ABSTRACT
Background
The aim of this study was to describe the trends in the incidence, prevalence and survival of patients on kidney replacement therapy (KRT) for end-stage kidney disease (ESKD) across Europe from 2008 to 2017.
Methods
Data from renal registries in 9 countries and 16 regions that provided individual patient data to the ERA Registry from 2008 to 2017 were included. These registries cover 34% of the general population in Europe. Crude and standardized incidence and prevalence per million population (pmp) were determined. Trends over time were studied using Joinpoint regression. Survival probabilities were estimated using Kaplan–Meier analysis and hazard ratios (HRs) using Cox regression analysis.
Results
The standardized incidence of KRT was stable [annual percentage change (APC): −1.48 (−3.15; 0.21)] from 2008 (146.0 pmp) to 2011 (141.6 pmp), followed by a slight increase [APC: 1.01 (0.43; 1.60)] to 148.0 pmp in 2017, although trends in incidence varied across countries. This increase was primarily due to a rise in the incidence of KRT in men older than 65 years. Moreover, as a cause of kidney failure, diabetes mellitus is increasing. The standardized prevalence increased from 2008 (990.0 pmp) to 2017 (1166.8 pmp) [APC: 1.82 (1.75; 1.89)]. Patient survival on KRT improved in the time period 2011–13 compared with 2008–[adjusted HR: 0.94 (0.93; 0.95)].
Conclusion
This study showed an overall increase in the incidence and prevalence of KRT for ESKD as well as an increase in the KRT patient survival over the last decade in Europe.
Keywords: dialysis, incidence, kidney transplantation, prevalence, survival
KEY LEARNING POINTS.
What is already known about this subject?
Worldwide, the prevalence of patients on kidney replacement therapy (KRT) for end-stage kidney disease (ESKD) has been estimated at 3.9 million in 2017 and continues to rise, which is amplified by improved KRT survival in many parts of the world.
The incidence rate of patients on KRT has slowed down or fallen in Europe, the USA and Canada since the 1990s, although with substantial international differences.
It is unknown how the trends in KRT incidence, prevalence and survival have evolved in Europe over the last decade. Therefore, the aim of this ERA Registry study is to examine the trends in KRT incidence, prevalence and survival across European countries from 2008 to 2017.
What this study adds?
The crude and standardized KRT incidence was stable from 2008 to 2011, thereafter a slight rise in the incidence was shown from 2011 to 2017, while the crude and standardized KRT prevalence increased during the entire study period.
The increase in standardized KRT incidence was mainly found in men aged 65 years and older and in patients with diabetes mellitus, hypertension and glomerulonephritis/sclerosis as primary renal disease. The increase in standardized KRT prevalence was found in all age groups and both in men and women.
Survival improved over time, both in patients on dialysis and after a first kidney transplantation (time period 2011–13 compared with the time period 2008–10), except in kidney transplant recipients aged 65 years and older.
What impact this may have on practice or policy?
The increasing number of patients in need of KRT in European countries calls for resources and actions to maintain access to high-quality KRT care, including kidney transplantation.
Diabetes mellitus remained the leading cause for ESKD requiring KRT and the incidence continued to increase during the study period. Monitoring of patients with diabetes mellitus and prevention of complications remain key for delaying the progression to KRT.
Overall kidney transplant survival outcomes have improved despite increased recipient age and the more frequent use of lower-quality donor kidneys. Still, ongoing efforts are needed to enhance the kidney transplant rate.
INTRODUCTION
Worldwide the number of patients on kidney replacement therapy (KRT) has been estimated at 3.9 million in 2017, with the highest prevalence in high-income regions like Europe, North America and Japan [1–3]. In the first decade of this century, after years of continuous growth, the KRT registries in the USA and Europe have shown signs of stabilization and sometimes falling incidence rates [4–7]. In other large-scale KRT registries like in Australia and New Zealand [8] and Canada [9], the increase in incidence rates also slowed down after the 1990s. Nevertheless, KRT prevalence continued to rise worldwide [2] and patient survival continued to improve in many parts of the world [4, 9, 10].
The aim of this study was to examine the trends in the incidence and prevalence of patients on KRT for end-stage kidney disease (ESKD) across European countries from 2008 to 2017, and secondly, it reports the trends in patient and graft survival comparing the 2008–10 and 2011–13 patient cohorts using data from the ERA Registry.
MATERIALS AND METHODS
Patient population
The ERA Registry collects individual patient and aggregated data from population-based national and regional KRT registries across Europe [4]. These registries provide annual data on KRT for patients with ESKD, including data on patient demographics, primary renal disease (PRD) and treatment modalities. In this study, we use individual patient data that were derived for the time period 2008 to 2017 from 9 national registries (Austria, Denmark, Finland, France, Greece, Iceland, The Netherlands, Norway, Sweden) and 16 regional registries (Dutch-speaking and French-speaking Belgium; the UK including England, Northern Ireland and Wales; the UK Scotland; and the Spanish regions of Andalusia, Aragon, Asturias, Basque country, Cantabria, Castile and Léon, Castile-La Mancha, Catalonia, Community of Madrid, Extremadura, Galicia and Valencia). For most national and regional KRT registries, the data represent the complete general population for the entire study period (corresponding to a percentage coverage of 100%), except for France (incidence and prevalence: 86%), The Netherlands in 2016 (incidence 94% and prevalence 96%) and 2017 (incidence: 93% and prevalence: 97%) and the UK, England in 2014–17 (incidence: 98% and prevalence: 99%); any lower percentage in coverage was taken into account in the analyses. For France, the following 20 of the 24 regions (including Corse and Reunion overseas) with a coverage of 100% throughout the study period were included: Auvergne, Limousin, Lorraine, Rhône-Alpes, Bretagne, Champagne-Ardenne, Languedoc-Roussillon, Nord-Pas-de-Calais, Provence-Alpes-Côte-d'Azur, Basse-Normandie, Bourgogne, Centre, Midi-Pyrénées, Corse, Haute-Normandie, Île-de-France, Picardie, Poitou-Charentes, Alsace and Reunion. For the Netherlands, an opt-out registration system was used for the inclusion of patients in their registry, while in UK, England one center has not submitted data since 2014. Overall, the 25 registries participating in this study represent a combined general population of approximately 240 million people corresponding to around 34% of the general population in Europe (geographically defined, including the European parts of Russia and Turkey) in 2017. The participating countries are from Northern (N = 6), Western (N = 4) and Southern (N = 2) Europe, as countries from Eastern Europe only started to participate in the ERA Registry with individual patient data after 2008, or provide aggregated data [11]. Informed consent was obtained in accordance with national and/or regional regulations for each individual registry. Compliance with ethical standards was confirmed by the medical ethical committee of the Amsterdam Medical Centre (W21_123 No. 21.136).
In the ERA Registry, data quality is controlled by manual and automated data checks using standardized (data-handling) protocols and close correspondence on data interpretation with participating KRT registries. To account for delayed reporting, the period 2008–17 was selected in the 2018 database. Only in case of the UK, the 2017 database was used, therefore delayed reporting could not be taken into account and the maximum survival follow-up was set at 31 December 2017. Data on children were unavailable for the renal registries of Dutch- and French-speaking Belgium; the Spanish regions of Cantabria, Castile and Léon, and Castile-La Mancha; and the UK including England, Northern Ireland and Wales. The exclusion of data on children from these registries will result in an underestimation of approximately 2 prevalence per million population (pmp) for incidence and 13 pmp for prevalence in these regions, but this will not influence trends. Data were complete except for primary renal disease (PRD), for which we used a category ‘missing’. Details on data collection and processing methods have been published in the ERA Registry annual reports (available on https://www.era-online.org/en/registry/).
Incidence and prevalence
Incident counts were defined as the number of patients starting KRT (any form of dialysis or pre-emptive kidney transplantation) during a year and prevalent counts as the number of patients who were alive and receiving KRT (any form of dialysis or kidney transplantation) on December 31 of a year. The crude incidence and prevalence pmp were calculated by dividing the number of patients by the mid-year general population of the country or region, multiplied by one million. In case the percentage coverage of the general population by the renal registry was <100%, we corrected for this by adjusting the general population data with the lower coverage. Standardized incidence and prevalence pmp were determined using the age and sex distribution of a reference population (i.e. the EU28 population in 2015) [12]. Incidence pmp will be further indicated as incidence and prevalence pmp as prevalence. Analyses were stratified by age group, sex and PRD, with the latter being classified according to the ERA coding system [4]. Where the incidence or prevalence was calculated by age group, this was done by dividing the number of patients in a particular age group by the general population in that age group, resulting in an incidence or a prevalence per million age-related population (pmarp). Population data were obtained from the statistical office of the European Union (Eurostat) [12] or the national statistics agency in each country.
Time trends
Joinpoint regression analysis was used to determine the annual percentage change (APC) of time trends in the incidence and prevalence. With this approach, the incidence and prevalence are assumed to change at a constant percentage relative to the rate of the previous year, unless a joinpoint was detected. A joinpoint is detected in the year in which a statistically significantly change in the trend took place and therefore such a joinpoint could differ across the countries. To test whether joinpoints were statistically significant and should be added to the model, the Monte Carlo Permutation method was used [13]. We used a maximum of two joinpoints per model. To calculate the APC for each time interval, the weighted average of the slope or β coefficients of the fitted regression lines was calculated, and weighted for the length of the corresponding sub-intervals.
Patient and graft survival
Survival probabilities were analyzed for 1, 2 and 5 years for patients initiating KRT (overall and for patients on dialysis) in the time periods 2008–10 and 2011–13, and for 1, 2 and 5 years after kidney transplantation for patients receiving a first transplant during the same time periods.
For overall patient survival on KRT, patients were followed from the date of KRT initiation until death or censoring (due to recovery of kidney function, loss to follow-up or the end of the follow-up period). For patient survival on dialysis, patients were followed from the start date of dialysis until death or censoring (due to kidney transplantation, recovery of kidney function, loss to follow-up or the end of the follow-up period). Patient and graft survival after kidney transplantation were calculated from the date of first transplant until the occurrence of the event of interest (death in case of patient survival and graft failure or death in case of graft survival) or censoring (due to loss to follow-up or the end of the follow-up period). The maximum follow-up was set at 5 years after the start date or 31 December 2018, except for the UK, for which the maximum follow-up in the survival analyses was set at 31 December 2017.
Survival probabilities were calculated using the Kaplan–Meier method. Hazard ratios (HRs) comparing the survival in the time periods 2008–10 and 2011–13 were computed using a Cox regression model, which was performed unadjusted, and with adjustment for age, sex, PRD and country as fixed effect. The Cox proportional hazards assumption was met. Additionally, we analyzed the survival trends in patients aged younger than 65 years compared with those aged 65 years and older, and the KRT and dialysis patient survival in patients aged 85 years and older. All survival probabilities and crude and adjusted HRs are reported with 95% confidence intervals.
R statistical software 4.0.4 (R Foundation for Statistical Computation, Vienna), including the packages dplyr (version 1.0.5), survival (version 3.2.7) and survminer (version 0.4.9), was used for the calculation of incidence, prevalence and survival [14], whereas the Joinpoint Regression Program was used for trend analyses [15].
RESULTS
Incidence of KRT, 2008–17
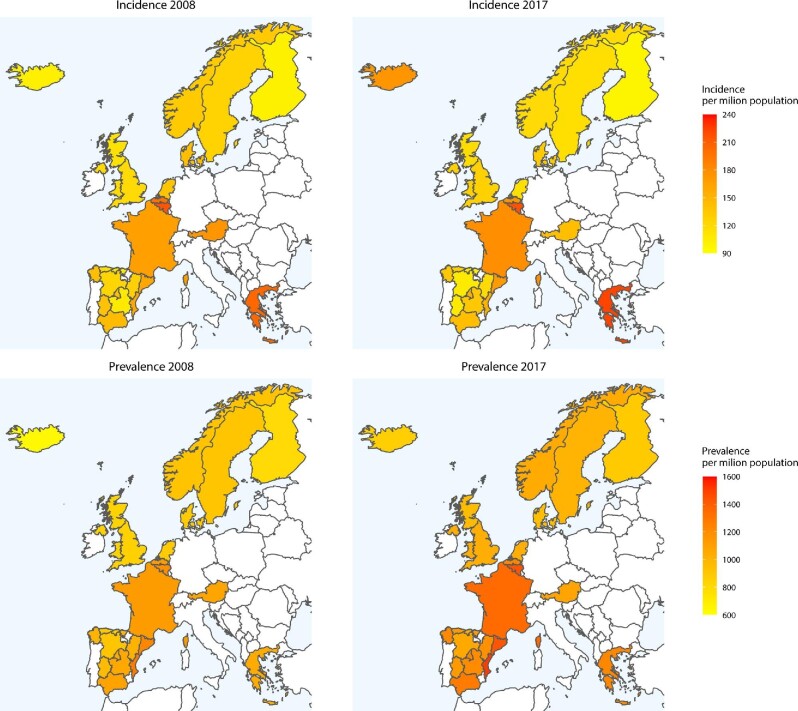
From 2008 to 2011, the overall crude incidence of KRT was stable from 133.9 to 132.1 pmp [APC: −0.71 (−2.37; 0.98)], followed by an increase from 132.1 pmp in 2011 to 147.2 pmp in 2017 [APC: 1.98 (1.40; 2.56)] with substantial variation between countries and regions (Supplementary data, Table S1). After standardization for age and sex, we found similar trends: a stable incidence from 2008 to 2011 of 146.0 to 140.6 pmp [APC: −1.48 (−3.15; 0.21)], and an increase from 140.6 pmp in 2011 to 148.0 pmp in 2017 [APC: 1.01 (0.43; 1.60)], also with considerable variation across individual countries and regions (Table 1 and Fig. 1). Some countries and regions showed a decline in standardized KRT incidence over the study period: Austria, Belgium (Dutch-speaking part), Norway, the Spanish regions of Aragon and Castile and Léon (2008–13), Sweden and the Netherlands, whereas others showed an increase: France and Greece (2010–17), and the Spanish regions of Andalusia (2011–17), Asturias, Castile-La Mancha, Catalonia and the Community of Madrid.
Table 1.
Standardized incidence pmp of patients starting KRT across countries in Europe in 2008–17
| Annual percentage change | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Time perioda | Time perioda | Time perioda |
| Austria | 174.6 | 169.5 | 156.4 | 155.7 | 155.5 | 157.7 | 144.8 | 156.9 | 152.7 | 141.0 | 2008–17: −1.71* (−2.65; −0.76) | ||
| Belgium—Dutch-speaking | 198.5 | 211.9 | 199.0 | 185.5 | 188.5 | 185.0 | 174.5 | 174.6 | 178.5 | 173.6 | 2008–17: −1.96* (−2.75; −1.16) | ||
| Belgium—French-speaking | 216.3 | 223.3 | 217.6 | 214.3 | 218.6 | 208.7 | 202.7 | 212.6 | 213.3 | 217.3 | 2008–17: −0.37 (−1.02; 0.29) | ||
| Denmark | 140.4 | 147.5 | 131.7 | 126.0 | 135.0 | 127.4 | 144.2 | 124.0 | 130.7 | 131.5 | 2008–17: −0.84 (−2.23; 0.56) | ||
| Finland | 100.5 | 87.2 | 87.4 | 86.5 | 83.6 | 88.6 | 85.3 | 95.5 | 102.1 | 97.5 | 2008–12: −3.51 (−9.04; 2.34) | 2012–17: 4.08 (−0.17; 8.51) | |
| France | 165.7 | 168.3 | 168.5 | 165.2 | 167.2 | 171.9 | 172.8 | 175.8 | 172.5 | 177.7 | 2008–17: 0.72* (0.39; 1.05) | ||
| Greece | 204.6 | 206.6 | 187.4 | 196.5 | 200.6 | 203.4 | 203.2 | 207.8 | 225.4 | 223.0 | 2008–10: −4.39 (−11.91; 3.77) | 2010–17: 2.27* (1.15; 3.39) | |
| Iceland | 102.3 | 122.8 | 143.9 | 129.0 | 85.7 | 84.8 | 70.8 | 84.2 | 107.8 | 174.5 | 2008–10: 19.04 (−47.23; 168.54) | 2010–14: −17.95 (−5.37; 23.23) | 2014–17: 34.56 (−10.14; 102.1) |
| Norway | 132.6 | 137.0 | 121.1 | 117.4 | 120.2 | 116.3 | 115.2 | 111.6 | 116.5 | 119.6 | 2008–17: −1.54* (−2.68; −0.39) | ||
| Spain—Andalusia | 144.8 | 139.8 | 135.2 | 130.4 | 135.1 | 140.8 | 138.3 | 137.3 | 148.1 | 143.2 | 2008–11: −2.86 (−6.91; 1.37) | 2011–17: 1.64* (0.18; 3.11) | |
| Spain—Aragon | 128.3 | 128.4 | 136.4 | 122.6 | 132.0 | 128.1 | 120.2 | 112.5 | 121.5 | 119.1 | 2008–17: −1.27* (−2.36; −0.17) | ||
| Spain—Asturias | 116.6 | 123.2 | 113.7 | 128.6 | 109.0 | 140.1 | 126.7 | 139.1 | 168.7 | 130.0 | 2008–17: 2.70* (0.08; 5.39) | ||
| Spain—Basque country | 106.8 | 129.4 | 108.0 | 107.2 | 115.2 | 116.0 | 105.5 | 113.0 | 117.1 | 103.7 | 2008–17: −0.47 (−2.20; 1.29) | ||
| Spain—Cantabria | 110.2 | 109.9 | 134.5 | 108.9 | 108.1 | 85.5 | 99.7 | 104.5 | 98.6 | 109.0 | 2008–17: −1.57 (−4.27; 1.21) | ||
| Spain—Castile and Léon | 123.6 | 114.5 | 111.1 | 108.9 | 108.4 | 97.8 | 106.1 | 104.2 | 108.3 | 106.5 | 2008–13: −3.48* (−5.22; −1.71) | 2013–17: 1.63 (−0.94; 4.28) | |
| Spain—Castile-La Mancha | 106.3 | 102.9 | 120.0 | 105.2 | 108.7 | 110.0 | 130.8 | 117.3 | 144.4 | 135.2 | 2008–17: 3.14* (1.17; 5.14) | ||
| Spain—Catalonia | 159.9 | 164.1 | 148.8 | 151.0 | 155.1 | 162.4 | 164.3 | 172.2 | 172.5 | 168.9 | 2008–17: 1.14* (0.10; 2.20) | ||
| Spain—Community of Madrid | 139.7 | 132.9 | 126.3 | 128.2 | 124.5 | 142.2 | 147.9 | 143.6 | 151.9 | 152.3 | 2008–17: 1.78* (0.33; 3.26) | ||
| Spain—Extremadura | 138.9 | 110.6 | 138.4 | 104.9 | 123.0 | 121.4 | 111.8 | 138.6 | 106.4 | 107.3 | 2008–17: −1.45 (−4.24; 1.42) | ||
| Spain—Galicia | 143.2 | 127.1 | 127.1 | 134.3 | 124.1 | 129.0 | 128.4 | 128.4 | 130.6 | 134.2 | 2008–17: −0.27 (−1.33; 0.81) | ||
| Spain—Valencian region | 157.5 | 163.2 | 161.8 | 148.4 | 159.1 | 142.8 | 163.0 | 158.1 | 188.0 | 152.9 | 2008–17: 0.47 (−1.44; 2.42) | ||
| Sweden | 126.5 | 130.0 | 124.0 | 125.2 | 115.9 | 117.6 | 119.6 | 118.4 | 120.3 | 115.3 | 2008–17: −1.04* (−1.70; −0.39) | ||
| The Netherlands | 136.5 | 132.7 | 130.2 | 128.3 | 129.6 | 123.6 | 121.4 | 123.2 | 121.7 | 114.7 | 2008–17: −1.60* (−2.00; −1.19) | ||
| UK—England, Northern Ireland and Wales | 119.1 | 119.7 | 115.9 | 117.4 | 116.3 | 118.3 | 124.1 | 127.2 | 126.2 | 127.3 | 2008–12: −0.75 (−3.27; 1.84) | 2012–15: 3.13 (−4.93; 11.87) | 2015–17: −0.04 (−7.85; 8.43) |
| UK—Scotland | 113.0 | 110.5 | 105.6 | 100.0 | 102.9 | 98.4 | 105.7 | 116.2 | 106.7 | 118.0 | 2008–12: −3.18 (−7.7; 1.56) | 2012–17: 3.21 (−0.22; 6.76) | |
| All countries | 146.0 | 146.8 | 142.0 | 140.6 | 141.6 | 143.2 | 144.7 | 147.3 | 149.1 | 148.0 | 2008–11: −1.48 (−3.15; 0.21) | 2011–17: 1.01* (0.43; 1.60) | |
This table shows the standardized incidence pmp of patients starting KRT for 25 countries and regions across Europe for each year between 2008 and 2017. The incidence is reported per million population (pmp), and standardized using the age and sex distribution of the EU28 population in 2015. ‘All countries’ represents the overall standardized incidence in all participating countries and regions together. To reflect the trends over time, the APC with corresponding 95% confidence interval is given for each time period. aTime periods are defined by a statistically significant join point and could therefore differ between countries and regions. Bold numbers and an asterisk (*) indicate whether the APC was statistically significant (P < .05).
APC, annual percentage change; KRT, kidney replacement therapy; pmp, per million population.
FIGURE 1:
Standardized incidence and prevalence of kidney replacement therapy (KRT) per million population (pmp) in countries and regions in Europe over time (comparison of 2008 and 2017). For standardization the age and sex distribution of the EU28 population in 2015 was used. Dark red represents a higher incidence and prevalence pmp (more patients starting or receiving KRT in that country/region in that year compared with other countries/regions), while yellow represents a relatively lower incidence and prevalence pmp (fewer patients starting or receiving KRT in that country/region in that year compared with other countries/regions).
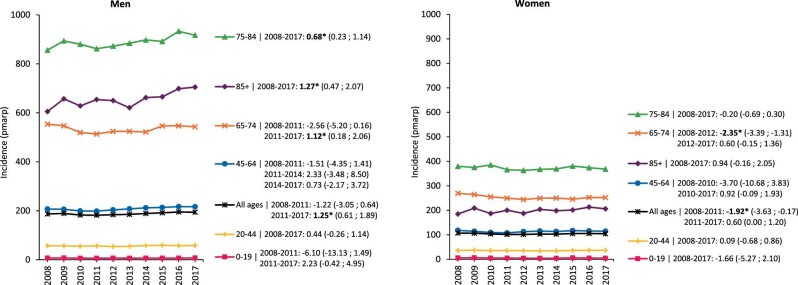
In women, the crude KRT incidence was much lower than in men; nevertheless, the rise in incidence after 2011 was twice as high in men [APC: 2.40 (1.76; 3.04)] as in women [APC: 1.24 (0.64; 1.85)] (Supplementary data, Fig. S1). Also, after standardization for age the rise in KRT incidence after 2011 was only found among men [APC: 1.25 (0.61; 1.89)], which was mainly attributable to an increase in men older than 65 years of age (Fig. 2). Even more specific, this increase in standardized KRT incidence was particularly substantial for men aged 85 years and older [APC: 1.27 (0.47; 2.07)].
FIGURE 2:
Standardized incidence of kidney replacement therapy (KRT) per million age-related population (pmarp) in men and women, stratified by age group. For standardization the age and sex distribution of the EU28 population in 2015 was used. Trends are indicated by the APC with corresponding 95% confidence interval. Bold numbers and an asterisk (*) indicate whether the APC was statistically significant (P < .05).
Analyses by PRD category showed that diabetes mellitus was the leading cause of ESKD requiring KRT, and that the standardized incidence continued to increase from 2011 onward [APC: 1.66 (0.49; 2.83)]. Furthermore, from 2010 onward the standardized incidence of hypertension [APC: 0.62 (0.13; 1.10)] and glomerulonephritis/sclerosis [APC: 1.64 (0.07; 3.23)] as causes of kidney failure increased over time. Missing data on PRD were infrequently encountered from 2008 (1.4 pmp) to 2016 (2.2 pmp) but became slightly more common in 2017 (5.2 pmp). This increase was mainly due to the higher number of missing PRD documentation in the UK (including England, Northern Ireland and Wales) at the end of the study period (Fig. 3).
FIGURE 3:
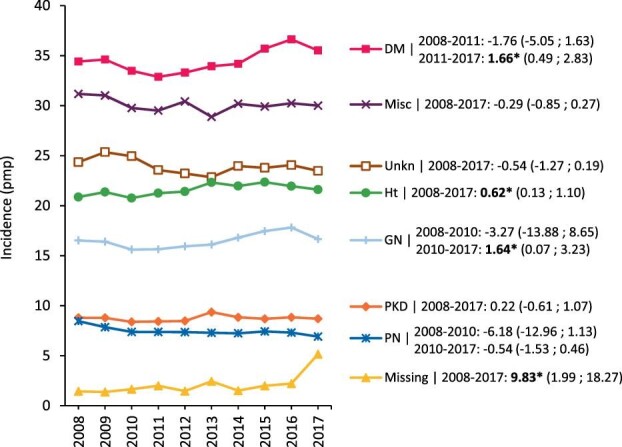
Standardized incidence of kidney replacement therapy (KRT) per million population (pmp), stratified by primary renal disease (PRD). For standardization the age and sex distribution of the EU28 population in 2015 was used. Trends are indicated by the APC with corresponding 95% confidence interval. Bold numbers and an asterisk (*) indicate whether the APC was statistically significant (P < .05). Mapping of the 2012 primary renal disease (PRD) codes to the old PRD codes might have influenced the trend of renal vascular disease (RVD); DM, diabetes mellitus; represents DM type I, DM type II and DM type unknown; GM, glomerulonephritis/sclerosis; HT, hypertension; Misc, miscellaneous; PKD, polycystic kidneys, adult type; PN, pyelonephritis; Unkn, unknown (PRD not identified).
Prevalence of KRT, 2008–17
Over the study period the crude prevalence of KRT increased from 922.4 to 1154.0 pmp [APC: 2.50 (2.44; 2.56); Supplementary data, Table S2]. This increase was visible in all individual countries and regions. Similarly, there was a rise in the overall standardized prevalence from 990.0 to 1166.8 pmp, although this was less pronounced [APC: 1.82 (1.75; 1.89); Table 2, Fig. 1].
Table 2.
Standardized prevalence pmp of patients receiving KRT across countries in Europe in 2008–17
| Annual percentage change | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Time perioda | Time perioda | Time perioda |
| Austria | 1077.6 | 1061.2 | 1053.9 | 1049.6 | 1067.5 | 1085.9 | 1089.6 | 1086.1 | 1083.0 | 1081.1 | 2008–11: −0.73 (−1.47; 0.01) | 2011–14: 1.36 (−0.15; 2.88) | 2014–17: −0.43 (−1.17; 0.32) |
| Belgium—Dutch-speaking | 1121.2 | 1155.0 | 1171.1 | 1185.2 | 1197.0 | 1202.4 | 1207.6 | 1213.3 | 1214.3 | 1223.3 | 2008–11: 1.87* (1.29; 2.45) | 2011–17: 0.44* (0.25; 0.63) | |
| Belgium—French-speaking | 1187.6 | 1225.2 | 1255.3 | 1280.2 | 1311.0 | 1332.6 | 1355.0 | 1384.3 | 1414.7 | 1435.8 | 2008–10: 2.85* (1.99; 3.72) | 2010–17: 1.94 * (1.82; 2.05) | |
| Denmark | 879.1 | 887.3 | 885.2 | 889.8 | 900.2 | 902.4 | 925.2 | 928.4 | 939.8 | 955.1 | 2008–11: 0.27 (−0.65; 1.2) | 2011–17: 1.17* (0.85; 1.48) | |
| Finland | 789.8 | 798.3 | 810.4 | 813.2 | 815.5 | 823.4 | 830.2 | 841.8 | 869.7 | 884.6 | 2008–14: 0.75* (0.46; 1.04) | 2014–17: 2.19* (1.32; 3.07) | |
| France | 1121.9 | 1154.0 | 1187.7 | 1211.9 | 1223.0 | 1249.4 | 1279.9 | 1305.3 | 1327.6 | 1352.4 | 2008–17: 2.03* (1.89; 2.17) | ||
| Greece | 1044.5 | 1067.8 | 1070.6 | 1081.1 | 1112.6 | 1134.5 | 1151.3 | 1165.2 | 1201.0 | 1221.3 | 2008–17: 1.75* (1.56; 1.94) | ||
| Iceland | 634.2 | 655.7 | 721.1 | 789.8 | 817.8 | 809.0 | 785.0 | 740.9 | 749.6 | 863.0 | 2008–12: 7.50* (2.87; 12.34) | 2012–15: 4.09 (−16.56; 10.24) | 2015–17: 6.94 (−6.96; 22.92) |
| Norway | 922.4 | 951.2 | 964.7 | 976.2 | 986.8 | 994.8 | 1008.6 | 1014.9 | 1022.3 | 1042.2 | 2008–10: 2.22* (1.03; 3.42) | 2010–17: 1.02* (0.86; 1.17) | |
| Spain—Andalusia | 1086.7 | 1109.0 | 1131.4 | 1142.7 | 1180.4 | 1202.3 | 1220.1 | 1227.6 | 1253.3 | 1274.8 | 2008–17: 1.78* (1.63; 1.94) | ||
| Spain—Aragon | 1009.7 | 1002.6 | 1038.4 | 1048.9 | 1088.4 | 1099.5 | 1105.6 | 1109.6 | 1135.6 | 1182.1 | 2008–17: 1.71* (1.39; 2.02) | ||
| Spain—Asturias | 895.6 | 911.5 | 914.1 | 941.5 | 954.6 | 1001.0 | 1015.8 | 1032.7 | 1099.0 | 1126.7 | 2008–17: 2.62* (2.19; 3.04) | ||
| Spain—Basque country | 977.1 | 1005.2 | 1010.9 | 1032.5 | 1059.3 | 1073.3 | 1079.2 | 1089.0 | 1112.2 | 1118.5 | 2008–17: 1.49* (1.29; 1.69) | ||
| Spain—Cantabria | 869.1 | 888.8 | 914.2 | 935.2 | 950.7 | 963.7 | 970.9 | 987.9 | 1016.6 | 1028.8 | 2008–11: 2.42* (1.37; 3.47) | 2011–17: 1.59* (1.23; 1.94) | |
| Spain—Castile and Léon | 907.7 | 916.6 | 922.9 | 935.4 | 955.8 | 960.9 | 978.2 | 994.8 | 1022.3 | 1055.9 | 2008–15: 1.33* (1.11; 1.54) | 2015–17: 3.22* (1.61; 4.86) | |
| Spain—Castile-La Mancha | 1051.5 | 1045.0 | 1076.9 | 1096.6 | 1104.2 | 1109.3 | 1131.9 | 1148.7 | 1176.7 | 1208.1 | 2008–17: 1.53* (1.29; 1.77) | ||
| Spain—Catalonia | 1226.2 | 1257.1 | 1274.0 | 1289.2 | 1314.7 | 1340.8 | 1361.1 | 1392.0 | 1419.7 | 1442.4 | 2008–17: 1.80* (1.71; 1.88) | ||
| Spain—Community of Madrid | 1044.7 | 1066.1 | 1078.9 | 1091.8 | 1106.5 | 1126.3 | 1161.6 | 1182.5 | 1210.0 | 1228.4 | 2008–12: 1.40* (0.91; 1.88) | 2012–17: 2.17* (1.82; 2.51) | |
| Spain—Extremadura | 963.2 | 988.7 | 1034.4 | 1046.6 | 1071.6 | 1093.3 | 1106.7 | 1130.7 | 1125.5 | 1133.5 | 2008–10: 3.62* (1.07; 6.24) | 2010–15: 1.84* (1.04; 2.65) | 2015–17: 0.08 (−2.39; 2.60) |
| Spain—Galicia | 1007.0 | 1027.7 | 1046.7 | 1070.6 | 1097.3 | 1120.4 | 1144.0 | 1163.7 | 1180.0 | 1212.9 | 2008–17: 2.08* (1.99; 2.16) | ||
| Spain—Valencian region | 1274.8 | 1293.9 | 1310.6 | 1319.9 | 1345.5 | 1363.3 | 1427.7 | 1453.4 | 1506.2 | 1513.2 | 2008–17: 2.06* (1.72; 2.41) | ||
| Sweden | 906.6 | 921.2 | 936.8 | 953.2 | 951.3 | 959.3 | 971.9 | 977.7 | 992.0 | 1004.8 | 2008–17: 1.05* (0.91; 1.19) | ||
| The Netherlands | 877.2 | 907.2 | 924.5 | 938.4 | 953.0 | 971.4 | 984.7 | 997.0 | 1038.8 | 1029.9 | 2008–17: 1.79* (1.57; 2.02) | ||
| UK—England, Northern Ireland and Wales | 843.8 | 872.2 | 892.1 | 918.7 | 933.6 | 954.3 | 977.5 | 1003.8 | 1019.5 | 1031.9 | 2008–17: 2.27* (2.1; 2.43) | ||
| UK—Scotland | 841.5 | 854.0 | 861.8 | 862.8 | 881.4 | 887.3 | 904.3 | 928.2 | 940.5 | 961.2 | 2008–13: 1.05* (0.66; 1.43) | 2013–17: 2.02* (1.47; 2.57) | |
| All countries | 990.0 | 1014.8 | 1034.5 | 1052.8 | 1069.0 | 1088.2 | 1109.6 | 1129.4 | 1151.4 | 1166.8 | 2008–17: 1.82* (1.75; 1.89) | ||
This table shows the standardized prevalence pmp of patients receiving KRT for 25 countries and regions across Europe for each year between 2008 and 2017. The prevalence is reported per million population (pmp), and standardized using the age and sex distribution of the EU28 population in 2015. ‘All countries’ represents the overall standardized prevalence rates in all participating countries and regions together. To reflect the trends over time, the APC with corresponding 95% confidence interval is given for each time period. aTime periods are defined by a statistically significant joinpoint and could therefore differ between countries and regions. Bold numbers and an asterisk (*) indicate whether the APC was statistically significant (P < .05).
APC, annual percentage change; KRT, kidney replacement therapy; pmp, per million population.
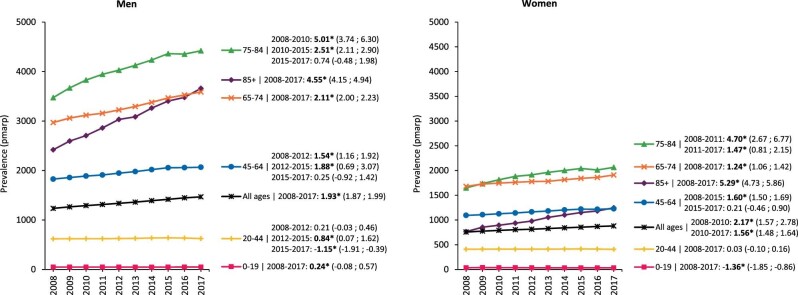
The crude prevalence of KRT increased in both sexes, but more prominently in men [APC: 2.74 (2.69; 2.79)] than in women [APC: 2.12 (2.04; 2.20); Supplementary data, Fig. S2], and this finding was also observed after standardization (Fig. 4). Analyses by age group and sex showed that the increase in standardized prevalence was more pronounced among individuals aged 75 years and older (Fig. 4).
FIGURE 4:
Standardized prevalence of KRT pmarp (per million age-related population) in men and women, stratified by age group. For standardization the age and sex distribution of the EU28 population in 2015 was used. Trends are indicated by the APC with corresponding 95% confidence interval. Bold numbers and an asterisk (*) indicate whether the APC was statistically significant (P < .05).
Patient and graft survival
In the time period 2011–13 patient survival probabilities on KRT at 1, 2 and 5 years were 84.6%, 74.7% and 51.7%, respectively. This was superior to the time period 2008–10 (adjusted HR: 0.94 [0.93; 0.95]) with survival probabilities at 1, 2 and 5 years of 83.7%, 73.5% and 50.6%, respectively. Patient survival on dialysis and graft survival after the first kidney transplant had improved, while patient survival after a first kidney transplant had not (Table 3).
Table 3.
Survival on KRT over time stratified by dialysis and kidney transplantation
| Survival probabilities | Hazard ratios for mortality | |||||
|---|---|---|---|---|---|---|
| N | 1 year | 2 years | 5 years | Unadjusted HR | Adjusted HR | |
| Patient survival on KRT | ||||||
| Time period 2008–10 | 92 586 | 83.7 (83.5; 83.9) | 73.5 (73.2; 73.8) | 50.6 (50.3; 51.0) | 1 (ref) | 1 (ref) |
| Time period 2011–13 | 94 465 | 84.6 (84.4; 84.8) | 74.7 (74.5; 75.0) | 51.7 (51.4; 52.0) | 0.96 (0.95; 0.98) | 0.94 (0.93; 0.95) |
| Patient survival on dialysis | ||||||
| Time period 2008–10 | 88 185 | 82.7 (82.4; 83.0) | 71.1 (70.8; 71.4) | 41.3 (41.0; 41.7) | 1 (ref) | 1 (ref) |
| Time period 2011–13 | 89 404 | 83.5 (83.3; 83.8) | 72.2 (71.9; 72.5) | 42.0 (41.6; 42.3) | 0.97 (0.96; 0.99) | 0.95 (0.94; 0.96) |
| Patient survival after first kidney transplant | ||||||
| Time period 2008–10 | 25 459 | 96.6 (96.4; 96.9) | 94.9 (94.7; 95.2) | 88.9 (88.5; 89.3) | 1 (ref) | 1 (ref) |
| Time period 2011–13 | 28 159 | 96.8 (96.6; 97.0) | 94.9 (94.6; 95.2) | 88.3 (87.9; 88.6) | 1.06 (1.00; 1.11) | 0.96 (0.91; 1.01) |
| Graft survival after first kidney transplant | ||||||
| Time period 2008–10 | 25 459 | 91.6 (91.3; 92.0) | 89.0 (88.6; 89.4) | 80.1 (79.6; 80.5) | 1 (ref) | 1 (ref) |
| Time period 2011–13 | 28 159 | 92.3 (92.0; 92.6) | 89.5 (89.1; 89.8) | 80.2 (79.7; 80.7) | 0.99 (0.95; 1.03) | 0.95 (0.91; 0.98) |
This table shows the crude survival probabilities and crude and adjusted HRs with corresponding 95% confidence intervals comparing the survival of the time periods 2008–10 and 2011–13 with the time period 2008–10 used as a reference. Bold numbers indicate whether the HRs were statistically significantly (P < .05) different from the reference. A HR below 1 indicates improvement in survival over time (i.e. the more recent time period 2011–13 had better survival compared with the period 2008–10).
HR, hazard ratio; KRT, kidney replacement therapy.
Stratification into younger (<65 years) and older (≥65 years) patients showed that in younger patients survival had improved both on dialysis and after kidney transplantation. However, in older patients only patient survival on KRT and on dialysis were better in the most recent cohort (Supplementary data, Table S3). An additional analysis including patients aged 85 years and older also showed improvements in KRT and dialysis patient survival over time (adjusted HR: 0.92 [0.88–0.96] and 0.92 [0.86–0.96], respectively).
DISCUSSION
This study shows that the overall crude and standardized KRT incidence was initially stable over the period 2008–11, but since 2011 there has been a slight rise in the incidence. The overall increase was primarily due to a rise in the incidence of KRT in men, especially in those aged 65 years and older. The increase in the incidence of KRT was also caused by the increase in patients on KRT with diabetes mellitus, hypertension and glomerulonephritis/sclerosis as causes of kidney failure. The trends in the incidence of KRT for ESKD between 2008 and 2017 varied substantially across individual European countries and regions. The prevalence of KRT rose over the entire study period and in all age groups. Five-year survival improved slightly over time, with survival gains observed for patients on dialysis and after kidney transplantation, except for kidney transplant outcomes in transplant recipients aged 65 years and older.
Incidence
Previous studies from the ERA Registry have shown that the overall standardized incidence of KRT in Europe increased substantially from 1990 to 2000 [5, 6], flattened from 2000 to 2008 [4, 5] and declined from 2008 until 2011 [4]. In the current ERA Registry study, we found a similar decline for this latter period (2008–11), although it was not statistically significant, and was therefore referred to as a stable incidence. A secondary analysis of our dataset showed that this difference between studies was mainly due to a different selection of countries. The incidence was stable between 2008 and 2011, but thereafter we found that the incidence slightly increased from 2011 to 2017. Also in the USA, there was a considerable increase in the 1990s followed by a stabilization, whereas no increase in the standardized incidence was seen from 2011 to 2017 [7]. In other parts of the world, such as Australia and New Zealand and Canada, a significant increase in the standardized incidence occurred in the nineties [16, 17]. From the early 2000s onwards, the annual standardized incidence began to fluctuate in Australia and New Zealand [18], while in Canada the standardized incidence continued to increase, albeit at a slower pace [19]. In Europe, the deviation in trend coincided with the economic crisis of 2007–08, which may have influenced the access to KRT [20] or possibly resulted in alternative treatment (i.e. conservative care) of the elderly population. However, the consequences of this crisis for public health and healthcare remain controversial, and the relationship with the deviation in trend in KRT incidence is uncertain [21, 22].
We found that the trends in KRT incidence varied between individual European countries and even across regions within a country. For example, the Spanish region of Castile-La Mancha showed a marked increase in the standardized KRT incidence from 106.3 to 135.2 pmp in 2008–17, while the Spanish region of Castile and Léon showed a substantial decline from 123.6 to 97.8 pmp in 2008–13. The variation in KRT incidence trends between European countries and regions may be explained by several factors. In general practice, variation may exist in the introduction of protocols regarding the detection of chronic kidney disease or timely referral to a nephrologist [23]. In nephrology, changes in practice patterns may have influenced the levels of estimated glomerular filtration rate and the degree of uremic symptoms at the onset of KRT [24, 25], the use of supportive therapies [26, 27] or the choice for conservative care. Over the clinical course of chronic kidney disease, variation may arise in the timing and effect of tertiary prevention strategies, like the use of renal protective therapies, alerts for drug toxicity and attention for lifestyle changes [28–30]. At center- or country-level, changes in the organization of care (e.g. capacity and resources, financing and education), for example, increased emphasis on conservative care might also affect time trends in KRT incidence [23, 31, 32].
The rise in KRT incidence was mainly attributable to an increase in men older than 65 years of age. This increase in KRT incidence was even noted among men aged 85 years and older, while no increase was found among women in the same age groups. Sex disparities in the choice for conservative care exist (older women choose conservative care more frequently) [32–34] and may have increased over time. However, potential reasons behind these sex differences in treatment choices at older age (like differences in medical care or education, family support and clinical symptoms) require further study. In this perspective, the benefits of KRT regarding quality of life—especially among the oldest patients—compared with conservative care remain a subject of debate [35, 36]. Another explanation for the different trends in KRT incidence between older men and women may be that in the general population men have more risk factors (such as cardiovascular diseases, malignancies and diabetes) and lifestyle associated with development and progression of kidney disease than women [37, 38]. This is also reflected in the sex-specific difference in comorbidity patterns at the start of KRT, with a higher burden in men [39].
The rise in the standardized KRT incidence might also be due to an increase in the number of patients with diabetes mellitus, hypertension and glomerulonephritis/sclerosis as causes of kidney failure. The continuous increase in the frequency of diabetic kidney disease follows the epidemic of diabetes mellitus type 2 in the ageing general population [38], after a period of stable standardized incidence of KRT for ESKD due to diabetic nephropathy in some countries [40, 41]. Another explanation for the increase is the incidence of KRT for patients with diabetes mellitus as the cause of ESKD might be that the survival of patients with diabetes mellitus in the general population has improved, resulting in more people on dialysis, even at higher age. For example, the introduction of novel drugs such as Glucagon-like peptide-1 (GLP1) receptor agonists and Dipeptidyl peptidase-4 (DPP-4) inhibitors may alter the clinical course and may increase the survival of diabetic patients [42–44]. The same might be expected from the more recently introduced SGLT2 inhibitors and potentially from mineralocorticoid receptor antagonists in the future [45–48]. Therefore, all efforts in monitoring and prevention of complications remain key to success. The same applies to patients with hypertension and glomerulonephritis/sclerosis. Still, in Europe the incidence of KRT due to these primary renal diseases is relatively low in comparison with other parts of the world [49]. For example, the overall standardized incidence of KRT for ESKD due to diabetes in 2017 was 128.1 pmp in the USA [41] compared with a ‘modest’ 35.3 pmp in the European countries and regions participating in this study. In contrast, the standardized incidence of KRT for ESKD due to glomerulonephritis in the USA and Europe has become more similar over time, as the incidence declined from 31.2 pmp in 2010 to 26.0 pmp in 2017 in the USA [50], while increasing from 15.6 pmp in 2010 to 16.7 pmp in 2017 in Europe.
Like previous studies from the ERA Registry [4, 5], the current analysis shows an ongoing increase in the crude and standardized prevalence in all participating countries and regions. A similar trend was found in the crude and standardized prevalence in the USA [7], Australia and New Zealand [8, 51], and in Canada [9]. A rise in prevalence simply means that the number deaths of patients on KRT in one year is lower than the number of patients starting KRT in that same year. This phenomenon is amplified by increasing survival rates, mainly in dialysis patients. A further increase in KRT prevalence in Europe is therefore expected [2].
Survival
In this study, we found improved overall patient survival on KRT in the most recent time period 2011–13, compared with the previous time period 2008–10, both for dialysis patients and after first kidney transplantation, except in kidney transplant recipients aged 65 years and older. However, we found that survival gains over time were smaller compared with previous published ERA Registry studies. This might at least partly be due to an increase in the number of patients with comorbidities at onset of KRT in more recent years, although such an increase was not confirmed in our previous published study on comorbidity patterns in the period from 2005 to 2014 where the participating countries only partially correspond to this study [39]. The survival improvement for patients on dialysis may at least in part be explained by the implementation of new treatment strategies (e.g. high-flux dialysis) and novel drugs in recent years [52]. In line with this, we showed in another study that the excess mortality risk (the risk beyond the mortality risk in the general population) decreased in patients on dialysis between 2002 and 2015, but increased in kidney transplant recipients [53]. In that study the largest reduction in excess mortality risk in dialysis patients was due to a decrease in cardiovascular diseases as cause of death, while in kidney transplant recipients there was a substantial increase in excess mortality risk due to malignancies. However, trends in kidney transplant outcomes should be viewed in perspective of absolute survival probabilities, which are already high—and the lack of further improvement of transplant outcomes in our study might partially be due to a ceiling effect. The positive news is that the overall transplant outcomes have improved, despite the increased recipient age and the more frequent use of lower quality donor kidneys [54]; this may possibly be outweighed by advances over the last decade in graft preservation [55], desensitization protocols [56], kidney paired donation programs [57] and other types of (local) quality improvement [58]. In addition, future strategies aimed at increasing patient survival should focus on issues beyond acute rejection or graft loss, such as the effects of a more holistic patient management approach. Kidney transplantation remains the treatment of choice for ESKD with respect to costs, quality of life and survival [59, 60], although a survival benefit was inconclusive for those aged 65 years or older who received an expanded criteria donor transplant [61]. Therefore, high kidney transplant rates should remain a priority, even if this means a further increase in the prevalence of KRT.
This study has several strengths and limitations. We included data from a very large patient cohort assembled from multiple European national and regional KRT registries representing countries in Northern, Western and Southern Europe that have provided individual patient data for many years. Extensive data quality control was conducted to ensure optimal quality. Still, the interpretation and assignment of PRD coding might vary between participating KRT registries [62]. Data on race and ethnicity and comorbidities are not available for all countries in the ERA Registry. We were however able to adjust for age and primary renal disease, which accounts for a large part of the confounding effect of comorbidity [63]. Finally, the results are based on patients using KRT for ESKD but not on ESKD patients who are not being treated with KRT or those receiving conservative care.
CONCLUSIONS
With increasing incidence and survival on KRT over the last decade, the prevalence of KRT in Europe continues to rise. Variation in incidence trends across individual countries and regions might be explained by—for example—differences and changes over time in clinical practice. A substantial increase in KRT incidence was found among men older than 65 years of age, possibly reflecting changes in treatment selection or comorbidity patterns compared with women of similar age. The KRT incidence also increased due to expansion of diabetes mellitus, hypertension and glomerulonephritis/sclerosis causing kidney failure. Even though overall patient survival on KRT improved, there was no change in kidney transplant outcomes in older patients. Continued efforts are required to monitor and reduce complications of primary renal diseases in order to delay the progression to KRT. In addition, we need to ensure future access to high quality KRT care including kidney transplantation. Even if this will further increase the prevalence of KRT, higher transplant rates are needed.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry (OEDTR) (F. Engler, R. Kramar, G. Mayer and the Austrian Society of Nephrology); Dutch speaking Belgian Society of Nephrology (NBVN) (M. Couttenye and F. Schroven); French speaking Belgian Society of Nephrology (GNFB) (J.M. des Grottes); Renal Registry Danish Nephrology Registry (DNS) (K. Hommel); Finnish Registry for Kidney Diseases (P. Finne and H. Niemelä); France: The Epidemiology and Information Network in Nephrology (REIN) (C. Couchoud); Hellenic Renal Registry (G. Moustakas); Icelandic End-Stage Renal Disease Registry; Norwegian Renal Registry (A. Åsberg); Swedish Renal Registry (SRR) (K.G. Prütz, M. Evans, S. Schön, T. Lundgren and H. Rydell); Dutch Renal Registry (RENINE) (L. Heuveling and S. Vogelaar); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (SRR) (all of the Scottish renal units); and the regional registries of Andalusia (SICATA) [P. Castro de la Nuez (on behalf of all users of SICATA)], Aragon (F. Arribas Monzón), Asturias (P. Beltrán, M. Rodríguez, J.R. Quirós and RERCA Working Group), Basque country (UNIPAR) (Á. Magaz, J. Aranzabal, M. Rodrigo and I. Moina), Cantabria (J.C. Ruiz San Millán), Castile and León (M.A. Palencia García), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia (RMRC) (J. Comas and J. Tort), Community of Madrid (M.I. Aparicio de Madre and F. Tornero Molina), Extremadura [all the renal units (Nephrology and Dialysis)], Galicia (E. Bouzas-Caamaño) and Valencian region (O. Zurriaga); and the other ERA Registry committee members not mentioned above for their advice in the analysis and the drafting of this paper: C. Wanner, P. Ambühl; M. Arnol, J. Harambat, J.E. Sánchez-Alvarez, S.S. Sørensen and E. Vidal; and R. Boenink, and M. Astley in the AMC Registry office for data collection and management. The ERA Registry is funded by the European Renal Association (ERA). This article was written by J.A. Huijben et al. on behalf of the ERA Registry, which is an official body of the ERA. This ERA Registry study was funded by the ERA. The funders had no role in the design of the study and the collection, analyses, interpretation of data and in writing the manuscript.
Contributor Information
Jilske A Huijben, Department of Medical Informatics, ERA Registry, Amsterdam UMC, Academic Medical Center, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Anneke Kramer, Department of Medical Informatics, ERA Registry, Amsterdam UMC, Academic Medical Center, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV - Nephrology and Hypertension, Medical University Innsbruck, Austria.
Johan de Meester, Department of Nephrology, Dialysis and Hypertension, Dutch-speaking Belgian Renal Registry (NBVN), Sint-Niklaas, Belgium.
Frederic Collart, French-Belgian ESRD Registry (GNFB), Brussels, Belgium.
Olga Lucía Rodríguez Arévalo, Valencia Region Renal Registry, Dirección General de Salut Publica i Adiccions, Valencia, Spain; Doctoral student of the Technologies for Health and Well-being program, Universidad Politécnica de Valencia, Valencia, Spain.
Jaakko Helve, Finnish Registry for Kidney Diseases, Helsinki, Finland; Abdominal Center Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Mathilde Lassalle, REIN Registry, Agence de la Biomédecine, Saint-Denis La Plaine, France.
Runolfur Palsson, Division of Nephrology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland; Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland.
Marc ten Dam, Dutch Registry RENINE, Nefrovisie, Utrecht, The Netherlands.
Anna Casula, UK Renal Registry, the Renal Association, Bristol, UK.
Shona Methven, Department of Renal Medicine, Aberdeen Royal Infirmary, Foresterhill Health Campus, Aberdeen, UK.
Alberto Ortiz, School of Medicine, IIS-Fundacion Jimenez Diaz, University Autonoma of Madrid, FRIAT and REDINREN, Madrid, Spain.
Pietro Manuel Ferraro, U.O.S. Terapia Conservativa della Malattia Renale Cronica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Università Cattolica del Sacro Cuore, Rome, Italy.
Mårten Segelmark, Department of Clinical Sciences, Division of Nephrology, Lund University and Skane University Hospital, Lund, Sweden; Swedish Renal Registry, Department of Internal Medicine, Ryhov County Hospital, Jönköping, Sweden.
Pablo Ucio Mingo, Coordinador Autonómico de Trasplantes de Castilla y León, Dirección General de Planificación y Asistencia Sanitaria, Valladolid, Castilla y León, Spain.
Mustafa Arici, Department of Nephrology, Faculty of Medicine, Hacettepe University, Ankara, Turkey.
Anna Varberg Reisæter, Department of Transplantation Medicine, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Maria Stendahl, Swedish Renal Registry, Department of Internal Medicine, Ryhov County Hospital, Jönköping, Sweden.
Vianda S Stel, Department of Medical Informatics, ERA Registry, Amsterdam UMC, Academic Medical Center, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Kitty J Jager, Department of Medical Informatics, ERA Registry, Amsterdam UMC, Academic Medical Center, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.
CONFLICT OF INTEREST STATEMENT
K.J.J. reports grants from the European Renal Association (ERA). All other authors declare no competing interests. The results presented in this paper have not been published previously in whole or part.
AUTHORS’ CONTRIBUTIONS
A.K. and K.J.J. designed the study protocol and supervised the study. J.A.H. and A.K. analyzed the data. J.A.H. drafted the tables and figures. J.A.H., V.S.S., A.K. and K.J.J. interpreted the data and drafted the manuscript. All authors were involved in data collection for the ERA Registry and reviewed and approved the final version of the manuscript.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham Ret al. . A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 2. Liyanage T, Ninomiya T, Jha Vet al. . Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–1982 [DOI] [PubMed] [Google Scholar]
- 3. Nitta K, Masakane I, Hanafusa Net al. . Annual dialysis data report for 2017, JSDT Renal Data Registry: survey methods, facility data, incidence, prevalence, and mortality. Ren Replace Ther 2020; 6: 41 [Google Scholar]
- 4. Pippias M, Jager KJ, Kramer Aet al. . The changing trends and outcomes in renal replacement therapy: data from the ERA-EDTA registry. Nephrol Dial Transplant 2016; 31: 831–841 [DOI] [PubMed] [Google Scholar]
- 5. Kramer A, Stel V, Zoccali Cet al. . An update on renal replacement therapy in Europe: ERA-EDTA registry data from 1997 to 2006. Nephrol Dial Transplant 2009; 24: 3557–3566 [DOI] [PubMed] [Google Scholar]
- 6. Stengel B, Billon S, Van Dijk PCet al. . Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990-1999. Nephrol Dial Transplant 2003; 18: 1824–1833 [DOI] [PubMed] [Google Scholar]
- 7. Johansen KL, Chertow GM, Foley RNet al. . US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2021; 77: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australia and New Zealand Dialysis and Transplant Registry. 30th annual ANZDATA report 2007. https://www.anzdata.org.au/report/anzdata-30th-annual-report-2007 (8 August 2021, date last accessed)
- 9. Moist LM, Fenton S, Kim JSet al. . Canadian organ replacement register (CORR): reflecting the past and embracing the future. Can J Kidney Health Dis 2014;1: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. United States Renal Data System . 2015 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Volume 2 - End-stage Renal Disease (ESRD) in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015 [Google Scholar]
- 11. United Nations Statistics Division . Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/Published 1999. Updated 2020 (8 August 2021, date last accessed)
- 12. Statistical Office of the European Communities. (1990). EUROSTAT: Regional statistics: Reference guide. Luxembourg: Eurostat. URL https://ec.europa.eu/eurostat/ (8 August 2021, date last accessed). [Google Scholar]
- 13. Kim HJ, Fay MP, Feuer EJet al. . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–351 [DOI] [PubMed] [Google Scholar]
- 14. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021; URL https://www.R-project.org/ (8 August 2021, date last accessed). [Google Scholar]
- 15. Joinpoint Regression Program, Version 4.8.0.1 - April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. https://surveillance.cancer.gov/joinpoint/download (20 July 2021, date last accessed) [Google Scholar]
- 16. Canadian Institute for Health Information . Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2006 to 2015: End-Stage Kidney Disease and Kidney Transplants—Data Tables. Ottawa, ON: CIHI [Google Scholar]
- 17. ANZDATA Registry Report 1998 Australia and New Zealand Dialysis and Transplant Registry, Chapter 2: New patients, Adelaide, South Australia.http://www.anzdata.org.au (8 August 2021, date last accessed). [Google Scholar]
- 18. ANZDATA Registry . 43rd Report, Chapter 1: Incidence of Renal Replacement Therapy for End Stage Kidney Disease. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2020; http://www.anzdata.org.au (8 August 2021, date last accessed). [Google Scholar]
- 19. Canadian Institute for Health Information . Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2010 to 2019: End-Stage Kidney Disease and Kidney Transplants—Data Tables. Ottawa, ON: CIHI, 2020 [Google Scholar]
- 20. Caskey FJ, Kramer A, Elliott RFet al. . Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011; 26: 2604–2610 [DOI] [PubMed] [Google Scholar]
- 21. Catalano R. Health, medical care, and economic crisis. N Engl J Med 2009; 360: 749–751 [DOI] [PubMed] [Google Scholar]
- 22. Stuckler D, Basu S, Suhrcke Met al. . The public health effect of economic crises and alternative policy responses in Europe: an empirical analysis. Lancet 2009; 374: 315–323 [DOI] [PubMed] [Google Scholar]
- 23. Wauters JP, Bosson JL, Forneris Get al. . Patient referral is influenced by dialysis centre structure in the Diamant Alpin Dialysis cohort study. Nephrol Dial Transplant 2004; 19: 2341–2346 [DOI] [PubMed] [Google Scholar]
- 24. Couchoud C, Guihenneuc C, Bayer Fet al. . The timing of dialysis initiation affects the incidence of renal replacement therapy. Nephrol Dial Transplant 2010; 25: 1576–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van de Luijtgaarden MW, Noordzij M, Tomson Cet al. . Factors influencing the decision to start renal replacement therapy: results of a survey among European nephrologists. Am J Kidney Dis 2012; 60: 940–948 [DOI] [PubMed] [Google Scholar]
- 26. Liabeuf S, McCullough K, Young EWet al. . International variation in the management of mineral bone disorder in patients with chronic kidney disease: results from CKDopps. Bone 2019; 129: 115058. [DOI] [PubMed] [Google Scholar]
- 27. Wong MMY, Tu C, Li Yet al. . Anemia and iron deficiency among chronic kidney disease stages 3-5ND patients in the chronic kidney disease outcomes and practice patterns study: often unmeasured, variably treated. Clin Kidney J 2020; 13: 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saweirs WW, Goddard J.. What are the best treatments for early chronic kidney disease? A background paper prepared for the UK consensus conference on early chronic kidney disease. Nephrol Dial Transplant 2007; 22: ix31–ix38 [DOI] [PubMed] [Google Scholar]
- 29. Wouters OJ, O'Donoghue DJ, Ritchie Jet al. . Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol 2015; 11: 491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sane Schepisi M, Di Napoli A, Asciutto Ret al. . The 2008 financial crisis and changes in lifestyle-related behaviors in Italy, Greece, Spain, and Portugal: a systematic review. Int J Environ Res Public Health 2021; 18: 8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Rijn MHC, Alencar de Pinho N, Wetzels JFet al. . Worldwide disparity in the relation between CKD prevalence and kidney failure risk. Kidney Int Rep 2020; 5: 2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morton RL, Turner RM, Howard Ket al. . Patients who plan for conservative care rather than dialysis: a national observational study in Australia. Am J Kidney Dis 2012; 59: 419–427 [DOI] [PubMed] [Google Scholar]
- 33. Chandna SM, Carpenter L, Da Silva-Gane Met al. . Rate of decline of kidney function, modality choice, and survival in elderly patients with advanced kidney disease. Nephron 2016; 134: 64–72 [DOI] [PubMed] [Google Scholar]
- 34. Carrero JJ, Hecking M, Chesnaye NCet al. . Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018; 14: 151–164 [DOI] [PubMed] [Google Scholar]
- 35. Wongrakpanich S, Susantitaphong P, Isaranuwatchai Set al. . Dialysis therapy and conservative management of advanced chronic kidney disease in the elderly: a systematic review. Nephron 2017; 137: 178–189 [DOI] [PubMed] [Google Scholar]
- 36. Brown EA, Johansson L.. Epidemiology and management of end-stage renal disease in the elderly. Nat Rev Nephrol 2011; 7: 591–598 [DOI] [PubMed] [Google Scholar]
- 37. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1223–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392: 1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ceretta ML, Noordzij M, Luxardo Ret al. . Changes in co-morbidity pattern in patients starting renal replacement therapy in Europe-data from the ERA-EDTA registry. Nephrol Dial Transplant 2018; 33: 1794–1804 [DOI] [PubMed] [Google Scholar]
- 40. van Dijk PR, Kramer A, Logtenberg SJet al. . Incidence of renal replacement therapy for diabetic nephropathy in the Netherlands: Dutch diabetes estimates (DUDE)-3. BMJ Open 2015; 5: e005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. United States Renal Data System . 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 42. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298: 194–206 [DOI] [PubMed] [Google Scholar]
- 43. Richter B, Bandeira-Echtler E, Bergerhoff Ket al. . Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008; 2: CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGill JB, Sloan L, Newman Jet al. . Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 2013; 36: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ortiz A, Ferro CJ, Balafa Oet al. . Mineralocorticoid receptor antagonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. Nephrol Dial Transplant 2021: 1–16 [DOI] [PubMed] [Google Scholar]
- 46. Bhatt DL, Szarek M, Pitt Bet al. . Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021; 384: 129–139 [DOI] [PubMed] [Google Scholar]
- 47. Wheeler DC, Stefansson BV, Jongs Net al. . Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021; 9: 22–31 [DOI] [PubMed] [Google Scholar]
- 48. Perkovic V, Jardine MJ, Neal Bet al. . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 49. Stel VS, Awadhpersad R, Pippias Met al. . International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrology (Carlton) 2019; 24: 1064–1076 [DOI] [PubMed] [Google Scholar]
- 50. United States Renal Data System . 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Reference tables incidence A2. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019 [Google Scholar]
- 51. Australia and New Zealand Dialysis and Transplant Registry. 43rd annual ANZDATA report. https://www.anzdata.org.au/report/anzdata-43rd-annual-report-2020-data-to-2019/ (8 August 2021, date last accessed)
- 52. Evans M, Xu H, Rydell Het al. . Association between implementation of novel therapies and improved survival in patients starting hemodialysis: the Swedish renal registry 2006-2015. Nephrol Dial Transplant 2020; 36: 1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boenink R, Stel VS, Waldum-Grevbo BEet al. . Data from the ERA-EDTA registry were examined for trends in excess mortality in European adults on kidney replacement therapy. Kidney Int 2020; 98: 999–1008 [DOI] [PubMed] [Google Scholar]
- 54. Pippias M, Stel VS, Arnol Met al. . Temporal trends in the quality of deceased donor kidneys and kidney transplant outcomes in Europe: an analysis by the ERA-EDTA registry. Nephrol Dial Transplant 2021; 37: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moers C, Smits JM, Maathuis MHet al. . Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 2009; 360: 7–19 [DOI] [PubMed] [Google Scholar]
- 56. Montgomery RA, Lonze BE, King KEet al. . Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 2011; 365: 318–326 [DOI] [PubMed] [Google Scholar]
- 57. Maggiore U, Oberbauer R, Pascual Jet al. . Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant 2015; 30: 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matesanz R, Coll E, Dominguez-Gil Bet al. . Benchmarking in the process of donation after brain death: a methodology to identify best performer hospitals. Am J Transplant 2012; 12: 2498–2506 [DOI] [PubMed] [Google Scholar]
- 59. Mohnen SM, van Oosten MJM, Los Jet al. . Healthcare costs of patients on different renal replacement modalities - Analysis of Dutch health insurance claims data. PLoS One 2019; 14: e0220800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med 2021; 385: 729–743 [DOI] [PubMed] [Google Scholar]
- 61. Hellemans R, Kramer A, De Meester Jet al. . Does kidney transplantation with a standard or expanded criteria donor improve patient survival? Results from a Belgian cohort. Nephrol Dial Transplant 2021; 36: 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Venkat-Raman G, Tomson CR, Gao Yet al. . New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 2012; 27: 4414–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Manen JG, van Dijk PC, Stel VSet al. . Confounding effect of comorbidity in survival studies in patients on renal replacement therapy. Nephrol Dial Transplant 2007; 22: 187–195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.