Abstract
KRAS and TP53 mutations are frequently observed in extrahepatic biliary cancer. Mutations of KRAS and TP53 are independent risk factors for poor prognosis in biliary cancer. However, the exact role of p53 in the development of extrahepatic biliary cancer remains elusive. In this study, we found that simultaneous activation of Kras and inactivation of p53 induces biliary neoplasms that resemble human biliary intraepithelial neoplasia in the extrahepatic bile duct and intracholecystic papillary-tubular neoplasm in the gall bladder in mice. However, inactivation of p53 was not sufficient for the progression of biliary precancerous lesions into invasive cancer in the context of oncogenic Kras within the observation period. This was also the case in the context of additional activation of the Wnt signaling pathway. Thus, p53 protects against formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras.
Keywords: BilIN, ICPN, biliary cancer, Kras, p53
Biliary tract cancer (BTC) includes cholangiocarcinoma (CCA) and gall bladder carcinoma (GBC). According to the World Health Organization (WHO) databases, the global mortality for CCA increased worldwide [1]. The 5-year survival rate of biliary cancer remains only 5% to 15% [2, 3]. Recently, biliary intraepithelial neoplasm (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB) were defined as precursor lesions of invasive adenocarcinoma by the WHO classification [4–6]. BilIN is defined as a microscopically identifiable, pre-invasive neoplastic lesion of the biliary lining epithelia of the bile duct. IPNB is defined as a grossly visible, intraductal, preinvasive papillary or villous epithelial neoplasm covering fine fibrovascular stalks [7]. Papillary lesion like IPNB in the gall bladder (GB) is intracholecystic papillary-tubular neoplasm (ICPN) [8]. However, the molecular mechanism of formation of these precursor lesions is not fully understood.
Whole-genome sequencing (WGS) studies have revealed high incidence of mutations in KRAS (17–18%) and TP53 (26–32%) in biliary cancer [9–11]. The incidence of KRAS mutation and TP53 mutation is 31.5% and 36.9%, respectively, in extrahepatic cholangiocarcinoma [12]. Mutations of KRAS codon12 occurred in about 50% of early BilIN [13]. These findings suggest the possible roles of mutations in Kras and p53 in biliary tumorigenesis.
To clarify the exact role of each molecule in which mutations are observed in biliary cancer, genetically engineered mouse model (GEM) is a powerful tool. Recently, we have shown that Hnf1bCreER mouse is one of the useful biliary-specific driver CreER mouse lines for gene manipulation in the extrahepatic biliary duct (EHBD) and GB, and that concurrent activation of the Kras and canonical Wnt pathways in the adult biliary epithelial cells induces BilIN and ICPN in the EHBD and GB, respectively [14].
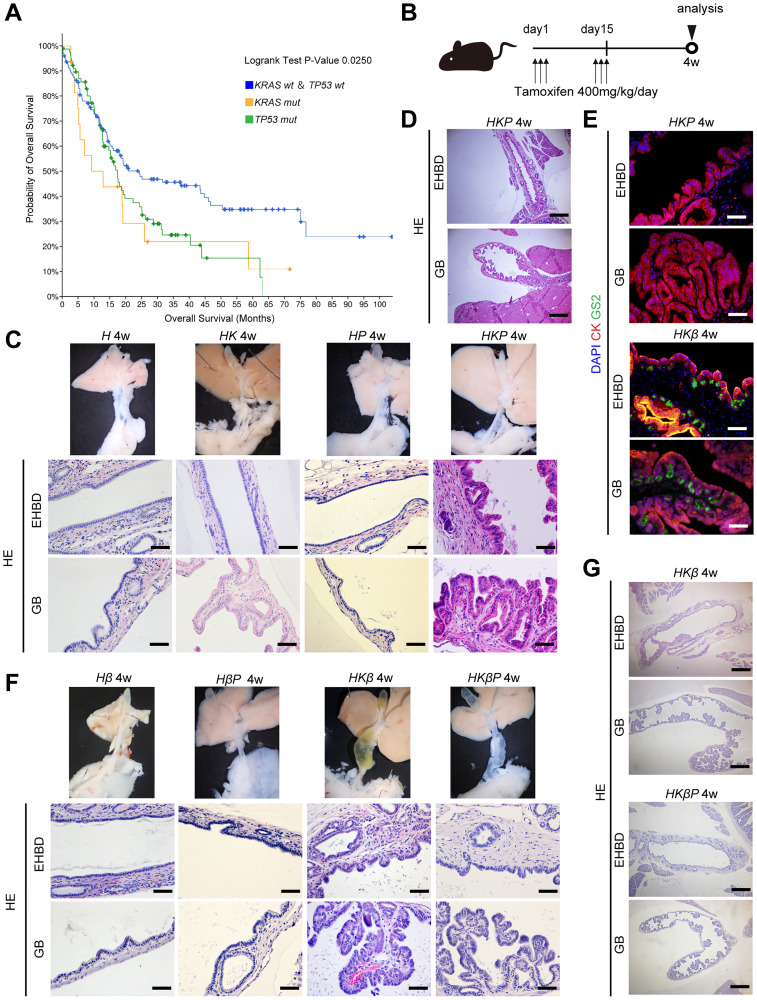
It is well-known that p53 deficiency promotes the progression of precancerous lesions into invasive adenocarcinoma in the context of oncogenic Kras in the pancreas [15]. Therefore, we hypothesized that inactivation of p53 also promotes tumorigenesis in the extrahepatic biliary system. To determine whether mutation of KRAS or TP53 affects the progression of human cholangiocarcinoma, we first performed prognosis analyses of cholangiocarcinoma using the The Cancer Genome Atlas (TCGA) database. Overall survival was compared among the group with mutation of KRAS, group with mutation of TP53, and group without KRAS or TP53 mutation. Mutations of KRAS and TP53 were each negatively correlated with overall survival in human biliary cancer patients (Figure 1A). Additionally, overall survival was comparable between the group with mutation of KRAS and TP53 and the group without mutation of KRAS and TP53. These data indicated that mutations of KRAS and TP53 were independent risk factors for poor prognosis in human biliary cancer.
Figure 1. Simultaneous activation of Kras and inactivation of p53 induced ICPN and BilIN that resemble human ICPN and BilIN.
(A) Kaplan-meier curve of overall survival for group with mutation of KRAS and TP53 and group without mutation of KRAS or TP53. (B) Tamoxifen administration schema for the experiments using Hnf1bCreER-line mice. All mice were sacrificed 4 weeks after the last tamoxifen administration. (C) Macroscopic (upper) and microscopic (lower) images of the EHBD and GB in H, HK, HP, and HKP mice 4 weeks after the last tamoxifen administration. (black scale bars = 50 μm). (D) Low-power field of microscopic images of the EHBD and GB in HKP mice 4 weeks after the last tamoxifen administration (black scale bars = 500 μm). (E) Coimmunostaining for DAPI (blue), GS-II (green), and CK19 (red) in the EHBD and GB in HKP and HKβP mice 4 weeks after the last tamoxifen administration. (F) Macroscopic (upper) and microscopic (lower) images of the EHBD and GB in Hβ, HKβ, HβP, and HKβP mice 4 weeks after the last tamoxifen administration. (All black or white scale bars = 50 μm). (G) Low-power field of microscopic images of the EHBD and GB in HβP and HKβP mice 4 weeks after the last tamoxifen administration. (black scale bars = 500 μm).
We next investigated the functional role of Kras and p53 in tumorigenesis of the biliary system using the Hnf1bCreER mouse line [14]. We crossed KrasG12D mice and/or Tp53flox/flox mice with Hnf1bCreER mice to generate Hnf1bCreER(H), Hnf1bCreER; KrasG12D (HK), Hnf1bCreER; Tp53flox/flox (HP), and Hnf1bCreER; KrasG12D; Tp53flox/flox (HKP) mice. Four weeks after the last tamoxifen administration, the biliary tract was analyzed (Figure 1B). Macroscopically, the EHBD in H, HK, HP, and HKP mice looked normal (Figure 1C). H&E staining revealed an almost normal appearance of the GB and EHBD epithelial cells in H, HK and HP mice. In contrast, HKP mice displayed microscopic papillary neoplasms which resembled human BilIN in the EHBD and papillary neoplasm which resembled human ICPN in the GB (Figure 1C, 1D). Neoplastic changes were also observed in the peribiliary glands of the EHBD in HKP mice. Hyperchromasia, nuclear stratification, and partial loss of nuclear polarity were observed in the epithelial cells of the BilIN and ICPN lesions in HKP mice. Immunohistochemistry (IHC) for mucin was next performed to assess the subtypes of BilIN and ICPN in HKP mice. Muc1 was positive in biliary epithelial cells in HKP mice, whereas muc2 and muc5AC were negative. In our previous report, Griffonia simplicifolia lectin II (GSII lectin) was useful as an alternate marker for muc6 in mice [14]. Staining of GSII revealed that GSII was not expressed in BilIN and ICPN in HKP mice (Figure 1E). In our recent report, concurrent activation of the Kras and canonical Wnt pathways induces GSII-positive or gastric BilIN and ICPN (Figure 1E). In contrast, BilIN and ICPN in HKP mice did not represent the gastric type. These data suggested that activation of the Wnt pathway induces biliary precancerous lesions into the gastric type, whereas p53 inactivation does not. These data indicated that concurrent activation of Kras and inactivation of p53 induces BiliN in the EHBD and ICPN in the GB in HKP mice. However, inactivation of p53 was not sufficient for the progression of precancerous lesions into adenocarcinoma in the extrahepatic biliary system within the observation period. Longer-term analysis was not possible, because HKβ and HKβP mice died due to lung cancers at 6 to 8 weeks of age after tamoxifen treatment.
We next investigated whether inactivation of p53 promotes biliary precancerous lesions into adenocarcinoma in the context of activated Kras and Wnt signaling in mice. To this end, we crossed KrasG12D mice and/or Tp53flox/flox mice and Ctnnb1lox(ex3)/+ mice with Hnf1bCreER mice to generate Hnf1bCreER; Ctnnb1lox(ex3)/+ (Hβ), Hnf1bCreER; Ctnnb1lox(ex3)/+ Tp53flox/flox (HβP), Hnf1bCreER; KrasG12D Ctnnb1lox(ex3)/+ (HKβ), and Hnf1bCreER; KrasG12D; Ctnnb1lox(ex3)/+; Tp53flox/flox (HKβP) mice. Four weeks after the last tamoxifen administration, the biliary tract was analyzed (Figure 1B). Macroscopically, the EHBD in Hβ and HβP mice appeared normal, whereas the EHBD was dilated in HKβ and HKβP mice. H&E staining revealed an almost normal appearance of the GB and EHBD epithelial cells in Hβ and HβP mice. In contrast, HKβ and HKβP mice displayed microscopic papillary neoplasms, which resembled human BilIN in the EHBD and ICPN in the GB (Figure 1F, 1G). However, neoplastic grade of BilIN and ICPN was not different between HKβ and HKβP mice. These data indicated that inactivation of p53 did not accelerate the progression of biliary precancerous lesions into adenocarcinoma even in the context of activated Kras and Wnt signaling. Longer-term analysis was not possible, because HKβ and HKβP mice died due to massive intestinal adenomas at 6 to 8 weeks of age after tamoxifen treatment.
In conclusion, p53 protects against formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras in mice, however, inactivation of p53 is not sufficient for the progression into invasive cancer in the extrahepatic biliary system.
ACKNOWLEDGMENTS
The authors thank all members of the Fukuda laboratory for technical assistance and helpful discussions.
Abbreviations
- BilIN
biliary intraepithelial neoplasia
- BTC
biliary tract cancer
- CCA
cholangiocarcinoma
- EHBD
extrahepatic bile duct
- GB
gall bladder
- GBC
gall bladder carcinoma
- GEMs
genetically engineered mouse models
- ICPN
intracholecystic papillary-tubular neoplasm
- IHC
immunohistochemistry
- TCGA
The Cancer Genome Atlas
- WGS
whole-genome sequencing
- WHO
World Health Organization
Author contributions
M.N., K.M. and A.F. conceived and designed the study. M.N., K.M., S.N., M.N., Y.H., T.M., and Y.N. conducted the experiments and analyzed the data. T.T. diagnosed murine neoplasia of biliary tract. M.N. and K.M. wrote the manuscript, and A.F. and H.S. revised it.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
FUNDING
This work was supported in part by Grants-in-Aid KAKENHI (19H03639, 19K16712, 19K22619, 20K22841, 20H03659, 21K15948, and 21K20801). It was also supported by Japan Agency for Medical Research and Development, the Project for Cancer Research and Therapeutic Evolution (18cm0106142h0001, 20cm0106177h0001, 20cm0106375h0001) and AMED-PRIME (20gm6010022h0003), Moonshot Research and Development Program (JPMJMS2022-1), and COI-NEXT (JPMJPF2018). It was also supported by Princess Takamatsu Cancer Research Fund (17-24924), the Mochida Foundation (2017bvAg), the Mitsubishi Foundation (201910037), the Uehara Foundation (201720143), the Naito Foundation (22205-1), the Kobayashi Foundation (203200700019), the Simizu Foundation (203180700103), the Japan Foundation for Applied Enzymology (203190700054), the SGH Foundation (203200700056), the Kanae Foundation (203190700083), the Bristol Myers Squibb (200190700011), the Ichiro Kanehara Foundation (20KI037), Takeda Science Foundation, and the Takeda Foundation (201749741, 203200700045).
REFERENCES
- 1. Bertuccio P, et al. J Hepatol. 2019; 71:104–14. 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 2. Banales JM, et al. Nat Rev Gastroenterol Hepatol. 2020; 17:557–88. 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hundal R, et al. Clin Epidemiol. 2014; 6:99–109. 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zen Y, et al. Mod Pathol. 2007; 20:701–9. 10.1038/modpathol.3800788. [DOI] [PubMed] [Google Scholar]
- 5. Ohtsuka M, et al. Int J Hepatol. 2014; 2014:459091. 10.1155/2014/459091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakanuma Y, et al. J Hepatobiliary Pancreat Sci. 2018; 25:181–87. 10.1002/jhbp.532. [DOI] [PubMed] [Google Scholar]
- 7. Nakanuma Y, et al. Ann Diagn Pathol. 2022; 61:152055. 10.1016/j.anndiagpath.2022.152055. [DOI] [PubMed] [Google Scholar]
- 8. Adsay V, et al. Am J Surg Pathol. 2012; 36:1279–301. 10.1097/PAS.0b013e318262787c. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura H, et al. Nat Genet. 2015; 47:1003–10. 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 10. Wardell CP, et al. J Hepatol. 2018; 68:959–69. 10.1016/j.jhep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 11. Jusakul A, et al. Cancer Discov. 2017; 7:1116–35. 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lendvai G, et al. Pathol Oncol Res. 2020; 26:3–15. 10.1007/s12253-018-0491-8. [DOI] [PubMed] [Google Scholar]
- 13. Hsu M, et al. Cancer. 2013; 119:1669–74. 10.1002/cncr.27955. [DOI] [PubMed] [Google Scholar]
- 14. Nagao M, et al. Cancer Res. 2022; 82:1803–17. 10.1158/0008-5472.CAN-21-2176. [DOI] [PubMed] [Google Scholar]
- 15. Hingorani SR, et al. Cancer Cell. 2005; 7:469–83. 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]