAS, and anxiety and depression, results of Cox analysis revealed that a PSQI > 5 was a great risk factor of moderate-to-severe exacerbation, frequent exacerbation, and severe exacerbation during one-year follow up. The global PSQI score was a significant risk factor of moderate-to-severe exacerbation and frequent exacerbation, but not severe exacerbation during the one-year follow-up period (Table 4). The ROC curve exhibited that future exacerbation of COPD patients was predicted by baseline PSQI score (AUC: 0.69, 95% CI: 0.64–0.73, p < 0.001) (Fig. 4).
Table 4.
Risk of sleep quality for future exacerbation during one- year follow-up using Cox regression
| Moderate to severe exacerbation | Frequent exacerbation | Severe exacerbation | ||||
|---|---|---|---|---|---|---|
| HR (95CI%) | P- value | HR (95CI%) | P- value | HR (95CI%) | P- value | |
| PSQI>5* | 1.692(1.213–2.357) | 0.002 | 1.766(1.115–2.797) | 0.015 | 1.124(1.018–1.301) | 0.031 |
| PSQI# | 1.070(1.030–1.111) | <0.001 | 1.080(1.020–1.142) | 0.008 | 1.010(0.983–1.026) | 0.102 |
Notes: Multivariate cox analysis of model 1(*) and model2 (#) were adjusted for age, sex, CAT score, mMRC score, CCQ score, GOLD group, exacerbation in the past year, inhalation drug, risk of OSAS, anxiety and depression.
Abbreviations: CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard rate? mMRC, modified medical research council dyspnea scale; MCID, minimum clinically important difference; OSAS, obstructive sleep apnea syndrome ; PSQI, Pittsburgh sleep quality index.
Fig. 4.
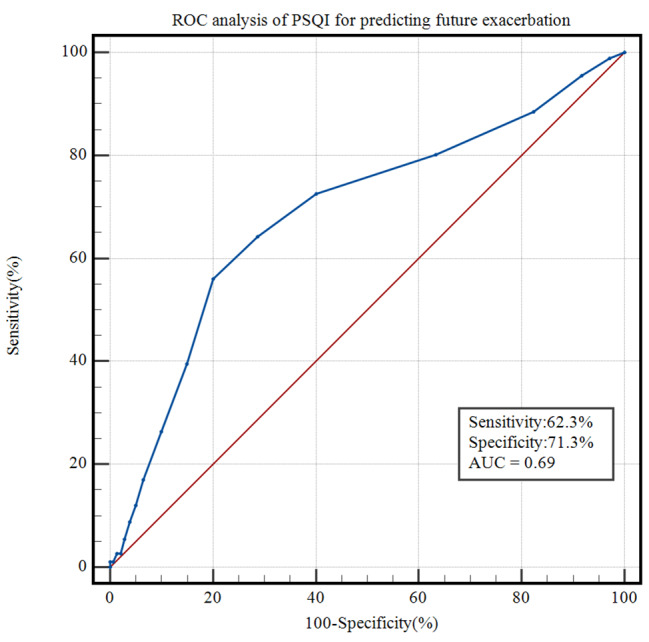
ROC curve of sleep quality for predicting future exacerbation
AUC of PSQI score for predicting future exacerbation is 0.69(95% CI: 0.64–0.73, P<0.001), the sensitivity and the specificity is 62.3% and 71.3%
Abbreviations: AUC, Area under of ROC curve; PSQI, Pittsburgh sleep quality index; ROC, Receiver operating characteristic.
Occurrence of future exacerbation between different GOLD groups or different inhalation therapies in COPD patients with different sleep quality
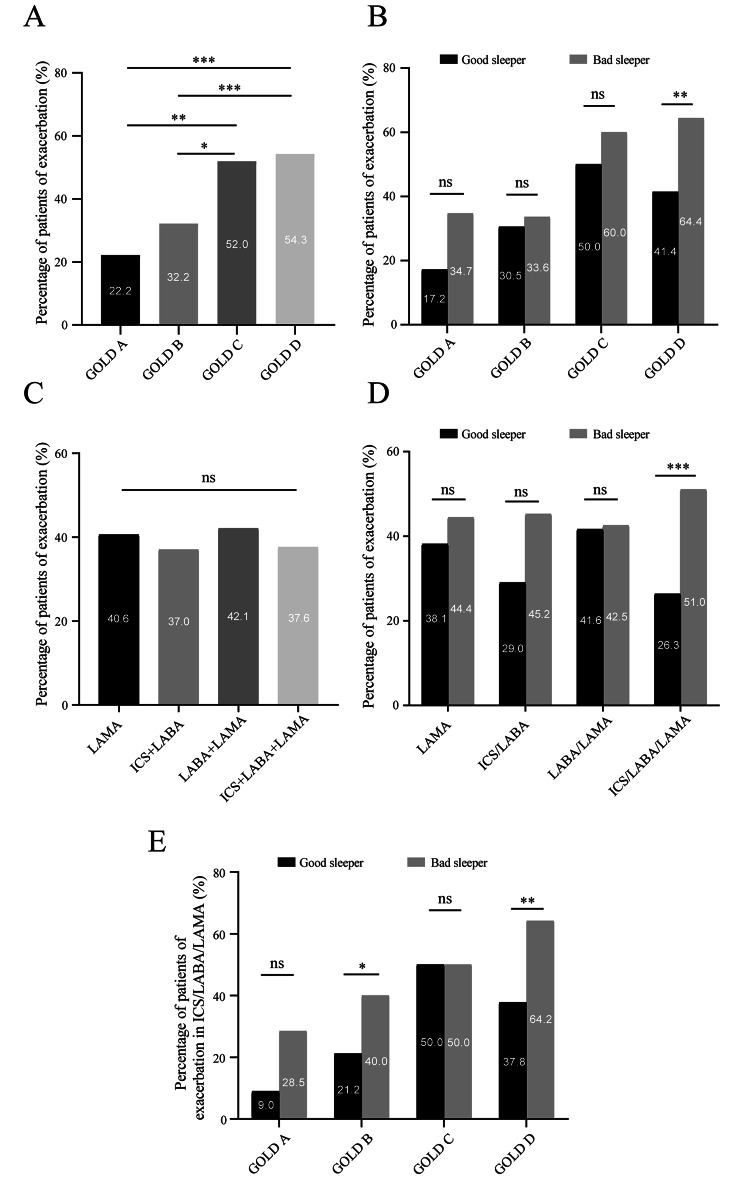
Patients in GOLD C and GOLD D groups experienced higher incidence of exacerbation during the one-year visit than GOLD A and B groups. There was no significant difference in incidence of exacerbation in GOLD A, B, and C groups between patients with different sleep quality. However, more patients with impaired sleep quality experienced future exacerbation in GOLD D group than good sleepers (Fig. 5A and B).
Fig. 5.
Comparison of the incidence of exacerbation between different GOLD group or different main inhalation therapy in COPD patients with different sleep quality
Incidence of future exacerbation of different GOLD group in COPD patients. (B) Incidence of future exacerbation of different GOLD group in COPD patients with different sleep quality. (C) Incidence of future exacerbation of different inhaled medication in COPD patients. (D) Incidence of future exacerbation of different inhaled medication in COPD patients with different sleep quality. (E) Incidence of future exacerbation of different GOLD different in treatment of ICS/LABA/LAMA. ns indicates P-values > 0.05;*indicates P-values < 0.05, **indicates P-values < 0.01,*** indicates P-values < 0.001
Furthermore, there was no difference in incidence of future exacerbation in patients with different inhaled medication treatment. More poor sleepers experienced future exacerbation in the one-year follow up with ICS/LABA/LAMA (p < 0.001), whereas there was no difference in incidence of exacerbation of LAMA, ICS/LABA, and LABA/LAMA between patients with poor sleep quality and good sleep quality (Fig. 5C and D).
The study further analysed the incidence of future exacerbation of different GOLD groups with ICS/LABA/LAMA. More poor sleepers experienced future exacerbation in GOLD B (p = 0.046) and D (p = 0.009) groups with ICS/LABA/LAMA than good sleepers (Fig. 5E).
Discussion
The study indicated that fewer patients with poor sleep quality attained symptom improvement than good sleepers. In our study, we used MCID (CAT improved ≥ 2) to evaluate the change in symptom. MCID was the CAT responsive to short-term changes in COPD patients and is an indicator to assess symptom improvement [25]. The mean total PSQI score, Sleep Quality score, and Daily Disturbance score, and proportions of PSQI > 5 were higher in patients without MCID compared with patients with MCID. This was the first study to find the correlation between sleep quality of COPD patients and change in CAT at 6 months. In addition, a PSQI ≤ 5 was a significant factor related with related with clinically significant reduction in CAT score in multivariate logistic regression. Previous studies also demonstrated that patients with poor sleep quality may experience worse quality of life and disease outcomes, including exacerbation and mortality [20]. However, short-term clinically important deterioration (including change of CAT score) was associated with long-term exacerbation and mortality risk [26, 27]. Therefore, it may be consistent with the result of this study, in which COPD patients with good sleep quality are more likely to obtain MCID in CAT and patients with sleep disturbance are less likely to obtain MCID in the short term. However, previous studies have not specifically investigated whether poor sleep quality affected improvement in symptoms. Multivariate logistic regression also revealed that a higher baseline CAT score was one of the independent correlation factors for MCID. Although patients with sleep disturbances had higher baseline CAT scores, they were less likely to attain improvement in CAT, with smaller proportions of MCID in the short term; therefore, it will be an important subtype to focus on.
Results of this study also indicated that poor sleepers had higher baseline CAT and CCQ scores and more proportions with an mMRC > 1 than good sleepers. These results are similar with the reported study that patients with CAT ≥ 10 points, or mMRC ≥ 2 points, in GOLD B and D groups were more likely to have impaired sleep quality [11].
This study also presented that the mean PSQI score was 5.8 ± 3.4 and 49.5% of COPD patients with a PSQI > 5 had impaired sleep quality. This result is consistent with previous studies in which the mean PSQI score was 6.4 ± 3.9 and 51.0% of COPD patients had impaired sleep quality [6]. The results also demonstrated that 48.6% of COPD patients attained MCID at 6 months. A recent study also indicated that 51% of patients treated with inhalation therapy achieved a MCID of any two measures such as CAT, St. George’s Respiratory Questionnaire (SGRQ), Self-Administered Computerized-Transition Dyspnea Index (SAC-TDI), and Evaluating Respiratory Symptoms (E-RS) at the 24th week [28].
Furthermore, the study elucidated that patients with future exacerbation had higher total PSQI scores and greater proportions of PSQI > 5 than patients without future exacerbation. It was mostly in line with the published study in which individuals with exacerbation during the 18-month follow-up period had a higher baseline global PSQI score and were also more likely to be poor sleepers [21]. Cox regression analysis demonstrated that poor sleep quality was a greater risk factor of moderate-to-severe exacerbation, frequent exacerbation, and severe exacerbation during one-year follow up. The global PSQI score was a significant risk factor of moderate- to-severe exacerbation and frequent exacerbation, but not severe exacerbation. A reported study using the Negative Binomial Regression Model also revealed that a higher baseline global PSQI score and a PSQI score > 5 were associated with a greater risk of symptom-based or event-based exacerbation [21]. The difference is that the result in our study analysed the relationship between sleep quality and risk of moderate-to-severe exacerbation, severe exacerbation, and frequent exacerbation in Cox analysis, while the previous study focused on correlation between sleep quality and symptom-based exacerbation and event-based exacerbation in the Negative Binomial Model. Besides, our study is the first study to illustrate that sleep quality has capacity for predicting future exacerbation using ROC curve, with an AUC of 0.69.
There are some limitations of this study. Firstly, recent study revealed that OSAS may have impact on sleep quality. This study did not exclude COPD patients with OSAS, but our study assessed the risk of OSAS in patients and adjusted the risk of OSAS in multivariate analysis. Secondly, it has not been definitively established that a PSQI > 5 is the best cut-off for poor sleepers in a COPD population. Although a PSQI > 5 was originally developed for healthy individuals, many studies have already used the cut-off value in COPD patients. Future work is needed as we come to understand more about the relationship between sleep and COPD.
Conclusions
COPD patients with impaired sleep quality were less likely to obtain symptom improvement and were at increased risk of future exacerbation than patients with good sleep quality. Impaired sleep quality may also affect the symptom improvement and future exacerbation of patients with different inhaled medication or in different GOLD groups. This study may provide a clinical basis of relation between sleep quality and choice of inhalation medication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to all the patients and clinicians who contributed to this study, especially the research assistants Guo-lin Zhong from the Second Xiangya Hospital, Central south university and thanks to the support and encouragement of Tongxing Lin.
Abbreviations
- BMI
Body Mass Index
- COPD
Chronic Obstructive Pulmonary Disease;
- CAT
COPD Assessment Test
- FEV1
Forced Expiratory Volume in one second
- FVC
Forced Vital Capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HADS
Hospital Anxiety and Depression Scale
- ICS
inhaled corticosteroids
- IQR
interquartile range
- LABA
long-actingβ-2-agonist
- LAMA
long-acting muscarinic antagonist
- MCID
minimal clinically important difference
- mMRC
modified Medical Research Council
- OSAS
obstructive sleep apnea syndrome
- PSQI
Pittsburgh sleep quality index
- ROC
The receiver operating characteristic
- SGRQ
St George’s Respiratory Questionnaire
Author Contribution
Ping Chen is the guarantor and takes responsibility for the content of this manuscript. Ling Lin was involved with the active management of the study patients. Ling Lin, Song Qing and Ping Chen wrote the main manuscript text and Jiaxi Duan prepared Figs. 1, 2, 3, 4 and 5. All authors contributed to observe and supervise the research process and reviewed the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC, Grants 81970044 to Prof Ping Chen), Xiangya Mingyi grant (2013).
Data Availability
All publications discussed in the manuscript are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of the Second Xiangya Hospital of Central South University. All patients gave informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Global regional. National incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YW, Camp PG, Coxson HO, et al. A comparison of Pain, fatigue, Dyspnea and their impact on Quality of Life in Pulmonary Rehabilitation participants with chronic obstructive Pulmonary Disease. Copd. 2018;15(1):65–72. doi: 10.1080/15412555.2017.1401990. [DOI] [PubMed] [Google Scholar]
- 6.Zeidler MR, Martin JL, Kleerup EC et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS).Sleep. 2018;41(5). [DOI] [PMC free article] [PubMed]
- 7.Silva JLRJ, Conde MB, Corrêa KS, et al. Sleep-disordered breathing in patients with COPD and mild hypoxemia: prevalence and predictive variables. J Bras Pneumol. 2017;43(3):176–82. doi: 10.1590/s1806-37562016000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–75. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding B, Small M, Bergström G, et al. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:589–99. doi: 10.2147/COPD.S122485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budhiraja R, Parthasarathy S, Budhiraja P, et al. Insomnia in patients with COPD. Sleep. 2012;35(3):369–75. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ierodiakonou D, Bouloukaki I, Kampouraki M, et al. Subjective sleep quality is associated with disease status in COPD patients. The cross-sectional greek UNLOCK study. Sleep Breath. 2020;24(4):1599–605. doi: 10.1007/s11325-020-02039-8. [DOI] [PubMed] [Google Scholar]
- 12.Xu WQ, Lin LH, Ding KR, et al. The role of depression and anxiety in the relationship between poor sleep quality and subjective cognitive decline in chinese elderly: exploring parallel, serial, and moderated mediation. J Affect Disord. 2021;294:464–71. doi: 10.1016/j.jad.2021.07.063. [DOI] [PubMed] [Google Scholar]
- 13.Gümüştakim R, Ayhan Baser D, Cevik M, et al. Evaluation of sleep quality, insomnia severity and OSAS risk in primary care population: a descriptive study. Int J Clin Pract. 2021;75(2):e13786. doi: 10.1111/ijcp.13786. [DOI] [PubMed] [Google Scholar]
- 14.Nunes DM, Mota RM, de Pontes Neto OL, et al. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187(3):159–63. doi: 10.1007/s00408-009-9147-5. [DOI] [PubMed] [Google Scholar]
- 15.Vukoja M, Kopitovic I, Milicic D, et al. Sleep quality and daytime sleepiness in patients with COPD and asthma. Clin Respir J. 2018;12(2):398–403. doi: 10.1111/crj.12528. [DOI] [PubMed] [Google Scholar]
- 16.Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15(1):122. doi: 10.1186/s12931-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spina G, Spruit MA, Alison J, et al. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax. 2017;72(8):694–701. doi: 10.1136/thoraxjnl-2016-208900. [DOI] [PubMed] [Google Scholar]
- 18.Rafanan AL, Baquilod RA. Sleep-related Breathing Complaints in Chronic Obstructive Pulmonary Disease. Sleep Med Clin. 2022;17(1):99–109. doi: 10.1016/j.jsmc.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–83. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Kwak N, Choi SM, et al. Sleep duration and its Associations with Mortality and Quality of Life in Chronic Obstructive Pulmonary Disease: results from the 2007–2015 KNAHNES. Respiration. 2021;100(11):1043–9. doi: 10.1159/000516381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shorofsky M, Bourbeau J, Kimoff J, et al. Impaired Sleep Quality in COPD is Associated with Exacerbations: the CanCOLD Cohort Study. Chest. 2019;156(5):852–63. doi: 10.1016/j.chest.2019.04.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee GE. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2020 report. http://www.goldcopd.org.Last searched on Nov, 2019.
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Cole JC, Motivala SJ, Buysse DJ, et al. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–6. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 26.Naya IP, Tombs L, Muellerova H, et al. Long-term outcomes following first short-term clinically important deterioration in COPD. Respir Res. 2018;19(1):222. doi: 10.1186/s12931-018-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao YY, Liu C, Zeng YQ, et al. Modified and simplified clinically important deterioration: multidimensional indices of short-term disease trajectory to predict future exacerbations in patients with chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2020;14:1753466620977376. doi: 10.1177/1753466620977376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelmeier CF, Naya IP, Maltais F, et al. Treatment of COPD with Long-Acting Bronchodilators: Association between early and longer-term clinically important improvement. Int J Chron Obstruct Pulmon Dis. 2021;16:1215–26. doi: 10.2147/COPD.S295835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All publications discussed in the manuscript are available from the corresponding author on request.