ABSTRACT
Acute kidney injury (AKI) is a growing epidemic and is independently associated with increased risk of death, chronic kidney disease (CKD) and cardiovascular events. Randomized-controlled trials (RCTs) in this domain are notoriously challenging and many clinical studies in AKI have yielded inconclusive findings. Underlying this conundrum is the inherent heterogeneity of AKI in its etiology, presentation and course. AKI is best understood as a syndrome and identification of AKI subphenotypes is needed to elucidate the disease's myriad etiologies and to tailor effective prevention and treatment strategies. Conventional RCTs are logistically cumbersome and often feature highly selected patient populations that limit external generalizability and thus alternative trial designs should be considered when appropriate. In this narrative review of recent developments in AKI trials based on the Kidney Disease Clinical Trialists (KDCT) 2020 meeting, we discuss barriers to and strategies for improved design and implementation of clinical trials for AKI patients, including predictive and prognostic enrichment techniques, the use of pragmatic trials and adaptive trials.
Keywords: AKI, biomarkers, outcome, machine learning, pragmatic trial
INTRODUCTION
Acute kidney injury (AKI) is a growing epidemic and is independently associated with increased morbidity and mortality, including in critically ill and perioperative populations. Studies have shown that even modest postoperative changes in serum creatinine levels are associated with poor clinical outcomes, including chronic kidney disease (CKD), cardiovascular events and death [1, 2]. As a condition that affects 13.3 million people worldwide, causes 1.7 million deaths globally per year and lacks effective treatments other than supportive care [3, 4], AKI has a widespread impact on the entire medical community [5]. AKI is associated with an estimated $24 billion in hospitalization costs in the USA and accounts for 1% of the overall National Health Service budget in the UK, reflecting an enormous impact on society [6].
While animal studies investigating the prevention and treatment of perioperative AKI have seen varying levels of success, human studies remain largely inconclusive [7, 8]. Underlying this conundrum is the inherent heterogeneity of AKI in its etiology, presentation and course. Understanding the etiology-specific mechanism of renal injury is crucial in developing targeted treatment and prevention strategies. While recent consensus guidelines have standardized criteria defining AKI [Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) guidelines, Acute Kidney Injury Network (AKIN) criteria, Kidney Disease: Improving Global Outcomes (KDIGO)], these rely on imperfect biomarkers like serum creatinine and urine output that merely approximate kidney function, and detectable changes in these biomarker levels frequently lag behind the inciting injury and correlate poorly with the trajectory of disease [9]. In this narrative review of developments in AKI trials based on the Kidney Disease Clinical Trialists (KDCT) 2020 meeting, we explore barriers to and strategies for improved design and implementation of clinical trials for AKI patients.
LEARNING FROM PRIOR TRIALS: LESSONS FROM STOP-AKI AND REVIVAL
Safety, Tolerability, efficacy and quality of life Of huma recombinant alkaline Phosphatase in patients with sepsis-associated Acute Kidney Injury (STOP-AKI) was a randomized, double-blind, placebo-controlled, four-arm, parallel-group and dose-finding adaptive phase 2a/b study investigating the safety, tolerability and efficacy of human recombinant alkaline phosphatase (recAP) in the treatment of patients with sepsis-associated AKI. The trial involved 53 recruiting sites across 11 countries in North America and Europe and ultimately enrolled 301 patients admitted to the ICU with sepsis and AKI [10].
The study's primary objective was to evaluate the effect of recAP on kidney function and the primary endpoint was the mean time-corrected area under the curve (AUC) of endogenous creatinine clearance (ECC) for the first 7 days of illness. RecAP did not significantly improve short-term kidney function at 7 days compared with placebo, however the authors observed a delayed improvement in kidney function, which was statistically significant at 21- and 28-day timepoints. In reviewing exploratory endpoints, the 1.6 mg/kg recAP group exerted a differential treatment effect and a 40% reduction in mortality compared with placebo at Day 28 and this mortality difference persisted through Day 90.
There are several possible explanations for the lack of significance in short-term improvement of kidney function across recAP and placebo groups. Despite randomization, there was a slight imbalance in baseline kidney function between groups, favoring placebo. It has previously been shown that the degree of initial kidney dysfunction can prognosticate the extent of eventual recovery and survival [11] and as such, the more impaired starting kidney function in the recAP group may account for the absence of more pronounced improvement in the first week of treatment. The 1.6 mg/kg recAP group featured 7.7–9.9 mL/min/1.73 m2 lower initial ECC, which accounts for approximately 50% of the assumed treatment effect of 19 mL/min/1.73m2 used to power the study. The results were therefore affected by survivorship selection bias, in that severely ill patients randomized to the recAP treatment group survived in greater numbers than comparably ill patients randomized to the placebo group. This mortality benefit allowed for survival of patients with lower ECC in the treatment arm compared with the placebo arm. In other words, comparable patients may have survived with impaired renal function in the treatment group and died earlier—before assessment of renal function—in the control group. The 7-day timeframe may therefore have been too short since differences in recovery of ECC only became apparent at later timepoints (at Day 21 and 28). In the setting of sepsis, kidney injury is heterogeneous throughout the renal parenchyma and ECC is a delayed and non-specific metric of function [12]. An intervention like recAP that is thought to reduce damage and improve organ function could prevent maladaptive repair mechanisms such as fibrosis, which may only show signs of ECC recovery after several weeks. Finally, creatinine and its clearance are of limited precision in estimating kidney function in critically ill patients [13].
The trialists behind the ongoing REVIVAL Study (REcombinant human alkaline phosphatase in sepsis associated-AKI surVIVAL trial) learned from the pitfalls encountered in the STOP-AKI trial and they adapted the study's protocol accordingly. REVIVAL is a randomized, double-blind, placebo-controlled, two-arm, parallel-group, multi-center, phase 3 trial to investigate the efficacy and safety of recAP for treatment of patients with sepsis-associated AKI (Table 1) [14].
Table 1.
Lessons from the completed STOP-AKI and ongoing REVIVAL trials of recombinant alkaline phosphatase (recAP) in the treatment of patients with sepsis-associated AKI
| Lessons from the STOP-AKI trial | Takeaways |
|---|---|
| The mean time-corrected area under the curve (AUC) of ECC over days 1–7 does not appear to be a suitable endpoint for therapeutics aimed at treating AKI. | ● Use patient-centered outcomes [e.g. all-cause mortality, Major Adverse Kidney Events at 90 days (MAKE90)] ● Allow for longer follow-up time to better assess renal recovery |
| Baseline differences between treatment arms can influence outcome measures even in large, randomized, placebo-controlled studies. | ● Proceed conservatively with power calculations and favor larger sample sizes whenever possible ● Consider stratifying on important prognostic covariates at baseline ● Explore for inter-group imbalance before unblinding and adjust accordingly |
| Survivorship bias should be considered, as mortality benefit can dramatically skew softer endpoints (e.g. end-organ surrogate metrics) when sicker patients survive following treatment and predominate in the treatment arm compared with the placebo arm. | ● Use hard, patient-centered outcomes—including death—as the primary endpoint in high-risk populations (e.g. in critically ill patients) |
| Strive for homogenous patient populations. | ● Use enrichment methods to detect patients most likely to respond to treatment in early phase trials ● Ensure large sample size to detect potential heterogeneity of treatment effects |
| Optimize site selection and recruitment. Simplifying the patient screening process and study design can improve recruitment metrics. | ● Consider pragmatic trial design ● Limit burdensome data collection and monitoring whenever feasible ● Implement consecutive screening methods |
Compared with its STOP-AKI predecessor, REVIVAL focuses on a more homogenous patient population, using refined criteria to exclude patients with only mild AKI and to instead include patients requiring vasopressor support who are at higher risk of death (see ‘Prognostic enrichment’ section below). An optimized protocol allows for earlier diagnosis of AKI and more expedient progression to treatment with recAP. Systematic consecutive screening is performed for all patients with sepsis receiving vasopressor infusions who exhibit AKI and do not meet exclusion criteria. Instead of four arms (0.4, 0.8 and 1.6 mg/kg dosing groups versus placebo), REVIVAL features only two arms: 1.6 mg/kg recAP dose versus placebo control.
In another important contrast to STOP-AKI, REVIVAL's primary endpoint is all-cause mortality at 28 days. This raises important questions regarding the selection of clinical endpoints (see ‘Optimizing outcome selection’ section below) [15]. While AKI may be a suitable endpoint for safety analysis or preventive studies, it may not be associated with important patient-centered outcomes like survival in patients with established AKI. For this reason, endpoints such as the sustained loss of kidney function or the use of Major Adverse Kidney Events (MAKE) are increasingly used, which combine survival and renal recovery endpoints (Table 2) [16, 17]. The authors of REVIVAL anticipate enrollment of 1400 patients in the main study population from 120 sites across 13 countries in North America, Europe and Japan, lasting 2–3 years, with preliminary results available in 2023.
Table 2.
Proposed endpoints for AKI trials
| Endpoint | Advantages | Limitations | Suggested applications |
|---|---|---|---|
| AKI stage 1 | ● Sensitive (frequent) endpoint, accommodating smaller sample size ● Associated with mortality in certain settings |
● Not a patient-centered outcome ● May lack specificity ● Has been associated with better outcomes (mortality) in certain settings (e.g. in heart failure) ● Not conventionally used as an outcome for Phase 3 licensing studies |
● Primary endpoint for preventive studies ● Secondary endpoint for safety studies |
| Severe AKI | ● Specific to kidney damage ● Associated with mortality |
● Occurs less frequently, requiring larger sample size ● Not a patient-centered outcome ● Not conventionally used as an outcome for Phase 3 licensing studies |
● Primary endpoint for preventive strategies ● Secondary endpoint for safety studies |
| Change in glomerular filtration rate | ● Sensitive (frequent) endpoint, accommodating smaller sample size | ● Not a patient-centered outcome ● Requires accurate measurement |
● Primary or secondary endpoint for early phase trials |
| Renal recovery | ● Sensitive (frequent) endpoint, accommodating smaller sample size ● Associated with mortality |
● Definition of this outcome may vary, allowing for potential misclassification |
● Primary or secondary endpoint for therapeutic studies in patients with recognized AKI |
| Sustained loss of renal function | ● Sensitive (frequent) endpoint, accommodating smaller sample size | ● Requires accurate measurement | ● Primary or secondary endpoint in patients with recognized AKI |
| Renal replacement therapy (RRT) | ● Associated with mortality ● Associated with resource utilization ● Patient-centered outcome |
● Indications vary between institutions and providers; use of RRT is a clinical decision and not a pathophysiologic entity per se ● Susceptible to survivorship bias |
● Secondary endpoint for therapeutic studies in patients with recognized AKI ● Recommend defining strict criteria for initiation ● Consider combining with ‘days alive’ |
| Death (28-day all-cause mortality) | ● Hard, patient-centered outcome | ● Cause of death may not directly result from AKI ● Rare event requiring large sample size |
● Primary or secondary endpoint for therapeutic studies in patients with recognized AKI |
| Major Adverse Kidney Events at 90 days (MAKE-90) | ● Patient-centered outcome ● Occurs more frequently than individual component events |
● May be driven by death and not directly related to AKI ● Low incidence in certain populations |
● Primary or secondary endpoint for therapeutic studies in patients with recognized AKI |
| Chronic kidney disease | ● Patient-centered outcome | ● Low incidence can require large sample size ● Requires longer follow-up time ● Costly |
● Primary or secondary endpoint for therapeutic studies in patients with recognized AKI |
| Cardiovascular events | ● Patient-centered outcome | ● May not directly result from AKI ● Susceptible to survivorship bias, especially short-term cardiovascular events |
● Primary or secondary endpoint for therapeutic studies in patients with recognized AKI ● Consider combining with death or ‘days alive’ |
UNDERSTANDING AKI: NEW PERSPECTIVES
AKI is a common phenomenon affecting critically ill patients, however current diagnostic criteria suffer from limited early detection. Among patients presenting to the emergency department with sepsis, for example, 50% were found to have already developed AKI and an additional 19% developed AKI within 7 days of admission [18]. Even hemodynamically stable patients presenting with acute illness appear to be exceedingly susceptible, as 34% of patients presenting with community-acquired pneumonia without evidence of shock were found to develop AKI. By the time AKI is detected, renal insults have already occurred, thwarting attempts at meaningful prevention [9, 19].
Underlying the conundrum of AKI are the myriad pathophysiological mechanisms leading to a similar clinical phenotype, many of which may fester subclinically despite the resolution of grossly evident clinical findings. The Kidney Precision Medicine Project (KPMP) employs strict inclusion and exclusion criteria to collect and analyze renal biopsies, but even with these stringent criteria in place, significant heterogeneity exists among AKI cases [20]. Interestingly, despite clinical recovery at the time of discharge, near-contemporaneous biopsies reveal subclinical cellular processes suggestive of ongoing insults to and remodeling of the renal parenchyma that could ostensibly predispose to CKD. Identification of crosstalk patterns between immune cells and epithelial and stromal cells may reveal specific targets for precision therapies.
Evidence is emerging to support an additional common subtype of acute renal injury: perioperative AKI. This variant involves etiologically unique insults to the renal parenchyma, many of which are iatrogenic sequelae of surgery and anesthesia. Several perioperative risks have been well described, including basic demographic predictors, type and urgency of surgery, and cardiovascular comorbidities. In the setting of anticipated perioperative insult, prevention of AKI is paramount, yet randomized clinical trials to date have failed to identify effective prophylaxis. A Perioperative Ischemic Evaluation Study-2 sub-study analysis demonstrated no significant difference in perioperative AKI treated with either aspirin or clonidine versus placebo [21]. The RELIEF trial failed to demonstrate improvement in surgical outcomes with a restrictive fluid balance compared with less restrictive fluid resuscitation, however the former treatment group was found to be associated with double the rate of perioperative AKI [22]. Hypotension and subsequent hypoperfusion of the renal parenchyma are thought to predispose to kidney injury, yet the threshold effect remains unclear, with some studies suggesting mere minutes of mean arterial pressure less than 55 mmHg to be associated with AKI while others posit that longer periods of hypotension are needed to induce injury [23–25]. Similarly, a debate regarding the renal impact of relative versus absolute hypotension persists in the literature. Studies have demonstrated that patients treated with individualized blood pressure management (i.e. targeting within 10% of the patient's baseline pressure) compared with treatment of absolute hypotension reduced the risk of post-operative organ dysfunction, although reduced rates of AKI in the individualized management group did not achieve statistical significance [26]. Large observational studies from perioperative databases, however, have demonstrated absolute—not relative—hypotension to be associated with postoperative AKI [27].
THE AKI SPECTRUM: IMPLICATIONS FOR PATIENT AND POPULATION SELECTION
There are multiple known factors that influence the development and manifestation of AKI, most notably sepsis, hypotension, hypovolemia, nephrotoxic drugs, heart failure, liver failure and/or surgical insult. The pathophysiology and clinical presentation can vary dramatically, thus contributing to the syndrome's notorious heterogeneity. Indeed, AKI is more aptly described as a syndrome rather than an individual disease, as it is associated with multiple risk factors, disease modifiers and outcomes and its trajectory varies considerably based on the severity and etiology of insult. For instance, hemodynamic factors likely play a greater role in perioperative AKI than they do in septic AKI. Fluid restriction and hypotension (both absolute and relative) have been shown to increase the risk of perioperative AKI, while hemodynamic optimization appears to reduce this risk. By contrast, the impact of hemodynamic optimization remains unclear in septic AKI, in which renal insults may be primarily caused by circulating mediators [damage- and pathogen-associated molecular patterns (DAMPs, PAMPs)], rather than from hypoperfusion [28]. Interestingly, all stages of hospital-acquired AKI appear to be associated with increased mortality compared with community-acquired cases, which reinforces the importance of etiology and underlying conditions in delineating clinical outcomes [29].
A direct effects-based approach is perhaps the most common design for clinical trials investigating AKI, wherein a homogenous population undergoing a procedure is randomized to different interventions and subsequent rates of AKI are then evaluated and compared between the groups. The PRESERVE trial, for example, was a randomized trial with a two-by-two factorial design comparing sodium bicarbonate versus normal saline and N-acetylcysteine versus placebo in patients at high risk for AKI following contrast angiography [30]. The results were ultimately neutral, finding no significant differences between the groups, and although 10% of all enrolled patients experienced AKI, only a fraction of these ultimately suffered the persistent loss of renal function.
The heterogeneity of AKI underlies clinical and biological differences observed in patient characteristics and these differences are associated with inter-individual variation in the incidence of illness, risk of outcomes and response to treatments.
Prognostic enrichment
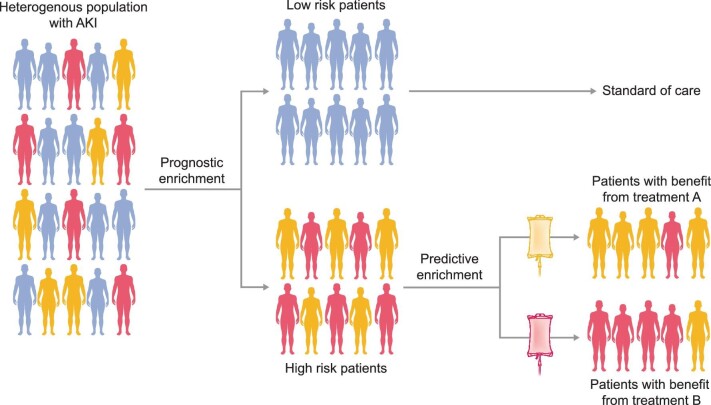
A simplified approach to prognostic and predictive enrichment is often feasible and allows trialists to better control population heterogeneity, such as in septic patients (Figure 1) [31]. A heterogeneous cohort of patients presenting with a disease of interest (e.g. AKI) are separated into low- and high-risk mortality subphenotypes based on prognostic enrichment [12, 32]. While the low-risk subphenotype proceeds with standard care, the high-risk group can be further stratified by predictive enrichment into different endotypes—subtypes of AKI defined by distinct functional or pathophysiologic mechanisms—to tailor mechanism-specific treatments. Factors influencing subphenotypes of AKI could be classified into three major sets of variables: clinical, physiologic and biochemical. For most clinical trials investigating AKI, the disease stage often represents the key variable of prognostic enrichment [33]. This measure of illness severity is known to be associated with worse clinical outcomes, as evidenced by the Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (ELAIN) and Initiation in Kidney Injury (AKIKI) timing-of-dialysis trials [34, 35]. An example of a prognostic enrichment strategy is in the ongoing REVIVAL study [14]. Based on results from the preceding STOP-AKI trial, a lower baseline ECC correlated with a log-linear higher relative hazard ratio for mortality in Cox regression [36]. Consequently, in the subsequent REVIVAL trial, only patients with sepsis-associated-AKI and an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73m2 at baseline will be enrolled in the study, as these patients are expected to benefit most from treatment with recAP.
FIGURE 1:
Schematic approach to prognostic and predictive enrichment, in this example, of patients with sepsis-associated AKI. A heterogeneous cohort of patients presenting with sepsis are separated into low- and high-risk mortality subphenotypes based on prognostic enrichment. While the low-risk subphenotype proceeds with standard care, the high-risk group can be further stratified by predictive enrichment into different endotypes—subtypes of AKI defined by a distinct functional or pathobiological mechanism—to tailor mechanism-specific treatments.
Another emerging criterion for prognostic enrichment in the perioperative setting is the physiologic premise of the renal reserve, as determined by delivering a protein load and measuring both resting and ‘stressed’ glomerular filtration rates and then calculating the difference to find the reserve function [37]. Patients with reduced reserve function at baseline are more likely to experience AKI and other adverse clinical outcomes. Biomarker-based prognostic enrichment is emerging as an increasingly popular approach to clinical trials. The PrevAKI Trial stratified patients undergoing cardiac surgery who were at high risk for AKI based on the urinary ratio of tissue inhibitor metalloproteinase-2 (TIMP-2) to insulin-like growth factor binding protein-7 (IGFBP-7) and these groups demonstrated significantly different rates of postoperative AKI [38].
Predictive enrichment
Molecular subphenotyping is another promising approach to prognostic and predictive enrichment in clinical trials. AKI subphenotypes identified by latent class analysis and defined by the ratio of angioprotein-2/-1 and soluble Tumor Necrosis Factor Receptor-1 (sTNFR1) levels were found to better stratify the risk of poor clinical outcome compared with KDIGO criteria [39]. Interestingly, the Vasopressin and Septic Shock Trial (VASST) of 2008 showed no difference in 28-day mortality among patients in septic shock when comparing the use of vasopressin versus norepinephrine vasopressor infusions. However, when VASST patients were later stratified according to these molecular subphenotypes, the high-risk subphenotype suggested a significant improvement in 90-day mortality with the use of vasopressin compared with norepinephrine [39].
An even more nuanced approach to predictive enrichment involves genomic determinants. A study of Caucasian patients undergoing cardiac surgery with cardiopulmonary bypass analyzed the association between allelic frequencies of guanine-thymine (GT) repeats in the heme oxygenase-1 gene promoter (HMOX1) and post-operative rates of AKI, finding that patients with the longer polymorphism were associated with increased risk compared with patients with shorter repeats [40].
Approaches to predictive enrichment based on biologic determinants have explored mitochondrial dysfunction in AKI. A placebo-controlled phase 1 study of oral nicotinamide, a precursor of nicotinamide adenine dinucleotide (NAD+) biosynthesis, demonstrated dose-related increases in NAD+ metabolites, whose reductive properties are thought to be protective and were subsequently found to be associated with reduced incidence of AKI [41].
By contrast, the Euphrates Randomized Clinical Trial serves as an example of timing determinant-based predictive enrichment in evaluating AKI as a metric of organ dysfunction in sepsis. In this study, septic patients were deemed eligible for enrollment based on the timing of continuous vasopressor support (≥2 h, <30 h) and were then randomized to either real or sham polymyxin B hemoperfusion to selectively remove endotoxin from circulation [42].
Subphenotyping in AKI trials: next steps
Use of machine learning models
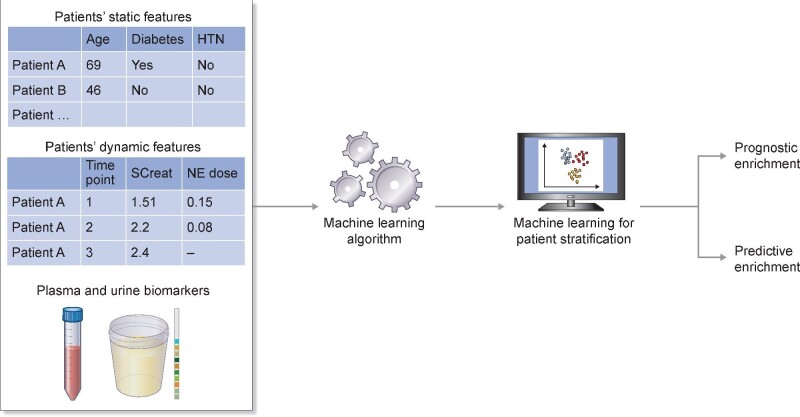
Moving forward, the field requires a more streamlined clinical stratification of high-risk patients. This may require stricter and more explicitly standardized definitions for AKI and its recovery patterns (e.g. including the acute kidney disease stage in patients not recovering within 7 days) in order to better delineate risk profiles predictive of outcome [33]. Ultimately, this push to better identify high-risk patients may involve machine learning (ML) algorithms and real-time integration with electronic medical records. The advantage of ML algorithms is that they flexibly integrate many predictors as well as complex interactions into a composite signature, which can be higher powered than that of individual biomarkers. Recent works in ML for AKI demonstrate promising results [43–46]. In retrospective analyses, the ML models were able to predict AKI with lead times of 2–7 days with areas under the curve (AUCs) exceeding 0.8. Indeed, ML models appear to outperform simple models that depend on a single biomarker (e.g. serum creatinine) by accounting for and analyzing detailed patient histories, such as trends in vital signs and lab values. Nevertheless, these models have only been evaluated on observational datasets. To truly evaluate their clinical utility, we must conduct randomized clinical trials with ML-based risk stratification of patients and demonstrate clinically significant improvement in patient outcomes (Figure 2). Moreover, many of these ML models can only formulate predictions given detailed measurements for an immense number of variables. Unfortunately, only a subset of these measurements is typically available due to differences in clinical practice patterns and hospital resources. Additionally, several ML models are described as ‘black box’ algorithms, meaning that the contribution of each predictor is not directly available to the user. Given that high-dimensional ML models are difficult to transport across medical sites and deploy for clinical trials, more work is needed to simplify these models in order to improve their usability and generalizability.
FIGURE 2:
Illustration of the use of machine learning for prognostic and predictive enrichment in clinical trials applied to acute kidney injury. Machine learning can be used to determine enrollment criteria for a clinical trial involving prognostic/predictive enrichment (table abbreviations: HTN, hypertension; SCreat, serum creatinine; NE, norepinephrine).
Use of adaptive enrichment trial design
The field may also benefit from ‘adaptive enrichment designs’ that learn and incorporate a signature for patients who are most likely to benefit from treatment and subsequently update entry criteria to enrich this patient subgroup within the context of a single trial [47]. When there is a subgroup that truly benefits from treatment, these designs can improve power compared with a standard, non-enriched design. The adaptive approach is advantageous when the development of reliable biomarkers is difficult using available data (due to problems of confounding, small sizes in early-phase randomized trials, the high-dimensionality of patient covariates, etc.), in which case a non-adaptive enrichment design may be less appropriate. Using statistical methods that appropriately account for mid-trial adaptations [48], adaptive enrichment trials efficiently combine the tasks of development and validation of targeted agents, and can considerably reduce the total time spent on both tasks. Finally, clinicians and researchers alike need to more closely monitor patients’ clinical courses using both kidney-specific and systemic biomarkers. This will ultimately require universal accessibility of said biomarkers by means of point-of-care testing with rapid, actionable results, allowing for dynamic profiling of disease progression. The NIH's Kidney Precision Medicine Project (KPMP) will shed new light on the intricacies of AKI and its disease mechanisms, but the KPMP must be paired with process-of-care studies as well as with pooled data from existing clinical trials.
Exploration of novel biomarkers
Identification of AKI is based primarily on increased serum creatinine (sCr) and decreased urine output. Decreased urine output lacks specificity, and increased sCr lacks sensitivity and can be significantly delayed beyond the timing of the actual insult, thus obscuring the disease's true timeline and trajectory [13]. AKI diagnosed solely by reduced urine output is problematic and is rarely used in clinical studies. Furthermore, the kinetics of increased sCr in AKI have numerous confounders including dilution by intravenous fluid resuscitation and acute changes in creatinine generation, rendering sCr-based AKI outcomes imprecise reflections of actual renal injury or of significant changes in glomerular filtration. It is incumbent on the nephrology and critical care communities to work together to develop biomarkers that are more specific, more sensitive and allow for improved subphenotyping of AKI. The Kidney Health Initiative (KHI) is working diligently in this capacity, developing a ‘Roadmap’ to explore unmet needs in the study and treatment of AKI [20].
In an effort to accelerate biomarker development, the KHI Roadmap has identified key use cases for biomarkers and how they could advance patient care, milestones for success in biomarker development, major challenges slowing biomarker development and strategies to overcome these challenges and build momentum for biomarker development and adoption [49]. Stakeholders in the success of biomarker development and adoption are myriad and include clinicians, government agencies, industry professionals, diagnostic companies and patients. A helpful biomarker should (i) predict which patients are more likely to develop AKI in response to a drug or procedure, predict which patients may respond to a drug or intervention to treat AKI, or predict progression of AKI to CKD and end-stage renal disease (ESRD) or other important clinical outcomes (e.g. death, cardiovascular events), (ii) assess the presence of kidney injury early in the disease course (i.e. diagnostic biomarker) and/or (iii) measure AKI recovery in response to a drug or intervention (i.e. prognostic biomarker).
To meet these ambitious goals of biomarker development, we must overcome important hurdles to technical implementation. To our knowledge, only two trials to date have used AKI biomarkers for predictive enrichment, with very low discriminative value [38, 50]. Community efforts must be focused, selecting one or two specific use cases and choosing to pursue a limited set of biomarkers within these cases. Biomarkers should be optimized to address community needs, which requires improved precision in defining AKI subphenotypes by using panels of complementary biomarkers [39, 29, 51]. Focused workshops and targeted funding should be employed to incentivize biomarker development and a consortium of research groups should be established to pool biomarker data and collaborate on validation efforts in AKI trials. Finally, clinicians and researchers alike must educate patients and the non-medical community about the potential benefits of biomarker use in AKI and the profound ways in which effective biomarkers could advance patient care by improving our understanding of this disease.
Optimizing outcome selection
While AKI and its prevention remain valuable endpoints, many studies have moved to harder, patient-centered outcomes (Table 2). An important limitation in choosing AKI defined by serum creatinine as a primary endpoint for renal function is the inconsistent association observed between this event and mortality. Following initiation of angiotensin-converting enzyme inhibitors or in the setting of heart failure, for example, slight increases in serum creatinine are frequently associated with survival [52–55]. Prevention of perioperative AKI in the setting of cardiac surgery, however, does not appear to be associated with improved survival [56]. Hemodilution as well as muscle atrophy and decreased creatinine production can distort the accuracy of serum creatinine in estimating renal function and given these inherent limitations, the use of cystatin C may be a more accurate assay in evaluating AKI recovery [57]. AKI recovery, or alternatively progression to CKD (defined by non-recovery 3 months after AKI diagnosis) including ESRD, are important outcomes given their impacts on survival, quality of life and cost of subsequent care. Given the modest incidence of renal non-recovery [58], choosing this endpoint may require substantially larger sample sizes to ensure adequate statistical power compared with studies involving more common outcomes. Patient-centered outcomes also include Major Adverse Kidney Events (MAKE), a combined endpoint of death, indication for renal replacement therapy and/or non-recovery of renal function. This combined endpoint encompasses multiple events, thereby increasing the overall event rate and thus the power to detect differences between groups. However, while death and non-recovery are obvious patient-centered outcomes, indication for renal replacement therapy (RRT) as an outcome per se is encumbered by enormous heterogeneity in practice [59]. The criteria prompting a valid indication for RRT as well as the decision to initiate this therapy can vary dramatically across providers and institutions and therefore this endpoint, although clinically important, is often challenging to interpret. An alternative endpoint may be the presence of severe AKI characterized by Stage 2 or 3 disease as defined by objective laboratory measures, thereby circumventing the heterogeneity in defining valid RRT indications and the misclassification that could potentially ensue. Endpoints in AKI studies are most often evaluated 28–90 days after trial enrollment. Notably, the 90-day timepoint mirrors the timeline for diagnosis of CKD following AKI (Table 2). Finally, all-cause mortality remains an important patient-centered outcome. As this endpoint accounts for the many contributors beyond AKI directly affecting the risk of death, a large sample size is required to detect signals.
A PRAGMATIC APPROACH TO CLINICAL TRIALS IN AKI
Clinical trials historically enroll highly selected patients, have complex protocols with multiple study procedures and include many outcomes that often require external adjudication. Trials with these characteristics can be slow and expensive, and offer limited generalizability. This scenario describes an explanatory trial, which uses ‘ideal experimental conditions’ to test a causal hypothesis and assess efficacy. Pragmatic trials, by contrast, rely on real-world conditions to inform choices between treatment or strategy approaches, thereby assessing effectiveness. Ultimately there is a tradeoff between achieving high internal validity in explanatory trials and high generalizability in pragmatic trials [60]. One of the potential pitfalls of clinical trials in AKI is the unrealistic effect size estimation of a given intervention leading to falsely negative results. These trials may therefore miss an opportunity to identify efficacy (or harm) in the studied population. Pragmatic trials involving a larger number of patients may partially overcome this issue. Additionally, moving beyond the frequentist statistical approach by using Bayesian analysis and identification of heterogeneity of treatment effects can refine our ability to identify potential benefit or harm among the studied population and subgroups.
In contrast to conventional explanatory trials, pragmatic trials offer interventions via clinical care delivery by non-trialist providers who are otherwise performing routine care. Pragmatic trials offer the possibility of evaluating treatments or strategies whose impact on outcome is unclear. Outcomes are often evaluated indirectly via data collected during routine clinical care. Given these imperfect experimental conditions, statistical noise is anticipated. Cluster randomization—an approach that is often preferred or required for pragmatic trials—requires the incorporation of the intra-cluster correlation in outcomes into the sample size determination, which typically increases the required sample size compared with trials employing individual-level randomization. These limitations notwithstanding, advantages of a pragmatic approach include highly generalizable findings, sustainable interventions and efficient trial conduct.
Pragmatic trials are therefore ideal for (i) evaluating interventions with established efficacy to assess effectiveness for the population of interest under real-world conditions, (ii) investigating an intervention being used clinically despite the absence of established efficacy (i.e. to assess its effectiveness compared to other alternatives that are also in clinical use), or (iii) evaluation of uptake improvement for interventions with already established efficacy and effectiveness.
The coronavirus disease 2019 (COVID-19) pandemic has demonstrated how pragmatic trials can improve our medical knowledge and lead to the rapid implementation of strategies in clinical practice [61, 62]. The use of shorter consent forms and streamlined enrollment procedures for critically ill patients with COVID-19 has facilitated the rapid creation of large sample sizes. We hope that similar efforts to streamline enrollment for pragmatic trials will be adopted by AKI trialists. Certain cluster randomized trials may even feature an institutional waiver of consent that can facilitate large multicenter studies across participating institutions. However, this approach poses a specific drawback for AKI trials, which require meticulous follow-up intervals involving specimen collection and biomarker evaluation. ‘Usual care’ often does not allow for the detailed level of protocol adherence needed for such studies, so informed consent is still needed in this case.
In practice, some trials combine elements of both pragmatic and explanatory designs [63]. The latter remains the gold standard for newly developed drugs or therapies and should be attempted whenever possible in these scenarios. Pragmatic trials should be ‘fit for purpose’ and should not be pragmatic merely for purposes of convenience.
Thinking differently about pragmatic trials in AKI
Pragmatic trials are particularly relevant in AKI, as there are many existing treatments, strategies and interventions with clinical equipoise ripe for investigation. Among these, there is no clear ‘gold standard’ and all approaches fall within the standard of care, posing minimal or limited risk to the patient.
In addition to the traditional stakeholders for a clinical trial, stakeholders for pragmatic trials often include health system leaders and on-the-ground clinicians. For a pragmatic trial to be successful, it is critical to consider the priorities and concerns of these stakeholders. Health system leaders typically want the trials to generate answers quickly and evaluate interventions that add value to the clinical experience while not distracting from competing initiatives or from the quotidian demands of care delivery. Clinicians appreciate minimal disruption to clinical workflow and seek answers to questions that are important to their practice.
Achieving fidelity to the protocol can pose significant challenges in pragmatic trials. Changing routine behaviors is difficult and engagement with on-the-ground clinicians is crucial in facilitating implementation. Some degree of flexibility is required, but not so much as to compromise the core trial design, thereby engendering a delicate balance that must be achieved. In this regard, there are many lessons to be drawn from quality improvement (QI) programs and implementation science. Further, while electronic health records (EHR) have enormous promise as sources of data, trialists would benefit from common data models, faster data cleaning and more expedient access to claims data [64, 65].
Alternatives to traditional adverse event reporting may be needed for pragmatic design. The trial outcomes themselves may provide sufficient information about safety and clinically acquired data that are not explicit outcomes can also serve as safety data. Institutional Review Boards (IRBs) and Data Safety Monitoring Boards may need to be educated about the goals of the trial, as these alternative means of safety monitoring may be perfectly appropriate in the correct setting.
If the intervention being studied poses no greater than minimal risk to the participant, the trial might meet regulatory criteria for waiving the requirement for informed consent [66]. Many pragmatic trials are successfully conducted in this manner, but to move beyond ‘minimal risk trials’, the research and clinical communities must come together to design an ethical consent process that does not require on-the-ground research teams. For example, the Pragmatic Trial of HIgher vs Lower Serum Phosphate Targets in Patients Undergoing Hemodialysis trial to investigate phosphate targets in hemodialysis patients has made use of electronic and remote consenting processes, which many IRBs have begun accommodating in the COVID-19 era of restricted face-to-face availability [67, 68].
Additional examples of effective pragmatic trials investigating AKI include the Isotonic Solutions and Major Adverse Renal Events Trial [69] and Saline Against Lactated Ringers or Plasmalyte in the Emergency Departmen trials [70], which compare balanced crystalloids to normal saline in critically ill adults. This was an open-label, cluster-randomized controlled trial that compared Major Adverse Kidney Events at 30 days (MAKE-30s) in patients receiving either 0.9% (normal) saline or balanced crystalloid and found the latter to be associated with reduced incidence of MAKE-30.
A group at the University of California, San Francisco (UCSF) and Los Angeles (UCLA) and funded by the International Anesthesia Research Society (IARS) is currently employing a pragmatic design to compare vasopressors in the treatment of hypotension during general anesthesia for major surgery (The Choice of Vasopressor for Treating Hypotension During General Anesthesia (VEGA-1)) [71]. VEGA-1 is a pragmatic cluster cross-over randomized trial that will compare phenylephrine versus norepinephrine in maintaining mean arterial pressure to preserve renal perfusion and prevent perioperative AKI. This trial combines an adaptive design and seeks to enroll 2000 patients and will inform the design of the subsequent VEGA-2 phase, including its sample size calculation, refinement of inclusion criteria and identification of risk-based subgroups for Bayesian and heterogeneous treatment effect analyses.
PATIENT AND FUNDER PERSPECTIVES
Patient and Public Involvement (PPI) is recognized as a key feature of a well-designed trial and itself requires detailed planning and funding [72]. In addition to enhancing recruitment and retention, PPI is a crucial feature of important ongoing studies in nephrology such as the Kidney Precision Medicine Project (KPMP) [20] and is a requirement for certain funding organisms like the Patient-Centered Outcomes Research Institute (PCORI). Funders endeavor to distinguish the actual value of an innovative therapy by differentiating it from the standard of care and assessing the translatable benefit to the patient, and this objective is often encumbered by conventional trial design, namely low statistical power, short study duration and the use of surrogate endpoints. From this perspective, impactful endpoints beyond conventional regulatory metrics are key. The endpoints for AKI trials are changing, moving from surrogate-based to patient-centered outcomes. While prevention of and recovery from AKI remain valuable endpoints, many studies have adopted more patient-centered outcomes, including MAKE (combined endpoint of death, indication for renal replacement therapy and/or non-recovery of renal function), mortality, sustained loss of renal function, indication for renal replacement therapy or dialysis, hospitalization and incidence of renal transplant. These endpoints are most often evaluated 28–90 days after trial enrollment, and the 90-day timepoint corresponds to the diagnostic timeline for CKD following AKI and persistent renal impairment (Table 2).
The rapid appraisal of new drugs and therapies coming to market is frequently associated with immature datasets, questionable surrogates and—ultimately—increased uncertainty, all of which complicate the payer's assessment of pricing for a given therapy's true clinical value. This conundrum has spurred the development of innovative reimbursement contracting involving value-based agreements, namely performance ‘money back’ guarantees. For trials ending too early to evaluate patient-centered endpoints, for example, conditional reimbursement models have evolved in which payments are made for disease-free survival and are reimbursed for disease recurrence. To better describe the clinical ‘value add’ of an innovative therapy, trials should pit the therapy against the available standard of care. Value-based agreements can help bridge value gaps on pricing as manufacturers design trials with values in mind rather than traditional regulatory endpoints.
CONCLUSIONS
Randomized controlled trials (RCTs) investigating AKI pose significant challenges, as AKI is primarily based on increased serum creatinine, a marker of renal function, which can be significantly delayed beyond the timing of actual insult, thus obscuring the disease's true timeline and trajectory. Indeed, AKI is best understood as a disease spectrum and etiology-specific biomarkers of renal injury are needed to better study the different pathophysiologic mechanisms of AKI and tailor more effective treatment and prevention strategies. Similarly, while conventional RCTs remain the ‘gold standard’ means of exploring important questions about AKI, they are not the definitive solution. Holding pre-defined parameters constant throughout the trial's execution increases the risk of negative or failed trials, even when the treatment is inherently effective. Flawed recruitment can also result in highly selective patient populations, which can limit the results’ external validity. Single-center RCTs feature nuanced idiosyncrasies in their enrollment, treatment and human research protocols that further limit generalizability. Furthermore, multiple treatments cannot be compared head-to-head within contemporary observation groups, complicating the use of RCTs in comparative effectiveness research. Many RCTs are underpowered, as the effect size in question is often overestimated to facilitate recruitment. To perform a worthwhile RCT, the effect size needs to be biologically plausible, statistically conservative and clinically meaningful. A priori determination of the main parameters of interest is mandatory in preventing the willful or inadvertent cherry-picking of statistically significant differences from multiple comparisons. Alternative trial designs should be considered when appropriate, including pragmatic trial protocols, Bayesian adaptive design and cluster randomization.
Contributor Information
Daniel Lazzareschi, Department of Anesthesia & Perioperative Care, Division of Critical Care Medicine, University of California, San Francisco (UCSF), San Francisco, CA, USA.
Ravindra L Mehta, Department of Medicine, University of California, San Diego, San Diego, CA, USA.
Laura M Dember, Renal-Electrolyte and Hypertension Division, Department of Medicine, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Pennsylvania, PA, USA.
Juliane Bernholz, AM-Pharma, Utrecht, The Netherlands.
Alparslan Turan, Department of Anesthesiology, Lerner College of Medicine of Case Western University, Cleveland, OH, USA; Department of Outcomes Research, Cleveland Clinic, Cleveland, OH, USA.
Amit Sharma, Bayer Pharmaceuticals, Cambridge, MA, USA.
Sachin Kheterpal, Department of Anesthesiology, University of Michigan, Ann Arbor, MI, USA.
Chirag R Parikh, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Omar Ali, Verpora Ltd, Nottingham, UK; University of Portsmouth, UK.
Ivonne H Schulman, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), Bethesda, MD, USA.
Abigail Ryan, Division of Chronic Care Management, Centers for Medicare & Medicaid Services, Woodlawn, MD, USA.
Jean Feng, Department of Epidemiology and Biostatistics, University of California, San Francisco (UCSF), San Francisco, CA, USA.
Noah Simon, Department of Biostatistics, University of Washington (UW), Seattle, WA, USA.
Romain Pirracchio, Department of Anesthesia & Perioperative Care, Division of Critical Care Medicine, University of California, San Francisco (UCSF), San Francisco, CA, USA.
Patrick Rossignol, INI-CRCT Network, Nancy, France; University of Lorraine, Inserm 1433 CIC-P CHRU de Nancy, Inserm U1116, Nancy, France.
Matthieu Legrand, Department of Anesthesia & Perioperative Care, Division of Critical Care Medicine, University of California, San Francisco (UCSF), San Francisco, CA, USA; INI-CRCT Network, Nancy, France.
CONFLICT OF INTEREST STATEMENT
J.B. is an employee of AM-Pharma. L.M.D. receives compensation for her role as Deputy Editor of the American Journal of Kidney Diseases and reports consulting for Merck, AstraZeneca and Cara Therapeutics. R.L.M. reports consulting for Baxter, AM Pharma, Sanofi, Akebia, Intercept, Mallinckrodt, Biomerieux, Sphingotec, GE Healthcare, CHF solutions, Novartis, Unicycive and investigator-initiated grants from Fresenius and Fresenius-Kabi. C.R.P. is a member of the advisory board of and owns equity in RenalytixAI. He also serves as a consultant for Genfit and Novartis. C.R.P. is supported by NIH grants R01HL085757, UH3DK114866, U01DK106962 and R01DK093770. P.R. reports consulting for Bayer, G3P, Idorsia and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer-Ingelheim, Corvidia, CVRx, Fresenius, Grunenthal, Novartis, Novo Nordisk, Relypsa Inc., a Vifor Pharma Group Company, Sanofi, Sequana Medical, Servier, Stealth Peptides and Vifor Fresenius Medical Care Renal Pharma; Cofounder: CardioRenal. I.H.S. is an employee of the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH). O.A. is Head of Payers at Verpora. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIDDK, NIH, or the Department of Health and Human Services. All other authors deny any conflict of interest.
REFERENCES
- 1. Legrand M, Rossignol P. Cardiovascular consequences of acute kidney injury. N Engl J Med 2020; 382: 2238–2247 [DOI] [PubMed] [Google Scholar]
- 2. Hoste EAJ, Bagshaw SM, Bellomo Ret al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423 [DOI] [PubMed] [Google Scholar]
- 3. Acute Kidney Injury [Internet] . International Society of Nephrology https://www.theisn.org/commitment-to-kidney-health/focus-areas/acute-kidney-injury/ (17 August 2021, date last accessed) [Google Scholar]
- 4. Mehta RL, Burdmann EA, Cerdá Jet al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 2016; 387: 2017–2025 [DOI] [PubMed] [Google Scholar]
- 5. Pickkers P, Darmon M, Hoste Eet al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med 2021; 47: 835–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silver SA, Chertow GM. The economic consequences of acute kidney injury. Nephron 2017; 137: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siew ED, Liu KD. Contrast-induced acute kidney injury in the PRESERVE Trial: lessons learned. Clin J Am Soc Nephrol 2018; 13: 949–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Legrand M, Liu KD. Kidney replacement therapy in the ICU: less is more (death)? Am J Kidney Dis 2021; 78: 614–616 [DOI] [PubMed] [Google Scholar]
- 9. Ostermann M, Zarbock A, Goldstein Set al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Net Open 2020; 3: e2019209. [DOI] [PubMed] [Google Scholar]
- 10. Pickkers P, Mehta RL, Murray PTet al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 2018; 320: 1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kellum JA, Sileanu FE, Bihorac Aet al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195: 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legrand M, Bokoch MP. The yin and yang of the renin-angiotensin-aldosterone system in acute kidney injury. Am J Respir Crit Care Med 2021; 203: 1053–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Legrand M, Kellum JA. Serum creatinine in the critically ill patient with sepsis. JAMA 2018; 320: 2369–2370 [DOI] [PubMed] [Google Scholar]
- 14. (Revival) Study to Investigate the Efficacy and Safety of Alkaline Phosphatase in Patients With Sepsis-Associated AKI - Full Text View - ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT04411472 (16 August 2021, date last accessed) [Google Scholar]
- 15. McCoy IE, Chertow GM. AKI-a relevant safety end point? Am J Kidney Dis 2020; 75: 508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palevsky PM, Molitoris BA, Okusa MDet al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 2012; 7: 844–850 [DOI] [PubMed] [Google Scholar]
- 17. Sd W, Pm P. Design of clinical trials in acute kidney injury: lessons from the past and future directions. Seminars in nephrology [Internet] 2016https://pubmed.ncbi.nlm.nih.gov/27085734/ (16 November 2021, date last accessed) [DOI] [PubMed] [Google Scholar]
- 18. Peerapornratana S, Manrique-Caballero CL, Gómez Het al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019; 96: 1083–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jamme M, Legrand M, Geri G. Outcome of acute kidney injury: how to make a difference? Ann Intensive Care 2021; 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Boer IH, Alpers CE, Azeloglu EUet al. Rationale and design of the Kidney Precision Medicine Project. Kidney Int 2021; 99: 498–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg AX, Kurz A, Sessler DIet al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 2014; 312: 2254–2264 [DOI] [PubMed] [Google Scholar]
- 22. Myles PS, Bellomo R, Corcoran Tet al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 2018; 378: 2263–2274 [DOI] [PubMed] [Google Scholar]
- 23. Khanna AK, Maheshwari K, Mao Get al. Association between mean arterial pressure and acute kidney injury and a composite of myocardial injury and mortality in postoperative critically ill patients: a retrospective cohort analysis. Crit Care Med 2019; 47: 910–917 [DOI] [PubMed] [Google Scholar]
- 24. Walsh M, Devereaux PJ, Garg AXet al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119: 507–515 [DOI] [PubMed] [Google Scholar]
- 25. Sun LY, Wijeysundera DN, Tait GAet al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015; 123: 515–523 [DOI] [PubMed] [Google Scholar]
- 26. Futier E, Lefrant J-Y, Guinot P-Get al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318: 1346–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathis MR, Naik BI, Freundlich REet al. Preoperative risk and the association between hypotension and postoperative acute kidney injury. Anesthesiology 2020; 132: 461–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peerapornratana S, Manrique-Caballero CL, Gómez Het al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019; 96: 1083–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dépret F, Hollinger A, Cariou Aet al. Incidence and outcome of subclinical acute kidney injury using penKid in critically ill patients. Am J Respir Crit Care Med 2020; 202: 822–829 [DOI] [PubMed] [Google Scholar]
- 30. Weisbord SD, Gallagher M, Jneid Het al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 2018; 378: 603–614 [DOI] [PubMed] [Google Scholar]
- 31. Legrand M, Oufella HA, De Backer Det al. The I-MICRO trial, Ilomedin for treatment of septic shock with persistent microperfusion defects: a double-blind, randomized controlled trial-study protocol for a randomized controlled trial. Trials 2020; 21: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 2020; 16: 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chawla LS, Bellomo R, Bihorac Aet al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 34. Zarbock A, Kellum JA, Schmidt Cet al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN Randomized Clinical Trial. JAMA 2016; 315: 2190–2199 [DOI] [PubMed] [Google Scholar]
- 35. Gaudry S, Hajage D, Schortgen Fet al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122–133 [DOI] [PubMed] [Google Scholar]
- 36. Peters E, Mehta RL, Murray PTet al. Study protocol for a multicentre randomised controlled trial: Safety, Tolerability, efficacy and quality of life Of a human recombinant alkaline phosphatase in patients with sepsis-associated Acute Kidney Injury (STOP-AKI). BMJ open 2016; 6: e012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Husain-Syed F, Ferrari F, Sharma Aet al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 2018; 105: 1094–1101 [DOI] [PubMed] [Google Scholar]
- 38. Meersch M, Schmidt C, Hoffmeier Aet al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017; 43: 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhatraju PK, Zelnick LR, Herting Jet al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 2019; 199: 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leaf DE, Body SC, Muehlschlegel JDet al. Length polymorphisms in Heme Oxygenase-1 and AKI after cardiac surgery. J Am Soc Nephrol 2016; 27: 3291–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poyan Mehr A, Tran MT, Ralto KMet al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 2018; 24: 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dellinger RP, Bagshaw SM, Antonelli Met al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA 2018; 320: 1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koyner JL, Adhikari R, Edelson DPet al. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol 2016; 11: 1935–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Churpek MM, Carey KA, Edelson DPet al. Internal and external validation of a machine learning risk score for acute kidney injury. JAMA Net Open 2020; 3: e2012892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomašev N, Glorot X, Rae JWet al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019; 572: 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rank N, Pfahringer B, Kempfert Jet al. Deep-learning-based real-time prediction of acute kidney injury outperforms human predictive performance. NPJ Digit Med 2020; 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thorlund K, Haggstrom J, Park JJet al. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 2018; 360: k698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simon N, Simon R. Adaptive enrichment designs for clinical trials. Biostatistics 2013; 14: 613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu KD, Goldstein SL, Vijayan Aet al. AKI!Now initiative: recommendations for awareness, recognition, and management of AKI. Clin J Am Soc Nephrol 2020; 15: 1838–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zarbock A, Küllmar M, Ostermann Met al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg 2021; 133: 292–302 [DOI] [PubMed] [Google Scholar]
- 51. Legrand M, Hollinger A, Vieillard-Baron Aet al. One-year prognosis of kidney injury at discharge from the ICU: a multicenter observational study. Crit Care Med 2019; 47: e953–e961 [DOI] [PubMed] [Google Scholar]
- 52. Legrand M, Mebazaa A, Ronco Cet al. When cardiac failure, kidney dysfunction, and kidney injury intersect in acute conditions: the case of cardiorenal syndrome. Crit Care Med 2014; 42: 2109–2117 [DOI] [PubMed] [Google Scholar]
- 53. Legrand M, De Berardinis B, Gaggin Het al. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One 2014; 9: e112313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Testani JM, Kimmel SE, Dries DLet al. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4: 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Testani JM, Coca SG, Shannon RPet al. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. Eur J Heart Fail 2011; 13: 1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lamy A, Devereaux PJ, Prabhakaran Det al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366: 1489–1497 [DOI] [PubMed] [Google Scholar]
- 57. Delgado C, Baweja M, Crews DCet al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 2021; Sep 23;32(12): 2994–3015. doi: 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chawla LS, Bellomo R, Bihorac Aet al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 59. Legrand M, Darmon M, Joannidis Met al. Management of renal replacement therapy in ICU patients: an international survey. Intensive Care Med 2013; 39: 101–108 [DOI] [PubMed] [Google Scholar]
- 60. Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375: 454–463 [DOI] [PubMed] [Google Scholar]
- 61. RECOVERY Collaborative Group , Horby P, Mafham Met al.Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; 383: 2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. RECOVERY Collaborative Group , Horby P, Lim WSet al.Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Legrand M, Futier E, Leone Met al. Impact of renin-angiotensin system inhibitors continuation versus discontinuation on outcome after major surgery: protocol of a multicenter randomized, controlled trial (STOP-or-NOT trial). Trials 2019; 20: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson FP, Shashaty M, Testani Jet al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015; 385: 1966–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilson FP, Martin M, Yamamoto Yet al. Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. BMJ 2021; 372: m4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Office for Human Research Protections (OHRP) . 2018 Requirements (2018 Common Rule) [Internet]. HHS.gov2017 https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/revised-common-rule-regulatory-text/index.html (16 August 2021, date last accessed) [Google Scholar]
- 67. Duke University . HiLo: Pragmatic Trial of Higher vs Lower Serum Phosphate Targets in Patients Undergoing Hemodialysis [Internet]. clinicaltrials.gov, 2021 https://clinicaltrials.gov/ct2/show/NCT04095039 (15 August 2021, date last accessed). [Google Scholar]
- 68. Edmonston DL, Isakova T, Dember LMet al. Design and rationale of HiLo: a pragmatic, randomized trial of phosphate management for patients receiving maintenance hemodialysis. Am J Kidney Dis 2021; 77: 920–930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Semler MW, Self WH, Wanderer JPet al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Self WH, Semler MW, Wanderer JPet al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 2018; 378: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. University of California, San Francisco . The Choice of Vasopressor for Treating Hypotension During General Anesthesia: a Pilot Pragmatic Cluster Cross-over Randomized Trial (the VEGA-1 Trial) [Internet]. clinicaltrials.gov, 2021 https://clinicaltrials.gov/ct2/show/NCT04789330 (15 August 2021, date last accessed) [Google Scholar]
- 72. Crocker JC, Ricci-Cabello I, Parker Aet al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ 2018; 363: k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]