Abstract
Background and Objective
Ruxolitinib is a tyrosine kinase inhibitor targeting the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways. Ruxolitinib is used to treat myelofibrosis, polycythemia vera and steroid-refractory graft-versus-host disease in the setting of allogeneic stem-cell transplantation. This review describes the pharmacokinetics and pharmacodynamics of ruxolitinib.
Methods
Pubmed, EMBASE, Cochrane Library and web of Science were searched from the time of database inception to march 15, 2021 and was repeated on November 16, 2021. Articles not written in English, animal or in vitro studies, letters to the editor, case reports, where ruxolitinib was not used for hematological diseases or not available as full text were excluded.
Results
Ruxolitinib is well absorbed, has 95% bio-availability, and is bound to albumin for 97%. Ruxolitinib pharmacokinetics can be described with a two-compartment model and linear elimination. Volume of distribution differs between men and women, likely related to bodyweight differences. Metabolism is mainly hepatic via CYP3A4 and can be altered by CYP3A4 inducers and inhibitors. The major metabolites of ruxolitinib are pharmacologically active. The main route of elimination of ruxolitinib metabolites is renal. Liver and renal dysfunction affect some of the pharmacokinetic variables and require dose reductions. Model-informed precision dosing might be a way to further optimize and individualize ruxolitinib treatment, but is not yet advised for routine care due to lack of information on target concentrations.
Conclusion
Further research is needed to explain the interindividual variability of the ruxolitinib pharmacokinetic variables and to optimize individual treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-023-01225-7.
Key Points
| Ruxolitinib is a tyrosine kinase inhibitor targeting the Janus Kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) pathways and is used to treat myelofibrosis, polycythemia vera and steroid refractory graft versus host disease in the setting of allogeneic stem cell transplantation. |
| We describe the pharmacokinetics and pharmacodynamics of ruxolitinib for myelofibrosis, polycythemia vera and steroid refractory graft versus host disease. |
| We show that the pharmacokinetics and pharmacodynamics are well known, but further research is needed to explain the interindividual variability of the pharmacokinetic variables and to optimize the individual treatment. |
Introduction
Tyrosine kinase inhibitors (TKIs) specifically bind to tyrosine kinases and thereby selectively inactivate downstream kinase signaling. TKIs are widely used in the treatment of solid tumors and hematological malignancies [1, 2]. Janus kinase (JAK) is a tyrosine kinase family of cytokine receptors (JAK1, JAK2 and JAK3). In conjunction with signal transducer and activator of transcription (STAT), the JAK family regulates erythropoiesis and thrombopoiesis [3, 4]. Under physiological conditions, JAK/STAT pathway activation leads to gene transcription of cytokines and growth factors, resulting in cell growth, differentiation and apoptosis. The JAK/STAT pathway thereby regulates hematopoiesis and modulates the immune system [4–8]. Ruxolitinib is a TKI that selectively targets JAK1/JAK2 and is used for the treatment of myelofibrosis (MF), polycythemia vera (PV) and steroid-refractory graft-versus-host disease (GVHD) in the setting of an allogeneic stem-cell transplantation. Ruxolitinib was first approved in 2011 by the US Food and Drug Administration (FDA) for MF, in 2014 for PV and in 2019 for GVHD. The European Medicines Agency (EMA) approved ruxolitinib in 2012 for MF, in 2015 for PV and in 2022 for GVHD.
The use of ruxolitinib for MF, PV and GVHD has been studied intensively. In addition, many new therapeutic applications of ruxolitinib are being investigated, especially in diseases where the immune-modulating effects of ruxolitinib could be of value, such as treatment of COVID-19 [9] and dermatological auto-immune diseases [10]. This review is focused on the pharmacokinetics and pharmacodynamics of ruxolitinib in patients with MF, PV and GVHD.
Disease Overview
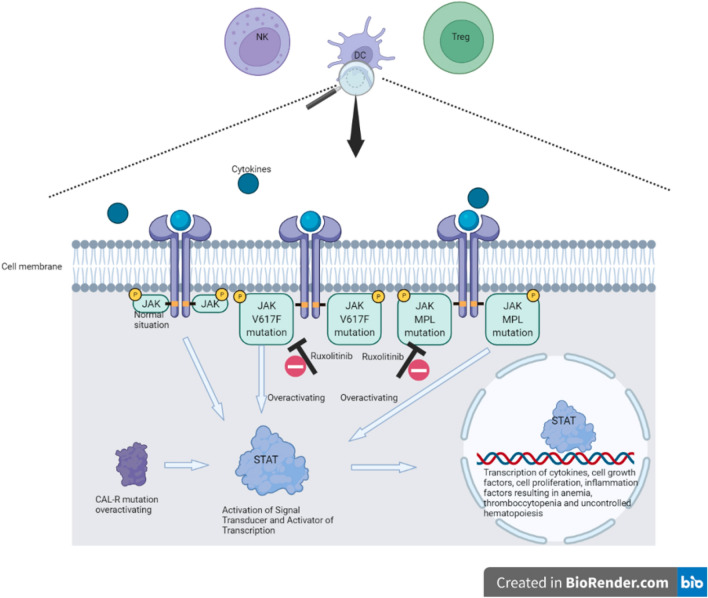
PV is a myeloproliferative neoplasm (MPN) and is characterized by hyperplastic hematopoiesis, leading to erythrocytosis, thrombocytosis and/or leukocytosis. Symptoms can be splenomegaly, fatigue, itching and flushing. The major causes of mortality and morbidity are vascular complications such as coronary heart disease and stroke [11, 12], caused by the hyperinflammatory state leading to a higher risk of thrombotic events [13]. The pathogenesis of PV is only partly understood. Nearly all PV patients have an overactivated JAK/STAT-pathway (Fig. 1). Several activating mutations in the genes coding for the JAK/STAT pathway have been discovered. Most frequent is an activating somatic mutation in the gene encoding for the JAK2 protein, the JAK2 V617F mutation, which can be found in 95% of PV patients [6, 12, 14]. In < 10% of the patients, PV transforms into MF over time [11, 12].
Fig. 1.
JAK/STAT dysregulation in myelofibrosis and polycythemia vera. CAL-R calreticulin gene, DC dendritic cell, JAK Janus kinase, NK natural killer cell, STAT signal transducer and activator of transcription, Treg regulatory T cells
Primary myelofibrosis (PMF) is another MPN and is characterized by initial hematopoietic hyperplasia and systemic inflammation, leading to ineffective and extramedullary hematopoiesis and splenomegaly resulting in reduced survival [8, 15–19]. MF progression leads to thrombocytopenia, anemia and complications related to these, such as fatigue, dyspnea and bleeding-related issues. Although most patients experience thrombocytopenia, thrombotic complications are common in this patient category, comparable to PV [8, 20, 21].
The pathogenesis of MF is partly understood. Most MF patients have an overactivated JAK/STAT pathway. Several mutations of the genes coding for the JAK/STAT pathway are found in MF. The most frequent mutation is JAK2 V617F, which is found in 65% of MF patients [17, 18, 21, 22]. Other JAK/STAT-pathway activating mutations that are found are mutations in the gene encoding the thrombopoietin receptor (MPL) and the endoplasmic reticulum chaperone protein, calreticulin (CALR) [23].
A third indication for ruxolitinib is GVHD. Allogeneic hematopoietic stem-cell transplantation (HSCT) is a treatment used for patients with hematological malignancies, inborn errors, or bone marrow failure syndromes. GVHD is a complication of allogeneic HSCT which presents as a systemic inflammatory condition primarily mediated by the immune system of the donor. GVHD with an onset in the first 3 months is acute GVHD (aGVHD) and GVHD with a later onset is chronic GVHD (cGVHD), although onset time is not the only difference between aGVHD and cGVHD [24, 25]. Corticosteroids are the first-line treatment for aGVHD and cGVHD. Patients with steroid-refractory GVHD (srGVHD) can be treated with cyclosporine, tacrolimus or sirolimus as second-line immunosuppressive treatment [26]. Ruxolitinib can be a successful treatment for srGVHD with response rates of 55% [27, 28]. Based on the pivotal REACH1, REACH2 and REACH3 trials [28–30], the FDA and EMA approved ruxolitinib to treat srGVHD.
Methods
Literature Search
For this review, conducted according to the PRISMA guidelines, four databases were assessed on March 15, 2021: PubMed, EMBASE, Cochrane Library and Web of Science. The search terms used were combinations of ‘INCB018424’, ‘ruxolitinib’, ‘INC424’, ‘pharmacology’, pharmacological and toxicological phenomena’, ‘Drug administration schedule’, ‘pharmacodynamics’, ‘pharmacological parameters’, ‘pharmacokinetics’, ‘adverse events’, ‘toxicology’, ‘dose’, ‘dosing’, ‘dosage’, and ‘interaction’. See supplement I in the electronic supplementary material for the exact search. The following publications were excluded: not written in English, animal or in vitro studies, letters to the editor, case reports, where ruxolitinib was not used for hematological diseases, or not available as full text. On November 16, 2021 the search was repeated to search for publications between March 15, 2021 and November 16, 2021.
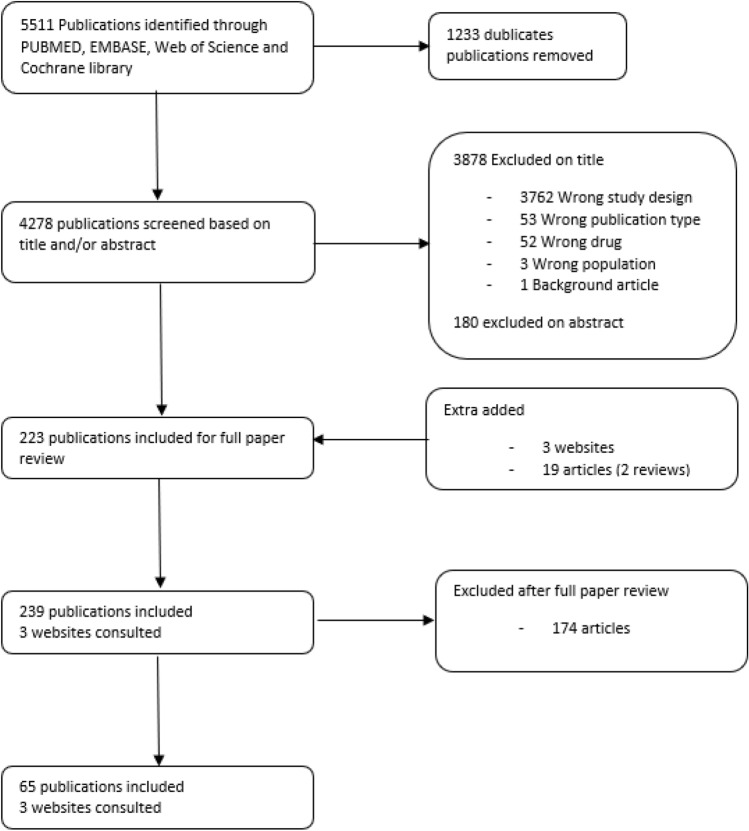
From the combined literature search, 5511 articles were identified, 1249 from PubMed, 2833 from EMBASE, 570 from Cochrane Library and 859 from Web of Science. Of these articles, 1233 duplicates were removed, which led to 4278 articles left for screening. Another 4058 articles were excluded based on their title and/or abstract, leaving 220 articles suitable for this review. Three websites and 19 extra articles were added (Fig. 2).
Fig. 2.
Prisma flow diagram
Results and Discussion
Pharmacokinetics
Absorption and Distribution
Ruxolitinib (base) has an acid dissociation constant (pKa) of 5.9 [31] and is considered a class I compound due to its high solubility (100.8 mg/L) in water of pH 1-8 [32, 33]. Ruxolitinib is formulated in immediate-release (IR) tablets, is almost completely absorbed and in healthy subjects reaches its maximum concentration (Cmax) on average within 1 h, ranging from 0.5 to 6.0 h and an absorption rate constant (Ka) of 3.43 h−1 [34]. Bio-availability of ruxolitinib is over 95%. The protein binding to albumin is approximately 97%. The plasma concentration over time is linear for a dose ranging from 5 mg to 200 mg [32, 35]. Table 1 provides an overview of the pharmacokinetic variables.
Table 1.
Overview of pharmacokinetic variables
| Healthy volunteers [34] | Healthy volunteers [30] | Healthy volunteers (liver) [40] | Healthy volunteers (renal) [40] | Healthy volunteers [36] | Pop-PK model for MF & PV patients [32] | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib dose | 5 mg | 10 mg | 25 mg | 50 mg | 25 mg | 25 mg | 25 mg | 25 mg | 10 mg, 25 mg and 50 mg |
| Single or multiple dose | Single dose | Single dose | Single dose | Single dose | Single dose | Single dose | Single dose | Single dose | Multiple dose |
| No. of patients | 12 | 6 | 6 | 6 | 6 | 32 | 8 | 9 | 414 |
| Cmax (nM) [mg/L] | 195 [0.060] (CV = 35.4%) | 368 [0.11] (CV = 29.9%) | 934 [0.28] (CV = 55.9%) | 1700 [0.52] (CV = 29.2%) | 1093 [0.33] (SD = 651 [0.20]) | 1350 [0.41] (CV = 46.2%) | 1110 [0.34] (CV = 28.9%) | 1100 [0.34] (SD = 332 [0.10]) | |
| Tmax (h) | 2.0 (range 0.50–3.0) | 1.8 (range 0.50–4.0) | 2.0 (range 0.50–6.0) | 1.0 (range 0.50–2.0) | 0.63 (SD = 0.31) | 0.5 (range 0.5–1.5) | 1.0 (range 05–1.5) | 0.9 (SD = 0.5) | |
| T½ (h) | 2.6 (CV = 40%) | 3.4 (CV = 41%) | 3.0 (CV = 22%) | 2.7 (CV = 21%) | 2.32 (SD = 0.37) | 2.7 (CV = 25%) | 3.7 (CV = 23%) | 2.8 (SD = 0.7) | |
| AUCinf (nM*h) [mg/L*h] | 823 [0.25] (CV = 31.7 %) | 1759 [0.54] (CV = 22.2%) | 4110 [1.25] (CV = 34.1%) | 6940 [2.11] (CV = 27.2%) | 3200 [0.97] (SD = 1361 [0.41]) | 3520 [1.07] (CV = 42.4%) | 4670 [1.42] (CV = 21.4%) | 4350 [1.32] (SD = 1990 [0.61]) | |
| Vz/F, (L) | 75.1 (CV = 48.2%) | 90.8 (CV = 36.3%) | 85.7 (CV = 23.7%) | 90.5 (CV = 43.2%) | 91.5 (CV = 29.4%) | 103 (CV = 23.2%) | 58.6 (RSE = 2.8%) (Vc/F) | ||
| CL/F (L/h) | 19.8 (CV = 31.2%) | 18.6 (CV = 20.4%) | 19.9 (CV = 37.7%) | 23.5 (CV = 59.2%) | 23.2 (CV = 49.6%) | 19.3 (CV = 20.5%) | 22.8 (SD = 10.3) |
Male: 22.1 (RSE = 3.4%) Female: 17.7 (RSE = 3.5%) |
|
AUCinf area under the curve to infinity, Cmax maximum drug concentration, CL/F clearance, CV coefficient of variation, MF myelofibrosis, Pop-PK population pharmacokinetics, PV polycythemia vera, RSE relative standard error, SD standard deviation, Tmax time to reach maximum concentration, T½ elimination half-life, Vz/F terminal volume of distribution
The ingestion of food (high fat and high calorie) decreases Cmax by 24.3% compared with ingestion without food. Also, time to reach Cmax (Tmax) is postponed with ingestion of food, while the area under the plasma concentration–time curve from zero to infinity (AUC0-∞) is not affected [36]. Furthermore, ruxolitinib pharmacokinetics can be described with a two-compartment model with a first-order absorption and linear elimination.
The use of ruxolitinib in patients with GVHD involving the gut could result in altered ruxolitinib absorption. Isberner et al. studied ruxolitinib exposure in patients with GVHD, of whom 31% had involvement of the gut, and found that ruxolitinib exposure was actually increased in GVHD patients in comparison with myelofibrosis patients. Since this difference was related to reduced ruxolitinib clearance, it is unlikely that ruxolitinib absorption is hampered in patients with GVHD of the gut [37].
Volume of distribution for the central compartment for a typical/average patient of 72.9 kg was 58.6 L, with a percent relative standard error (%RSE) of 2.80% and a peripheral volume of 11.2 L, %RSE 18.6% [34].
In order to decrease Cmax, and to reduce the risks of adverse events, a study with modified-release preparations was performed [38]. Two different sustained-release (SR) formulations of ruxolitinib, SR-1 and SR-2, were compared with the commercially available IR formulation. Both male and female healthy subjects as well as 38 patients with MF were included in this study and were given SR-1 or SR-2 ruxolitinib 25 mg IR.
The Cmax of IR ruxolitinib in healthy volunteers (6 males and 3 females) was 1100 nM (0.34 mg/L); Tmax was 0.9 h. The Cmax of SR-1 and SR-2 ruxolitinib was 333 nM (0.10 mg/L) and 394 nM (0.12 mg/L). Tmax of SR-1 and SR-2 ruxolitinib was prolonged compared with IR, 2.4 h and 2.9 h, respectively. The relative bioavailability, compared with IR, of SR-1 and SR-2 ruxolitinib was 76% and 87%. The 38 MF patients were treated for 16 weeks with SR (not specified which SR) ruxolitinib 25 mg or 50 mg once daily and thereafter continued treatment with IR ruxolitinib. Tmax was 2.0 h and 3.0 h for 25 mg SR and 50 mg SR ruxolitinib, respectively. Cmax was 464 nM (0.14 mg/L) and 730 nM (0.22 mg/L) for 25 mg SR and 50 mg SR, respectively. An interesting finding was that both clearance for SR (18.3 vs 34.8 L/h) and volume of distribution (Vc/F) during the terminal phase (218 vs 304 L) were lower in women than in men. For IR ruxolitinib, a sex-based difference in clearance was also found [38]. Two other studies found the same results for sex-based difference for volume of distribution [34, 38].
Metabolism
In a study by Shilling et al., six healthy male subjects were given 25 mg [14C]-ruxolitinib and radioactivity was measured in plasma. Ruxolitinib was predominant and nine metabolites of ruxolitinib could be identified. Metabolites were recovered both in urine and in feces [32]. These metabolites of ruxolitinib are pharmacologically active [34, 39]. Ruxolitinib is mainly metabolized by the cytochrome P450 (CYP) enzyme CYP3A4 [36, 39]. It is unknown if CYP3A5 has a role in ruxolitinib metabolism. CYP2C9 has a minor contribution to the metabolism of ruxolitinib [40, 41]. Shi et al. found eight metabolites of ruxolitinib. The metabolites contributed 13% of the pharmacological activity of ruxolitinib, whereas Chen et al. calculated 17% pharmacological activity of ruxolitinib [42]. In combination with the CYP3A4 inducer rifampicin, the metabolites contributed 31% to the pharmacological activity [39].
Excretion
The main route of elimination for ruxolitinib and its metabolites is renal. In the previously mentioned study by Shilling et al., using 25 mg [14C]-ruxolitinib given to healthy individuals, 74% of radioactivity was found in urine and 96% of radioactivity could be detected in urine and feces together. Unchanged [14C]-ruxolitinib was found for < 1% in both the urine and feces, which indicates that the primary elimination route of ruxolitinib is metabolic and the metabolites are predominantly renally eliminated [32]. In a study performed by Shi et al., ruxolitinib was administered in doses of 5–200 mg twice daily. Elimination half-life (T½) was approximately 3 h and clearance (CL/F) was 20 L/h and dose independent. The mean Cmax and AUC0-∞ increased in a linear manner indicating a non-saturable clearance [32, 36]. The previously discussed study with two different SR formulations and an IR formulation found that the T½ of SR-1 and SR-2 formulations was almost two times longer compared with the IR formulation, 5.3 and 6.1 h versus 2.8 h. The CL/F was almost the same for IR, SR-1 and SR-2, respectively 22.8 L/h, 27.2 L/h and 24.6 L/h [38]. These differences can be explained by the different characteristics of IR and SR formulations. Similar results were found in a study with healthy volunteers in Japan [43]. In a population-based pharmacokinetic study where data from three different studies in patients with MF and PV were pooled, T½ was 3.76 h for men and 4.07 h for women. Another population pharmacokinetic analysis found CL/F of 22.1 L/h for males and 17.7 L/h for females [34]. This difference between men and women might be explained by differences in body weight, although in both female and male patients there was no relationship between CL/F and body weight. The underlying disease might have played a role. Isberner et al. studied ruxolitinib in patients with GVHD and found a CL/F of 9.74 L/h, whereas Chen et al. studied healthy volunteers and found a CL/F ranging from 18.6 to 23.5 L/h [34, 37]. The authors did not find an explanation for why their findings differ from other studies [37]. In patients with PV, CL/F was 13.7–20.7 L/h, further indicating that the underlying disease might play a role [35].
Renal Dysfunction
Ruxolitinib is mainly excreted as metabolites via the kidneys [32]. Therefore, renal dysfunction can increase the exposure of active metabolites of ruxolitinib. In a study by Chen et al. [42], patients with various stages of renal impairment (mild, moderate, severe and patients on hemodialysis) were given a single dose of ruxolitinib 25 mg. Their major finding was that the pharmacokinetic variables (Tmax, T½, AUC0-∞, CL/F and Vz/F [terminal volume of distribution]) of ruxolitinib were not affected by renal dysfunction, while T½ and AUC0-∞ of the metabolites increased with severity of renal impairment [42].
Improvement of renal function has been reported in ruxolitinib-treated patients with PMF [44]. The study used estimated glomerular filtration rate (eGFR)-based renal function improvement measured as best percentage change in eGFR during treatment as compared with baseline. By choosing best percentage change in eGFR there could have been a positive bias overestimating the finding that ruxolitinib improves renal function. An alternative explanation is that the disease (MF, PV, GVHD) might be the cause of renal failure, and ruxolitinib thus indirectly improves renal function by reversing the disease effect on the kidney [45].
Liver Dysfunction
Ruxolitinib is metabolized by the liver, mostly by CYP3A4 [36, 39]. In a study by Chen et al., patients with various stages of hepatic impairment (none, mild, moderate and severe hepatic impairment based on the Child-Pugh classification) received a single dose of ruxolitinib 25 mg [42]. Cmax was unchanged in all groups, but AUC0-∞ in the mild, moderate and severe hepatic impairment groups was significantly higher compared with healthy subjects: 6590 nM*h (2.04 mg/L*h), 4510 nM*h (1.39 mg/L*h) and 5830 nM*h (1.80 mg/L*h) versus 3520 nM*h (1.09 mg/L*h), respectively. Furthermore, T½ was increased (from 2.7 to 4.5 h) in the mild hepatic impairment groups and increased to 4.9 h in the severe hepatic impairment group. Tmax also showed a prolongation in hepatic impairment.
Drug–Drug Interactions
GVHD patients that require ruxolitinib are usually treated with multiple other drugs like corticosteroids, anti-mold and anti-fungal drugs, antimicrobial drugs and immune suppressants. Many drugs in these classes are either a substrate, inductor or inhibitor of CYP3A4, the major enzyme involved in ruxolitinib metabolism, and drug–drug interactions (DDIs) can be expected. Table 2 summarizes the effects of CYP3A4 inhibition or induction on the pharmacokinetics of ruxolitinib.
Table 2.
Impact of CYP3A4 inhibition or induction on pharmacokinetic variables and parameters
| AUC (%) | T½ (%) | Cmax (%) | CL/F (%) | |
|---|---|---|---|---|
| CYP3A4 inhibition (erythromycin) [42] | + 27 | + 10 | + 8 | − 20 |
| CYP3A4 inhibition (ketoconazole) [42] | + 90 | + 60 | + 30 | − 50 |
| CYP3A4 inhibition (fluconazole) [43, 44] | + 234 | + 250 | + 50 | − 70 |
| CYP3A4 inhibition (voriconazole) [49] | + 100 | + 80 | + 50 | − 50 |
| CYP3A4 induction (rifampicin) [42] | − 71 | − 50 | − 30 to 50 | + 300 |
AUC area under the curve, CL/F apparent clearance, Cmax maximum drug concentration, CYP3A4 cytochrome P450 3A4, T½ elimination half-life.
Co-administration of erythromycin, a moderate CYP3A4 inhibitor, influences the pharmacokinetics of ruxolitinib. Healthy volunteers were given erythromycin which resulted in a 27% increase (2670 nM*h [0.83 mg/L*h]) of the area under the curve from zero to 24h (AUC0–24h) compared with ruxolitinib alone (2130 nM*h [0.66 mg/L*h]), a T½ increase of 10% (4.5 h vs 4.1 h), a Cmax increase of 8% (609 nM [0.19 mg/L] vs 562 nM [0.17 mg/L]), and a 20% reduction of CL/F (11.8 vs 15.0 L/h) [39].
In a study by Shi et al., the effect of CYP3A4 on the metabolism of ruxolitinib was evaluated. In this study, healthy volunteers were given the CYP3A4 inducer rifampicin, resulting in a 71% decrease (3710 nM*h [1.15 mg/L*h]) of the AUC0–24h compared with ruxolitinib alone (12100 nM*h [3.74 mg/L*h]) [39]. Furthermore, rifampicin decreased the T½ with almost 50% and increased the first-pass effect. For the major five metabolites of ruxolitinib (> 90% combined plasma AUC0–24h), pharmacokinetic analyses have been performed with and without rifampicin. The Cmax of the active metabolites increased 30–50% in combination with rifampicin [39].
Co-administration with fluconazole (CYP3A4 and CYP2C9 inhibitor) resulted in an increased Cmax of approximately 50%, increased AUC0-∞ with 234% and increased the T½ with a factor of 2.5. The CL/F decreased from 29.5 L/h to 8.85 L/h [40, 41]. An interaction study with ruxolitinib and voriconazole, a potent CYP3A4 inhibitor, showed similar pharmacokinetic results [46]. Patients were given ruxolitinib 5 mg followed by voriconazole 200 mg for 5 days. On day 5, the patients were given a single dose of ruxolitinib 5 mg again. Ruxolitinib in combination with voriconazole compared with ruxolitinib alone increased Cmax (0.073 mg/L vs 0.048 mg/L), T½ (5.5 h vs 3.0 h), area under the curve from time zero to last (AUClast) (210.1 ng/mL*h [0.210 mg/L*h] vs 100 ng/mL*h [0.100 mg/L*h]) and decreased CL/F (43.6 L/h vs 100 L/h) [46].
Ketoconazole, a CYP3A4 inhibitor, also influences the pharmacokinetics of ruxolitinib. Compared with ruxolitinib alone, ketoconazole in combination with ruxolitinib increases the Cmax (850 nM [0.26 mg/L] vs 644 nM [0.20 mg/L]), T½ (5.6 h vs 3.5 h), AUC0-∞ (4970 nM*h [1.54 mg/L*h] vs 2600 nM*h [0.80 mg/L*h]) and decreases CL/F (6.57 L/h vs 12.6 L/h), Tmax remains equal to 1.0 h [39].
In a study in human immunodeficiency virus (HIV)-positive patients, the concomitant use of the CYP3A4 inducer efavirenz increased the CL/F of ruxolitinib compared with concomitant use of integrase inhibitors [47]. Patients on efavirenz or integrase inhibitors were administered ruxolitinib 10 mg twice daily for 5 weeks. CL/F at week 1 in the efavirenz group and the integrase inhibitor group was 22.3 L/h and 13.8 L/h, respectively. CL/F at week 4/5 in the efavirenz group and the integrase inhibitor group was 20.8 L/h and 12.7 L/h, respectively [47].
The studies discussed above generally have studied DDIs between two single drugs. In clinical practice however, patients often receive combinations with either additive or opposed effects. Model informed precision dosing (see section 3.10) may then be of help.
Ruxolitinib (base) has an acid dissociation constant (pKa) of 5.9 [31], therefore it is unlikely that ruxolitinib absorption is affected by an increasing gastric/intestinal pH due to the use of proton pump inhibitors (PPI) or other drugs that increase the gastric/intestinal pH, such as magnesium or calcium preparations. As expected, use of a PPI did not affect the exposure of ruxolitinib [37]. It is also suggested that ruxolitinib is not a substrate for the efflux pump P-glycoprotein (P-gp) based on in-vitro data [39], and therefore drugs that inhibit P-gp have limited effects on ruxolitinib pharmacokinetics [32, 48]. This is contradicted by the results of Ebert et al., who found that there is a correlation between P-gp expression and the JAK-inhibiting effect of ruxolitinib [49].
Adverse Events and Toxicity
The most reported non-hematologic adverse events (AEs) of ruxolitinib are peripheral edema and diarrhea. Patients who were longer exposed to ruxolitinib often experience less AEs than patients with a shorter period of exposure. Another common AE in patients treated with ruxolitinib is infection, such as sepsis, viral infections, pneumonia and urinary tract infections [50, 51]. Immune responses and hematopoiesis are inhibited by ruxolitinib through JAK1/2 inhibition and patients’ blood count should be checked regularly [52]. The hematological AEs such as bone marrow depression and low blood cell counts can be explained by the mechanism of action of ruxolitinib as a JAK1/2 inhibitor. In the COMFORT-II trial, 55% of the patients experienced one or more serious adverse events (SAE), such as anemia, thrombocytopenia, abdominal pain and cardiac failure [13, 19]. Twenty-five percent of the patients’ AEs, such as anemia and thrombocytopenia, led to discontinuation of ruxolitinib. Ruxolitinib AEs are mostly treated by dose reductions, supportive care or with concomitant medication such as corticosteroids, antibiotics or blood transfusions [13, 38, 53, 54]. In the EXPAND study, similar results were found [55]. This study was performed with MF patients with low platelet count divided into two strata (75 to < 100 × 109/L and 50 to < 75 × 109/L). All 69 patients experienced at least one AE such as anemia, thrombocytopenia, decreased platelet count and fatigue, where thrombocytopenia was the most common reason to discontinue the treatment with ruxolitinib [55].
Renal failure has been observed during treatment with ruxolitinib [13]. Several other TKIs also cause renal failure, but the mechanism is unknown. A possibility is that the disease (MF, PV, GVHD) can be the cause of the renal failure and not necessarily the use of ruxolitinib [45]. Kidney function should be closely monitored during ruxolitinib treatment, because loss of kidney function decreases the clearance of ruxolitinib active metabolites which can be a reason to reduce the dose.
Ruxolitinib has no effect on the QTc interval. Punwani et al. performed a study where healthy subjects received placebo, a single dose of ruxolitinib 25 mg, a single dose of ruxolitinib 200 mg or a single dose of moxifloxacin 400 mg, a drug known for its QTc prolonging effect, which was used as positive control [56]. The overall change of QTc time ranged between − 3.09 and 3.28 ms for both ruxolitinib doses. The largest change in the single-dose ruxolitinib 25-mg group was 1.69 ms and for the single-dose ruxolitinib 200-mg group, 3.28 ms. Not one of the subjects in the ruxolitinib group had a change of QTc interval larger than 10 ms. The largest change in the moxifloxacin group was 10.65 ms and there was a positive linear relationship between moxifloxacin plasma concentration and prolongation of QTc time. There was no relationship between ruxolitinib plasma concentration and change in the QTc interval [56].
A study performed with tofacitinib, also a JAK1 and JAK2 inhibitor, for the treatment of rheumatoid arthritis found a concerning AE [57]. In a follow-up period of 4 years the incidence of cancer (excluding nonmelanoma skin cancer) was higher with tofacitinib compared with a tumor necrosis factor inhibitor with an incidence of 4.2% and 2.9%, respectively. Although tofacitinib is not used for the treatment of MF, PV or GVHD, it acts as a JAK inhibitor like ruxolitinib. Therefore, ruxolitinib might show the same finding over a longer period. To date, there have been no safety signals that ruxolitinib induces (secondary) cancer development.
Pharmacodynamics
Ruxolitinib has a pyrrolo pyrimidine structure (Fig. 3), and works by competitively inhibiting the ATP-binding catalytic site of JAK1 and JAK2 [2]. JAK1 and JAK2 antagonism is selective and reversible and indirectly inhibits STAT mediating the signaling of cytokines and growth factors, which are important for hematopoiesis and immune function (see Fig. 1). JAK1 and JAK2 can be found in several immune cells such as T-regulatory cells (Treg), natural killer cells (NK cells) and dendritic cells (DC). Half maximal inhibitory concentration (IC50) for ruxolitinib is defined as the ruxolitinib concentration at which 50% of STAT inhibition is achieved. The ex-vivo IC50 is 254 nM (0.079 mg/L) for a single dose and 225 nM (0.070 mg/L) in vivo [36]. Shi et al. found an IC50 of single-dose ruxolitinib 10 mg of 191 nM (0.059 mg/L) [39].
Fig. 3.
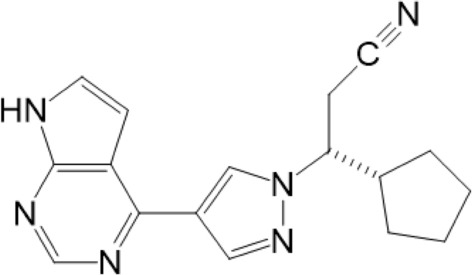
Chemical structure of ruxolitinib. Created with Chemwindow
Ruxolitinib has a chiral carbon atom with an R-enantiomer and an S-enantiomer. The S-enantiomer is approximately ten times less active than the R-enantiomer, with the R-enantiomer having an IC50 of 0.40 nM (0.00012 mg/L) compared with 5.0 nM (0.0015 mg/L) for the S-enantiomer, and only the R-enantiomer binds to the ATP-binding site on JAK [58]. The R-enantiomer is used in the registered ruxolitinib product Jakavi®.
Pharmacokinetic/Pharmacodynamic Relationships
There is a correlation between ruxolitinib dose and inhibition of phosphorylated STAT (pSTAT). The inhibition of pSTAT3 ranged from 40 to > 90%, with a single ruxolitinib dose of 5 mg and 200 mg, respectively. STAT inhibition is measured as a percent decrease of IL-6-stimulated STAT compared with baseline. For multiple dosing of ruxolitinib, the inhibition of pSTAT is also dose dependent and ranges from approximately 65–95% inhibition with 15 mg twice daily and 100 mg once daily, respectively. The time period during which ruxolitinib plasma concentrations are greater than IC50 (209 nM [0.065 mg/L]) with 15 mg and 50 mg twice daily is 8 h and 18 h of a 24-h period, respectively [36]. After a single dose of ruxolitinib 25 mg, pSTAT3 was inhibited to a maximum of 60–70% within 1 h, which declined to 25% inhibition at 12–16 h after administration. STAT inhibition is prolonged in patients with renal or hepatic impairment [42].
In these patients, STAT is also inhibited within 1 h after administration and is inhibited for 24 h. The inhibition of both JAK1/2 and STAT is important, however it remains unclear which of these proteins plays the dominant role. It is unknown for how long and for what percentage STAT should be inhibited to achieve the maximum pharmacodynamic effect.
Due to pharmacokinetic variation, there will be a subpopulation with higher exposure and probably also increased risk of AEs. There is preliminary evidence for a dose–response and exposure–response relationship in decreasing platelet count and spleen size reduction with increasing ruxolitinib exposure. No exposure–response relationship was found for efficacy and safety endpoints in the analysis of the two phase III trials of ruxolitinib in aGVHD and cGVHD [59]. However, this finding might be inconclusive since there was a limited exposure range [39]. The exposure–response relationship of ruxolitinib and platelet count was further characterized in a population pharmacokinetic/pharmacodynamic model. The model described for aGVHD an initial reduction in platelet count after start of ruxolitinib, which is manageable with dose reductions, and platelet recovery towards the end of treatment [39].
Isberner et al. performed a pharmacokinetic study in 29 patients with GVHD to investigate the effects of CYP3A4 and CYP2C9 on ruxolitinib exposure. Median trough serum concentrations were 0.0314 mg/L (range 0.0022–0.229 mg/L) at various dose levels and 0.0339 mg/L (range 0.0056–0.0998 mg/L) for patients at a daily dose of 20 mg. An interesting finding is that GVHD patients had higher ruxolitinib concentrations compared with MF patients. Patients with AEs requiring dose reductions had significantly higher serum trough concentrations (median trough concentration 0.0606 mg/L) at the initial daily dose of 20 mg compared with patients not requiring dose reductions (median trough concentration 0.0398 mg/L). After dose reduction in the patients with AEs, the median serum trough concentration was 0.0284 mg/L, which was no longer different from the median trough concentration in patients without AE-related dose reductions. The odds ratio of having at least three AEs was 8.8 for patients with a serum trough concentration above the threshold of 0.0211 mg/L [37]. This study indicates an exposure–toxicity relationship for ruxolitinib.
Studies usually focus on exposure in plasma. What matters is exposure at the site of action. The drug is distributed to the site of action, so there must be a relation between the concentration in the plasma and the concentration at the site of action, however the precise nature of this relationship is currently unknown.
A new technique that can be used to further investigate exposure at the tumor site (target exposure) is matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI). MALDI MSI can reveal the quantitative distribution of ruxolitinib in tissue with high spatial resolution [60]. With this technique it is possible to investigate the distribution of ruxolitinib on a cellular level in relation to a plasma concentration.
To get better insight into the dynamics of the adverse events (off-target exposure), relationships need to be investigated between plasma exposure (trough concentrations, peak concentrations and area under curve) of the parent drug and/or its metabolites and toxic effects. As the available data on ruxolitinib outcomes matures and the association evaluations continue, it is expected that future pharmacokinetic/pharmacodynamic modeling reports could further assist in understanding the pharmacological effects of ruxolitinib as well as provide guidance to dose optimization.
Recommended Dose
There are different dosing regimens for ruxolitinib depending on the indication (Table 3). The starting dose for PV is 10 mg twice daily, for MF it is 5–20 mg twice daily, for aGVHD 10 mg twice daily (EMA label) or 5 mg twice daily (FDA label) and for cGVHD 10 mg twice daily [13, 28, 61]. During treatment the dose is adjusted to individual tolerance based on safety and toxicity. Patients often use concomitant medications which might requiring dose reductions [35]. Dose reduction to 50% is advised for patients with moderate or severe renal impairment or any hepatic impairment and in case of a platelet count of 100–150 × 109/L [42].
Table 3.
Initial dose recommendations for ruxolitinib in adult patients according to indication
| Disease | Starting dose | Concomitant strong CYP3A4 inhibitor | Severe renal impairment (< 30 mL/min) | Liver impairment |
|---|---|---|---|---|
| MF | 5 mg, 10 mg, 15 mg or 20 mg BID depending on platelet count | Reduce dose by approximately 50% | Reduce dose by approximately 50% | Reduce dose by approximately 50% or reduce to 10 mg BID |
| PV | 10 mg BID | Reduce dose by approximately 50% | Starting dose 5 mg BID or reduce dose by approximately 50% | Reduce dose by approximately 50% |
| aGVHDa |
10 mg BID (EMA label) 5 mg BID (FDA label) |
Reduce dose by approximately 50% | Reduce dose by approximately 50% | Reduce dose by approximately 50% |
| cGVHDa | 10 mg BID | Reduce dose by approximately 50% | Reduce dose by approximately 50% | Reduce dose by approximately 50% |
aGVHD acute graft-versus-host disease, BID twice a day, cGVHD chronic graft-versus-host disease, CYP3A4 cytochrome P450 3A4, MF myelofibrosis, PV polycythemia vera
aApproved for use in patients > 12 years of age
Pediatrics
The indications for ruxolitinib have recently been extended to children aged ≥ 12 years for aGVHD and cGVHD treatment. Ruxolitinib has shown positive results in children compared with best available therapy with 85% overall response rate in grade 2–4 GVHD patients. Ruxolitinib dose in this pediatric population ranged between 2.5–10 mg twice daily and was based on bodyweight and tolerability. Infection incidence in children is lower compared with adults [62, 63]. Also, srGVHD pediatric patients showed a high overall response to ruxolitinib [64]. Furthermore, children treated with ruxolitinib show less liver toxicity than adults. Both might indicate that children can cope with a relative higher dose [65]. Two clinical trials (INC424F12201 and INC424G12201) are ongoing to investigate the pharmacokinetics of ruxolitinib in children.
Model-Informed Precision Dosing to Individualize Therapy
What is Model-Informed Precision Dosing?
Model-informed precision dosing (MIPD) is a science-based method that uses (1) pharmacokinetic and pharmacodynamic principles to identify how patients are different in terms of parameters such as CL, V, Emax and IC50 and give the dose needed to reach the target; and (2) measurement of a parameter—usually the concentration of the drug involved—in combination with a population pharmacokinetic model and appropriate software to calculate the dose to reach that target in case of deviations from the target. In the next sections population modeling and MIPD are discussed.
Population Pharmacokinetics and Physiologically Based Pharmacokinetic Modeling
To predict plasma concentrations for guiding therapy optimization (MIPD), explaining concentration-related adverse events or DDIs, population pharmacokinetic (popPK) modeling or physiologically based pharmacokinetic (PBPK) modeling can be used. Shi et al. constructed a general PBPK model for healthy volunteers using all published ruxolitinib pharmacokinetic, absorption, distribution, metabolism and excretion data. As a model for a typical study subject, a 30-year-old American male with a bodyweight of 78 kg was used. The PBPK model well described the plasma concentration profile of ruxolitinib 10 mg single dose compared with the known data, and the linear pharmacokinetic characteristics of ruxolitinib were reproduced [31]. DDI data obtained from the PBPK model with rifampicin, ketoconazole, erythromycin and fluconazole were comparable with the clinical observations [31, 34]. The PBPK model showed similar results for concomitant use with ketoconazole and erythromycin. Chen et al. conducted a popPK analysis of ruxolitinib in patients with MF and PV. Pharmacokinetic data of three clinical trials, with different dosing strategies, were used to make a popPK model. Data of 272 patients was used for modeling and data of 142 patients was used to validate the model. A two-compartment model with first-order absorption and linear elimination was used as a structural base. The popPK model predicted pharmacokinetic parameters representing those that were found in clinical studies. In the covariate analysis, gender was found to be a statistically significant predictor of CL/F, where males have a higher CL/F compared with women (22.1 L/h vs 17.7 L/h). This could be explained by the differences in bodyweight, however both males and females showed a lack of relationship between clearance and weight. Additional popPK analyses were performed and the addition of bodyweight as a covariate on clearance was not needed to describe the pharmacokinetics of ruxolitinib [34]. For CYP3A4 DDIs, another PBPK model has been developed for ruxolitinib combined with posaconazole in GVHD patients. After composing a posaconazole, ruxolitinib and DDI model, the authors created a posaconazole and ruxolitinib DDI model. The virtual model patient population was given posaconazole 300 mg once daily and ruxolitinib 10 mg twice daily. The model-predicted Cmax was 0.116 mg/L for ruxolitinib alone versus 0.140 mg/L combined with posaconazole, an increase of 21%. The model-predicted AUClast of ruxolitinib alone was 0.240 mg/L*h versus 0.382 mg/L*h combined with posaconazole, an increase of 59% [66]. These studies show that ruxolitinib popPK and PBPK models can help to predict ruxolitinib behavior in various clinical scenarios.
Evidence for Model-Informed Precision Dosing
MIPD might be of value for the treatment of MF, PV and GVHD for several reasons. The first reason is a high inter-patient variability in pharmacokinetics, where MIPD can be used to individualize the dose, such that exposure is comparable between patients for an optimal balance between efficacy and adverse events. A second reason is the occurrence of drug–drug interactions. Most TKIs are substrates for CYP3A4 enzymes and are susceptible to enzyme inhibition and enzyme induction increasing pharmacokinetic variability and exposure. Currently, MIPD is not routinely used for ruxolitinib, because optimal serum or plasma concentrations balanced between efficacy and toxicity are unknown. In clinical practice, ruxolitinib is dosed on effect, adverse events, renal impairment, liver impairment and toxicity.
Some preliminary exposure–effect studies have been performed with ruxolitinib. There is a relationship between plasma concentration and the inhibition of STAT [36]. It has not yet been investigated what the duration of STAT inhibition is in relation to the clinical effect. There are correlations between inhibition of STAT and ruxolitinib blood-serum concentrations [36, 39].
For TKIs in general, there is increasing evidence and rationale to use MIPD in clinical practice [67]. A large study performed by the Dutch Pharmacology Oncology Group (DPOG) showed that over half of patients using oral anticancer drugs were underdosed at some point during their treatment. In 56.6% of these patients, MIPD was used to attain the serum concentration target again without additional toxicities [68]. Taken together, the evidence is currently too limited to support ruxolitinib MIPD in routine clinical practice; however, for complex individual cases, ruxolitinib MIPD might be of value.
Conclusion
The current knowledge on the pharmacology of ruxolitinib is described in this review. Pharmacodynamics and pharmacokinetics, liver and kidney dysfunction, DDIs, adverse events, toxicity and MIPD of ruxolitinib are summarized for MF, PV and GVHD. No great differences in the pharmacokinetics of ruxolitinib were found between studies, except for the volume of distribution. This can be explained by the different methods used to determine the volume of distribution. There are differences in clearance between men and women due to body weight, although this is not conclusive. Liver impairment modulates the pharmacokinetics of ruxolitinib by increasing AUC, T½ and Tmax, whereas kidney impairment modulates the pharmacokinetics of the active metabolites (AUC and T½), requiring dose adjustment. The inhibition of STAT is also prolonged in patients with kidney impairment. Ruxolitinib is metabolized by CYP3A4 and therefore has several clinically relevant DDIs with other drugs that influence CYP3A4. Furthermore, it could be useful to better understand the pharmacokinetic/pharmacodynamic differences between MF, PV and GVHD patients to optimize ruxolitinib treatment and to use MIPD in a beneficial way.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of Interest
TA, TOM, LM, MLH and DT do not have any conflict of interest to disclose.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publication of this article.
Availability of Data and Material
Not applicable because this is a review article.
Code Availability
Not applicable.
Authors Contribution
TA produced an initial draft of the manuscript and conducted a literature search under guidance and supervision from TOM, LM, MLH and DT.
References
- 1.O'Brien Z, Moghaddam MF. Small molecule kinase inhibitors approved by the FDA from 2000 to 2011: a systematic review of preclinical ADME data. Expert Opin Drug Metab Toxicol. 2013;9(12):1597–1612. doi: 10.1517/17425255.2013.834046. [DOI] [PubMed] [Google Scholar]
- 2.Attwood MM, Fabbro D, Sokolov AV, Knapp S, Schiöth HB. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat Rev Drug Discov. 2021;20(11):839–861. doi: 10.1038/s41573-021-00252-y. [DOI] [PubMed] [Google Scholar]
- 3.Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham LC, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25(2):e328–e334. doi: 10.1634/theoncologist.2019-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Y, Alexer M, Gadina M, O'Shea JJ, Meylan F, Schwartz DM. JAK-STAT signaling in human disease: from genetic syndromes to clinical inhibition. J Allergy Clin Immunol. 2021;148(4):911–925. doi: 10.1016/j.jaci.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curto-Garcia N, Harrison CN. An updated review of the JAK1/2 inhibitor (ruxolitinib) in the Philadelphia-negative myeloproliferative neoplasms. Future Oncol. 2017;14(2):137–150. doi: 10.2217/fon-2017-0298. [DOI] [PubMed] [Google Scholar]
- 6.Bryan JC, Verstovsek S. Overcoming treatment challenges in myelofibrosis and polycythemia vera: the role of ruxolitinib. Cancer Chemother Pharmacol. 2016;77(6):1125–1142. doi: 10.1007/s00280-016-3012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014;13(7):967–976. doi: 10.1517/14740338.2014.916273. [DOI] [PubMed] [Google Scholar]
- 8.Plosker GL. Ruxolitinib: a review of its use in patients with myelofibrosis. Drugs. 2015;75(3):297–308. doi: 10.1007/s40265-015-0351-8. [DOI] [PubMed] [Google Scholar]
- 9.Botta S, Indrien A, Garofalo E, Biamonte F, Bruni A, Pasqua P et al. COVID-19: High-JAKing of the inflammatory ''flight'' by ruxolitinib to avoid the cytokine storm. Front Oncol. 2020;10:599502. [DOI] [PMC free article] [PubMed]
- 10.Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):p736–p744. doi: 10.1016/j.jaad.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeage K. Ruxolitinib: a review in polycythaemia vera. Drugs. 2015;75(15):1773–1781. doi: 10.1007/s40265-015-0470-2. [DOI] [PubMed] [Google Scholar]
- 12.Colafigli G, Scalzulli E, Pepe S, Di Prima A, Efficace F, Martelli M. The advantages and risks of ruxolitinib for the treatment of polycythemia vera. Expert Rev Hematol. 2020;13(10):1067–1072. doi: 10.1080/17474086.2020.1816819. [DOI] [PubMed] [Google Scholar]
- 13.Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–1707. doi: 10.1038/leu.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum S, Martins F, Alberio L. Ruxolitinib in the treatment of polycythemia vera: patient selection and special considerations. J Blood Med. 2016;7:205–215. doi: 10.2147/JBM.S102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arana YC, Tam CS, Verstovsek S. Efficacy and safety of ruxolitinib in the treatment of patients with myelofibrosis. Future Oncol. 2015;11(5):719–733. doi: 10.2217/fon.14.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankar A, Gupta V. Investigational non-JAK inhibitors for chronic phase myelofibrosis. Expert Opin Investig Drugs. 2020;29(5):461–474. doi: 10.1080/13543784.2020.1751121. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ali HK, Vannucchi AM. Managing patients with myelofibrosis and low platelet counts. Ann Hematol. 2016;96(4):537–548. doi: 10.1007/s00277-016-2697-8. [DOI] [PubMed] [Google Scholar]
- 18.Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18(12):3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- 19.Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa R, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139–1145. doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CW, Shih LY, Xiao Z, Jie J, Hou HA, Du X, et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk Lymphoma. 2014;56(7):2067–2074. doi: 10.3109/10428194.2014.969260. [DOI] [PubMed] [Google Scholar]
- 21.Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AJ, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31(10):1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantarjian HM, Silver RT, Komrokji RS, Mesa RA, Tacke R, Harrison CN, et al. Ruxolitinib for myelofibrosis-An update of its clinical effects. Clin Lymphoma Myeloma Leukemia. 2013;13(6):638–645. doi: 10.1016/j.clml.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose P, Abou Zahr A, Verstovsek S. Investigational Janus kinase inhibitors in development for myelofibrosis. Expert Opin Investig Drugs. 2017;26(6):723–734. doi: 10.1080/13543784.2017.1323871. [DOI] [PubMed] [Google Scholar]
- 24.de Kort EA, van Dorp S, Blijlevens NMA, van de Velden WJFM. Corticosteroid replacement by ruxolitinib in patients with acute GVHD experiencing severe steroid-induced side effects. Bone Marrow Transplant. 2020;55(1):253–255. doi: 10.1038/s41409-019-0526-0. [DOI] [PubMed] [Google Scholar]
- 25.Ali H, Salhotra A, Modi B, Nakamura R. Ruxolitinib for the treatment of graft-versus-host disease. Expert Rev Clin Immunol. 2020;16(4):347–359. doi: 10.1080/1744666X.2020.1740592. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Li Q, Hu GY, Shen XL, Tan MA. Ruxolitinib for treatment of steroid-refractory graft-versus-host disease in adults: a systematic review and meta-analysis. Expert Rev Hematol. 2020;13(5):565–575. doi: 10.1080/17474086.2020.1738214. [DOI] [PubMed] [Google Scholar]
- 27.Hill LQ, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Therap Adv Hematol. 2018;9(1):21–46. doi: 10.1177/2040620717741860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagasia M, Pareles MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Results from REACH1, a single-arm phase 2 study of ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-vs-host disease. Blood. 2018;132:601. doi: 10.1182/blood-2018-99-116342. [DOI] [Google Scholar]
- 29.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederweiser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 30.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228–238. doi: 10.1056/NEJMoa2033122. [DOI] [PubMed] [Google Scholar]
- 31.Shi JG, Fraczkiewicz G, Williams WV, Yeleswaram S. Predicting drug-drug interactions involving multiple mechanisms using physiologically based pharmacokinetic modeling: a case study with ruxolitinib. Clin Pharmacol Ther. 2014;97(2):177–185. doi: 10.1002/cpt.30. [DOI] [PubMed] [Google Scholar]
- 32.Shilling AD, Nedza FM, Emm T, Diamond S, McKeever E, Punwani N, et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab Dispos. 2010;38(11):2023–2031. doi: 10.1124/dmd.110.033787. [DOI] [PubMed] [Google Scholar]
- 33.National Library of Medicine. Pubchem. Compound summary; Ruxolitinib https://pubchem.ncbi.nlm.nih.gov/compound/Ruxolitinib. Accessed 2 Dec 2021
- 34.Chen X, Williams WV, Or V, Yeleswaram S. Population pharmacokinetic analysis of orally-administered ruxolitinib (INCB018424 Phosphate) in patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF) or post-essential thrombocythemia myelofibrosis (PET MF) J Clin Pharmacol. 2013;53(7):721–730. doi: 10.1002/jcph.102. [DOI] [PubMed] [Google Scholar]
- 35.European Medicines Agency. Committee for Human Medical Products. Assessment report. https://www.ema.europa.eu/en/documents/assessment-report/jakavi-epar-public-assessment-report_en.pdf. Accessed 13 Nov 2021.
- 36.Shi JG, Chen X, McGee RF, Man RR, Emm T, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644–1654. doi: 10.1177/0091270010389469. [DOI] [PubMed] [Google Scholar]
- 37.Isberner N, Kraus S, Grigoleit GU, Aghai F, Kurlbaum M, Zimmermann S, et al. Ruxolitinib exposure in patients with acute and chronic graft versus host disease in routine clinical practice—a prospective single-center trial. Cancer Chemother Pharmacol. 2021;88:973–983. doi: 10.1007/s00280-021-04351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verstovsek S, Yeleswaram S, Hou K, Chen X, Erickson-Vitanen S. Sustained-release ruxolitinib: findings from a phase 1 study in healthy subjects and a phase 2 study in patients with myelofibrosis. Hematol Oncol. 2018;36(4):701–708. doi: 10.1002/hon.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol. 2012;52(6):809–818. doi: 10.1177/0091270011405663. [DOI] [PubMed] [Google Scholar]
- 40.Aslanis V, Umehara K, Huth F, Ouatas T, bharathy S, Butler AA, et al. Multiple administrations of fluconazole increase plasma exposure to ruxolitinib in healthy adult subjects. Cancer Chemother Pharmacol. 2019;84(4):749–757. doi: 10.1007/s00280-019-03907-1. [DOI] [PubMed] [Google Scholar]
- 41.Umehara K, Huth F, Jin Y, Schiller H, Aslanis V, Heimbach T, et al. Drug-drug interaction (DDI) assessments of ruxolitinib, a dual substrate of CYP3A4 and CYP2C9, using a verified physiologically based pharmacokinetic (PBPK) model to support regulatory submissions. Drug Metab Pers Ther. 2019;34(2):20180042. [DOI] [PubMed]
- 42.Chen XJ, Shi JG, Emm T, Scherle PA, McGee RF, Lo Y, et al. Pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 Phosphate) in renal and hepatic impairment patients. Clin Pharmacol Drug Dev. 2014;3(1):34–42. doi: 10.1002/cpdd.77. [DOI] [PubMed] [Google Scholar]
- 43.Ogama Y, Mineyama T, Yamamoto A, Woo M, Shimada N, Amagasaki T, et al. A randomized dose-escalation study to assess the safety, tolerability, and pharmacokinetics of ruxolitinib (INC424) in healthy Japanese volunteers. Int J Hematol. 2013;97(3):351–359. doi: 10.1007/s12185-013-1280-5. [DOI] [PubMed] [Google Scholar]
- 44.Strati P, Abdelrahim M, Selamet U, Page VD, Pierce SA, Verstovsek S, et al. Ruxolitinib therapy is associated with improved renal function in patients with primary myelofibrosis. Ann Hematol. 2019;98(7):1611–1616. doi: 10.1007/s00277-019-03708-9. [DOI] [PubMed] [Google Scholar]
- 45.Babushok DV, Nelson EJ, Morrissette JJD, Joshi S, Palmer MB, Frank D, et al. Myelofibrosis patients can develop extramedullary complications including renal amyloidosis and sclerosing hematopoietic tumor while otherwise meeting traditional measures of ruxolitinib response. Leuk Lymphoma. 2018;60(3):852–855. doi: 10.1080/10428194.2018.1509319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Chen P, Dou L, Li F, Li M, Xu L, et al. Co-administration with voriconazole doubles the exposure of ruxolitinib in patients with hematological malignancies. Drug Des Dev Ther. 2022;16(1177):817–825. doi: 10.2147/DDDT.S354270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurwitz SJ, Tao S, Gavegnanp C, Jiang Y, Tressler RL, Tsibris A, et al. Pharmacokinetics of ruxolitinib in HIV suppressed individuals on antiretroviral agent therapy from the ACTG A5336 study. J Clin Pharmacol. 2021;61(12):1555–1566. doi: 10.1002/jcph.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alim K, Bruyère A, Lescoat A, Jouan E, Lecureur V, Le Vée M, et al. Interactions of janus kinase inhibitors with drug transporters and consequences for pharmacokinetics and toxicity. Expert Opin Drug Metab Toxicol. 2021;17(3):259–271. doi: 10.1080/17425255.2021.1862084. [DOI] [PubMed] [Google Scholar]
- 49.Ebert C, Perner F, Wolleschak D, Schnöder TM, Fischer T, Heidel FH. Expression and function of ABC-transporter protein ABCB1 correlates with inhibitory capacity of Ruxolitinib in vitro and in vivo. Haematologica. 2016;101(3):e81–e85. doi: 10.3324/haematol.2015.136754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2017;93(3):339–347. doi: 10.1002/ajh.24976. [DOI] [PubMed] [Google Scholar]
- 51.Polverelli N, Breccia M, Benevolo G, Martino B, Tieghi A, Latagliata R, et al. Risk factors for infections in myelofibrosis: role of disease status and treatment. A multicenter study of 507 patients. Am J Hematol. 2017;92(1):37–41. doi: 10.1002/ajh.24572. [DOI] [PubMed] [Google Scholar]
- 52.Mesa RA, Komrokji RS, Verstovsek S. Ruxolitinib dose management as a key to long-term treatment success. Int J Hematol. 2016;104(4):420–429. doi: 10.1007/s12185-016-2084-1. [DOI] [PubMed] [Google Scholar]
- 53.Zeiser R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 54.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder S, et al. Ruxolitinib for corticosteroid-refractory graft-versus-host disease: analysis of 95 patients treated at multiple medical centers. Oncol Res Trea. 2015;38:43–44. [Google Scholar]
- 55.Guglielmelli P, Kiladjian JJ, Vannucchi AM, Duan M, Meng H, Pan L, et al. Efficacy and safety of ruxolitinib in patients with myelofibrosis and low platelet count (50 × 109/L to <100 × 109/L) at baseline: the final analysis of EXPAND. Therap Adv Hematol. 2022;13:1–13. doi: 10.1177/20406207221118429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Punwani N, Yeleswaram S, Chen X, Bowman J, Soloviev M, Williams W. Evaluation of the effect of ruxolitinib on cardiac repolarization: a thorough QT study. Clin Pharmacol Drug Dev. 2013;3(3):207–214. doi: 10.1002/cpdd.90. [DOI] [PubMed] [Google Scholar]
- 57.Ytterberg SR, Bhatt DI, Mikuls TR, Koch GG, Fleischmann R, RIvas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 58.Davis RR, Li B, Yun SY, Chan A, Nareddy P, Gunawan S, et al. Structural insights into JAK2 inhibition by ruxolitinib, fedratinib, and derivatives thereof. J Med Chem. 2021;64(4):2228–2241. doi: 10.1021/acs.jmedchem.0c01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Food and Drug Administration. Center for drug evaluation and research. Office of clinical pharmacology genomics group review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202192Orig1s000ClinPharmR.pdf. Accessed 1 Dec 2022.
- 60.Schulz S, Becker M, Groseclose MR, Schadt S, Hopf C. Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Curr Opin Biotechnol. 2019;55:51–59. doi: 10.1016/j.copbio.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Kiladjian JJ, Zachee P, Hino M, Pane F, Masszi T, Harrison CN, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. The lancet. Haematology. 2020;7(3):e226–e237. doi: 10.1016/S2352-3026(19)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang W, Zhu G, Qin M, Li Z, Wang B, Yang J, et al. The effectiveness of ruxolitinib for acute/chronic graft-versus-host disease in children: a retrospective study. Drug Des Devel Ther. 2021;15:743–752. doi: 10.2147/DDDT.S287218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mozo Y, Bueno D, Sisinni L, Fernández-Arroyo A, Rosich B, Martínez AP, et al. Ruxolitinib for steroid-refractory graft versus host disease in pediatric HSCT: high response rate and manageable toxicity. Pediatr Hematol Oncol. 2021;38(4):331–345. doi: 10.1080/08880018.2020.1868637. [DOI] [PubMed] [Google Scholar]
- 64.Uygun V, Karasu G, Daloglu H, Öztürkmen S, Kilic SC, Yalcin K, et al. Ruxolitinib salvage therapy is effective for steroid-refractory graft-versus-host disease in children: A single-center experience. Pediatr Blood Cancer. 2020;67(4):e28190. doi: 10.1002/pbc.28190. [DOI] [PubMed] [Google Scholar]
- 65.Marcuzzi A, Rimondi E, Melloni E, Gonelli A, Grasso AG, Barbi E, et al. New applications of JAK/STAT inhibitors in pediatrics: current use of ruxolitinib. Pharmaceuticals (Basel) 2022;15(3):374. doi: 10.3390/ph15030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerner B, Aghai-Trommeschlaeger F, Kraus S, Grigoleit GU, Zimmermann S, Kurlbaum M, et al. A physiologically-based pharmacokinetic model of ruxolitinib and posaconazole to predict CYP3A4-mediated drug-drug interaction frequently observed in graft versus host disease patients. Pharmaceutics. 2022;14(12):2556. doi: 10.3390/pharmaceutics14122556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verheijen RB, Yu H, SChellens JHM, Beijnen JH, Steeghs N, Huitema ADR, Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765–776. doi: 10.1002/cpt.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groenland SL, van Eerden RAG, Westerdijk K, Meertens M, Koolen SLW, Moes D, et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: a prospective multicenter study. Ann Oncol. 2022;33(10):1071–1082. doi: 10.1016/j.annonc.2022.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.