Abstract
Background
Although the clinical benefit of obtaining a remission in proteinuria in nephrotic patients with focal segmental glomerulosclerosis (FSGS) is recognized, the long-term value of maintaining it and the impact of relapses on outcome are not well described.
Methods
We examined the impact of remissions and relapses on either a 50% decline in kidney function or end-stage kidney disease (combined event) using time-dependent and landmark analyses in a retrospective study of all patients from the Toronto Glomerulonephritis Registry with biopsy-proven FSGS, established nephrotic-range proteinuria and at least one remission.
Results
In the 203 FSGS individuals with a remission, 89 never relapsed and 114 experienced at least one relapse. The first recurrence was often followed by a repeating pattern of remission and relapse. The 10-year survival from a combined event was 15% higher in those with no relapse versus those with any relapse. This smaller than anticipated difference was related to the favourable outcome in individuals whose relapses quickly remitted. Relapsers who ultimately ended in remission (n = 46) versus in relapse (n = 68) experienced a 91% and 32% 7-year event survival (P < .001), respectively. Using time-varying survival analyses that considered all periods of remission and relapse in every patient and adjusting for each period's initial estimated glomerular filtration rate, the state of relapse was associated with a 2.17 (95% confidence interval 1.32–3.58; P = .002) greater risk of experiencing a combined event even in this FSGS remission cohort.
Conclusion
In FSGS, unless remissions are maintained and relapses avoided, long-term renal survival remains poor. Treatment strategies addressing remission duration remain poorly defined and should be an essential question in future trials.
Keywords: FSGS, landmark analyses, remission, relapse, time-dependent survival analyses
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Regardless of the aetiology of focal segmental glomerulosclerosis, complete and partial remissions in proteinuria remain the best surrogates of an improved long-term renal outcome.
Although relapses and remissions occur, the burden of relapses on outcome remains undefined.
What this study adds?
In a retrospective analysis of 203 individuals with at least one remission, we found that relapses were frequent and as severe as seen in the initial nephrotic period.
Nevertheless, provided it was followed by a remission, it did not carry a worse renal prognosis than in those who never relapsed.
However, the cumulative state of unresponsive relapse was associated with an adverse renal outcome.
What impact this may have on practice or policy?
These results support the importance of long-term maintenance of remissions.
Treatment strategies targeting prolonging remission durations have not been addressed and should be an essential question in future trials.
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is the leading glomerular histology of end-stage kidney disease (ESKD) in the USA, with evidence that the incidence is continuing to increase [1, 2]. Significant gains in understanding the pathogenesis have been made in recent years, with many clinical, genetic and histological descriptions. However, their association with clinical outcomes remains unclear, reflecting the heterogeneous nature of the disease [3–6]. The current classification system of FSGS lesions proposes separation into primary (autoimmune), secondary (maladaptive, toxic or viral), genetic and of undetermined cause [7]. There is currently no gold standard for primary FSGS, as clinical and pathology characteristics for each form overlap. Primary FSGS is an exclusion diagnosis suggested when abrupt nephrotic-range proteinuria exists and secondary causes have been eliminated [7, 8]. Although diffuse foot process effacement appears to be highly specific for the autoimmune form, this feature has been identified mostly in subjects with overt nephrotic syndrome and it is uncertain how sensitive this finding is in primary FSGS with moderate proteinuria [9]. Similarly, >50 gene variants have been associated with the FSGS lesion, but the presence of some with weaker associations with the pathogenesis of the disease does not always preclude the possibility of a response to immunosuppression [5]. Finally, whether ‘FSGS of undetermined cause’ can represent a less severe form of autoimmune FSGS has never been formally studied.
Complete and partial remission (CR and PR, respectively) of proteinuria remain the best surrogates of an improved-long term renal outcome [10, 11]. However, the impact of subsequent relapses and remissions following an initial PR or CR on renal survival is uncertain. This clinical phenotype is not well recognized nor reported in the literature. Recent studies have shown the advantages of remission duration on long-term kidney survival in immunoglobulin A (IgA) and membranous nephropathy [12, 13]. Our question was whether the remission duration had a similar impact on long-term kidney outcome in a cohort of patients with presumed primary FSGS as defined by individuals who have been nephrotic, lack evident secondary causes and have achieved a remission in proteinuria.
MATERIALS AND METHODS
Setting and population
This is a cohort study using data from the Toronto Glomerulonephritis Registry [14]. We reviewed all cases and only patients with defined pathologic findings suggestive of primary FSGS were considered as reported by a nephropathologist specializing in glomerulonephritis. Since its inception, when the Toronto Glomerulonephritis Registry receives a renal biopsy pathology report, a secondary review is performed prior to classification to ensure that the description is compatible with immunologically mediated FSGS. For this study, a tertiary review of all biopsy descriptions was done, according to current standards. The histologic requirements included focal and segmental consolidation of the tuft by increased extracellular matrix obliterating the capillary lumen, hyalinosis with or without glomerular adhesions with either negative immunofluorescence or only segmental IgM and/or complement 3. In addition, electron microscopy (EM) findings had to be consistent with FSGS, with EM showing diffuse foot process effacement (FPE, >75%) when the biopsy was performed during a nephrotic period with hypoalbuminemia and prior to any treatment [6]. However, this was not a criterion when the biopsy was done with lower levels of proteinuria, albumin >35 g/l or during immunosuppressive therapy. As well, we reviewed all medical charts, including demographics, laboratory tests and radiologic evaluations, to rule out other potential secondary cases, including other types of glomerulonephritis, malignant hypertension, reflux nephropathy, single kidney and kidney biopsies from transplanted patients. Finally, in addition to these histologically defined FSGS patients, only those >16 years of age at presentation, with at least 12 months of observation and who had documented nephrotic-range proteinuria of >3.5 g/day and who had experienced a remission in proteinuria were included in the study. We could not verify confidently the presence of oedema from retrospective charts. We did not include an albuminemia threshold for presumed primary FSGS, as some patients with multiple remissions had nephrotic periods associated with marked hypoalbuminemia prior to a remission and at other times did not have this pattern. The ethics committee approved this retrospective study. This study was carried out in accordance with the Declaration of Helsinki.
Exposures
The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration formula [15]. The exposure was defined as the presence of either partial or complete remission of proteinuria following a period of nephrotic-range proteinuria [10]. PR was defined as a reduction in baseline proteinuria of 50% and achieving a value of <3.5 g/day and CR was defined as a proteinuria ≤0.3 g/day. Remissions were not ascribed if the eGFR was ≤15 ml/min at the corresponding proteinuria time point. A relapse was a sustained proteinuria increase to ≥3.5 g/day.
Outcome
The outcome of interest was a combined outcome of survival from a 50% decline in initial kidney function or ESKD defined by the onset of dialysis or kidney transplant or by a persistent eGFR ≤15 ml/min/1.73 m2.
Covariates
Demographic variables included sex, race (database data), age and body mass index (BMI) at the first clinical assessment suggestive of renal disease. Clinical and laboratory parameters collected included both initial and follow-up information on systolic and diastolic blood pressure (BP), weight, serum creatinine and 24-h urine proteinuria and creatinine. Serum albumin measurements throughout the observation period in Ontario, Canada used the bromocresol green method, where levels ≤35 g/l defined hypoalbuminemia [16]. Also recorded was exposure to immunosuppressive agents and antihypertensive medications, including renin–angiotensin system (RAS) blockade with angiotensin-converting enzyme inhibitor and angiotensin receptor blocker classes of drug.
Statistical analyses
Normally distributed variables were assessed with histograms and q-q plots. Continuous variables were presented as mean ± standard deviation (SD) and compared using t-tests and one-way analysis of variance. Variables not meeting the normality criterion were summarized as median and interquartile range (IQR) and inferences were made using Mann–Whitney and Kruskal–Wallis tests as appropriate. Categorical variables were reported as percentages and significance was assessed using the Pearson chi-squared test.
We studied the value of maintaining a remission using Kaplan–Meier curves and Cox regression including time-dependent covariates. We initially compared, in the entire remission cohort, the outcome in patients who never relapsed with those who did relapse using the time of the first remission as the starting point and a time-dependent expression of the first relapse.
Recognizing that patients with at least one relapse could experience subsequent remissions and relapses, each of variable duration, we performed additional analyses. To better assess the benefit of maintaining remissions during the follow-up, we examined the survival of individuals at the specific landmark times of 6, 12 and 24 months following their first remission to see how their condition (i.e. in remission or relapse) at these times was associated with kidney outcome. We also compared the survival from a combined event starting from the last recorded remission in three groups: those who relapsed but ended in remission, those who relapsed and ended in relapse and those who never relapsed (where the last remission is also the first). This allowed us to capture all patient outcomes many years beyond the landmark times, particularly in those who relapsed and never remitted. Finally, to address simultaneously all states within a patient trajectory, we delineated the periods in remission and in relapse. We then performed a time-varying survival analysis by patient assessing for the entire follow-up the hazard ratio (HR) of experiencing an event in the state of relapse compared with the state of remission.
For each analysis we had to revise the definition of a combined event since the first eGFR and the subsequent 50% decline threshold changed according to the starting time of interest. We also adjusted all HRs by the starting eGFR and, if applicable, the eGFR at a time-dependent expression of an event. In particular, the time-varying survival analysis was adjusted by the starting eGFR with each change in status.
Two-tailed P-values <.05 were considered statistically significant and 95% confidence intervals (CIs) are reported. Analyses were performed using SPSS version 26 (IBM, Armonk, NY, USA).
RESULTS
Cohort characteristics
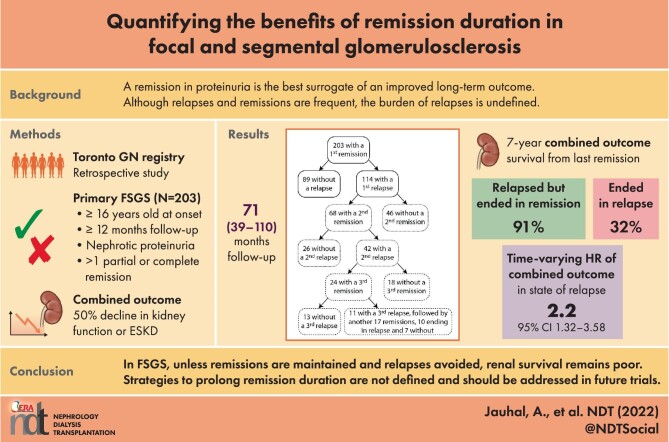
There were 1005 patients with a histologic pattern of FSGS as documented by academic nephropathologists in the Toronto Glomerulonephritis Registry from its creation until January 2019: 421 had <12 months follow-up; 96 were <16 years of age; 12, after tertiary review, were adjudicated as a potential secondary form of FSGS; and 41 had missing information. In the remaining 435 patients, 104 were never nephrotic and 128 never had a remission, leaving 203 patients as the remission cohort. These patients were followed for a mean of 71 months (range 39–110). The number of available measurements per individual for eGFR, proteinuria, serum albumin and BP were 15 (range 9–22), 11 (7–29), 10 (5–15) and 13 (7–18), respectively. The year of diagnosis included 69 cases before 1990, 78 from 1990–2000 and 56 after 2000.
Women accounted for a little more than a third of the remission cohort. Patients presented clinically with an eGFR of 69 ± 29 ml/min/1.73 m2, proteinuria of 5.8 g/day (IQR 3.6–10.0) and serum albumin of 29 ± 10 g/l (Table 1), including 71% with hypoalbuminemia. We assessed renal biopsy findings relative to their clinical findings during the period prior to remission: 100 subjects had a renal biopsy done during nephrotic proteinuria with hypoalbuminemia and their pathology findings showed diffuse FPE. However, 85 had their biopsy performed without nephrotic proteinuria or hypoalbuminemia and 18 had their biopsy done >3 months prior to the first clinical assessment available to the Toronto Glomerulonephritis Registry. While all of these had optical microscopy consistent with primary FSGS, not all available EM photographs showed diffuse FPE. Nevertheless, all of these 85 patients subsequently developed nephrotic-range proteinuria, all had a remission and 43/85 eventually developed hypoalbuminemia.
Table 1:
FSGS remission cohort characteristics.
| Characteristics at the time of the first available clinical assessmenta (n = 203) | Values | Characteristics at follow-up | Values |
|---|---|---|---|
| Sex (female), % | 37 | Follow-up (months), median (IQR) | 71 (39–110) |
| Race, % (AA, Asian, Cauc, other), % | 11, 10, 62, 17 | BP (mmHg), mean ± SD | 131/81 ± 14/7 |
| Age, (years), mean ± SD | 41 ± 16 | MAP (mmHg), mean ± SD | 97 ± 8 |
| eGFR, (ml/min/1.73 m2), mean ± SD | 69 ± 29 | Number of BP medications, median (IQR) | 1.2 (0.7–2.0) |
| BMI, median (IQR) | 27 (23–32) | Use of RASB, % | 72 |
| Proteinuria (g/day), median (IQR) | 5.8 (3.6–10.0) | Only PR/at least one CR, n/n | 139/64 |
| Serum albumin, (g/l)L, mean ± SD | 29 ± 10 | Time-averaged proteinuriab (g/day), median (IQR) | 3.5 (2.2–4.9) |
| Hypoalbuminemia, % | 71 | Immunosuppressive treatment, % | 81 |
| Systolic BP, (mmHg), mean ± SD | 141 ± 25 | Patients with 1, 2 and 3 remissions, n | 203, 68, 24 |
| Diastolic BP, (mmHg), mean ± SD | 86 ± 13 | Time to first remission (months), median (IQR) | 9 (4–20) |
| MAP, (mmHg), mean ± SD | 104 ± 15 | Patients with 0, 1 and 2 relapses, n | 89, 114, 42 |
| Number of BP medications, median (IQR) | 0 (0–1) | Time remission to first relapse (months), median (IQR) | 8 (4–23) |
| Use of RAS blocker, % | 26 | Combined events, n (%) | 65 (32) |
| ESKD, n (%) | 31 (15) | ||
AA, African American; Cauc, Caucasian.
aThe first available clinical assessment in not necessarily the time of the most severe nephrotic syndrome.
bFor each patient, an average proteinuria was determined for each 6-month period of follow-up. Time-averaged proteinuria represents the average of every period's mean from the first assessment to last follow-up.
Throughout the follow-up, 81% received immunosuppression, often in combination with other immunosuppressive agents. Patient exposure to immunosuppression included steroid monotherapy (46%), cyclophosphamide (13%), rituximab (0.5%), mycophenolate mofetil (MMF) or azathioprine (3%) and calcineurin inhibitors (CNIs; 19%). In addition, 72% received RAS blockers. The time-averaged BP was 131/81 ± 14/7 mmHg and time-averaged proteinuria was 3.5 g/day (IQR 2.5–4.9) with 1.2 antihypertensives (IQR 0.7–2.0).
Renal outcomes
Sixty-five patients experienced a combined event with a 10-year survival of 49% and 31 progressed to ESKD with a 10-year survival of 73% (Supplementary Fig. 1). Of the 138 patients who did not experience an event, 12 were still followed; 126 were considered lost to follow-up, as most were returned to their referring nephrologist or primary care physician after a median of 66 months (IQR 39–110). In these, only eight patients had their last available eGFR as <30 ml/min/1.73 m2 (and only one <20 ml/min/1.73 m2). There were no patients lost to follow-up because of death.
Remissions and relapses
Over the follow-up time, 32% of patients had at least one CR while 68% had only PR. The flow in the number of remissions and relapses within this cohort is illustrated in Fig. 1. Prior to the first remission, 21% received no immunosuppression, 55% received steroids only and 2% received MMF or azathioprine, 7% received cyclophosphamide and 15% received CNI, with or without concomitant steroids. In those receiving steroid monotherapy (n = 111), the time from the start of immunosuppression to first remission was 3 months (IQR 1–6). In these, 44/111 had a first remission >4 months from the start of steroids. At the time of remission, 27 were still on steroids and 17 had already stopped.
Figure 1:
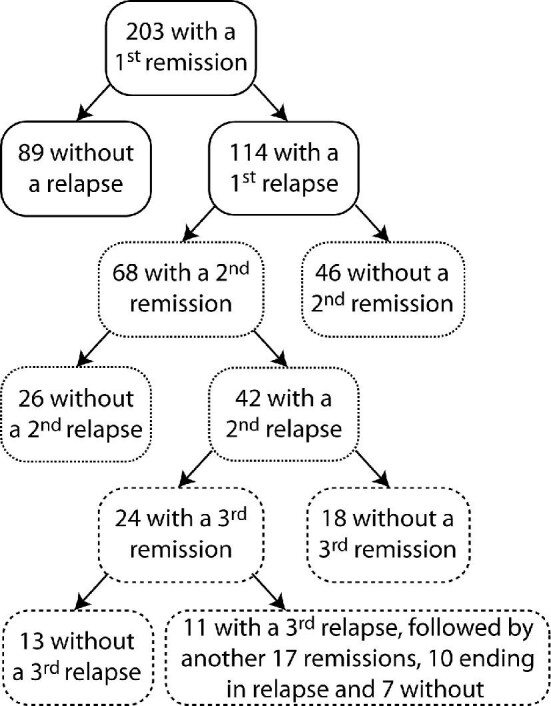
First, second, third and subsequent remissions and relapses. Flow diagram of remissions and relapses of the 203 individuals who obtained a remission in proteinuria and their status changes over time.
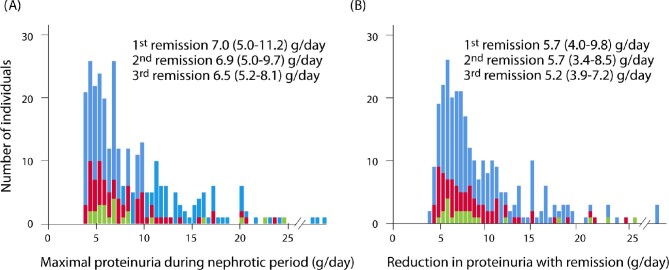
Of the 203 patients included in the study, 68 (33%) and 23 (11%) had a second and third remission following relapse. The peak proteinuria prior to the first, second and third remissions and the subsequent reduction of proteinuria from maximal (peak) to nadir measurements were comparable (Fig. 2). Other clinical characteristics of first, second and third remissions were also similar except for a greater use of RAS blockers while nephrotic and a more rapid time to both the start of immunosuppression and the time to obtain a PR with subsequent events. However, the time from start of immunosuppression to PR was similar between first, second and third remissions (Table 2).
Figure 2:
Maximal proteinuria and its reduction during the first, second and third remission events. (A) Maximal proteinuria while nephrotic. (B) Maximal reduction in proteinuria with a remission (defined by the maximal proteinuria before remission minus the lowest value obtained after). These findings illustrate the magnitude of fluctuations in FSGS with (A) relapses and (B) remissions is similar, although the numbers (y-axis) are smaller with each event. Blue: first remission; red: second remission; green: third remission.
Table 2:
Comparisons of parameters of individuals at the time of first, second and third remissions.
| Characteristics | First remission (n = 203) | Second remission (n = 68) | Third remission (n = 24) | P-value |
|---|---|---|---|---|
| Nephrotic period prior to the remission | ||||
| Initial age (years), mean ± SD | 42 ± 16 | 44 ± 14 | 48 ± 15 | .77 |
| Initial eGFR (ml/min/1.73 m2), mean ± SD | 69 ± 30 | 72 ± 30 | 68 ± 30 | .23 |
| Maximal proteinuria (g/day), median (IQR) | 7.0 (5.0–11.2) | 6.9 (5.0–9.7) | 6.5 (5.2–8.1) | .73 |
| Nadir albumin (g/l), mean ± SD | 28 ± 9 | 30 ± 8 | 29 ± 9 | .60 |
| Initial MAP (mmHg), mean ± SD | 104 ± 15 | 97 ± 11 | 105 ± 14 | .002 |
| Initial number of BP medications, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (1–2) | .09 |
| Use of RAS blocker, % | 32 | 48 | 57 | .01 |
| Immunosuppressive treatment, % | 79 | 75 | 79 | .75 |
| Time to PR (months), median (IQR) | 9 (4–20) | 6 (4–19) | 4 (2–8) | .006a |
| Time to start immunosuppression (months), median (IQR) | 1 (0–5) | 0 (0–1) | 0 (0–1) | .001 |
| Time from start of immunosuppression to PR (months), median (IQR) | 4 (2–11) | 4 (3–11) | 3 (2–8) | .44 |
| Remission period | ||||
| Complete remission, % | 27 | 29 | 42 | .33 |
| eGFR at start of remission (ml/min/1.73 m2), mean ± SD | 65 ± 31 | 66 ± 33 | 64 ± 30 | .99 |
| Nadir proteinuria (g/day), median (IQR) | 1.0 (0.3–1.9) | 1.3 (0.3–2.1) | 0.8 (0.2–1.5) | .34 |
| MAP at start of remission (mmHg), mean ± SD | 96 ± 11 | 94 ± 12 | 100 ± 15 | .13 |
| Number of BP meds at start of remission, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .67 |
| Use of RAS blocker at start of remission, % | 56 | 66 | 71 | .15 |
| Relapse, % | 56 | 62 | 46 | .39a |
a P-value obtained by logrank test.
There were 54 patients (27%) who maintained serum albumins >35 g/l despite nephrotic-range proteinuria. Of these, 52% never relapsed, 33% relapsed once and 15% relapse twice or more, a similar percentage as seen in those with hypoalbuminemia (41%, 35% and 24%; P =.30). In those with two or more remissions with detailed measurements of albumin, the level of albuminemia changed significantly within patients from least to most severe nephrotic event (Supplementary Fig. 2).
There where 38 patients (19%) who experienced remission(s) in proteinuria without the use of immunosuppression. At the onset of their first nephrotic period, these had a similar BMI, eGFR and mean arterial pressure (MAP) but lower levels of proteinuria, higher serum albumin and took longer to reach remission (Table 3) than the treated patients. They also experienced fewer complete remissions and tended to lose more eGFR while nephrotic than those treated with immunosuppression. However, they were just as likely to relapse as individuals who had received immunosuppression.
Table 3:
Comparison of remitting patients with and without immunosuppression.
| Characteristics | With immunosuppression (n = 165) | Without immunosuppression (n = 38) | P-value |
|---|---|---|---|
| Presenting nephrotic event | |||
| Initial age (years), mean ± SD | 41 ± 15 | 45 ± 17 | .19 |
| Initial BMI, median (IQR) | 26 (23–30) | 28 (25–33) | .19 |
| eGFR (ml/min/1.73 m2), mean ± SD | 69 ± 29 | 70 ± 27 | .86 |
| Maximal proteinuria (g/day), median (IQR) | 8.0 (5.4–12.0) | 5.0 (4.0–6.8) | <.001 |
| Nadir albumin (g/l), mean ± SD | 27 ± 9 | 36 ± 6 | <.001 |
| Initial MAP (mmHg), mean ± SD | 104 ± 15 | 103 ± 11 | .73 |
| Initial number of BP medications, median (IQR) | 1 (0–2) | 1 (1–2) | .93 |
| Use of RAS blocker, % | 32 | 32 | .98 |
| Time to partial remission (months), median (IQR) | 7.3 (3.8–17.3) | 14.0 (8.0–26.4) | .007 |
| First remission | |||
| Complete remission, % | 31 | 11 | .01 |
| eGFR at start of remission (ml/min/1.73 m2), mean ± SD | 66 ± 31 | 60 ± 28 | .31 |
| Decrease in eGFR from presentation (ml/min/1.73 m2), mean ± SD | 3 ± 26 | 9 ± 17 | .06 |
| Nadir proteinuria (g/day), median (IQR) | 0.9 (0.3–1.9) | 1.3 (0.6–1.9) | .08 |
| Maximal albumin (g/l), mean ± SD | 40 ± 5 | 42 ± 5 | .22 |
| MAP at start of remission (mmHg), mean ± SD | 96 ± 11 | 98 ± 10 | .24 |
| Number of BP medications at start of remission, median (IQR) | 1 (1–2) | 2 (0–2) | .79 |
| Use of RAS blocker at start of remission, % | 56 | 53 | .68 |
| Total number of remissions (1, 2, ≥3), % | 66, 21, 13 | 68, 26, 6 | .33 |
| Total relapses (0, 1, ≥2%), n | 42, 37, 21 | 53, 29, 18 | .47 |
There were 14 patients who experienced multiple remission events that occurred sometimes with and sometimes without immunosuppression. In paired analyses, the proteinuria decreased from 6.6 g/day (IQR 4.7–10.9) to 1.4 (0.5–3.0) with a minimal albumin of 32 g/l (IQR 21–36) in treated events, similar to 6.2 g/day (IQR 4.8–8.4) to 1.8 (0.7–2.8) with a minimal albumin of 35 g/l (IQR 27–39) in untreated events.
Characteristics and outcomes of patients who never relapse
Within the full remission cohort, there were 89 patients who never relapsed and 114 who had at least one relapse (Table 4). There was no identifying difference between the groups in their nephrotic period regarding the severity of the nephrotic syndrome or the time necessary to reach a PR. However, there were distinct differences in their first remission characteristics in terms of the percent of CR, nadir of proteinuria and peak albumin compared with those who never relapsed. Individuals who never relapsed reached a nadir proteinuria of 0.5 g/day (IQR 0.2–1.0) and maximal albumin of 43 ± 4 g/l, significantly better compared with those who relapsed following their first remission, whose nadir proteinuria was 1.6 g/day (IQR 0.7–2.5) and maximal albumin was 39 ± 5 g/l.
Table 4:
Comparison of patients that never versus ever relapsed.
| Characteristics | Never relapse (n = 89) | Relapse (n = 114) | P-value |
|---|---|---|---|
| Presenting nephrotic event | |||
| Initial age (years), mean ± SD | 43 ± 17 | 41 ± 15 | .48 |
| Initial BMI, median (IQR) | 27 (24–32) | 27 (23–32) | .76 |
| eGFR (ml/min/1.73 m2), mean ± SD | 67 ± 30 | 70 ± 28 | .38 |
| Maximal proteinuria (g/day), median (IQR) | 6.9 (4.7–10.1) | 7.1 (5.1–11.6) | .16 |
| Nadir albumin (g/l), mean ± SD | 29 ± 10 | 28 ± 9 | .38 |
| Initial MAP (mmHg), mean ± SD | 106 ± 16 | 103 ± 14 | .16 |
| Initial number of BP medications, median (IQR) | 1 (0–2) | 1 (0–2) | .24 |
| Use of RAS blocker, % | 35 | 30 | .45 |
| Immunosuppressive treatment, % | 78 | 81 | .58 |
| Time to partial remission (months), median (IQR) | 10 (4–19) | 8 (4–20) | .74 |
| First remission period | |||
| Complete remission, % | 40 | 17 | <.001 |
| eGFR at start of remission (ml/min/1.73 m2), mean ± SD | 63 ± 31 | 66 ± 30 | .50 |
| Nadir proteinuria (g/day), median (IQR) | 0.5 (0.2–1.0) | 1.6 (0.7–2.5) | <.001 |
| Maximal albumin (g/l), mean ± SD | 43 ± 4 | 39 ± 5 | <.001 |
| MAP at start of remission (mmHg), mean ± SD | 95 ± 10 | 97 ± 12 | .19 |
| Number of BP medications at start of remission, median (IQR) | 1 (1–2) | 1 (0–2) | .83 |
| Use of RAS blocker at start of remission, % | 57 | 54 | .68 |
The adjusted time-dependent Cox regression showed a greater survival from a combined event in the group who never relapsed compared with those who experienced at least one recurrence (P = .001), but only by an actuarial 15% at the 10-year time point following the first remission (Supplementary Fig. 3). However, the relapse group was heterogeneous, with variable numbers of remissions and relapses. Ultimately within this group of 114 individuals who had an initial relapse, 46 ended their follow-up in remission and 68 in relapse (Fig. 1).
Risk of a combined event with relapses using landmark and time-varying survival analyses
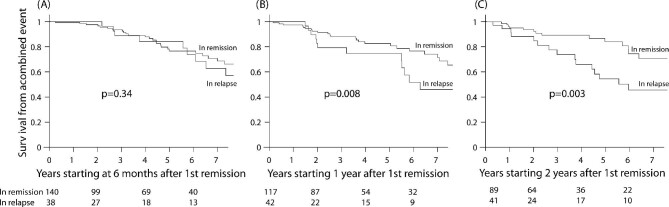
To help clarify the quantitative value of remission, we determined the status of patients at 6, 12 and 24 months following the start time of their first remission and assessed the risk of a combined event after these landmarks (Fig. 3). The patient's status at the time points of 1 and 2 years (remission or relapse at that point) were more predictive of outcomes compared with the outcome based on the status at 6 months. The HR, adjusted for the eGFR specific for the start of each landmark, was not different at 6 months [HR 1.18 (95% CI 0.84–1.65), P = .34], but they were significantly different at 12 months [HR 1.56 (95% CI 1.12–2.18), P = .008] and 24 months [HR 1.71 (95% CI 1.20–2.45), P = .003]. This translated into a significant 23% and 25% greater survival from a combined event at 7 years following these landmarks times.
Figure 3:
Survival from a combined event following the remission status at different landmarks following first remission. The P-values were obtained with Cox regression adjusted for the initial eGFR. The patient's status at the time points of 1 and 2 years (remission or relapse at that point) were more predictive of outcomes compared with the outcome based on the status at 6 months.
We also addressed the survival from a combined event starting from the time of the last recorded remission in each of the 203 patients. We compared the survival in those who never relapsed (n = 89), those who relapsed but ultimately ended in remission (n = 46) and those who ended their follow-up in relapse (n = 68) using a time-dependent expression of a relapse. In all, starting from the last remission event, those who ended in relapse had a 32% 7-year survival from a combined event compared to an average 86% survival in the other two groups (P < .001; Fig. 4).
Figure 4:
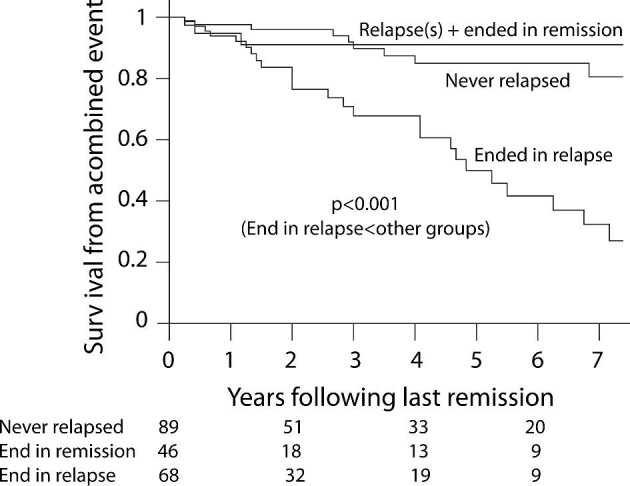
Survival from a combined event following the last remission obtained. The P-value was obtained with Cox regression using a time-dependent expression of the last relapse adjusted for the eGFR.
Given the number of remissions and relapses within each patient, we also performed a time-varying analysis to address all states during follow-up. We carefully delineated for each individual the time in each remission and in each relapse and we noted for each period the starting eGFR. We found a 2.17 (95% CI 1.32–3.58; P = .002; adjusted for eGFR) greater risk of experiencing a combined event over time while in relapse in reference to being in remission. A graphic outline of remissions and relapses over time within a sample of the patients is illustrated in Supplementary Fig. 4. When calculating the proportion of time in remission and in relapse for each individual, those who experienced a combined event spent a median 45% of their time in remission as opposed to 66% in those who did not experience a combined event (P = .002).
DISCUSSION
This study addressed the value of obtaining a remission in FSGS, but of equal importance, its maintenance. It demonstrates the clinical relevance of both the frequency and the magnitude of the remission and relapse pattern that can occur in FSGS patients and their impact on kidney survival. This pattern is distinct from other primary glomerular disease such as IgA and membranous nephropathy [2, 12, 13, 17]. Each relapse in proteinuria in FSGS was as severe as that seen in the initial nephrotic period and each subsequent remission was quantitatively the same as the first one. This finding translated into the observation that each relapse, provided it was followed promptly by a remission of equal magnitude, did not carry a worse kidney prognosis than those who never relapsed, in marked contrast to those who did not remit and ended in relapse. Finally, by comparing nephrotic periods within the same patient, some of them presented with and some without hypoalbuminemia, supporting that primary FSGS can be seen with normal albuminemia.
This relapsing and remitting clinical phenotype for adult-onset FSGS is not well documented in the literature. In earlier studies, relapses were mainly described in the setting of steroid responsive, frequent relapsing and steroid dependent, mostly in the paediatric population presumed to have minimal change disease and treated expectantly [18, 19]. However, with subsequent relapses or treatment resistance, kidney biopsies were often performed and commonly focal and segmental sclerosis lesions were found. A similar pattern has been described in adults [20, 21]. In contrast to the close relationship between the duration of first remission and long-term outcome in other primary glomerulonephritis, the relapsing/remitting pattern in FSGS is more compatible with the one seen with the more severe and systemic types of glomerular disease, such as anti-neutrophil cytoplasmic antibody vasculitis and lupus nephritis.
The findings of our study are limited by the nature of retrospective observational studies. Differences in management, including newer therapies, have been introduced during the time span of the study from 1975 to 2019. Although this has led to some improvement in the long-term outcome of patients with FSGS, it remains poor [2, 17]. This was seen even in our remission cohort, when subsequent relapses were not followed by a remission. Also, selection bias may be present, as it is more likely that refractory disease will be referred to our unit that specifically focuses on glomerulonephritis. This is less likely since our registry has always catered to patients at all stages of the disease. This is supported by the many patients who had <12 months of follow-up and were returned to their referring physician for monitoring. Only one patient was lost to follow-up with an eGFR <20 ml/min/1.73 m2 and there were no competing events such as death. Also, the definitions of complete and partial remission may change in the future, but we did apply the practice definitions as per the most recent Kidney Disease: Improving Global Outcomes guidelines [11], which are the current standard of care. While it is possible that the best definition of a partial remission is different, our classification at last follow-up clearly illustrates the advantage of attaining and maintaining partial remission using this definition.
The roles of hypoalbuminemia, diffuse foot process effacement and spontaneous remission in primary FSGS need further comments. Regarding the value of hypoalbuminemia, we found that some patients presented with levels that would place individuals in the ‘FSGS of undetermined cause’ category, where autoimmunity is uncertain. Interestingly, however, in the situation where patients with multiple nephrotic events and remissions can serve as their own controls, we observed that some primary autoimmune FSGS (defined classically by abrupt onset of overt nephrotic syndrome with a remission to immunosuppression) can relapse with a less severe picture, and vice versa. This would support that primary FSGS can present with nephrotic-range proteinuria without hypoalbuminemia and still be autoimmune in origin.
It is unknown if primary FSGS without overt nephrotic syndrome still displays diffuse foot process effacement, as this specific lesion was studied when proteinuria was very high [9]. This finding may be insufficiently sensitive to apply to less severe forms of primary FSGS, and multiple studies have found exceptions to this rule [22]. Therefore, in our inclusion criteria, diffuse foot process effacement was a requirement only when the biopsy was performed prior to immunosuppression exposure and while experiencing features of overt nephrotic syndrome. Many did not have the full nephrotic syndrome at the time of biopsy, but most developed these features later (or earlier) in their course. This underlines the difficulties in studying the value of FSGS lesions when a mild presentation warrants a renal biopsy but the subsequent clinical picture worsens.
Interestingly, some patients treated with steroids at first intention required >4 months to respond. Recent findings illustrate that this is not rare [23]. Others do not remit a second time, making it difficult to classify patients. Finally, there were a few patients with remissions obtained without immunosuppression. While this is atypical for primary FSGS and supports a secondary cause, this observation has been described in two randomized controlled trials in FSGS resistant to corticosteroids where some subjects assigned to the placebo control group experienced a partial remission [24, 25]. Another observational cohort study described 28 FSGS patients with recent-onset nephrotic syndrome, absence of family history or evident secondary causes, of which 20 were untreated and 14 had a spontaneous remission [26]. Our patients who experienced a remission without immunosuppression were much less nephrotic but experienced a similar rate of relapse, which would be unexpected with secondary causes. Their BMI was also similar to that of treated subjects. Finally, there were a few patients who experienced both immunosuppression-induced and spontaneous remissions, further supporting that spontaneous remission can exist in primary FSGS.
Differentiating primary or immune-mediated from secondary disease is difficult due to the lack of a gold standard [27–32]. While our selection criteria improve the likelihood that the included cases were immune-mediated variants of FSGS, these features cannot completely rule out non-immune-mediated factors [33–37]. Using more rigid inclusion criteria, such as an albumin threshold, to increase diagnosis specificity would potentially come at the cost of excluding some primary FSGS with milder presentation. We feel additional support for an immune-mediated mechanism was that all 203 patients in our remission cohort had a significant reduction in proteinuria, and subsequent relapses were accompanied by a comparable increase in proteinuria as in the first nephrotic episode. All subsequent proteinuria remission also had similar reductions as the first one, making dominant secondary causes less likely. Nevertheless, because we lack a diagnostic gold standard for cases of primary FSGS, clinicians should be wary of using immunosuppression in milder cases, where no evidence of benefits has been reported and where unknown secondary causes may still exist. However, if, with careful monitoring, significant worsening of the clinical or laboratory features ensues, such treatments should be considered.
In summary, in FSGS patients, unless remissions are maintained and relapses avoided, long-term renal survival remains poor. Current guidelines emphasize the importance of obtaining a remission in proteinuria, but our data suggest that unless it is maintained and relapses avoided, long-term renal survival will remain unfavourable. This part of patient management remains poorly defined and should be part of all future treatment trials [38].
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Toronto Glomerulonephritis Registry registrars N. Ryan, P. Ling, P. Lam and M. Romano and the following nephrologists for their contributions and support: S. Albert, R. Aslahi, P. Aujla, N. Barrese, M. Barua, M. Berall, A. Berbece, S. Bhandhal, D. R. Birbrager, P. Boll, G. Buldo, C. Cardella, C. Chan, P. Chan, A. Charest, D. Cherney, M. Chidambaram, S. Chow, E. Cole, M. Cummings, S. Donnelly, A. Dunn, A. Elfirjani, S. Fenton E. Fong, J. Fung, J. Goldstein, Z. Harel, G. Hercz, S. V. Jassal, S. Kajbaf, K. Kamel, A. Kang, S. Karanicolas, V. Ki, S. J. Kim, D. H. Kim, A. Konvalinka, K. Kundhal, V. Langlois, P. Lekas, I. Lenga, C. Licht, J. Lipscombe, C. Lok, J. Ly, M. Manogaran, R. McQuillan, P. McFarlane, H. Mehta, D. Mendelssohn, J. A. Miller, G. Nagai, B. Nathoo, G. Nesrallah, M. Pandes, S. Pandeya, R. Parekh, R. Pearl, Y. Pei, D. Perkins, J. Perl, A. Pierratos, R. Prasad, S. Radhakrishnan, M. Rao, R. Richardson, J. Roscoe, A. Roushdi, J. Sachdeva, D. Sapir, J. Sasal, J. Schiff, J. Scholey, M. Schreiber, X. Shan, N. Siddiqui, T. Sikaneta, C. V. Silva Gomez, S. Singh, R. Singhal, A. Sohal, A. Steele, S. Suneja, E. Szaky, D. Tam, P. Tam, L. Teskey, K. Tinckam, R. Ting, S. Tsui, P. A. Turner, D. Wadehra, J. A. Wadgymar, R. Wald, A. Walele, L. Warner, C. Wei, J. Weinstein, C. Whiteside, S. Wijeyasekaran, G. Wong, G. Wu, T. Yassa, D. Yuen and J. Zaltzman.
Contributor Information
Arenn Jauhal, Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada.
Heather N Reich, Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada.
Michelle Hladunewich, Division of Nephrology, Department of Medicine, Sunnybrook Health Science Center, University of Toronto, Toronto, Ontario, Canada.
Moumita Barua, Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada.
Bettina E Hansen, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada.
David Naimark, Division of Nephrology, Department of Medicine, Sunnybrook Health Science Center, University of Toronto, Toronto, Ontario, Canada.
Stéphan Troyanov, Division of Nephrology, Department of Medicine, Hôpital du Sacré-Coeur de Montréal, University of Montreal, Montreal, Quebec, Canada.
Daniel C Cattran, Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada.
The Toronto Glomerulonephritis Registry group:
N Ryan, P Ling, P Lam, M Romano, S Albert, R Aslahi, P Aujla, N Barrese, M Barua, M Berall, A Berbece, S Bhandhal, D R Birbrager, P Boll, G Buldo, C Cardella, C Chan, P Chan, A Charest, D Cherney, M Chidambaram, S Chow, E Cole, M Cummings, S Donnelly, A Dunn, A Elfirjani, S Fenton E Fong, J Fung, J Goldstein, Z Harel, G Hercz, S V Jassal, S Kajbaf, K Kamel, A Kang, S Karanicolas, V Ki, S J Kim, D H Kim, A Konvalinka, K Kundhal, V Langlois, P Lekas, I Lenga, C Licht, J Lipscombe, C Lok, J Ly, M Manogaran, R McQuillan, P McFarlane, H Mehta, D Mendelssohn, J A Miller, G Nagai, B Nathoo, G Nesrallah, M Pandes, S Pandeya, R Parekh, R Pearl, Y Pei, D Perkins, J Perl, A Pierratos, R Prasad, S Radhakrishnan, M Rao, R Richardson, J Roscoe, A Roushdi, J Sachdeva, D Sapir, J Sasal, J Schiff, J Scholey, M Schreiber, X Shan, N Siddiqui, T Sikaneta, C V Silva Gomez, S Singh, R Singhal, A Sohal, A Steele, S Suneja, E Szaky, D Tam, P Tam, L Teskey, K Tinckam, R Ting, S Tsui, P A Turner, D Wadehra, J A Wadgymar, R Wald, A Walele, L Warner, C Wei, J Weinstein, C Whiteside, S Wijeyasekaran, G Wong, G Wu, T Yassa, D Yuen, and J Zaltzman
FUNDING
The Toronto Glomerulonephritis Registry is supported in part by the McCann Fund of the Toronto General Hospital Foundation.
AUTHORS’ CONTRIBUTIONS
A.J., S.T. and D.C. designed the study and drafted the manuscript, including the figures. A.J., S.T., H.R., M.H. and D.C. gathered the data. A.J., S.T., B.H., D.N. and D.C. analysed the results. A.J., S.T., H.R., M.H., M.B. and D.C. edited the manuscript. All authors revised and gave final approval.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
A.J., S.T., D.N. and B.H. have no disclosures to report. D.C. has been on scientific advisory boards or received honoraria or consulting fees from Calliditas, Chinook, Novartis, Forsee, Alnylam, Alexion, Genetech, Aurinia, Dimerix and Vera. M.B. has received honoraria from Natera for genetic testing in renal disease. H.R. has received consulting fees or honoraria for lectures from Calliditas, Novartis, Chinook, Travere and Omeros. M.H. has received grants or consulting fees from Calliditas, Pfizer, Ionis, Genentech, GlaxoSmithKline and Alnylam. Part of this material was published in abstract form for the World Congress of Nephrology in Montreal, Canada in April 2021.
REFERENCES
- 1. Sim JJ, Batech M, Hever Aet al. . Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000–2011 among a racially and ethnically diverse US population. Am J Kidney Dis 2016;68:533–44. [DOI] [PubMed] [Google Scholar]
- 2. Kitiyakara C, Eggers P, Kopp JB.. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 2004;44:815–25. Available from: http://www.ajkd.org/article/S0272638604010819/fulltext. [PubMed] [Google Scholar]
- 3. Cattran DC, Reich HN, Beanlands HJet al. . The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 2008;23:2247–53. Available from: https://academic.oup.com/ndt/article/23/7/2247/1858573. [DOI] [PubMed] [Google Scholar]
- 4. D'Agati VD, Kaskel FJ, FalkRJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011;365:2398–411. [DOI] [PubMed] [Google Scholar]
- 5. Yao T, Udwan K, John Ret al. . Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol 2019;14:213–23. Available from: https://pubmed.ncbi.nlm.nih.gov/30647093/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Agati VD, Fogo AB, Bruijn JAet al. . Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004;43:368–82. Available from: https://pubmed.ncbi.nlm.nih.gov/14750104/. [DOI] [PubMed] [Google Scholar]
- 7. De Vriese AS, Wetzels JF, Glassock RJet al. . Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol 2021;17:619–30. Available from: https://pubmed.ncbi.nlm.nih.gov/34017116/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2017;12:502–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deegens JKJ, Dijkman HBPM, Borm GFet al. . Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 2008;74:1568–76. Available from: https://pubmed.ncbi.nlm.nih.gov/18813290/. [DOI] [PubMed] [Google Scholar]
- 10. Troyanov S, Wall CA, Miller JAet al. . Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol 2005;16:1061–8. [DOI] [PubMed] [Google Scholar]
- 11. Kidney Disease: Improving Global Outcomes Glomerulonephrtis Work Group K . KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:1–274. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO-GN-Guideline.pdf [Google Scholar]
- 12. Canney M, Barbour SJ, Zheng Yet al. . Quantifying duration of proteinuria remission and association with clinical outcome in IgA nephropathy. J Am Soc Nephrol 2021;32:436–47. Available from: https://jasn.asnjournals.org/content/32/2/436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattran DC, Kim ED, Reich Het al. . Membranous nephropathy: quantifying remission duration on outcome. J Am Soc Nephrol 2017;28:995–1003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Regional program for the study of glomerulonephritis . Central Committee of the Toronto Glomerulonephritis Registry. Can Med Assoc J 1981;124:158–61. [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CHet al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. https://pubmed.ncbi.nlm.nih.gov/19414839/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seimiya M, Ohno S, Asano Het al. . Change in albumin measurement method affects diagnosis of nephrotic syndrome. Clin Lab 2014;60:1663–7. Available from:https://pubmed.ncbi.nlm.nih.gov/25651712/ [DOI] [PubMed] [Google Scholar]
- 17. Heaf JG, Sørensen SS, Hansen A. Increased incidence and improved prognosis of glomerulonephritis: a national 30-year study. Clin Kidney J 2021;14:1594–602. Available from: https://pubmed.ncbi.nlm.nih.gov/34084455/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponticelli C, Edefonti A, Ghio Let al. . Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant 1993;8:1326–32. Available from: https://academic.oup.com/ndt/article/8/12/1326/1823293 [PubMed] [Google Scholar]
- 19. Rydel JJ, Korbet SM, Borok RZet al. . Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis 1995;25:534–42. [DOI] [PubMed] [Google Scholar]
- 20. Gipson DS, Gibson K, Gipson PEet al. . Therapeutic approach to FSGS in children. Pediatr Nephrol 2007;22:28–36. https://pubmed.ncbi.nlm.nih.gov/17109140/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruggenenti P, Ruggiero B, Cravedi Pet al. . Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014;25:850–63. Available from: www.jasn.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sethi S, Glassock RJ, Fervenza FC. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant 2015;30:375–84. Available from: https://pubmed.ncbi.nlm.nih.gov/24589721/ [DOI] [PubMed] [Google Scholar]
- 23. Rood IM, Bavinck A, Lipska-Ziętkiewicz BSet al. . Later response to corticosteroids in adults with primary focal segmental glomerular sclerosis is associated with favorable outcomes. Kidney Int Rep 2022;7:87–98. Available from: http://www.kireports.org/article/S2468024921014996/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cattran DC, Appel GB, Hebert LAet al. . A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 1999;56:2220–6. Available from: https://pubmed.ncbi.nlm.nih.gov/10594798/. [DOI] [PubMed] [Google Scholar]
- 25. Lieberman K V., Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 1996;7:56–63. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/8808110/ [DOI] [PubMed] [Google Scholar]
- 26. Deegens JK, Assmann KJ, Steenbergen EJet al. . Idiopathic focal segmental glomerulosclerosis: a favourable prognosis in untreated patients? Neth J Med 2005;63:393–8. https://uhn.idm.oclc.org/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med6&AN=16301760http://nt2yt7px7u.search.serialssolutions.com/?sid=OVID:Ovid+MEDLINE%28R%29+%3C2005+to+2007%3E&genre=article&id=pmid:16301760&id=doi:& [PubMed] [Google Scholar]
- 27. Maas RJH, Deegens JKJ, Wetzels JFM.. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol 2013;28:1041–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23515666/. [DOI] [PubMed] [Google Scholar]
- 28. Huang J, Liu G, Zhang YMet al. . Plasma soluble urokinase receptor levels are increased but do not distinguish primary from secondary focal segmental glomerulosclerosis. Kidney Int 2013;84:366–72. Available from: https://pubmed.ncbi.nlm.nih.gov/23447064/ [DOI] [PubMed] [Google Scholar]
- 29. Meijers B, Maas RJH, Sprangers Bet al. . The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int 2014;85:636–40. Available from: https://pubmed.ncbi.nlm.nih.gov/24402090/ [DOI] [PubMed] [Google Scholar]
- 30. Trachtman H, Futterweit S, Singhal PCet al. . Circulating factor in patients with recurrent focal segmental glomerulosclerosis postrenal transplantation inhibits expression of inducible nitric oxide synthase and nitric oxide production by cultured rat mesangial cells. J Investig Med 1999;47:114–20. Available from: https://pubmed.ncbi.nlm.nih.gov/10198566/. [PubMed] [Google Scholar]
- 31. Sharma R, Sharma M, McCarthy ETet al. . Components of normal serum block the focal segmental glomerulosclerosis factor activity in vitro. Kidney Int 2000;58:1973–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11044217/. [DOI] [PubMed] [Google Scholar]
- 32. Schlöndorff D. Are serum suPAR determinations by current ELISA methodology reliable diagnostic biomarkers for FSGS. Kidney Int 2014;85:499–501. Available from: https://pubmed.ncbi.nlm.nih.gov/24583981/ [DOI] [PubMed] [Google Scholar]
- 33. De Vriese AS, Sethi S, Nath KAet al. . Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 2018;29:759–74. Available from: www.jasn.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hommos MS, De Vriese AS, Alexander MPet al. . The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc 2017;92:1772–81. Available from: https://pubmed.ncbi.nlm.nih.gov/29110886/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kambham N, Markowitz GS, Valeri AMet al. . Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498–509. Available from: https://pubmed.ncbi.nlm.nih.gov/11260414/ [DOI] [PubMed] [Google Scholar]
- 36. Thomas DB, Franceschini N, Hogan SLet al. . Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 2006;69:920–6. Available from: https://pubmed.ncbi.nlm.nih.gov/16518352/ [DOI] [PubMed] [Google Scholar]
- 37. Miao J, Pinto EVF, Hogan MCet al. . Identification of genetic causes of focal segmental glomerulosclerosis increases with proper patient selection. Mayo Clin Proc 2021;96:2342–53. Available from: https://uhn.idm.oclc.org/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=34120753http://nt2yt7px7u.search.serialssolutions.com/?sid=OVID:Ovid+MEDLINE%28R%29+%3CDecember+Week+2+2021%3E&genre=article&id=pmid:34120753& [DOI] [PubMed] [Google Scholar]
- 38. American Society of Nephrology . Kidney Health Initiative. Current projects. https://khi.asn-online.org/projects/project.aspx?ID=74 (20 August 2022, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.