ABSTRACT
Background
Fibrosis is a suggested cause of graft failure and mortality among kidney transplant recipients (KTRs). Accumulating evidence suggests that collagen type VI is tightly linked to fibrosis and may be a marker of systemic fibrosis and ageing. We studied whether plasma endotrophin, a pro-collagen type VI fragment, is associated with graft failure and mortality among KTRs.
Methods
In cohort A (57% male, age 53 ± 13 years), we measured plasma endotrophin in 690 prevalent KTRs ≥1 year after transplantation. The non-overlapping cohort B included 500 incident KTRs with serial endotrophin measurements before and after kidney transplantation to assess trajectories and intra-individual variation of endotrophin.
Results
In cohort A, endotrophin was higher in KTRs compared with healthy controls. Concentrations were positively associated with female sex, diabetes, cardiovascular disease, markers of inflammation and kidney injury. Importantly, endotrophin was associated with graft failure {hazard ratio [HR] per doubling 1.87 [95% confidence interval (CI) 1.07–3.28]} and mortality [HR per doubling 2.59 (95% CI 1.73–3.87)] independent of potential confounders. Data from cohort B showed that endotrophin concentrations strongly decrease after transplantation and remain stable during post-transplantation follow-up [intra-individual coefficient of variation 5.0% (95% CI 3.7–7.6)].
Conclusions
Plasma endotrophin is strongly associated with graft failure and mortality among KTRs. These findings suggest a key role of abnormal extracellular matrix turnover and fibrosis in graft and patient prognosis among KTRs and highlight the need for (interventional) studies targeting the profibrotic state of KTRs. The intra-individual stability after transplantation indicates potential use of endotrophin as a biomarker and outcome measure of fibrosis.
Trial registration
Keywords: collagen, extracellular matrix, fibrosis, inflammation, kidney transplantation, mortality
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Long-term outcomes after kidney transplantation remain limited due to the risks of graft failure and premature mortality.
Fibrosis and accelerated aging are suggested causes of both adverse outcomes.
An abnormal extracellular matrix signature, particularly an increased abundance of collagen type VI, was recently identified as a key feature in aging and chronic (kidney) disease.
What this study adds?
Plasma concentrations of the pro-collagen type VI fragment endotrophin, which is related to abnormal extracellular matrix turnover and fibrosis, are strongly elevated among KTRs.
Endotrophin concentrations are strongly and independently associated with a higher risk of graft failure and mortality.
The intra-individual coefficient of variation in endotrophin measurements is low.
What impact this may have on practice or policy?
The independent associations of endotrophin with clinical outcomes suggest a key role of abnormal extracellular matrix turnover in graft and patient prognosis after kidney transplantation.
The striking magnitude of these associations, particularly with mortality, together with existing preclinical evidence, suggests that therapies targeting the extracellular matrix may hold therapeutic potential with great effect sizes.
Its intra-individual stability after transplantation suggests that endotrophin may hold potential as a scientific and/or clinical marker of biological aging and systemic fibrosis.
INTRODUCTION
Long-term outcomes after kidney transplantation remain limited. First, graft failure remains a continuous threat to kidney transplant recipients (KTRs), and its occurrence necessitates the start of dialysis or retransplantation [1]. Second, KTRs remain at increased risk of premature death compared with the general population, even after successful transplantation [2]. Inflammation, fibrosis and accelerated aging are regarded as important underlying causes for both graft failure and premature death in the KTR population [3–5]. Recent study results in mice have shown that an abnormal extracellular matrix signature is a key feature in aging and (kidney) disease [6]. One of the most prominent and consistent features of this abnormal extracellular matrix was the increased abundance of collagen type VI.
Interestingly, collagen type VI and the pro-collagen type VI fragment endotrophin [7] have already been linked to pro-inflammatory and profibrotic processes in the past. For example, a role for endotrophin is suggested in liver and lung fibrosis, and it has been shown that knockout of collagen type VI reduces ischaemia-induced injury and improves long-term cardiac performance [8–12]. More recently, it was shown that endotrophin is present in fibrotic kidneys, but not in histologically normal kidneys, in KTRs and patients with chronic kidney disease (CKD) [13, 14] and that circulating endotrophin correlates with the extent of renal interstitial fibrosis (IF) and tubular atrophy (TA) in patients with immunoglobulin A nephropathy and anti-neutrophil cytoplasmic antibody–associated vasculitis [15]. Plasma endotrophin, as a marker of abnormalities in the extracellular matrix, may therefore be a valuable non-invasive marker for fibrosis and aging. Indeed, endotrophin is consistently associated with adverse outcomes, including the development of end-stage kidney disease, kidney disease progression, cardiovascular events and all-cause mortality, in multiple populations [13, 16–20].
Interestingly, endotrophin is not an inactive end product of fibrosis, but supposedly also plays active detrimental biological roles in stimulating fibrosis, inflammation and metabolic dysfunction [21–23], rendering it a potential therapeutic target. However, the actual role of endotrophin in fibrosis and extracellular matrix abnormalities remains unclear, and its importance in graft and patient prognosis in KTRs is unexplored.
We therefore studied clinical and biochemical parameters associated with endotrophin in a cohort of KTRs at least 1 year after transplantation. In addition, we assessed the associations of plasma endotrophin with graft failure and mortality. Finally, we assessed individual trajectories of plasma endotrophin concentrations before and after kidney transplantation to identify changes in fibrotic states after kidney transplantation and to assess the intra-individual variation of endotrophin measurements over time after transplantation.
MATERIALS AND METHODS
This study is in accordance with the guidelines for STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) [24] and the STROBE checklist is provided in Supplementary Table 1. The flow of the study populations is visualized in Supplementary Fig. 1.
Study population—cohort A
We used data and samples from a previously described cohort including 707 adult KTRs with a functioning kidney graft ≥1 year after transplantation who visited the outpatient clinic of the University Medical Center Groningen (UMCG), Groningen, The Netherlands, between November 2008 and June 2011 [25]. All included patients provided written informed consent. This study was approved by the UMCG institutional review board (METc 2008/186) and adheres to the Declarations of Helsinki and Istanbul (ClinicalTrials.gov identifier: NCT02811835). The primary endpoints of the study were mortality and death-censored graft failure, which was defined as a return to dialysis or retransplantation. The continuous surveillance system of the UMCG ensured up-to-date information regarding patient and graft status, and no patients were lost to follow-up.
In the same study performed in the UMCG, an additional population of potential kidney donors before donation was included as a healthy control group.
Study population—cohort B
Another non-overlapping cohort (cohort B) consisted of 500 patients participating in the ongoing, prospective TransplantLines Biobank and Cohort Study [26]. From June 2015, this study included patients prior to their kidney transplantation, with follow-up including biobanking at 3 and 6 months and 1, 2 and 5 years after kidney transplantation in the UMCG. All patients provided written informed consent. The TransplantLines Biobank and Cohort Study was approved by the UMCG institutional review board (METc 2014/077), adheres to the UMCG Biobank Regulation and is in accordance with the Declarations of Helsinki and Istanbul (ClinicalTrials.gov identifier: NCT03272841).
Data collection and definitions
Clinical characteristics, including demographics and medical history, were extracted from patient files. Diabetes was defined using the American Diabetes Association definition. Kidney function was estimated using the creatinine-based estimated glomerular filtration rate (eGFR), according to the creatinine-based Chronic Kidney Disease Epidemiology Collaboration formula.
Biochemical analyses
Plasma endotrophin (PRO-C6) was measured using an enzyme-linked immunosorbent assay (ELISA) developed at Nordic Bioscience (Herlev, Denmark), that detects an active fragment of collagen type VI, which is released upon deposition in the extracellular matrix. Other clinical chemistry assays including parameters of kidney function, glucose homeostasis and inflammation were performed using routine spectrophotometric methods (Roche Diagnostics, Basel, Switzerland). Markers of tubular damage including urinary liver-type fatty acid–binding protein (uL-FABP), plasma neutrophil gelatinase–associated lipocalin (pNGAL) and urinary epidermal growth factor:creatinine ratio (uEGF:Cr) were determined according to methods described in detail previously [27–29].
Statistical analyses
Clinical and biochemical parameters at baseline of cohort A are presented as mean ± standard deviation (SD), median [interquartile range (IQR)] or count (%), depending on the data distribution. Plasma endotrophin concentrations among KTRs and healthy controls were visualized using a density plot and statistically tested for difference using a Mann–Whitney U-test. Furthermore, we presented baseline characteristics of patients depending on plasma endotrophin concentrations, where a cut-off was arbitrarily defined as the 95th percentile of endotrophin in healthy controls. To assess associations of clinical and biochemical parameters with plasma endotrophin among KTRs, univariable linear regression analyses were performed in cohort A, with log2 plasma endotrophin as a dependent variable. We then adjusted for age, sex, creatinine and donor status to identify other independent potential determinants of plasma endotrophin concentrations. Finally, we performed a backwards linear regression model, where variables with a P-value <.10 in univariable analyses were included in the initial model, followed by stepwise exclusion of variables with a P-value >.05. Normality of the residuals was evaluated by visual inspection of Q-Q plots, where variables were transformed using a log2 transformation if necessary to reach assumptions for linear regression. Regression coefficients are presented as standardized β values (St. β), referring to the number of SDs the dependent variable changes per SD increase of the independent variable, thus allowing for comparison of the strength of the associations of different variables.
Kaplan–Meier curves were used to visualize differences in graft and patient survival between tertiles of plasma endotrophin concentrations in cohort A. The significances of differences between the tertiles were assessed using logrank tests. Cox proportional hazards regression analyses were used to assess associations of endotrophin with death-censored graft failure and death in cohort A, where the associations were adjusted for known determinants of mortality and graft failure [30], and predefined variables that may be in the causal path of endotrophin, such as markers of inflammation and fibrosis [27–29]. In all prognostic analyses, multiple imputation was used to account for missing values other than values on plasma endotrophin, where the number of imputed values is reported in the table footnotes. The imputed values were visually checked for biological plausibility. Schoenfeld residuals were visually checked and tested, and the final models did not violate the assumption for proportionality of hazards (P = .08 and P = .28 for the final models for graft failure and mortality, respectively). Hazard ratios (HRs) are presented per doubling of endotrophin, with 95% confidence intervals (CIs). To visualize the associations of endotrophin with graft failure and mortality, log2-transformed endotrophin was individually plotted against the risks of graft failure and mortality.
In addition, in cohort B, we determined serial measurements of plasma endotrophin over time to identify individual trajectories and intra-individual variation of endotrophin concentrations. We included patients with one or more samples at the time points pretransplantation, at 3 and 6 months and at 1 and 2 years after kidney transplantation. Individual trajectories and population means were visualized. The statistical significance of differences in circulating concentrations before and at 3 months after transplantation were assessed using paired t-tests with exclusion of patients with missing endotrophin either pretransplantation or at 3 months after transplantation. The stability of circulating endotrophin concentrations over time was assessed by calculating the intra-individual coefficient of variation of log2 plasma endotrophin using the intra-individual SD divided by the mean among participants with no missing data for the time points 3 months, 6 months and 1 year after transplantation. All data were analysed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses, P-values <.05 were considered statistically significant.
RESULTS
Baseline characteristics—cohort A
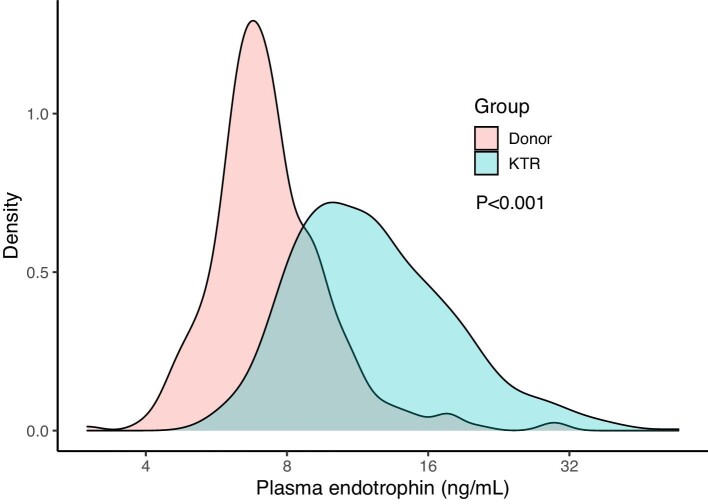
All 690 KTRs [age 53 ± 13 years, 43% female, median 5.4 years (IQR 1.9–12.0) after transplantation] with available plasma samples were included in the current analyses. Plasma endotrophin concentrations at baseline were 11.7 ng/ml (IQR 9.2–15.6), which was higher compared with a healthy control group of 324 potential kidney donors [54% female, age 54 ± 13 years, endotrophin 7.1 ng/ml (IQR 6.2–8.5), P < .001; Fig. 1]. In total, 99% of cohort A used prednisolone, whereas 83% used a proliferation inhibitor and 57% of used a calcineurin inhibitor. More detailed baseline characteristics of the KTRs in cohort A are presented in Table 1. The 95th percentile of endotrophin concentrations among healthy controls was 12.0 ng/ml. Baseline characteristics of KTRs below and above this cut-off are presented in Supplementary Table 2.
Figure 1:
Density plot of plasma endotrophin in donors versus KTRs. P-value indicates the significance of the difference between donors and KTRs as calculated using a Mann–Whitney U-test.
Table 1:
Population characteristics at baseline (N = 690).
| Characteristics | Values |
|---|---|
| Plasma endotrophin (ng/ml), median (IQR) | 11.7 (9.2–15.6) |
| Clinical characteristics | |
| Female sex, n (%) | 295 (43) |
| Age (years), mean (SD) | 53 (13) |
| Primary renal disease, n (%) | |
| Unknown | 108 (16) |
| Glomerulonephritis | 182 (26) |
| Interstitial nephritis | 87 (13) |
| Cystic kidney disease | 143 (21) |
| Other congenital/hereditary disease | 39 (6) |
| Renal vascular disease | 38 (5) |
| Diabetic nephropathy | 29 (4) |
| Other multisystem diseases | 46 (7) |
| Other | 18 (3) |
| Body mass index (kg/m2), mean (SD) | 26.7 (4.8) |
| Systolic blood pressure (mmHg), mean (SD) | 136 (17) |
| Diabetes, n (%) | 161 (23) |
| History of cardiovascular disease, n (%) | 168 (24) |
| Current smoking, n (%) | 84 (13) |
| Pre-emptive transplantation, n (%) | 107 (16) |
| Time after transplantation (years), median (IQR) | 5.4 (1.9–12.0) |
| History of rejection, n (%) | 183 (27) |
| History of delayed graft function, n (%) | 51 (7) |
| Anti-HLA class II antibodies, n (%) | 119 (17) |
| Donor age (years), mean (SD) | 43 (15) |
| Living donor, n (%) | 235 (34) |
| Laboratory measurements | |
| Haemoglobin (mmol/l), mean (SD) | 8.23 (1.07) |
| Sodium (mmol/l), mean (SD) | 140.9 (2.8) |
| Potassium (mmol/l), mean (SD) | 3.98 (0.47) |
| Creatinine (µmol/l), median (IQR) | 125 (100–160) |
| eGFR (ml/min/1.73 m2), mean (SD) | 52 (20.4) |
| Urea (mmol/l), median (IQR) | 9.5 (7.2–13.3) |
| HbA1c (%), median (IQR) | 5.8 (5.5–6.2) |
| Leukocyte count (×109/l), mean (SD) | 8.1 (2.6) |
| hs-CRP (mg/l), median (IQR) | 1.6 (0.7–4.5) |
| Albumin (g/l), mean (SD) | 43.0 (3.0) |
| Urinary protein excretion (g/24 h), median (IQR) | 0.21 (0.01–0.38) |
| uL-FABP (µg/24 h), median (IQR) | 2.07 (0.91–7.15) |
| uEGF:Cr ratio (ng/mg), median (IQR) | 6.4 (4.1–10.7) |
| pNGAL (µg/l), median (IQR) | 171 (133–232) |
| Medication, n (%) | |
| Prednisolone | 684 (99) |
| Calcineurin inhibitor | 395 (57) |
| Cyclosporine | 270 (39) |
| Tacrolimus | 125 (18) |
| Proliferation inhibitor | 574 (83) |
| Mycophenolic acid | 452 (66) |
| Azathioprine | 122 (18) |
| mTOR inhibitor | 24 (3) |
Diabetes was defined according to the American Diabetes Association criteria. Data on smoking status were missing in 46 patients (6.6%), data on donor age were missing in 19 patients (2.7%), data on eGFR were missing in 16 patients (2.3%) and data on hs-CRP were missing in 43 patients (5.8%). All other variables had missing data for <10 patients.
mTOR, mammalian target of rapamycin.
Association of endotrophin with clinical and biochemical parameters
Univariable linear regression analyses showed positive associations of weight, body mass index and systolic blood pressure with plasma endotrophin, but these associations were lost after adjustments (Table 2). In multivariable regression analyses adjusted for age, sex, creatinine and donor status, plasma endotrophin was higher in females, patients with a history of diabetes or cardiovascular disease, anti-human leucocyte antigen (HLA) class II antibodies, calcineurin inhibitors use and patients with a deceased donor kidney. Endotrophin was negatively associated with haemoglobin and positively associated with markers of worse kidney function. Higher plasma endotrophin concentrations were also independently associated with higher urinary protein excretion, high-sensitivity C-reactive protein (hs-CRP), pNGAL and uL-FABP.
Table 2:
Linear regression analysis of log2 plasma endotrophin.
| Univariable | Adjusted for age, sex, creatinine, donor status | Backwards modelb | ||||
|---|---|---|---|---|---|---|
| Baseline variables | St. β (95% CI) | P-value | St. β (95% CI) | P-value | St. β (95% CI) | P-value |
| Recipient | ||||||
| Female sex | 0.06 (−0.07–0.08) | .4 | 0.41 (0.30–0.52) | <.001 | 0.33 (0.22–0.44) | <.001 |
| Age | 0.00 (−0.07–0.08) | .9 | 0.07 (0.01–0.12) | .017 | – | – |
| Primary renal disease | ||||||
| Unknown | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Glomerulonephritis | 0.14 (−0.10–0.38) | .2 | 0.06 (−0.11–0.23) | .5 | – | – |
| Interstitial nephritis | 0.04 (−0.24–0.32) | .8 | 0.05 (−0.15–0.25) | .6 | – | – |
| Cystic kidney disease | 0.28 0.03–0.53) | .027 | 0.08 (−0.10–0.26) | .4 | – | – |
| Other congenital/hereditary disease | 0.05 (−0.31–0.42) | .8 | −0.02 (−0.28–0.25) | .9 | – | – |
| Renal vascular disease | 0.15 (−0.22–0.52) | .4 | −0.04 (−0.30–0.22) | .8 | – | – |
| Diabetic nephropathy | 0.87 (0.47–1.28) | <.001 | 0.56 (0.27–0.86) | <.001 | – | – |
| Other multisystem diseases | −0.05 (−0.39–0.30) | .8 | −0.04 (−0.29–0.21) | .7 | – | – |
| Other | 0.19 (−0.30–0.69) | .4 | 0.19 (−0.17–0.54) | .3 | – | – |
| Body mass index | 0.13 (0.05–0.20) | .001 | 0.08 (0.03–0.13) | .004 | – | – |
| Systolic blood pressure | 0.09 (0.01–0.16) | .022 | 0.03 (−0.03–0.08) | .3 | – | – |
| Diabetes | 0.26 (0.08–0.44) | .004 | 0.29 (0.16–0.42) | <.001 | 0.21 (0.09–0.33) | .001 |
| History of cardiovascular disease | 0.17 (−0.00–0.35) | .053 | 0.16 (0.03–0.29) | .015 | – | – |
| Current smoking | 0.13 (−0.10–0.36) | .3 | 0.08 (−0.09–0.25) | .3 | – | – |
| Pre-emptive transplantation | −0.32 (−0.52–−0.11) | .002 | −0.12 (−0.29–0.04) | .15 | – | – |
| Time after transplantationa | 0.08 (0.01–0.16) | .033 | 0.10 (0.04–0.15) | .001 | 0.14 (0.08–0.20) | <.001 |
| History of rejection | −0.41 (0.25–0.58) | <.001 | 0.21 (0.08–0.33) | .001 | – | – |
| History of delayed graft function | 0.40 (0.12–0.69) | .005 | 0.07 (−0.13–0.28) | .5 | – | – |
| Anti-HLA class II antibodies | 0.42 (0.22–0.61) | <.001 | 0.21 (0.07–0.35) | .004 | 0.20 (0.06–0.33) | .004 |
| Donor age | 0.10 (0.02–0.17) | .014 | −0.07 (−0.13 to −0.00) | .036 | – | – |
| Living donor | −0.35 (−0.51 to −0.19) | <.001 | −0.27 (−0.39 to −0.16) | <.001 | – | – |
| Laboratory measurements | ||||||
| Haemoglobin | −0.37 (−0.44 to −0.30) | <.001 | −0.12 (−0.18 to −0.06) | <.001 | – | – |
| Sodium | −0.13 (−0.20 to −0.06) | <.001 | −0.06 (−0.11 to −0.00) | .044 | – | – |
| Potassium | 0.28 (0.21–0.35) | <.001 | 0.10 (−0.04–0.15) | .001 | – | – |
| Creatininea | 0.65 (0.60–0.71) | <.001 | 0.71 (0.65–0.76) | <.001 | 0.33 (0.23–0.43) | <.001 |
| eGFR | −0.65 (−0.70 to −0.59) | <.001 | 0.21 (0.01–0.41) | .040 | – | – |
| Urea | 0.68 (0.62–0.73) | <.001 | 0.33 (0.24–0.42) | <.001 | 0.19 (0.10–0.28) | <.001 |
| HbA1c | 0.04 (−0.03–0.12) | .3 | 0.12 (0.06–0.17) | <.001 | – | – |
| Leucocyte counta | 0.02 (−0.06–0.09) | .6 | 0.02 (−0.03–0.08) | .4 | – | – |
| hs-CRPa | 0.20 (0.13–0.28) | <.001 | 0.14 (0.09–0.20) | <.001 | 0.13 (0.07–0.18) | <.001 |
| Urinary protein excretiona | 0.32 (0.25–0.39) | <.001 | 0.12 (0.06–0.18) | <.001 | – | – |
| uL-FABPa | 0.44 (0.37–0.51) | <.001 | 0.14 (0.07–0.20) | <.001 | 0.10 (0.04–0.16) | .001 |
| uEGF:CR ratioa | −0.55 (−0.61 to −0.48) | <.001 | −0.14 (−0.23 to −0.06) | .001 | – | – |
| pNGALa | 0.59 (0.53–0.65) | <.001 | 0.29 (0.23–0.36) | <.001 | 0.20 (0.13–0.26) | <.001 |
| Medication | ||||||
| Prednisolone | −0.80 (−1.55 to −0.06) | .035 | −0.49 (−1.02–0.05) | .074 | – | – |
| Calcineurin inhibitor | 0.47 (0.32–0.61) | <.001 | 0.22 (0.11–0.33) | <.001 | 0.25 (0.13–0.37) | <.001 |
| Proliferation inhibitor | −0.37 (−0.57–0.17) | <.001 | −0.20 (−0.34 to −0.06) | .007 | – | – |
| mTOR inhibitor | −0.33 (−0.74–0.08) | .12 | −0.17 (−0.46–0.12) | .2 | – | – |
Variables were log2 transformed.
b R 2 of the final model was 0.624.
mTOR: mammalian target of rapamycin.
In a backwards model, female sex and serum creatinine showed the strongest (positive) associations with endotrophin (St. β 0.33, P < .001 and St. β 0.33, P < .001, respectively). In addition, a history of diabetes; time after transplantation; anti-HLA class II antibodies; higher hs-CRP, urea, uL-FABP and pNGAL; and calcineurin inhibitor use were associated with higher endotrophin concentrations.
Associations of endotrophin with graft failure
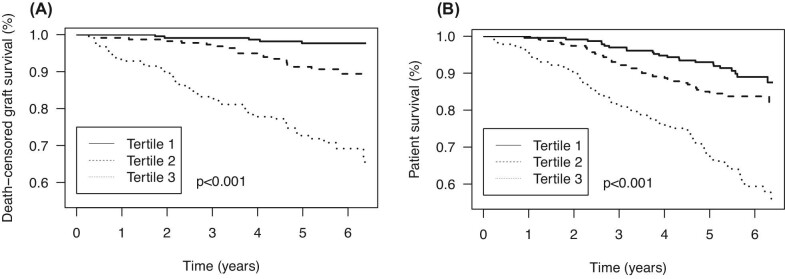
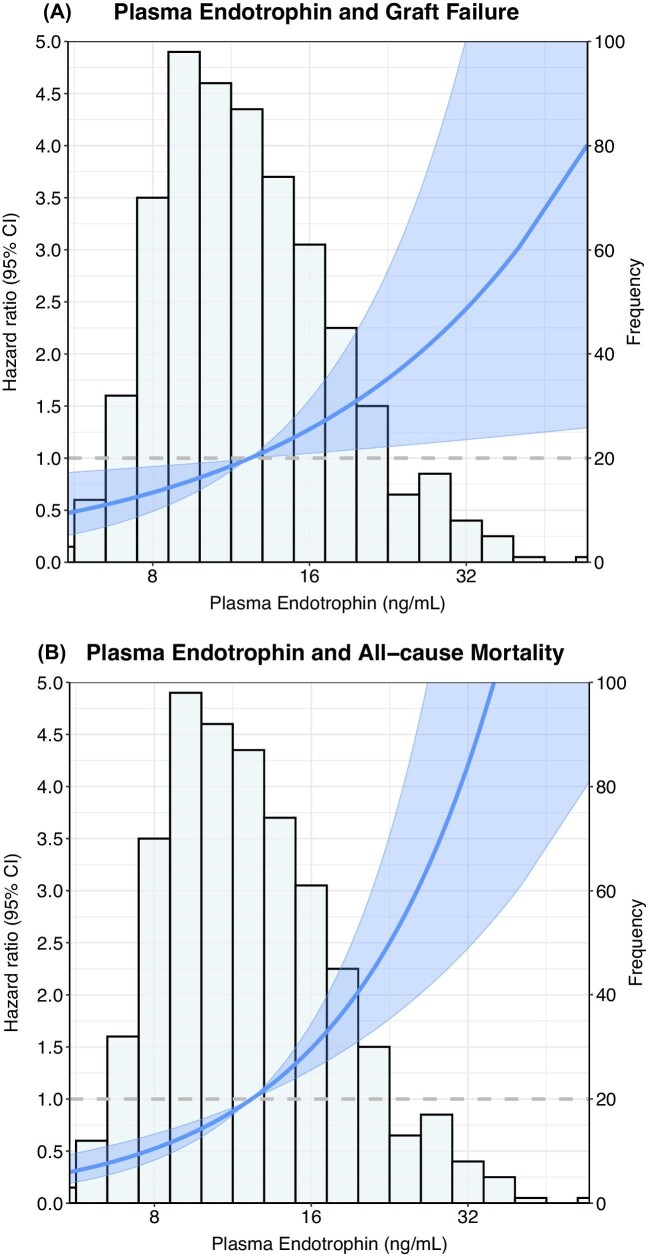
During follow-up of 5.3 years (IQR 4.5–6.0), 84 patients (12.2%) experienced graft failure. Graft failure occurred in 5 (0.7%), 20 (2.9%) and 59 (8.6%) patients in the first, second and third tertile of plasma endotrophin concentrations, respectively (Plogrank < .001; Fig. 2A). Plasma endotrophin was associated with graft failure [HR 4.91 (95% CI 3.59–6.70), P < .001; Table 3). This association weakened after adjustment for eGFR, urinary protein excretion and other confounders, but did not disappear [HR 1.87 (95% CI 1.07–3.28), P = .030], as visually presented in Fig. 3A. No interactions of endotrophin with age and sex were present for this association.
Figure 2:
Kaplan–Meier plot for (A) death-censored graft failure and (B) all-cause mortality per tertile of plasma endotrophin. Tertile 1: plasma endotrophin <9.8 ng/ml; tertile 2: plasma endotrophin 9.9–13.9 ng/ml; tertile 3: plasma endotrophin >13.9 ng/ml. P-value represents the evidence against the null hypothesis of no difference in survival across tertiles, as calculated using the logrank test.
Table 3:
Cox regression analysis of the association of plasma endotrophin with graft failure and all-cause mortality.
| Graft failure | All-cause mortality | |||
|---|---|---|---|---|
| Model | HR per doubling (95% CI) | P-value | HR per doubling (95% CI) | P-value |
| Crude | 4.91 (3.59–6.70) | <.001 | 2.45 (1.91–3.15) | <.001 |
| Model 1 | 4.92 (3.58–6.76) | <.001 | 2.89 (2.20–3.81) | <.001 |
| Model 2 | 2.35 (1.48–3.73) | <.001 | 3.14 (2.22–4.45) | <.001 |
| Model 3 | 2.19 (1.37–3.50) | .001 | 2.86 (1.99–4.11) | <.001 |
| Model 4 | 2.07 (1.28–3.34) | .003 | 2.87 (1.98–4.17) | <.001 |
| Model 5 | 1.89 (1.16–3.08) | .011 | 2.64 (1.81–3.85) | <.001 |
| Model 6 | 1.96 (1.14–3.36) | .015 | 2.60 (1.74–3.87) | <.001 |
| Model 7 | 1.87 (1.07–3.28) | .030 | 2.59 (1.73–3.87) | <.001 |
Model 1 adjusted for age, sex and time after transplantation. Model 2 adjusted for variables in model 1 and eGFR. Model 3 adjusted for variables in model 2 and log2 24-h urinary protein excretion. Model 4 adjusted for variables in model 3 and anti-HLA class II antibodies and use of calcineurin inhibitors. Model 5 adjusted for variables in model 4 and history of diabetes and history of cardiovascular disease. Model 6 adjusted for variables in model 5 and log2 hs-CRP. Model 7 adjusted for variables in model 6 and uEGF:Cr ratio and pNGAL.
In total, 84 patients (12.2%) encountered death-censored graft failure and 146 patients (21.1%) died during a median follow-up time of 5.4 years (IQR 4.8–6.1). Addition of log2 plasma endotrophin significantly augmented the model for graft failure including age, sex, time after transplantation, eGFR, log2 24-h urinary protein excretion, anti-HLA class II antibodies, calcineurin inhibitor use, log2 hs-CRP, history of diabetes mellitus, history of cardiovascular disease, uEGF:Cr ratio and pNGAL (Plikelihood ratio = .032). Addition of log2 plasma endotrophin also significantly augmented the model for mortality including age, sex, time after transplantation, eGFR, log2 24-h urinary protein excretion, anti-HLA class II antibodies, calcineurin inhibitor use, log2 hs-CRP, history of diabetes mellitus, history of cardiovascular disease, uEGF:Cr ratio and pNGAL (Plikelihood ratio < .001).
Figure 3:
Graphical representation of the associations of plasma endotrophin with (A) death-censored graft failure and (B) all-cause mortality. The lines show the adjusted HR and the shaded area corresponds to the pointwise 95% CI based on a Cox proportional hazards regression analyses. The model was adjusted for age, sex, eGFR, 24-h urinary protein excretion, anti-HLA class II antibodies, use of calcineurin inhibitors, history of diabetes, history of cardiovascular disease and log2 hs-CRP and is presented in relation to the histogram of plasma endotrophin.
Associations of endotrophin with all-cause mortality
During follow-up of 5.4 years (IQR 4.8–6.1), 146 patients (21.1%) died. Death occurred in 24 (10.4%) patients in the first tertile, 37 (16.1%) in the second tertile and 85 (37.0%) in the third tertile of endotrophin concentrations (Plogrank < .001; Fig. 2B). Plasma endotrophin was strongly associated with a higher risk of mortality [HR 2.45 (95% CI 1.91–3.15), P < .001], which remained essentially unchanged after adjustment for age, sex, eGFR, urinary protein excretion, presence of anti-HLA class II antibodies, calcineurin inhibitor use, history of diabetes, cardiovascular disease and hs-CRP, as visually presented in Fig. 3B. Importantly, this association did not significantly change after additional adjustment for markers of tubular injury, including pNGAL, uL-FABP and uEGF:Cr. No interactions of endotrophin with age and sex were present for the association with mortality.
Population characteristics and trajectories of endotrophin concentration—cohort B
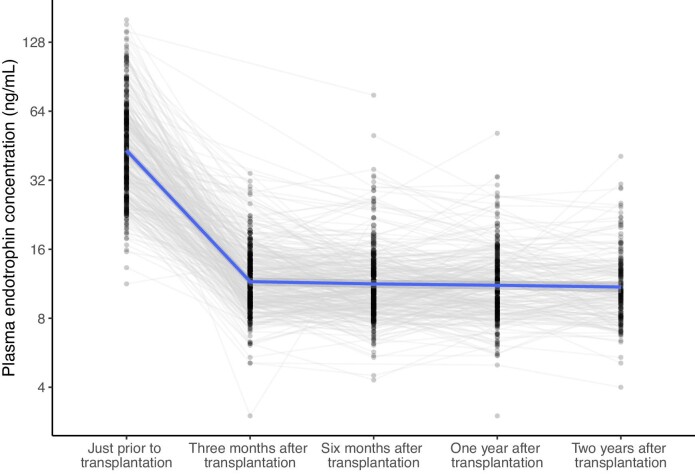
The non-overlapping cohort B consisted of 500 participants (age at transplantation 55 ± 14 years) with at least one measurement before and/or at specified time points after kidney transplantation. Plasma endotrophin concentrations before transplantation were 41.80 ng/ml (IQR 29.90–59.90). These concentrations decreased significantly to 11.50 ng/ml (IQR 9.40–14.00) at 3 months after transplantation (Ppairedt-test < .001) and then remained rather stable during follow-up (Fig. 4). The stability of endotrophin concentrations after transplantation is further illustrated by the low intra-individual coefficient of variation of 5.0% (IQR 3.7–7.6) among the 205 participants with complete data at 3 months, 6 months and 1 year after transplantation.
Figure 4:
Graphical representation of the trajectories of plasma endotrophin concentrations before and at several time points after transplantation. Plasma endotrophin concentrations decrease significantly after transplantation (Ppairedt-test < .001). Number of cases was 342, 359, 346, 306 and 182 for time points prior to transplantation, 3 months, 6 months, 1 year and 2 years after transplantation, respectively.
DISCUSSION
In cohort A including prevalent KTRs, plasma endotrophin concentrations were higher compared with healthy controls, in females and patients with a medical history of diabetes or cardiovascular disease. In addition, higher endotrophin concentrations were associated with worse kidney function, more proteinuria and increased markers of inflammation and tubular damage. Importantly, a doubling of plasma endotrophin was associated with an approximate doubling of the risks of both graft failure and all-cause mortality, independent of potential confounders. Analyses in cohort B showed that endotrophin concentrations are high before transplantation, strongly decrease after transplantation and then remain stable during follow-up, yet are still at markedly higher concentrations than those observed in healthy controls.
Fibrosis and inflammation are important causes of kidney graft dysfunction and graft failure [3]. Potential causes of fibrosis and inflammation may be found in alterations in collagen deposition in the extracellular matrix of the kidney [31]. Indeed, a recent study has shown that the signature of matrix proteins including an increased abundance of collagen type VI, was consistently associated with (kidney) aging and disease [6]. Upregulation of collagen type VI is therefore suggested to be a hallmark or even a causal factor of tissue fibrosis and metabolic dysfunction [21]. Fibrosis and abnormal extracellular matrix may therefore be identified by measuring endotrophin, a cleaved fragment of the C5 domain released during deposition of collagen type VI. Previous studies have indeed confirmed the association of endotrophin with CKD and kidney fibrosis in KTRs and other populations [13, 15, 32]. Other evidence suggests pro-inflammatory effects of endotrophin in patients with CKD [22]. Previously, pretransplant endotrophin was shown to predict delayed graft function after transplantation [33]. The current study confirms that endotrophin concentrations are elevated among KTRs compared with our healthy control group, which may be attributed in part to impaired kidney function as well as metabolic dysfunction in KTRs [21]. In addition, the study further underlines the relationship between collagen type VI and inflammation, since hs-CRP and pNGAL were independently associated with endotrophin concentrations, among others. In addition, factors that are known to induce stress and fibrosis in the kidney, such as a history of diabetes, cardiovascular disease, graft rejection and a longer time after transplantation were all associated with endotrophin. Moreover, the associations of body mass index suggest a link between adipose tissue and endotrophin. This hypothesis is supported by preclinical evidence that showed collagen type VI is upregulated in dysfunctional adipose tissue [21, 34, 35]. In addition, studies have suggested that a loss of muscle is also associated with higher endotrophin [36]. Notably, females generally have higher fat mass and lower muscle mass compared with males, potentially contributing to the observed positive association of female sex with endotrophin in multivariable regression models. The associations with pNGAL, uL-FABP and uEGF:Cr further support the notion of endotrophin as a reflection of kidney injury [27–29].
Since fibrosis and inflammation in the kidney graft are known causes of graft dysfunction, it is biologically plausible that collagen type VI upregulation may be associated with graft outcome [3]. Indeed, our study shows that endotrophin is robustly associated with a higher risk of graft failure, independent of adjustments for known confounders. In Cox regression, we also adjusted for variables potentially in the causal path of endotrophin expression. For example, a history of rejection and anti-HLA class II antibodies may induce fibrosis, which may then be reflected by increased endotrophin concentrations. However, the association of endotrophin with graft failure was robust, even after adjustment for these variables in the causal path. Notably, the associations of endotrophin with graft failure also remained after additional adjustment for markers of tubular injury that were shown to be important prognostic factors in previous studies [27–29]. We speculate that where these markers mainly reflect kidney injury, they do not particularly reflect fibrosis and abnormal extracellular matrix turnover. This may be the reason why endotrophin, which is tightly linked to those processes in particular, provides additional information on top of existing markers.
In addition to local effects on the kidney, upregulation of collagen type VI also has systemic effects. For instance, the increased expression of collagen type VI is well-established in Dupuytren's disease and lung and liver fibrosis [8–11]. Other studies have reported that collagen type VI promotes tumour growth, and a lack of collagen type VI improves cardiac function and remodelling after myocardial infarction [12, 37]. Consequently, it has been suggested that endotrophin and collagen type VI formation may mechanistically contribute to fibrosis, accelerated aging and increased risk of mortality. Indeed, associations of endotrophin with clinical outcome have been reported in patients with CKD, diabetes and other diseases [16–19, 38]. Our findings are fully in line with these studies, since our analyses show strong associations between endotrophin and all-cause mortality independent of kidney function, urinary protein excretion and tubular injury. This further suggests that fibrosis and abnormalities in the extracellular matrix, as measured using plasma endotrophin, are clinically relevant with regard to patient outcome.
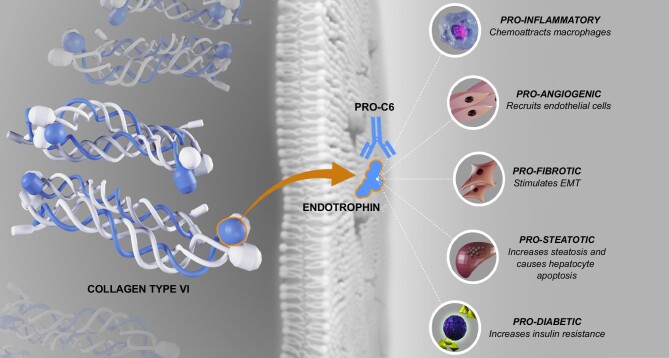
Part of this association may be explained by the notion that endotrophin is a marker of collagen type VI deposition and abnormalities in the extracellular matrix and therefore reflects ongoing fibrosis and biological aging [7]. However, it has also been suggested that endotrophin has direct pathogenic effects, which is corroborated by promising preclinical studies that have already shown that antibodies neutralizing endotrophin counteract tumour growth, hepatic fibrosis and metabolic dysfunction [21, 37, 39–41]. In addition to neutralizing antibodies, endotrophin may potentially be targeted with other drugs such as glucagon-like peptide 1 analogues or renin–angiotensin–aldosterone blockers [42, 43]. All in all, this evidence suggests that endotrophin may not only be a marker for risk stratification among KTRs, but it may also be a promising future target of therapy. It must be noted, however, that fibrosis is an extremely complex process with both beneficial and detrimental consequences throughout the body and that the mechanistic role of endotrophin in biological aging and fibrosis needs further investigation. Nevertheless, these study results suggest that fibrosis and collagen turnover are very important in graft and patient prognosis among KTRs. Indeed, fibrosis and abnormalities in the extracellular matrix may be key targets to improve long-term outcomes in this patient population, with potentially great effect sizes. Part of these fibrosis processes are ongoing in the kidneys, as reflected by the associations of endotrophin with tubular damage markers and graft failure. However, the current observation of associations of endotrophin with mortality independent of kidney function, along with other recent studies, raises the hypothesis that endotrophin may be a marker of ageing and systemic fibrosis that is not organ specific. The interplay between collagen type VI, endotrophin, inflammation and fibrosis is visually presented in Fig. 5.
Figure 5:
Schematic representation of the release of endotrophin from mature type VI collagen following release in the extracellular space and signalling roles of endotrophin [21, 23, 39–41, 44–46].
The data from cohort B regarding individual plasma endotrophin trajectories show that endotrophin concentrations are much higher in patients before transplantation. This may in part be the result of the low kidney function before transplantation, as well as the profibrotic state of these patients with end-stage kidney disease. Circulating endotrophin concentrations strongly decrease after kidney transplantation, which may be explained by kidney function improvement and normalization of extracellular matrix turnover, with an overall improving metabolic profile. However, as observed in both cohort A and cohort B, plasma endotrophin concentrations do not return to levels observed in our healthy control group, indicating that fibrosis is still increased after transplantation. The low intra-individual variation of plasma endotrophin after transplantation is reassuring with regard to the validity of the measurement. This observation highlights that endotrophin concentrations do not vary each day, but are stable, rendering it likely that endotrophin reflects the overall long-term fibrotic state. This may render endotrophin a valuable outcome measure to assess fibrosis in research or to assess treatment effect.
Although these results strongly suggest an important role for extracellular matrix turnover, fibrosis and accelerated biological aging in the prognosis of KTRs, we cannot provide causal evidence due to the observational nature the study. Although our results were robust, and preclinical studies are suggestive of pathophysiological pathways, it remains to be determined whether the association between endotrophin and kidney graft failure and mortality is causal. Future studies including data on biopsies may further substantiate the role of endotrophin in kidney fibrosis and systemic fibrosis. Additionally, this study was performed in a transplant population in the Netherlands, including a mainly Caucasian population. Ideally our results should be replicated in different cohorts, including patients from different races.
CONCLUSION
Plasma concentrations of the pro-collagen type VI fragment endotrophin are elevated among KTRs and are strongly and independently associated with graft failure and mortality. The low intra-individual coefficient of variation after transplantation indicates potential clinical and scientific use. These findings suggest a key role of abnormal extracellular matrix turnover and fibrosis in graft and patient prognosis among KTRs and highlight the need for targeted (interventional) studies.
Supplementary Material
ACKNOWLEDGEMENTS
Fig. 5 was designed by Fotis Mouselimis, whose contribution is gratefully acknowledged.
Contributor Information
Daan Kremer, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Firas F Alkaff, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Division of Pharmacology and Therapy, Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine Universitas Airlangga, Surabaya, Indonesia.
Adrian Post, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Tim J Knobbe, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Martin Tepel, Odense University Hospital, Department of Nephrology, Odense, Denmark; Institute of Molecular Medicine, Cardiovascular and Renal Research, University of Southern Denmark, Odense, Denmark.
Olivier Thaunat, Hospices Civils de Lyon, Hôpital Edouard Herriot, Service de Transplantation, Néphrologie et Immunologie Clinique, Lyon, France.
Stefan P Berger, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Jacob van den Born, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Federica Genovese, Nordic Bioscience, Herlev, Denmark.
Morten A Karsdal, Nordic Bioscience, Herlev, Denmark.
Daniel G K Rasmussen, Nordic Bioscience, Herlev, Denmark.
Stephan J L Bakker, Division of Nephrology, Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
FUNDING
The work was supported by a grant from the European Union, Eurostars (Project E!12850 PRO-C6-Rec, Innovation Fund Denmark, 9046-00025B, 2019). The TransplantLines Biobank and Cohort Study was supported by unrestricted grants from Astellas BV and Chiesi Pharmaceuticals BV. In addition, this collaboration project is co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the PPP allowance made available by Top Sector Life Sciences & Health to stimulate public–private partnerships.
AUTHORS’ CONTRIBUTIONS
M.T., S.P.B., J.v.d.B., O.T., D.G.K.R. and S.J.L.B. conceived and designed the study. D.K., F.F.A. and S.J.L.B. retrieved and validated data and performed the statistical analyses. D.K. wrote the draft manuscript. F.G., M.A.K. and D.G.K.R. planned, supervised and interpreted endotrophin measurements. All authors revised the report for important intellectual content.
DATA AVAILABILITY STATEMENT
Public sharing of individual participant data was not included in the informed consent of the TransplantLines Biobank and Cohort Study, but data can be made available to interested researchers upon reasonable request by sending an e-mail to the data manager of the TransplantLines Biobank and Cohort study (datarequest.transplantlines@umcg.nl).
CONFLICT OF INTEREST STATEMENT
This study was supported by a grant from the European Union, Eurostars, Project ProC6 Rec, Innovation Fund Denmark, 9046-00025B, 2019. The TransplantLines Biobank and Cohort Study was supported by unrestricted grants from Astellas BV and Chiesi Pharmaceuticals BV. In addition, this collaboration project is co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the PPP allowance made available by the Top Sector Life Sciences & Health to stimulate public–private partnerships. F.G., M.A.K. and D.G.K.R. are full-time employees at Nordic Bioscience and F.G. and M.A.K. hold stock. Nordic Bioscience is a privately owned, small–medium-size enterprise partly focused on the development of biomarkers and owns the patent for the ELISA used to measure endotrophin levels. The funders had no role in data collection, analysis or interpretation; trial design; patient recruitment; or any aspect pertinent to the study or the decision to submit it for publication. There was no payment for writing this article by a pharmaceutical company or other agencies. No authors received fees, bonuses or other benefits for the work described in this article and Nordic Bioscience did not have any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The other authors declare no conflicts of interest.
REFERENCES
- 1. Heemann U, Abramowicz D, Spasovski Get al. Endorsement of the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: a European Renal Best Practice (ERBP) position statement. Nephrol Dial Transplant 2011;26:2099–106. [DOI] [PubMed] [Google Scholar]
- 2. Neuberger JM, Bechstein WO, Kuypers DRJet al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) group. Transplantation 2017;101(4 Suppl 2):S1–56. [DOI] [PubMed] [Google Scholar]
- 3. Gago M, Cornell LD, Kremers WKet al. Kidney allograft inflammation and fibrosis, causes and consequences. Am J Transplant 2012;12:1199–207. [DOI] [PubMed] [Google Scholar]
- 4. Viklicky O. Systemic inflammation in kidney transplant candidates: a hidden threat? Transpl Int 2019;32:916–7. [DOI] [PubMed] [Google Scholar]
- 5. Abedini S, Holme I, März Wet al. Inflammation in renal transplantation. Clin J Am Soc Nephrol 2009;4:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Randles MJ, Lausecker F, Kong Qet al. Identification of an altered matrix signature in kidney aging and disease. J Am Soc Nephrol 2021;32:1713–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aigner T, Hambach L, Söder Set al. The C5 domain of Col6a3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem Biophys Res Commun 2002;290:743–8. [DOI] [PubMed] [Google Scholar]
- 8. Specks U, Nerlich A, Colby TVet al. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med 1995;151:1956–64. [DOI] [PubMed] [Google Scholar]
- 9. Williams LM, McCann FE, Cabrita MAet al. Identifying collagen VI as a target of fibrotic diseases regulated by CREBBP/EP300. Proc Natl Acad Sci USA 2020;117:20753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahara T, Sollberg S, Muona Pet al. Type VI collagen gene expression in experimental liver fibrosis: quantitation and spatial distribution of mRNAs, and immunodetection of the protein. Liver 1995;15:78–86. [DOI] [PubMed] [Google Scholar]
- 11. Cescon M, Gattazzo F, Chen Pet al. Collagen VI at a glance. J Cell Sci 2015;128:3525–31. [DOI] [PubMed] [Google Scholar]
- 12. Luther DJ, Thodeti CK, Shamhart PEet al. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res 2012;110:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen DGK, Fenton A, Jesky Met al. Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci Rep 2017;7:17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stribos EGD, Nielsen SH, Brix Set al. Non-invasive quantification of collagen turnover in renal transplant recipients. PLoS One 2017;12:e0175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sparding N, Genovese F, Rasmussen DGKet al. Endotrophin, a collagen type VI-derived matrikine, reflects the degree of renal fibrosis in patients with IgA nephropathy and in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 2022;37:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pilemann-Lyberg S, Rasmussen DGK, Hansen TWet al. Markers of collagen formation and degradation reflect renal function and predict adverse outcomes in patients with type 1 diabetes. Diabetes Care 2019;42:1760–8. [DOI] [PubMed] [Google Scholar]
- 17. Fenton A, Jesky MD, Ferro CJet al. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS One 2017;12:e0175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen DGK, Hansen TW, von Scholten BJet al. Higher collagen VI formation is associated with all-Cause mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care 2018;41:1493–500. [DOI] [PubMed] [Google Scholar]
- 19. Holm Nielsen S, Edsfeldt A, Tengryd Cet al. The novel collagen matrikine, endotrophin, is associated with mortality and cardiovascular events in patients with atherosclerosis. J Intern Med 2021;290:179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staunstrup LM, Bager CL, Frederiksen Pet al. Endotrophin is associated with chronic multimorbidity and all-cause mortality in a cohort of elderly women. EBioMedicine 2021;68: 103391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun K, Park J, Gupta OTet al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun 2014;5:3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y, Gu X, Zhang Net al. Divergent functions of endotrophin on different cell populations in adipose tissue. Am J Physiol Endocrinol Metab 2016;311:E952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim M, Lee C, Seo DYet al. The impact of endotrophin on the progression of chronic liver disease. Exp Mol Med 2020;52:1766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Berg E, Engberink MF, Brink EJet al. Dietary protein, blood pressure and renal function in renal transplant recipients. Br J Nutr 2013;109:1463–70. [DOI] [PubMed] [Google Scholar]
- 26. Eisenga MF, Gomes-Neto AW, van Londen Met al. Rationale and design of TransplantLines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018;8:e024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yepes-Calderón M, Sotomayor CG, Kretzler Met al. Urinary epidermal growth factor/creatinine ratio and graft failure in renal transplant recipients: a prospective cohort study. J Clin Med 2019;8:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yepes-Calderón M, Sotomayor CG, Pena Met al. Urinary liver-type fatty acid-binding protein is independently associated with graft failure in outpatient kidney transplant recipients. Am J Transplant 2021;21:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kremer D, Post A, Gomes-Neto AWet al. Plasma neutrophil gelatinase-associated lipocalin and kidney graft outcome. Clin Kidney J 2021;15:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loupy A, Aubert O, Orandi BJet al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ 2019;366:I4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bülow RD, Boor P.. Extracellular matrix in kidney fibrosis: more than just a scaffold. J Histochem Cytochem 2019;67:643–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yepes-Calderón M, Sotomayor CG, Rasmussen DGKet al. Biopsy-controlled non-invasive quantification of collagen type VI in kidney transplant recipients: a post-hoc analysis of the MECANO trial. J Clin Med 2020;9:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tepel M, Alkaff FF, Kremer Det al. Pretransplant endotrophin predicts delayed graft function after kidney transplantation. Sci Rep 2022;12:4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan T, Muise ES, Iyengar Pet al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 2009;29:1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lastra G, Sowers JR.. Obesity and cardiovascular disease: role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm Mol Biol Clin Investig 2013;15:49–57. [DOI] [PubMed] [Google Scholar]
- 36. Sun S, Henriksen K, Karsdal MAet al. Collagen type III and VI turnover in response to long-term immobilization. PLoS One 2015;10:e0144525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bu D, Crewe C, Kusminski CMet al. Human endotrophin as a driver of malignant tumor growth. JCI Insight 2019;5:e125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chirinos JA, Zhao L, Reese-Petersen ALet al. Endotrophin, a collagen VI formation–derived peptide, in heart failure. NEJM Evid 2022;1:EVIDoa2200091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee C, Kim M, Lee JHet al. COL6A3-derived endotrophin links reciprocal interactions among hepatic cells in the pathology of chronic liver disease. J Pathol 2019;247:99–109. [DOI] [PubMed] [Google Scholar]
- 40. Park J, Scherer PE.. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest 2012;122:4243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park J, Morley TS, Scherer PE.. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol Med 2013;5:935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tuttle KR, Wilson JM, Lin Yet al. 949-P: dulaglutide improves kidney fibrosis biomarker levels in patients with type 2 diabetes and moderate-to-Severe chronic kidney disease. Diabetes 2020;69 (Suppl 1):949–P. [Google Scholar]
- 43. Eruzun H, Toprak İD, Arman Yet al. Serum endotrophin levels in patients with heart failure with reduced and mid-range ejection fraction. Eur J Intern Med 2019;64:29–32. [DOI] [PubMed] [Google Scholar]
- 44. Oh J, Kim CS, Kim Met al. Type VI collagen and its cleavage product, endotrophin, cooperatively regulate the adipogenic and lipolytic capacity of adipocytes. Metabolism 2021;114:154430. [DOI] [PubMed] [Google Scholar]
- 45. Funcke JB, Scherer PE.. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res 2019;60:1648–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karousou E, Luisa D'Angelo M, Kouvidi Ket al. Collagen VI and hyaluronan: the common role in breast cancer. Biomed Res Int 2014;2014:606458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Public sharing of individual participant data was not included in the informed consent of the TransplantLines Biobank and Cohort Study, but data can be made available to interested researchers upon reasonable request by sending an e-mail to the data manager of the TransplantLines Biobank and Cohort study (datarequest.transplantlines@umcg.nl).