Abstract
Objectives:
The objective of this review is to provide updated information on the epidemiology, correlating factors and treatment of chronic kidney disease associated restless legs syndrome (CKD-A-RLS) in both adult and pediatric population.
Materials and Methods:
We have reviewed the Medline search and Google Scholar search up to May 2022, using key words restless legs syndrome, chronic kidney disease and hemodialysis and kidney transplant. The reviewed articles were studied for epidemiology, correlating factors, as well as pharmacologic and non-pharmacologic treatment options.
Results:
Our search revealed 175 articles, 111 were clinical trials or cross- sectional studies and 64 were review articles. All 111 articles were retrieved and studied in detail. Of these, 105 focused on adults and 6 on children. A majority of studies on dialysis patients reported a prevalence between 15–30%, which is notably higher than prevalence of RLS in general population (5–10%). The correlation between presence of CKD-A-RLS with age, gender, abnormalities of hemogram, iron, ferritin, serum lipids, electrolytes and parathyroid hormones were also reviewed. The results were inconsistent and controversial. Limited studies have reported on the treatment of CKD-A-RLS. Non-pharmacological treatment focused on the effect(s) of exercise, acupuncture, massage with different oils and infra-red light whereas, pharmacologic treatment options include the effects of dopaminergic drugs, Alpha2-Delta ligands (gabapentin and pregabalin), vitamins E and C, and intravenous iron infusion.
Conclusion:
This updated review showed that RLS is two to three times more common in patients with CKD compared to the general population. More patients with CKD-A-RLS demonstrated increased mortality, increased incidence of cardiovascular accident, depression, insomnia and impaired quality of life than those with CKD without RLS. Dopaminergic drugs such as levodopa, ropinirole, pramipexole and rotigotine as well as calcium channel blockers (gabapentin and pregabalin) are helpful for treatment of RLS. High quality studies with these agents are currently underway and hopefully confirm the efficacy and practicality of using these drugs in CKD-A-RLS. Some studies have shown that aerobic exercise and massage with lavender oil can improve symptoms of CKD-A- RLS suggesting that these measures can be useful as adjunct therapy.
Keywords: Restless legs syndrome, Movement disorders, Renal failure, Hemodialysis, Sleep Disorders
Introduction
The definition and classification of chronic kidney disease (CKD) have evolved over time. The current international guidelines define this condition as decreased kidney function characterized by a glomerular filtration rate (GFR) of less than 60 ml/min per 1·73 m², or markers of kidney damage, or both, of at least 3 months duration, regardless of the underlying cause. CKD is very prevalent in the general adult population. Data from the United States estimate a prevalence of 13.1% among adults, which has increased over time. The burden of CKD is substantial. According to WHO global health estimates, 864 226 deaths (or 1·5% of deaths worldwide) were attributable to this condition in 2012. Ranked fourteenth in the list of leading causes of death, CKD accounted for 12·2 deaths per 100,000 people. Projections from the Global Health Observatory suggest that the death rate from CKD will continue to increase to reach 14 per 100,000 people by 2030. CKD is also associated with substantial morbidity. Worldwide, CKD accounted for 2,968,600 (1·1%) of disability-adjusted life-years and 2,546,700 (1·3%) of life-years lost in 2012. Patients with CKD require monitoring for complications such as metabolic abnormalities, anemia, CKD associated mineral bone disease, and cardiovascular diseases [1,2].
Patients with chronic kidney disease are commonly affected with various types of sleep disorders. Sleep disorders have been associated with increased cardiovascular risk and may contribute to the morbidity and mortality of people with advanced (stages 4 to 5) CKD and those treated with dialysis [3]. Within the spectrum of sleep disorders, restless legs syndrome (RLS) causes a disturbance in sleep through an irresistible desire to move one’s legs. Symptoms of RLS are more common in patients with CKD than in the general population [4,5].
Restless legs syndrome, also known as Willis-Ekbom disease (WED), is a sensorimotor disorder characterized by an irresistible urge to move the legs. The urge is usually accompanied by an uncomfortable sensation in the legs that occurs in the evening or night and is partially or totally relieved by movement [6]. Brain iron deficiency and dopaminergic neurotransmission abnormalities play a central role in the pathogenesis of RLS, along with other nondopaminergic systems, although the exact mechanisms are still unclear. The cause of most cases of RLS is unknown, and hence is called primary (idiopathic) RLS. Secondary RLS occurs in association with a variety of systemic disorders especially iron deficiency and chronic renal insufficiency [7].
The initial management approach to essential RLS should include measuring serum ferritin and transferrin-percent saturation, with iron-replacement therapy indicated when these measures are below the low-to-normal range. There is limited evidence of nonpharmacologic treatment in primary RLS. In moderate to severe RLS, pharmacologic treatment may be considered. There is strong evidence for efficacy of both Alpha2-Delta Ligands (gabapentin and pregabalin) and dopamine agonists in the therapy for RLS. Unfortunately, a growing body of evidence over the last decade has indicated disturbing side effects associated with dopaminergic therapies. Most significantly, a large proportion of RLS patients treated with dopaminergic drugs (direct agonists such as ropinirole) develop augmentation syndrome. Augmentation is characterized by earlier appearance of the symptoms during the day, often associated with more intensity. Prevalence rates for dopamine agonist–related augmentation vary from less than 10% in the short term to 42% to 68% after approximately 10 years of treatment. In addition, excessive daytime sleepiness with sleep attacks particularly in patients with comorbid parkinsonism, impulse control disorder symptoms, as well as dose related adverse effects, such as dizziness and drowsiness may develop in patients on dopamine agonist medications. Second-line therapies include intravenous iron infusion in those who are intolerant of oral iron intake and/or those having augmentation with intense, severe RLS symptoms, and opioids including tramadol, oxycodone, and methadone [8,9,10].
There is evidence that chronic RLS makes the patients prone to cardiac and cerebrovascular accidents although there is a need for more careful studies in this area [11].
Research Design
A Medline search and Google Scholar search was conducted up to May 1st, 2022, crossing the term restless legs syndrome and chronic kidney disease (CKD) and additionally with hemodialysis. The search included only articles published in English language. The number of relevant articles in adult and pediatric literature were presented in a Prisma monograph. Prevalence of RLS was investigated in CKD patients on dialysis and off dialysis as well in hemodialysis versus peritoneal dialysis and after kidney transplantation.
The search included data on presence or lack of correlation between CKD-associated- RLS (CKD-A-RLS) and gender, age, basic metabolic index, serum albumin, serum lipid profile and presence of comorbidities (such as diabetes and hypertension). Additionally, correlations were searched and recorded between CKD-A-RLS and a large number of metabolic and hormonal factors including serum electrolytes (in particular calcium and phosphorus), serum iron, hemoglobin, ferritin, transferrin saturation, parathyroid hormone level as well as stages of kidney dysfunction and markers of kidney dysfunction (glomerular filtration rate, serum creatinine and BUN). The search also included data supporting or refuting correlation between CKD-A-RLS with dialysis parameters such as frequency and duration of dialysis as well as type of dialysate used for treatment of renal failure. The issue of mortality in CKD-A-RLS syndrome was searched for and analyzed.
Data from blinded and open label studies on treatment of CKD-A-RLS were compiled and analyzed. The search included data from commonly used agents such as dopaminergic and antiepileptic drugs and certain opioids as well as newer and experimental drugs. Information from case reports was excluded. Important treatment protocols in progress were mentioned and briefly discussed.
The data from non- pharmacological clinical trials such as those related to the use of different exercise modalities, acupuncture and herbal treatments were noted and recorded. Fischer exact test was used for to detect statistical significance between small data values.
Results
The search, performed up to May1st 2022, disclosed 510 articles. Two authors reviewed the literature under this subject independently. After exclusion of duplications (231 duplications), 279 articles remained (Figure 1- Prisma). Of these, 104 articles were excluded (not relevant to the topic, in languages other than English, case reports). Of the remaining 175 articles, 111 were clinical trials or cross- sectional studies and 64 were review articles. All 111 articles were retrieved and studied in detail. Of these, 105 focused on adults and 6 on children.
Figure 1.
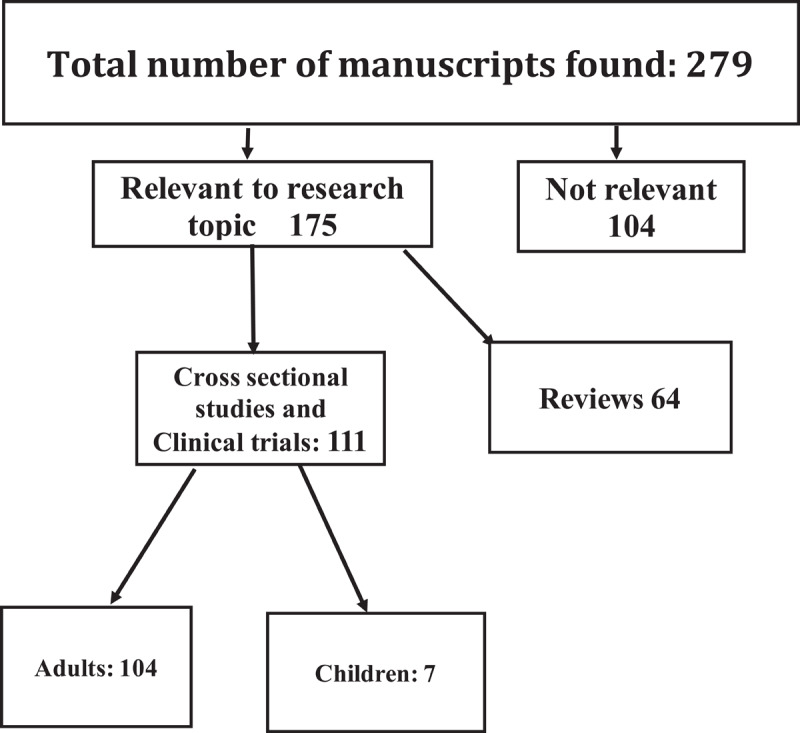
Research Prisma.
The reported prevalence of CKD-A-RLS varied among different studies. Majority of studies on dialysis patients reported a prevalence between 15–30%, notably higher than prevalence of RLS in general population (5–10%) [12]. Seven studies described much higher values, ranging from 51–84% [13,14,15,16,17,18,19]. One study also reported a high prevalence of CKD-A-RLS (37.1%) in non-dialyzed patients [20]. One study reported a very low incidence of CKD-A-RLS in Asian Indian population of only 1.5% (zero in controls) [21].
Seven studies reported on the prevalence of CKD-A-RLS after kidney transplant. In 6 of these studies, the prevalence value was considerably lower than that reported among dialysis patients and corresponded with RLS prevalence in general population [22,23,24,25,26]. six studies reported increased mortality in patients affected by CKD-A-RLS [26,27,28,29,30,31], whereas two studies found no significant change in mortality in the affected patients [32,33].
Several of the published manuscripts attempted to correlate presence of CKD-A-RLS with age, gender, abnormalities of hemogram, iron, ferritin, serum lipids, electrolytes (particularly calcium and phosphorus) and parathyroid hormones. The results were inconsistent and controversial. Although also controversial, more studies have found predominance of women and increased incidence of sleep apnea, cerebrovascular accidents, depression and poor quality of life among patients affected by CKD-A-RLS. The correlation between CKD-A-RLS and hemodialysis parameters was also controversial, but CKD-A-RLS was observed in more patients with advanced kidney failure (beyond stage 3).
Two studies compared the prevalence of CKD-A-RLS in peritoneal dialysis with hemodialysis [34,35]. In both studies, the prevalence of CKD-A-RLS was substantially higher in peritoneal dialysis, 50% and 33% versus 23% in hemodialysis. In one study, using the cool dialysate improved the symptoms of RLS in CKD [36].
Treatment of CKD-A-RLS
Limited studies have reported on the treatment of CKD-A-RLS; the data involves both non-pharmacological and pharmacological treatment. On non-pharmacological treatments, reports include the effect(s) of exercise, acupuncture, massage with different oils and infra-red light (Table 1).
Table 1.
Non- pharmacological Treatments of CKD-A-RLS.
|
| |||||
|---|---|---|---|---|---|
| MODE OF TREATMENT | NUMBER OF PATIENTS | TYPE OF STUDY | DURATION OF TREATMENT | RESULTS | SIDE EFFECTS |
|
| |||||
| Aerobic exercise [37] | Treatment 7 Control 7 |
Prospective, Open label | 16 weeks | Reduced IRLS score by 42% Improved QoL: P = 0.03 Functional ability: P = 0.02 Sleep quality P = 0.01 |
None |
|
| |||||
| Aerobic exercise [38] | Treatment 13 Control 13 All on hemodialysis |
Prospective, Open label | 16 weeks Bicycling 3 times/week |
IRLS scores improved on week 16; no improvement of quality of life | None |
|
| |||||
| Glycerin and lavender oil massage [39] | Glycerin 35 Lavender 35 Control 35 All on hemodialysis |
Prospective Open label |
45 minute, 3 times a week for one month | At the end of the study, both glycerin and lavender oil significantly improved symptoms (P < 0.05) | None |
|
| |||||
| Lavender oil and sweet orange oil massage [40] | Lavender 35 Sweet orange 35 Control 35 All on hemodialysis |
Double blind, Controlled | 3 weeks, 3 times a week |
At the end of the study, both glycerin and sweet orange significantly improved symptoms (P < 0.001) | None |
|
| |||||
| Lavender oil massage [41] | Lavender 21 Control 21 |
Double blind Controlled | 4 weeks | At the end of the study, lavender oil significantly improved symptoms (P < 0.0001) | None |
|
| |||||
| Lavender oil massage [42] | Lavender 31 Control 26 (baby oil) |
Placebo Controlled | 10 minutes massage, 3 times per week for 4 weeks | RLS severity decreased and QoL improved significantly in the lavender group (P < 0.001) | None |
|
| |||||
| Lavender oil massage [43] | Lavender 29 Control 30 |
Placebo Controlled | 10 minutes, 3 times per week | RLS severity significantly decreased in the lavender group (P < 0.0001) | None |
|
| |||||
| Application of infrared light at acuopoints [44] | Treatment 30 Control 30 |
Single blind Prospective | 3 times per week for 3 weeks | Reduced IRLSRS scores but only during treatment | None |
|
| |||||
Pharmacological treatments consist mainly of treatment with dopaminergic drugs (levodopa, ropinirole), and pramipexole, as well as treatment with calcium channel blockers structurally similar to gabaaminobutiric acid (gabapentin and pregabalin) (Table 2).
Table 2.
Pharmacological Treatment of CKD-A-RLS.
|
| ||||
|---|---|---|---|---|
| NAME OF THE DRUG (S) | DESIGN OF STUDY | NUMBER OF PATIENTS, DOSE | RESULTS | SIDE EFFECTS |
|
| ||||
| Levodopa [45] | Double blind, cross over for 4 weeks | 15, 100 & 200 mg |
Improved leg movements, PLM index, sleep quality and QoL (Ps < 0.03) | Dry mouth and headaches |
|
| ||||
| Gabapentin [46] | Double blind, crossover for 4 weeks (1 week wash out) | 15, 200 & 300 mg |
11 patients responded to gabapentin,1 to both placebo and gabapentin | Two drop-outs, one due to lethargy, one due to MI (unrelated to drug) |
|
| ||||
| Rotigotine patch, Stage 2 CKD [47] |
Single center Prospective, open label |
14, 1 mg, 2 mg |
Improved: severity of Symptoms (p < 0.003), QoL (P < 0.001, sleep (P < 0.001). | One patient:GI upset, no augmentation |
|
| ||||
| Gabapentin [48] | Single center, Retrospective |
59: GP 50 and 100 mg 125: controls |
No effect | In gabapentin group, 17% discontinued treatment |
|
| ||||
| Rotigotine patch [49] CKD/HD |
Double blind, placebo controlled | 15 Rotigotine 1 to 3 mg, 3 times daily for 3 weeks 10 Placebo |
At the end of study: 10 of 15 and 2 of 10 showed significant improvement of RLS score in Rotigotine and placebo groups, respectively. | Nausea 20% Vomiting 15% |
|
| ||||
| Low dose ropinirole vs aerobic exercise vs placebo 4 hours HD, 3 sessions/week [50] |
Partially, double blind/placebo controlled | 0.25 mg ropinirole, 2 h before sleep, 45 minutes cycling during each HD session Ropinirole 7 Exercise 15 Placebo 7 |
Ropinirole and aerobic exercise equally improved IRLS scores, QoL and depression. Ropinirole improved sleep quality | No side effects |
|
| ||||
| Gabapentin (GP) versus Levodopa (LD) [51] |
Double blind | GP: 42 LD: 40 GP: 200 mg/d LD: 110 mg/d Over 4 weeks |
Both reduced IRLS scores but GP was more effective (P < 0.016). Both improved quality of sleep | Transient hypotension in two patients who took Levodopa. Increased day time sleepiness GP > LD |
|
| ||||
| Gabapentin (GP) versus Levodopa (LD) [52] Hemodialysis: 3 times/week |
Observational Cross sectional |
GP: 14 LD: 12 GP: 200 mg/after each dialysis session LD: 110 mg/day 4 weeks treatment duration |
IRLSS score was significantly improved after both Gabapentin and Levodopa treatment (P = 0.0001). Gabapentin was superior to L-Dopa in improving quality of sleep and QoL |
Not mentioned |
|
| ||||
| Gabapentin versus levodopa [53] Hemodialysis 3 times/week |
Open label Prospective |
GP# 15 LD#15 Gabapentin: 200 mg after each dialysis session LD: 125 mg, 2 hours before sleep |
Both improved IRLSS scores. Gabapentin was superior to levodopa in improving sleep quality and latency, QoL (measured by SF36), general health and body pain | One patient dropped from the study due to gabapentin side effect (type not mentioned). |
|
| ||||
| Ropinirole versus levodopa-SR [54] Chronic hemodialysis patients |
Randomized, cross-over | 10 Levodopa 190 mg/day Ropinirole 1.45 mg/day Duration 14 weeks (4 weeks each trial with 6 weeks washout) |
At the end of study, ropinirole was superior to levodopa regarding improving scores of six item IRLS and increasing sleep time (P < 0.001) as well as improvement of clinical impression scorea (P < 0.01) | One patient taking levodopa withdrew from the study due to vomiting |
|
| ||||
GP: gabapentin; LD: levodopa; QOL-quality of life; HD: hemodialysis.
Additional therapeutic approaches for CKS-A-RLS include treatment with vitamins E and C which claimed alleviation of symptoms without side effects (Table 3).
Table 3.
Vitamin C and E treatment for CKD-A-RLS.
|
| |||||
|---|---|---|---|---|---|
| TYPE OF VITAMIN | NUMBER OF PATIENTS | STUDY DESIGN | DOSE AND DURATION OF TREATMENT | RESULTS | SIDE EFFECTS |
|
| |||||
| C and E [55] | 60 Divided in 4 groups each including 15 patients Group 1: C + P Group 2: E + P Group 3: C + E Group 4: double placebo |
Double blind-placebo controlled | Vitamin C: 200 mg/day Vitamin E: 400 mg/day All treatments for 8 weeks |
At 8 weeks, patients treated with vitamin C or E or both demonstrated significant reduction of IRLS score compared to placebo. The difference between vitamin treated groups was not significant. | none |
|
| |||||
| Intravenous Vitamin C [56] | 90 | Double blind placebo controlled | Intravenous Vitamin C, three times per week (given at the end of dialysis session for 8 weeks. | Quality of sleep and RLS scores improved significantly in patients who received vitamin C. | None |
|
| |||||
| Compared Vitamin C with pramipexole [57] | 45 Divided in three group each including 15 patients Group 1: Vitamin C Group 2: pramipexole Group 3-: placebo |
Double blind placebo controlled | Vitamin C: 250 mg Pramipexole: 0.18 mg Vitamin C, pramipexole and placebo were given once daily for 8 weeks |
Both Vitamin C and Pramipexole groups demonstrated significant improvement of IRLS scores after treatment (P < 0.001). | One patient in the pramipexole developed nausea and vomiting and excluded |
|
| |||||
Limited studies have been published on the therapeutic role of intravenous iron in dialysis patients with CKD-A-RLS (Table 4).
Table 4.
Intravenous Iron Therapy for RLS-A-CKS.
|
| |||||
|---|---|---|---|---|---|
| TREATMENT | NUMBER OF PATIENTS | STUDY DESIGN | DOSE, DURATION | RESULTS | SIDE EFFECTS |
|
| |||||
| Intravenous (IV) iron dextran [58] | 25 Iron dextran: 11 Placebo: 14 |
Double blind- placebo controlled | Iron dextran: 1000 mg Study duration: 4 weeks. IRLS intensity scores were assessed weekly |
At weeks 1 and 2 post-injection, patients in the Iron treated group showed significant reduction of IRLS scores (P = 0.01 and P = 0.03). At week 4, though still lower than placebo, the difference was not statistically significant | No difference in adverse effects between the two groups |
|
| |||||
| Intravenous iron sucrose [59] | 30 IV Iron Sucrose: 16 Placebo: 16 |
Randomized, placebo-controlled | Iron sucrose: 100 mg, three times/week for a total of 1000 mg. Study duration: 3 weeks | After two weeks, IRLS scores (compared to baseline) were significantly reduced compared to placebo group (P = 0.000). Improvement of IRLS score in the iron group continued for 4–24 weeks. | No adverse effects |
|
| |||||
| Intravenous iron sucrose [60] | 18 Iron sucrose:11 Placebo:7 |
Double blind- placebo controlled | 500 mg of Iron sucrose was administered IV in two successive days for a total of 1000 mg Study duration: 4 weeks Primary outcome for RLS symptom improvement was global rating scale (GRS) |
The trial was aborted half- way into the study since despite some improvement in GRS (at two weeks), authors predicted lack of robust response at the end of the study | Edema in either hands or feet (36%). Nausea or vomiting (36%). Hypotension (18%). dizziness (18%). abdominal pain (9%). All noted during infusion only. |
|
| |||||
GRS: Global rating Scale.
Among narcotic medications oxycodone (two blinded study [61,62]) (Table 5), and tramadol (two open label studies) have been reported to alleviate the symptoms of severe restless legs syndrome. No information on the use of oxycodone in CKD-A-RLS is available. Oxycodone needs to be used with caution in patients with kidney failure as the main mode of its elimination is renal [63].
Table 5.
Double-blind, placebo-controlled studies of narcotic treatment in restless legs syndrome-.
|
| ||||
|---|---|---|---|---|
| AUTHORS AND DATE | NUMBER OF PATIENTS | DRUG, DOSE, STUDY DURATION | RESULTS | SIDE EFFECTS |
|
| ||||
| *Walters et al, 1993 [61] | 11 | Oxycodone, 5 mg tablets, average dose 15.9 mg/day Study duration: two weeks |
Patients rated improvement on the scale of 1–4: leg sensations, daytime sleepiness, motor restlessness and PLS. All Significantly improved (P < 0.5), | Mild constipation: two patients, mild lethargy: 1 patient |
|
| ||||
| **Trenkwalder et al, 2009 [62] | 267 | Oxycodone/Naloxone (longacting), starting dose 5 mg/2.5 mg twice daily increasing up to 40/20 mg per research’s discretion. Study duration: 12 weeks |
Mean International RLS Study group severity rating scale Sum score significantly improved in oxycodone group At week 12 (P < 0.0001) |
Serious adverse effects: 3 in the double blind phase, 3 in the extension phase.(not specified). |
|
| ||||
* Double-blind, crossover; ** Double-blind, parallel design; PLS: periodic leg movements of sleep.
Six publications provided data on CKD-A- RLS in pediatric population with ages of 5 to 17 years [64,65,66,67,68,69]. In five of these studies [64,65,67,68,69], the prevalence of RLS was higher in CKD (15.3% to 35%) compared to normal population. All studies were open label. Three out of six were prospective. Information was taken in the clinic or via telephonic contact, often through the parents. In general, the symptoms were mild; only in two studies poor quality of sleep and impaired quality of life was mentioned. No other treatments were reported.
Discussion
Our search found a higher incidence of RLS in chronic kidney disease compared to the normal population; for most studies, the reported incidence ranged between 15–30%. A few studies that reported a much higher incidence for RLS in CKD (51–84%) [13,14,15,16,17,18,19] represent distinct populations, mostly from middle eastern countries [13,14,15,16,17]. One of these studies had been published in 1995 [18], well before establishment of the modern diagnostic RLS criteria (Table 6).
Table 6.
Diagnostic criteria defined for diagnosis of RLS by International Restless Legs Society, (last revision 2014).
|
| |
|---|---|
| 1 | An irresistible urge to move the legs, usually but not always accompanied by uncomfortable and unpleasant sensations in the legs |
|
| |
| 2 | Symptoms that begin or worsen during the periods of inactivity, such as lying down or sitting |
|
| |
| 3 | Symptoms are partially or totally relieved by movement |
|
| |
| 4 | Symptoms only occur and are worse in the evening or night than during the day |
|
| |
| 5 | The occurrence of the described features is not solely accounted for as symptoms primary to another medical or a behavioral condition (myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping). The criteria published earlier in 2003 lacks the 5th criteria. |
|
| |
The diversity of data found in this review is not surprising considering the fact that different investigators used different criteria for diagnosis of RLS (particularly before 2003). Furthermore, the studies represented findings in different stages of chronic kidney disease, hence not quite comparable. On the clinical sides, though the data is contradictory, considerably more studies reported positive correlations with increased mortality, increased cardiovascular complications, insomnia, and depression.
Non-pharmacological treatment, especially aerobic exercise and massage with lavender oil seems helpful in treatment of CKD-A-RLS. The Guideline Development Subcommittee of the American Academy of Neurology [70,71] recommends treating CKD-A-RLS with vitamin C and E based on one published class I study [55]. As a reducing agent, vitamin C plays an important role in iron metabolism. It increases absorption of iron from gastrointestinal tract and enhances the bioavailability of iron after intravenous iron injection. It also can mobilize iron from the reticuloendothelial system to transferrin [72,73].
Currently, dopaminergic medications (levodopa, ropinirole, pramipexole and rotigotine) and gabapentinoids (gabapentin and pregabalin) are recommended as the first line of drugs for treatment of essential RLS [74]. However, in case of CKD-A-RLS, more robust investigations for ropinirole and pramipexole are needed. Development of augmentation remains a worrisome issue with the use of dopaminergic drugs especially if long term therapy is contemplated. Currently, more practitioners prefer the use of direct dopamine agonists (ropinirole, pramipexole, rotigotine) over levodopa for treatment of CKD-A-RLS. One randomized controlled study has shown the superiority of ropinirole over levodopa for treatment of CKD-A-RLS (Table 2) [54]. The same preference applies to gabapentin over levodopa based on three small comparative studies (Table 2).
Due to renal clearance, dose adjustment is necessary when oral dopaminergic drugs or gabapentinoid medications (gabapentin and pregabalin) are going to be used for treatment of CKD-A-RLS. In case of gabapentin and pregabalin the following dosing schedule is recommended by Chincholkar et al [75] (Table 7).
Table 7.
Dose adjustment recommended for gabapentin and pregabalin in kidney failure [66].
|
| ||
|---|---|---|
| DRUG’S NAME | BASED ON CREATINE CLEARANCE (ML/MINUTE) | RECOMMENDED DOSE GIVEN IN THREE DIVIDED DOSES IN ALTERNATE DAYS |
|
| ||
| Gabapentin | 50–79 | 600–180 mg |
|
| ||
| 30–49 | 300–900 mg | |
|
| ||
| 15–29 | 150–600 | |
|
| ||
| <15 | 150–300 | |
|
| ||
| Pregabalin | Based on eGFR, mL/min/1.73 m2 | |
|
| ||
| 30–60 | Initially 75 mg, maximum 300 mg daily | |
|
| ||
| 15–30 | Initially 25–50 mg, maximum 300 mg in two divided doses | |
|
| ||
| <30 | Initially 25 mg once daily and maximum 75 mg once daily | |
|
| ||
Ropinirole was approved by FDA for treatment of restless legs syndrome in 2005 and pramipexole in 2006. In an open label study of 10 patients affected by advanced kidney disease and on dialysis, Miranda et al [76] reported a significant improvement of RLS severity scores (using the criteria set by International RLS Study Group) after treatment with pramipexole (mean dose of 0.25 mg/day). The mean time of follow up was 8 months. Currently, two randomized, double- blind studies are ongoing with aims of assessing the efficacy of ropinirole and pramipexole in CKD-A-RLS [77,78], the results of which will hopefully, be available soon.
Rotigotine as a skin patch has the advantage of bypassing drug absorption through the GI tract which is often affected in patients with chronic kidney disease. Renal clearance of rotigotine is also not influenced by kidney disease. Even in advanced kidney failure the level of unconjugated rotigotine does not change indicating no need for dose adjustment [79]. For these advantages, treatment with rotigotine deserves further investigation. Intravenous iron using iron dextran and iron sucrose have been helpful in reducing intensity of RLS in chronic kidney disease especially in case of iron deficiency [58,59,60].
Up to 65% of patients with CKD in clinical examination demonstrate evidence of peripheral Neuropathy [80]. Peritoneal and hemodialysis can improve mild peripheral neuropathy but their effect on severe peripheral neuropathy is not adequately studied [81]. A clinical trial with two years of follow up demonstrated failure of renal transplantation to improve CKD associated peripheral neuropathy [82].
Conclusion
This updated review showed that restless legs syndrome is two to three times more common in chronic kidney disease compared to the general population. When assessed, more patients with CKD-A-RLS demonstrated increased mortality, increased incidence of cardiovascular accident, depression, insomnia and impaired quality of life compared to CKD patients without RLS. Dopaminergic drugs such as levodopa, ropinirole, pramipexole and rotigotine as well as calcium channel blockers (gabapentin and pregabalin) are helpful for treatment of RLS. High quality studies with these agents are currently underway and hopefully confirm the efficacy and practicality of using these drugs in CKD-A-RLS. Treatment with Vitamin C and E is recommended for CKD-A-RLS. Some studies have shown that aerobic exercise and massage with lavender oil can improve symptoms of CKD-A- RLS suggesting that these measures can be useful as adjunct therapy.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017. Mar 25; 389(10075): 1238–1252. Epub 2016 Nov 23. PMID: 27887750. DOI: 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2.Ammirati AL. Chronic Kidney Disease. Rev Assoc Med Bras (1992). 2020. Jan 13; 66(Suppl 1): s03–s09. PMID: 31939529. DOI: 10.1590/1806-9282.66.s1.3 [DOI] [PubMed] [Google Scholar]
- 3.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011. May; 6(5): 986–94. Epub 2011 Mar 24. PMID: 21441128; PMCID: PMC3087794. DOI: 10.2215/CJN.05720710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak M, Winkelman JW, Unruh M. Restless Legs Syndrome in Patients with Chronic Kidney Disease. Semin Nephrol. 2015. Jul; 35(4): 347–58. PMID: 26355253. DOI: 10.1016/j.semnephrol.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Zhao C, Luo Q, Xia X, Yu X, Huang F. Prevalence of restless legs syndrome in chronic kidney disease: a systematic review and meta-analysis of observational studies. Ren Fail. 2016. Oct; 38(9): 1335–1346. Epub 2016 Oct 20. PMID: 27765002. DOI: 10.1080/0886022X.2016.1227564 [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003. Mar; 4(2): 101–19. PMID: 14592341. DOI: 10.1016/S1389-9457(03)00010-8 [DOI] [PubMed] [Google Scholar]
- 7.Venkateshiah SB, Ioachimescu OC. Restless legs syndrome. Crit Care Clin. 2015. Jul; 31(3): 459–72. Epub 2015 May 1. PMID: 26118915. DOI: 10.1016/j.ccc.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 8.Vlasie A, Trifu SC, Lupuleac C, Kohn B, Cristea MB. Restless legs syndrome: An overview of pathophysiology, comorbidities and therapeutic approaches (Review). Exp Ther Med. 2022. Feb; 23(2): 185. DOI: 10.3892/etm.2021.11108. Epub 2021 Dec 30. PMID: 35069866; PMCID: PMC8764906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossard TR, Trotti LM, Videnovic A, St Louis EK. Restless Legs Syndrome: Contemporary Diagnosis and Treatment. Neurotherapeutics. 2021. Jan; 18(1): 140–155. Epub 2021 Apr 20. PMID: 33880737; PMCID: PMC8116476. DOI: 10.1007/s13311-021-01019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anguelova GV, Vlak MHM, Kurvers AGY, Rijsman RM. Pharmacologic and Nonpharmacologic Treatment of Restless Legs Syndrome. Sleep Med Clin. 2020. Jun; 15(2): 277–288. PMID: 32386701. DOI: 10.1016/j.jsmc.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Walters AS, Moussouttas M, Siddiqui F, Silveira DC, Fuentes K, Wang L, Berger K. Prevalence of stroke in Restless Legs Syndrome: Initial Results Point to the Need for More Sophisticated Studies. Open Neurol J. 2010. Jun 15; 4: 73–7. PMID: 20721325; PMCID: PMC2923374. DOI: 10.2174/1874205X01004010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. International Restless Legs Syndrome Study Group. Sleep Med. 2014. Aug; 15(8): 860–73. DOI: 10.1016/j.sleep.2014.03.025 [DOI] [PubMed] [Google Scholar]
- 13.Zadeh Saraji N, Hami M, Boostani R, Mojahedi MJ. Restless leg syndrome in chronic hemodialysis patients in Mashhad hemodialysis centers. J Renal Inj Prev. 2016. Dec 25; 6(2): 137–141. PMID: 28497091; PMCID: PMC5423282. DOI: 10.15171/jrip.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasheminasab Zaware R, Mahmoodi Meymand MH, Rezaeian M, Mohammadi Kamalabadi N, Mostafavi SA, Abdolkarimi Dawarani MA, Jome Yazdian R, Bidaki R. Insomnia and Restless Leg Syndrome in Patients Undergoing Chronic Hemodialysis in Rafsanjan Ali Ibn Abitaleb Hospital. Nephrourol Mon. 2016. Jan 9; 8(1): e29527. PMID: 26981494; PMCID: PMC4780113. DOI: 10.5812/numonthly.29527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naini AE, Amra B, Mahmoodnia L, Taheri S. Sleep apnea syndrome and restless legs syndrome in kidney transplant recipients. Adv Biomed Res. 2015. Sep 28; 4: 206. PMID: 26605235; PMCID: PMC4627182. DOI: 10.4103/2277-9175.166142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Jahdali HH, Al-Qadhi WA, Khogeer HA, Al-Hejaili FF, Al-Ghamdi SM, Al Sayyari AA. Restless legs syndrome in patients on dialysis. Saudi J Kidney Dis Transpl. 2009. May; 20(3): 378–85. PMID: 19414938. [PubMed] [Google Scholar]
- 17.Hui DS, Wong TY, Ko FW, Li TS, Choy DK, Wong KK, Szeto CC, Lui SF, Li PK. Prevalence of sleep disturbances in chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000. Oct; 36(4): 783–8. PMID: 11007681. DOI: 10.1053/ajkd.2000.17664 [DOI] [PubMed] [Google Scholar]
- 18.Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995. Nov; 26(5): 751–6. PMID: 7485127. DOI: 10.1016/0272-6386(95)90438-7 [DOI] [PubMed] [Google Scholar]
- 19.Haider I, Anees M, Shahid SA. Restless legs syndrome in end stage renal disease patients on haemodialysis. Pak J Med Sci. 2014. Nov–Dec; 30(6): 1209–12. PMID: 25674109; PMCID: PMC4320701. DOI: 10.12669/pjms.306.5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung. 2006. Jan–Feb; 184(1): 43–9. PMID: 16598651. DOI: 10.1007/s00408-005-2563-2 [DOI] [PubMed] [Google Scholar]
- 21.Bhowmik D, Bhatia M, Tiwari S, Mahajan S, Gupta S, Agarwal SK, Dash SC. Low prevalence of restless legs syndrome in patients with advanced chronic renal failure in the Indian population: a case controlled study. Ren Fail. 2004. Jan; 26(1): 69–72. PMID: 15083925. DOI: 10.1081/JDI-120028557 [DOI] [PubMed] [Google Scholar]
- 22.Capelli I, Pizza F, Ruggeri M, Gasperoni L, Carretta E, Donati G, Cianciolo G, Plazzi G, La Manna G. Time evolution of restless legs syndrome in haemodialysis patients. Clin Kidney J. 2019. Dec 16; 14(1): 341–347. PMID: 33564437; PMCID: PMC7857816. DOI: 10.1093/ckj/sfz148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrastina M, Martinková J, Minar M, Zilinska Z, Valkovic P, Breza J. Impact of kidney transplantation on restless legs syndrome. Bratisl Lek Listy. 2015; 116(7): 404–7. PMID: 26286241. DOI: 10.4149/BLL_2015_077 [DOI] [PubMed] [Google Scholar]
- 24.Szentkiralyi A, Molnar MZ, Czira ME, Deak G, Lindner AV, Szeifert L, Torzsa P, Vamos EP, Zoller R, Mucsi I, Novak M. Association between restless legs syndrome and depression in patients with chronic kidney disease. J Psychosom Res. 2009. Aug; 67(2): 173–80. PMID: 19616146. DOI: 10.1016/j.jpsychores.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 25.Azar SA, Hatefi R, Talebi M. Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc. 2007. May; 39(4): 1132–3. PMID: 17524912. DOI: 10.1016/j.transproceed.2007.03.097 [DOI] [PubMed] [Google Scholar]
- 26.Winkelmann J, Stautner A, Samtleben W, Trenkwalder C. Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord. 2002. Sep; 17(5): 1072–6. PMID: 12360562. DOI: 10.1002/mds.10231 [DOI] [PubMed] [Google Scholar]
- 27.Stefanidis I, Vainas A, Giannaki CD, Dardiotis E, Spanoulis A, Sounidaki M, Eleftheriadis T, Liakopoulos V, Karatzaferi C, Sakkas GK, Zintzaras E, Hadjigeorgiou GM. Restless legs syndrome does not affect 3-year mortality in hemodialysis patients. Sleep Med. 2015. Sep; 16(9): 1131–8. Epub 2015 Jun 19. PMID: 26298790. DOI: 10.1016/j.sleep.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 28.Lin CH, Sy HN, Chang HW, Liou HH, Lin CY, Wu VC, Wu SL, Chang CC, Chiu PF, Li WY, Lin SY, Wu KD, Chen YM, Wu RM. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015. Jan; 22(1): 142–9. Epub 2014 Aug 21. PMID: 25142748. DOI: 10.1111/ene.12545 [DOI] [PubMed] [Google Scholar]
- 29.La Manna G, Pizza F, Persici E, Baraldi O, Comai G, Cappuccilli ML, Centofanti F, Carretta E, Plazzi G, Colì L, Montagna P, Stefoni S. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011. Jun; 26(6): 1976–83. Epub 2010 Nov 5. PMID: 21056943. DOI: 10.1093/ndt/gfq681 [DOI] [PubMed] [Google Scholar]
- 30.Unruh ML, Levey AS, D’Ambrosio C, Fink NE, Powe NR, Meyer KB; Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004. May; 43(5): 900–9. PMID: 15112181. DOI: 10.1053/j.ajkd.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 31.Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996. Sep; 28(3): 372–8. PMID: 8804235. DOI: 10.1016/S0272-6386(96)90494-1 [DOI] [PubMed] [Google Scholar]
- 32.DeFerio JJ, Govindarajulu U, Brar A, Cukor D, Lee KG, Salifu MO. Association of restless legs syndrome and mortality in end-stage renal disease: an analysis of the United States Renal Data System (USRDS). BMC Nephrol. 2017. Aug 1; 18(1): 258. PMID: 28764654; PMCID: PMC5540277. DOI: 10.1186/s12882-017-0660-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baiardi S, Mondini S, Baldi Antognini A, Santoro A, Cirignotta F. Survival of Dialysis Patients with Restless Legs Syndrome: A 15-Year Follow-Up Study. Am J Nephrol. 2017; 46(3): 224–230. Epub 2017 Sep 5. PMID: 28869939. DOI: 10.1159/000479938 [DOI] [PubMed] [Google Scholar]
- 34.Losso RL, Minhoto GR, Riella MC. Sleep disorders in patients with end-stage renal disease undergoing dialysis: comparison between hemodialysis, continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Int Urol Nephrol. 2015. Feb; 47(2): 369–75. Epub 2014 Oct 31. PMID: 25358390. DOI: 10.1007/s11255-014-0860-5 [DOI] [PubMed] [Google Scholar]
- 35.Al-Jahdali H. A comparison of sleep disturbances and sleep apnea in patients on hemodialysis and chronic peritoneal dialysis. Saudi J Kidney Dis Transpl. 2011. Sep; 22(5): 922–30. PMID: 21912020. [PubMed] [Google Scholar]
- 36.Kashani E, Mirhosseini Z, Rastaghi S, Rad M. The Effect of the Cool Dialysate on the Restless Leg Syndrome in Hemodialysis Patients: Randomized Triple-Blind Clinical Trial. Iran J Nurs Midwifery Res. 2019. May–Jun; 24(3): 200–205. PMID: 31057636; PMCID: PMC6485020. DOI: 10.4103/ijnmr.IJNMR_133_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakkas GK, Hadjigeorgiou GM, Karatzaferi C, Maridaki MD, Giannaki CD, Mertens PR, Rountas C, Vlychou M, Liakopoulos V, Stefanidis I. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J. 2008. Mar–Apr; 54(2): 185–90. PMID: 18356653. DOI: 10.1097/MAT.0b013e3181641b07 [DOI] [PubMed] [Google Scholar]
- 38.Mortazavi M, Vahdatpour B, Ghasempour A, Taheri D, Shahidi S, Moeinzadeh F, Dolatkhah B, Dolatkhah S. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Scientific World Journal. 2013. Nov 6; 2013: 628142. PMID: 24307876; PMCID: PMC3836425. DOI: 10.1155/2013/628142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirbagher Ajorpaz N, Rahemi Z, Aghajani M, Hashemi SH. Effects of glycerin oil and lavender oil massages on hemodialysis patients’ restless legs syndrome. J Bodyw Mov Ther. 2020. Jan; 24(1): 88–92. Epub 2019 Jun 29. PMID: 31987569. DOI: 10.1016/j.jbmt.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 40.Oshvandi K, Mirzajani Letomi F, Soltanian AR, Shamsizadeh M. The effects of foot massage on hemodialysis patients’ sleep quality and restless leg syndrome: a comparison of lavender and sweet orange essential oil topical application. J Complement Integr Med. 2021. Apr 12; 18(4): 843–850. PMID: 33838094. DOI: 10.1515/jcim-2020-0121 [DOI] [PubMed] [Google Scholar]
- 41.Amrollahi A, Rafiei A, Bahri A, Nasiriani K. Effects of aromatherapy massage on the severity of restless legs syndrome in hemodialysis patients: A randomized clinical trial. Ther Apher Dial. 2022. Jan 21; Epub ahead of print. PMID: 35060333. DOI: 10.1111/1744-9987.13802 [DOI] [PubMed] [Google Scholar]
- 42.Döner A, Taşcı S. Effect of massage therapy with lavender oil on severity of restless legs syndrome and quality of life in hemodialysis patients. J Nurs Scholarsh. 2021. Nov 14; Epub ahead of print. PMID: 34779137. DOI: 10.1111/jnu.12738 [DOI] [PubMed] [Google Scholar]
- 43.Hashemi SH, Hajbagheri A, Aghajani M. The effect of massage with lavender oil in restless legs syndrome in hemodialysis: a randomized controlled trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortazavi M, Vahdatpour B, Ghasempour A, Taheri D, Shahidi S, Moeinzadeh F, Dolatkhah B, Dolatkhah S. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Scientific World Journal. 2013. Nov 6; 2013: 628142. PMID: 24307876; PMCID: PMC3836425. DOI: 10.1155/2013/628142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trenkwalder C, Stiasny K, Pollmächer T, Wetter T, Schwarz J, Kohnen R, Kazenwadel J, Krüger HP, Ramm S, Künzel M, et al. L-dopa therapy of uremic and idiopathic restless legs syndrome: a double-blind, crossover trial. Sleep. 1995. Oct; 18(8): 681–8. PMID: 8560135. DOI: 10.1093/sleep/18.8.681 [DOI] [PubMed] [Google Scholar]
- 46.Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001. Jul; 38(1): 104–8. PMID: 11431189. DOI: 10.1053/ajkd.2001.25202 [DOI] [PubMed] [Google Scholar]
- 47.Esteve V, Carneiro J, Salazar G, Pou M, Tapia I, Fulquet M, Duarte V, Saurina A, Moreno F, Ramírez de Arellano M. Effects of rotigotine on clinical symptoms, quality of life and sleep hygiene adequacy in haemodialysis-associated restless legs syndrome. Nefrologia (Engl Ed). 2018. Jan–Feb; 38(1): 79–86. English, Spanish. Epub 2017 Dec 2. PMID: 29198453. DOI: 10.1016/j.nefro.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 48.Cheikh Hassan HI, Brennan F, Collett G, Josland EA, Brown MA. Efficacy and safety of gabapentin for uremic pruritus and restless legs syndrome in conservatively managed patients. Epub 2014 Sep 8. PMID: 25220049. DOI: 10.1016/j.jpainsymman.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 49.Dauvilliers Y, Benes H, Partinen M, Rauta V, Rifkin D, Dohin E, Goldammer N, Schollmayer E, Schröder H, Winkelman JW. Rotigotine in Hemodialysis-Associated Restless Legs Syndrome: A Randomized Controlled Trial. Am J Kidney Dis. 2016. Sep; 68(3): 434–43. Epub 2016 Feb 3. PMID: 26851201. DOI: 10.1053/j.ajkd.2015.12.027 [DOI] [PubMed] [Google Scholar]
- 50.Giannaki CD, Sakkas GK, Karatzaferi C, Hadjigeorgiou GM, Lavdas E, Kyriakides T, Koutedakis Y, Stefanidis I. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013. Sep 11; 14: 194. PMID: 24024727; PMCID: PMC3847208. DOI: 10.1186/1471-2369-14-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razazian N, Azimi H, Heidarnejadian J, Afshari D, Ghadami MR. Gabapentin versus levodopa-c for the treatment of restless legs syndrome in hemodialysis patients: a randomized clinical trial. Saudi J Kidney Dis Transpl. 2015. Mar; 26(2): 271–8. PMID: 25758874. DOI: 10.4103/1319-2442.152417 [DOI] [PubMed] [Google Scholar]
- 52.Ali M, Iram H, Nasim F, Solangi SA, Junejo AM, Un Nisa N, Solangi SA. Comparison of the Efficacy of Gabapentin Versus Levodopa-C for the Treatment of Restless Legs Syndrome in End-Stage Renal Disease on Hemodialysis Patients. Cureus. 2020. Dec 11; 12(12): e12034. PMID: 33457134; PMCID: PMC7797419. DOI: 10.7759/cureus.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of Restless Legs Syndrome in hemodialysis patients: an open-label study. Ren Fail. 2004. Jul; 26(4): 393–7. PMID: 15462107. DOI: 10.1081/JDI-120039823 [DOI] [PubMed] [Google Scholar]
- 54.Pellecchia MT, Vitale C, Sabatini M, Longo K, Amboni M, Bonavita V, Barone P. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: an open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004. Jul–Aug; 27(4): 178–81. PMID: 15319704. DOI: 10.1097/01.wnf.0000135480.78529.06 [DOI] [PubMed] [Google Scholar]
- 55.Sagheb MM, Dormanesh B, Fallahzadeh MK, Akbari H, Sohrabi Nazari S, Heydari ST, Behzadi S. Efficacy of vitamins C, E, and their combination for treatment of restless legs syndrome in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2012. May; 13(5): 542–5. Epub 2012 Feb 7. PMID: 22317944. DOI: 10.1016/j.sleep.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 56.Dadashpour S, Hajmiri MS, Roshani D. Effect of intravenous vitamin C supplementation on the quality of sleep, itching and restless leg syndrome in patients undergoing hemodialysis; A double-blind randomized clinical trial. J. Nephropharmacol. 2018; 7: 131–136. DOI: 10.15171/npj.2018.27 [DOI] [Google Scholar]
- 57.Rafie S, Jafari M. A comparative study on the effects of vitamin C and pramipexole on restless legs syndrome treatment in hemodialysis patients: A randomized, double blind, placebo-controlled trial. Int. J. Pharm. Res. Allied Sci. 2016; 5: 128–134 [Google Scholar]
- 58.Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004. Apr; 43(4): 663–70. PMID: 15042543. DOI: 10.1053/j.ajkd.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 59.Deng Y, Wu J, Jia Q. Efficacy of Intravenous Iron Sucrose in Hemodialysis Patients with Restless Legs Syndrome (RLS): A Randomized, Placebo-Controlled Study. Med Sci Monit. 2017. Mar 12; 23: 1254–1260. PMID: 28285317; PMCID: PMC5360424. DOI: 10.12659/MSM.900520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Earley CJ, Horská A, Mohamed MA, et al: A randomized, double-Blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009; 10: 206–11. DOI: 10.1016/j.sleep.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, Kavey N. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993. Jun; 16(4): 327–32. PMID: 8341893. DOI: 10.1093/sleep/16.4.327 [DOI] [PubMed] [Google Scholar]
- 62.Trenkwalder C, Beneš H, Grote L, García-Borreguero D, Högl B, Hopp M, Bosse B, Oksche A, Reimer K, Winkelmann J, Allen RP, Kohnen R; RELOXYN Study Group. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013. Dec; 12(12): 1141–50. Epub 2013 Oct 18. Erratum in: Lancet Neurol. 2013 Dec; 12(12): 1133. PMID: 24140442. DOI: 10.1016/S1474-4422(13)70239-4 [DOI] [PubMed] [Google Scholar]
- 63.de Biase S, Valente M, Gigli GL. Intractable restless legs syndrome: role of prolonged-release oxycodone-naloxone. Neuropsychiatr Dis Treat. 2016. Feb 23; 12: 417–25. PMID: 26966363; PMCID: PMC4770072. DOI: 10.2147/NDT.S81186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riar SK, Leu RM, Turner-Green TC, Rye DB, Kendrick-Allwood SR, McCracken C, Bliwise DL, Greenbaum LA. Restless legs syndrome in children with chronic kidney disease. Pediatr Nephrol. 2013. May; 28(5): 773–95. Epub 2013 Jan 20. PMID: 23334386. DOI: 10.1007/s00467-013-2408-9 [DOI] [PubMed] [Google Scholar]
- 65.Darwish AH, Abdel-Nabi H. Sleep disorders in children with chronic kidney disease. Int J Pediatr Adolesc Med. 2016. Sep; 3(3): 112–118. Epub 2016 Jun 29. PMID: 30805480; PMCID: PMC6372444. DOI: 10.1016/j.ijpam.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung V, Wertenteil S, Sasson S, Vento S, Kothare S, Trachtman H. Restless Legs Syndrome in Pediatric Patients With Nephrotic Syndrome. Glob Pediatr Health. 2015. May 11; 2: PMID: 27335958; PMCID: PMC4784599. DOI: 10.1177/2333794X15585994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis ID, Greenbaum LA, Gipson D, Wu LL, Sinha R, Matsuda-Abedini M, Emancipator JL, Lane JC, Hodgkins K, Nailescu C, Barletta GM, Arora S, Mahan JD, Rosen CL. Prevalence of sleep disturbances in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2012. Mar; 27(3): 451–9. Epub 2011 Oct 2. PMID: 21964556. DOI: 10.1007/s00467-011-2010-y [DOI] [PubMed] [Google Scholar]
- 68.Applebee GA, Guillot AP, Schuman CC, Teddy S, Attarian HP. Restless legs syndrome in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2009. Mar; 24(3): 545–8. Epub 2008 Dec 2. PMID: 19048298. DOI: 10.1007/s00467-008-1057-x [DOI] [PubMed] [Google Scholar]
- 69.Sinha R, Davis ID, Matsuda-Abedini M. Sleep disturbances in children and adolescents with non-dialysis-dependent chronic kidney disease. Arch Pediatr Adolesc Med. 2009. Sep; 163(9): 850–5. PMID: 19736340. DOI: 10.1001/archpediatrics.2009.149 [DOI] [PubMed] [Google Scholar]
- 70.Winkelman JW, Armstrong MJ, Allen RP, Chaudhuri KR, Ondo W, Trenkwalder C, Zee PC, Gronseth GS, Gloss D, Zesiewicz T. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016. Dec 13; 87(24): 2585–2593. Epub 2016 Nov 16. PMID: 27856776; PMCID: PMC5206998. DOI: 10.1212/WNL.0000000000003388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gronseth G, French J. Practice parameters and technology assessments: what they are, what they are not, and why you should care. Neurology. 2008. Nov 11; 71(20): 1639–43. PMID: 19001255. DOI: 10.1212/01.wnl.0000336535.27773.c0 [DOI] [PubMed] [Google Scholar]
- 72.Handelman GJ. Vitamin C neglect in hemodialysis: sailing between Scylla and Charybdis. Blood Purif. 2007; 25(1): 58–61. Epub 2006 Dec 14. PMID: 17170539. DOI: 10.1159/000096399 [DOI] [PubMed] [Google Scholar]
- 73.Tarng DC. Novel aspects of vitamin C in epoetin response. J Chin Med Assoc. 2007. Sep; 70(9): 357–60. PMID: 17908648. DOI: 10.1016/S1726-4901(08)70020-0 [DOI] [PubMed] [Google Scholar]
- 74.Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, Winkelman JW, Trenkwalder C, Sampaio C. Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)§. Mov Disord. 2018. Jul; 33(7): 1077–1091. Epub 2018 May 14. PMID: 29756335. DOI: 10.1002/mds.27260 [DOI] [PubMed] [Google Scholar]
- 75.Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020. May; 14(2): 104–114. Epub 2020 Mar 13. PMID: 32537149; PMCID: PMC7265598. DOI: 10.1177/2049463720912496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miranda M, Kagi M, Fabres L, Aguilera L, Alvo M, Elgueta L, Erazo S, Venegas P. Pramipexole for the treatment of uremic restless legs in patients undergoing hemodialysis. Neurology. 2004. Mar 9; 62(5): 831–2. PMID: 15007148. DOI: 10.1212/01.WNL.0000113752.14744.15 [DOI] [PubMed] [Google Scholar]
- 77.Collister D, Pohl K, Herrington G, Lee SF, Rabbat C, Tennankore K, Zimmermann D, Tangri N, Wald R, Manns B, Suri RS, Nadeau-Fredette AC, Goupil R, Silver SA, Walsh M. The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial. Can J Kidney Health Dis. 2020. Nov 26; 7: 2054358120968959. PMID: 33294203; PMCID: PMC7705292. DOI: 10.1177/2054358120968959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma TT, Yang Z, Zhu S, Zhao JH, Li Y, Sun FY, Zhao N, Xiong ZY, Xiong ZB, Dong J. Pramipexole in peritoneal dialysis patients with restless legs syndrome (RLS): a protocol for a multicentre double-blind randomised controlled trial. BMJ Open. 2020. Feb 18; 10(2): e033815. PMID: 32075834; PMCID: PMC7045231. DOI: 10.1136/bmjopen-2019-033815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cawello W, Ahrweiler S, Sulowicz W, Szymczakiewicz-Multanowska A, Braun M. Single dose pharmacokinetics of the transdermal rotigotine patch in patients with impaired renal function. Br J Clin Pharmacol. 2012. Jan; 73(1): 46–54. PMID: 21707699; PMCID: PMC3248255. DOI: 10.1111/j.1365-2125.2011.04053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004. Dec; 107(1): 1–16. PMID: 15567546. DOI: 10.1016/j.clineuro.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 81.Arnold R, Pussell BA, Kiernan MC, Krishnan AV. Comparative study to evaluate the effects of peritoneal and hemodialysis on peripheral nerve function. Muscle Nerve. 2016. Jun; 54(1): 58–64. Epub 2016 May 13. PMID: 26660121. DOI: 10.1002/mus.25016 [DOI] [PubMed] [Google Scholar]
- 82.Ferdousi M, Azmi S, Kalteniece A, Khan SU, Petropoulos IN, Ponirakis G, Alam U, Asghar O, Marshall A, Soran H, Boulton AJM, Augustine T, Malik RA. No evidence of improvement in neuropathy after renal transplantation in patients with end stage kidney disease. J Peripher Nerv Syst. 2021. Sep; 26(3): 269–275. Epub 2021 Jun 10. PMID: 34085731. DOI: 10.1111/jns.12456 [DOI] [PubMed] [Google Scholar]


