Abstract
Background
Gait impairment contributes to falls and frailty. Some studies suggest that cerebral small vessel disease (CSVD) is associated with gait impairment in the general population. We systematically reviewed and meta-analysed the literature on associations of CSVD with gait impairment and falls.
Methods
The protocol was published in PROSPERO (CRD42021246009). Searches of Medline, Cochrane and Embase databases were conducted on 30 March 2022. Cross-sectional and longitudinal studies of community-dwelling adults were included, reporting relationships between diagnosis or neuroimaging markers of CSVD and outcomes related to gait or falls. Partial correlation coefficients were calculated and pooled using a random-effects model for meta-analysis.
Results
The search retrieved 73 studies (53 cross-sectional; 20 longitudinal). Most studies reported an association between CSVD and gait impairments or falls risk: 7/7 studies on CSVD score or diagnosis, 53/67 studies on white matter hyperintensities (WMHs), 11/21 studies on lacunar infarcts, 6/15 studies on cerebral microbleeds and 1/5 studies on perivascular spaces. Meta-analysis of 13 studies found that higher WMH volume was mildly correlated with lower gait speed, in all studies (r = −0.23, 95% confidence interval: −0.33 to −0.14, P < 0.0001). However, there was significant heterogeneity between studies (I2 = 82.95%; tau2 = 0.02; Q = 79.37, P < 0.0001), which was unexplained by variation in age, sex, study quality or if the study adjusted for age.
Conclusions
Findings suggest that CSVD severity is associated with gait impairment, history of falls and risk of future falls. Prevention of CSVD should be part of a comprehensive public health strategy to improve mobility and reduce risk of falls in later life.
Keywords: cerebral small vessel disease, gait, falls, neuroimaging, systematic review, older people
Key Points
This systematic review and meta-analysis examines the literature on the associations of cerebral small vessel disease (CSVD) with gait impairment and falls.
Neuroimaging markers of cerebral small vessel disease (CSVD) (i.e. white matter hyperintensities, lacunar infarcts, cerebral microbleeds and enlarged perivascular spaces) were found to be associated with gait impairment and falls risk.
A meta-analysis found that higher white matter hyperintensity volume was significantly correlated with slower gait speed.
Prevention of CSVD should be part of a comprehensive public health strategy to improve mobility and reduce risk of falls in later life.
Introduction
Cerebral small vessel diseases (CSVDs) are a group of pathologies that affect the small arteries and veins of the brain, of which arteriolosclerosis and cerebral amyloid angiopathy are the two most common forms. On neuroimaging, CSVD can manifest as white matter hyperintensities (WMHs) of presumed vascular origin, lacunar infarcts, cerebral microbleeds (CMBs), cortical superficial siderosis (cSS) and enlarged perivascular spaces (PVSs) [1, 2].
The function of complex brain networks is vulnerable to the effects of CSVD, as CSVD-related injury can affect white matter pathways, subcortical networks or the cortex itself to produce dysfunction. For example, CSVD is a major contributor to age-related cognitive decline [3]. Several studies also suggest that CSVD can impair gait and contribute to risk of falls [4]. Gait is a complex function that requires the coordinated interaction of distributed brain regions [5]. However, there have been no recent systematic reviews or meta-analyses of the association between diagnosis and neuroimaging markers of CSVD and gait impairment or falls.
This systematic review examines the associations between CSVD and gait impairment, future gait decline and risk of falls. Additionally, a meta-analysis was conducted to investigate the relationship between the most common radiological feature of CSVD, WMH volume, and the most commonly reported gait measurement, gait speed. We hypothesised that there would be an association between greater CSVD severity and impaired gait.
Methods
This review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was published in PROSPERO (CRD42021246009). Ethics approval and informed consent were not required due to the nature of the study. Statistical code and data files are available by request to the corresponding author.
Search strategy and selection criteria
We searched Medline, Cochrane and Embase databases for relevant studies from inception to 29 March 2021 using search terms for gait (including gait metrics and assessment methods) and CSVD described in full in Supplementary Appendix A available in Age and Ageing online. The search was rerun on 30 March 2022, to include recent publications. Each study was reviewed by two independent reviewers for eligibility (EES and either BS or CRM). This included screening of titles, abstracts and full texts. Disagreements between reviewers were resolved via discussion.
We included studies that recruited adults (age >18 years) from the general population, or cases with the CSVD subtypes cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) or cerebral amyloid angiopathy (CAA), and reported an association between CSVD (based on radiological evidence of WMH, lacunar infarcts, CMBs, cSS or enlarged PVSs; a CSVD summary score; or a diagnosis of CADASIL or CAA) and one or more of these outcomes: quantitative gait measures (such as gait speed), gait scales (such as the Short Physical Performance Battery [SPPB]), self-report questionnaires, number of falls and risk of future falls. Included studies had to be journal articles published in the English language. We included cross-sectional studies and longitudinal studies of any duration. Clinic-based studies of patients with stroke, Alzheimer’s disease, mild cognitive impairment and other central nervous system diseases such as Parkinson’s disease or multiple sclerosis were excluded.
Data extraction
A predefined data extraction template was used to collect study information. This included general study characteristics (author, title, publication year, source study name, study design type [i.e. longitudinal or cross-sectional] and study duration for longitudinal studies), participant characteristics (sample size, age, female percent and inclusion/exclusion criteria), methods (CSVD definitions, neuroimaging modality used, gait analysis tools used, and gait variables measured, effect estimate and covariates), gait outcomes and falls outcomes. Because most covert brain infarcts in the general population are lacunes, we grouped infarcts not otherwise specified along with lacunar infarcts. Two reviewers (E.E.S. and either B.S. or C.R.M.) extracted data independently from articles, and any differences were resolved by consensus among the three reviewers.
Risk of bias assessment
Risk of bias was assessed by two reviewers (E.E.S. and either B.S. or C.R.M.) using modified versions of the Newcastle–Ottawa Quality Assessment Scale, adapted for cross-sectional studies (see Supplementary Appendix B available in Age and Ageing online) and longitudinal cohort studies (see Supplementary Appendix C available in Age and Ageing online), as appropriate. Discrepancies were resolved by consensus among the three reviewers.
Statistical analysis
After assessing possible ways to meta-analyse CSVD and gait measures, only the combination of WMH volume and gait speed produced a reasonable number of studies to meta-analyse. As such, a meta-analysis of 13 studies was conducted based on a random-effects model wherein partial correlation coefficients were used to describe the linear relationship between WMH volume and gait speed while controlling for the effects of additional variables [6]. This was calculated based on correlation coefficients as previously described [6] and pooled to obtain a summary score. If odds ratios, Spearman’s correlation coefficients or linear regression coefficients were reported, these were converted to correlation coefficients, with standardised regression coefficients first being reverted to unstandardised regression coefficients, if necessary [7–9]. For those studies that reported log-transformed WMH volumes, values were converted to raw WMH volumes [10]. The authors were contacted for missing information [11, 12].
Heterogeneity of the included studies was reported using Q-statistic, tau2 and I2. To investigate possible sources of heterogeneity, the following were conducted: the leave-one-out method, a meta-regression of select study-level characteristics and study quality and a subgroup analysis considering differences between studies that did and did not adjust for age. Publication bias was assessed using funnel plots and Egger’s linear regression test. In cases where multiple studies from the same source data were available, recent studies with larger sample sizes and results most relevant to the research questions were used. Statistical analyses were conducted using R (version 4.1.2) and the metafor package (version 3.0.2).
Results
Search yield
A flowchart of the inclusion of studies is shown in Figure 1. The search on 30 March 2022 included publications from June 1967 onwards and yielded a total of 1716 studies. After excluding duplicates (281) and irrelevant studies from title and abstract screening (1,234), 202 underwent full-text review, yielding 73 eligible studies. An overview of the eligible studies, broken down by sub-analyses of individual CSVD markers is displayed in Table 1. Study quality was generally good (see Appendices D–E); the most common shortcomings were sample size not being justified (cross-sectional studies), proportion of study non-respondents either being high (>20%) or insufficiently explained (cross-sectional studies) and no demonstration that study outcome was not present at the start of the study (longitudinal studies).
Figure 1.
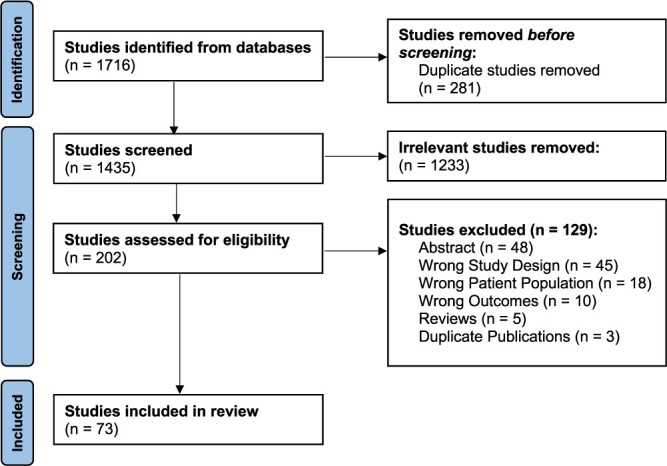
Selection of studies for inclusion in the systematic review.
Table 1.
Overview of sub-analyses within studies included in systematic review
| CSVD marker | Study design | Number of studies | Number of participants |
|---|---|---|---|
| CSVD burden score | Cross-sectional | 6 | 2,527 |
| Longitudinal | 0 | - | |
| WMH | Cross-sectional | 47 | 15,819 |
| Longitudinal | 20 | 13,343 | |
| Lacunar infarct | Cross-sectional | 14 | 5,188 |
| Longitudinal | 7 | 7,582 | |
| CMB | Cross-sectional | 12 | 4,940 |
| Longitudinal | 3 | 2,444 | |
| Enlarged PVS | Cross-sectional | 4 | 2,341 |
| Longitudinal | 1 | 331 | |
| CADASIL | Cross-sectional | 1 | 39 |
| Longitudinal | 0 | 0 |
Note: Longitudinal is defined here as studies where gait or falls were assessed at one or more time points after the baseline MRI.
Methodological comparisons
Of the studies reviewed, 53 were cross-sectional [3, 11–62] (see Table 2) and 20 were longitudinal [63–82] (see Table 3). Study participants in cross-sectional studies had mean ages ranging from 45 to 83.1 years (see Table 2) and mean ages ranging from 62.5 to 85.1 years in longitudinal studies (see Table 3).
Table 2.
Study characteristics of cross-sectional studies
| First author, year | Sample size | Age, mean (SD) | Female, number (%) | Neuroimaging modality | CSVD markers | Gait and falls measures | Quality score |
|---|---|---|---|---|---|---|---|
| Baloh, 1995 [13] | 54 | Patients: 82.0 (3.9); Controls: 81.2 (2.7) | 30 (56%) | 1.5T MRI | WMH grade (Rotterdam scale) | GAIT: Tinetti score | 5 |
| Ben Salem, 2008 [14] | 80 | 75.8, (4.1) | 47 (58.8%) | 1.5T MRI | WMH grade (Fazekas score) | GAIT: Tinetti score | 9 |
| Bhadelia, 2009 [15] | 173 | 72.83 (7.7) | 129 (74.57%) | 1.5T MRI | WMH volume; infarcts (presence/absence) | GAIT: Tinetti score | 9 |
| Blahak, 2009 [16] | 639 | 74.13 (5.0) | 351 (54.9%) | 0.5T and 1.5T MRI | ARWMC grade (Fazekas score); Lacunes (presence/absence) | GAIT: SPPB FALLS: Number of falls over the previous year |
8 |
| Bolandzadeh, 2014 [17] | 253 | 82.74 (2.7 | 147 (58%) | 3.0T MRI | WMH volume, adjusted for total brain volume | GAIT: Speed, 4 m | 8 |
| Carmelli, 2000 [18] | 390 | 72.3 (2.9) | 0 (0%; study of males only) | 1.5T MRI | WMH volume | GAIT: Time to walk 8 feet, time to rise from chair, balance score | 8 |
| Choi, 2012 [19] | 377 | CMB: 73.7 (7.7); No CMB: 72.0 (6.9); SI: 75.9 (6.0); No SI: 71.2 (6.8) | 168 (44.6%) | 1.5T MRI | WMH volume; silent infarcts: infarct (>3 mm) with no stroke; presence/absence; number, location; volume; CMB: presence/absence; number, location | GAIT: Speed, cadence, step length, step width and double support phase; single gait factor, 4.6 m FALLS: Standardised falls-risk z-score (computed using visual contrast sensitivity, body sway, quadriceps strength, reaction time and lower limb proprioception) |
9 |
| David, 2016 [20] | 141 | 63, range 59–69 | 76 (53.9%) | 1.5T MRI | WMH grade (Fazekas score) | GAIT: Speed, 10 m | 6 |
| de Laat, 2010 [23] | 431 | 65.2 (8.9) | 195 (45.2%) | 1.5T MRI | WMH volume; lacunar infarcts (presence/absence) | GAIT: Speed, stride length, cadence, stride width, double support percent; variability of stride length, stride time and stride width; Tinetti score and TUG test time, 5.6 m | 9 |
| de Laat, 2011a [22] | 485 | 65.6 (8.8) | 209 (43.10%) | 1.5T MRI | WMH volume; infarcts number; CMB number | GAIT: Speed, stride length, cadence, stride width, double support percentage, 5.6 m | 9 |
| de Laat, 2011b [21] | 429 | 65.2 (8.9) | 194 (45.2%) | 1.5T MRI | CSVD (WML or lacunar infarcts presence) | GAIT: Speed, stride length, stride width, cadence, 5.6 m | 6 |
| DiSalvio, 2020 [24] | 70 | 76 (5) | 40 (57.1%) | 3.0T MRI | WMH volume | GAIT: Speed, lower extremity strength and functioning, dynamic gait and balance, and dynamic standing balance and motor planning, 6 m | 5 |
| Finsterwalder, 2019 [25] | 39 | CADASIL: 50.0 (8.1) | 27 (69%) | 3.0T MRI | Diagnosis of CADASIL; PSMD; WMH volume | GAIT: Pace (gait velocity, cadence and stride length), rhythm (double support phase and swing phase) and variability (stride time variability, stride length variability and base of support variability) domains; dual task cost was calculated for all of the domains for the three dual tasks: Serial 7’s (calculatory dual task), naming animals (semantic dual task) and carrying a tray (motoric dual task), 6.7 m | 6 |
| Ghanavati, 2018 [26] | 62 | 80.0 (4.2) | 29 (46.8%) | 3.0T MRI | WMH volumes, corrected for intercranial volume | GAIT: Time to complete single and dual task (reciting alternate letters of the alphabet) walks, 20 m FALLS: Fall risk assessment based on visual contrast sensitivity, proprioception, quadriceps strength, simple reaction time and postural sway |
7 |
| Gouw, 2006 [27] | 574 | 74 (5) | 314 (55%) | 0.5T and 1.5T MRI | WMH grade (Fazekas score, Scheltens scale and volume) | GAIT: SPPB | 7 |
| Griebe, 2011 [28] | 34 | 69.4 (7.0) | 23 (68%) | 3.0T MRI | WMH (ARWMC scale; probability map for volume of lesions) | GAIT: SPPB | 6 |
| Guttmann, 2000 [29] | 28 | 81 (5.9) | 16/28 (57%) | 1.5T MRI | WMH volume | GAIT: SPPB, stabilogram diffusion analysis, altered sensory input test, post-translation stability test, functional base of support, gait speed and single-leg stance time | 8 |
| Hashimoto, 2014 [30] | 201 | 67.8 (6.5) | 109 (54.2%) | 1.5T MRI | WMH grade (Fazekas scale) | GAIT: TUG test, TUG test with dual task (serial 3’s) | 9 |
| Hou, 2021 [61] | 224 | 60.6 (10.5) | 80 (35.7%) | 3.0T MRI | CSVD score (4-point scale) | GAIT: speed, stride length, cadence, stride width, Tinetti test, TUG test, SPPB, 4 m | 6 |
| Jayakodi, 2021 [58] | 408 | 72.0 (7.0) | 176 (43.1%) | 1.5T MRI | CSVD score (4-point scale) | GAIT: Double support time variability, 4.6 m | 7 |
| Jenkins, 2020 [59] | 144 | 56 (4), range 48–63 | 61 (42.4%) | 3.0T MRI | WMH volume | GAIT: speed, cadence and stride width, 40 feet | 8 |
| Jokinen, 2021 [11] | 152 | 70.6 (2.9) | 95 (62.5%) | 3.0T MRI | WMH volume | GAIT: speed, single leg stance time, TUG test, SPPB, subjective walking difficulty, 8 m FALLS: falls in past 12 months |
7 |
| Karim, 2020 [31] | 269 | 82.9 (2.7) | 146 (57%) | 3.0T MRI | WMH volume | GAIT: Speed, 20 m | 9 |
| Kim, 2016 [32] | 129 | 73.8 (6.8) | 77 (59.7%) | 3.0T MRI | WMH volume; lacune number; CMB number | GAIT: Gait abnormality and gait severity | 9 |
| Koo, 2012 [33] | 125 | 71.9 (7.8) | 91 (72.8%) | 1.5T MRI | WMH volume | FALLS: Risk of falls | 6 |
| Li, 2020 [34] | 314 | 59.6 (2.7) | 54.10% | 3.0T MRI | CSVD score (4-point scale); WMH grade (Fazekas scale); Lacunes (presence/absence); CMB (presence/absence); PVS (presence/absence) | GAIT: Speed, stride time, cadence, stance phase time %, max swing velocity, stride length, heel strike angle, toe-off angle; Tinetti score and TUG test, 10 m | 8 |
| Ma, 2022 [60] | CSVD: 46; NC: 22 | CSVD: 71.2 (8.2); NC: 70.2 (6.7) | 33 (48.5%) | 3.0T MRI | WMH grade (Fazekas scale) | GAIT: Speed, stride time, stride length, cadence, swing time, stride time variability, stride length variability, speed variability, swing time variability, gait asymmetry, phase coordination index, 25 strides | 6 |
| Moscufo, 2011 [35] | 99 | 83 (4) | 57 (57.6%) | 3.0T MRI | WMH fraction (WMH volume/intracranial cavity) | GAIT: SPPB | 7 |
| Murray, 2010 [36] | 148 | median 79, IQR 76–83 | 83 (56.1%) | 3.0T MRI | WMH proportional volume (WMH volume/total WM volume*100) | GAIT: UPDRS gait and postural stability, speed, stride length, 4.88 m | 6 |
| Nadkarni, 2016 [37] | 179 | 83.1 (2.7) | 104 (58.1%) | 3.0T MRI | WMH volume, normalised to brain volume | GAIT: Speed, 4 m | 7 |
| Ogama, 2022 [62] | 91 | 73.2 (4.9) | 53 (58.2%) | 1.5T MRI | WMH volume | GAIT: Pace (speed, cadence, stride length), rhythm (stride time, double leg support time), postural control (walking angle, step width), variability (gait speed variability), 6.4 m | 7 |
| Pinter, 2017 [12] | 678 | 72.5 (0.7) | 319 (47.1%) | 1.5T MRI | CSVD score (4-point scale); WMH volume | GAIT: Speed, chair stands, standing balance, 6 m | 6 |
| Rasmussen, 2019 [38] | 904 | 45 (by study design) | 449 (49.7%) | 3.0T MRI | WMH volume | GAIT: Speed, 6 m | 9 |
| Rosano, 2006 [39] | 321 | 79.1 | 195 (60.7%) | 0.35T and 1.5T MRI | WMH grade (CVHS scale); Infarcts (presence/absence) | GAIT: Speed, stride length, base of support, double support time and latency, 4 m | 7 |
| Rosano, 2007 [40] | 331 | 78.3 (4.0) | Not reported | 0.35T and 1.5T MRI | WMH grade (10-point scale); infarcts (presence/absence) | GAIT: Step length variability, step width variability and stance time variability, 4 m | 9 |
| Rosario, 2016 [41] | 265 | 82.9 (2.7) | 152 (57%) | 3.0T MRI | WMH volume | GAIT: Speed, 4 m | 8 |
| Rosso, 2014 [42] | 265 | 82.9 (2.7) | 152 (57.4%) | 3.0T MRI | WMH volume | GAIT: Speed, step length, step length variability, 8 m | 8 |
| Sakakibara, 1999 [43] | 63 | 73 | 35 (55.6%) | 1.5T MRI | WMH grade (Brant-Zawadzki scale) | GAIT: Gait disorder | 2 |
| Sakurai, 2021 [44] | 34 | 78.4 (4.2) | 23 (54.8%) | 1.5T MRI | WMH volume normalised to ICV | GAIT: Speed, variability of double support time, stride time, stride length and step length, 5 m | 8 |
| Sartor, 2017 [45] | 101 | yPn = 57, range 50–69; oPn = 77, range 70–89 | 47 (46.5%) | Unknown | WMH grade (Fazekas score) | GAIT: Speed, dual task cost (serial 7s), 20 m | 7 |
| Seiler, 2017 [46] | 230 | 70.2 (4.9) | 153 (66.5%) | 3.0T MRI | WMH volume | GAIT: Speed; SPPB, 8 m | 6 |
| Smith, 2015 [47] | 803 | 58 (8) | 474 (59%) | 1.5T and 3.0T MRI | Lacunes (presence/absence, number and location); CMB (presence/absence, number and location); WMH volume, normalised to sex-specific average intracranial volume | GAIT: TUG test | 9 |
| Sorond, 2011 [48] | 42 | 78.3 (6.5) | 23 (54.8%) | 1.5T and 3.0T MRI | WMH volume | GAIT: Speed, 4 m | 5 |
| Starr, 2003 [49] | 97 | 78–79 years | 39 (40.2%) | 1.0T MRI | WMH grade (Fazekas scale) | GAIT: Time able to balance on one leg, walking time, unspecified walking distance | 7 |
| Stijntjes, 2016 [50] | 297 | 65.4 (6.8) | 150 (50.5%) | 3.0T MRI | WMH volume; CMB (presence/absence); lacunar infarcts (presence/absence) | GAIT: Standing balance duration, chair rise duration, speed, 4 m | 8 |
| Su, 2017 [52] | 770 | 57.2 (9.3) | 501 (65.1%) | 3.0T MRI | WMH grade (Fazekas scale); Lacunes (presence/absence); CMB (presence/absence); perivascular spaces (4-point scale) | GAIT: SPPB | 8 |
| Su, 2018 [51] | 770 | 57.2 (9.3) | 501 (65.1%) | 3.0T MRI | CSVD group: High WMH (Fazekas scale ≥2) and ≥1 lacune: Controls remainder of the population | GAIT: SPPB | 5 |
| Valkanova, 2018 [3] | 178 | 69 (5.1) | 44 (25%) | 3.0 T MRI | WMH volume | GAIT: Speed, stride length, stride time, gait speed variability, stride length variability, stride time variability and double stance percent, 10 m | 7 |
| Verlinden, 2016 [53] | 2,330 | 65.9 (9.2) | 1,283 (55.1%) | 1.5T MRI | WMH volume | GAIT: 7 gait domains: rhythm (cadence and single support time), phases (double support time and single support %), variability (stride length and time), pace (stride length and velocity), tandem (errors in tandem walking), turning (turning time and step count) and base of support (stride width and its variability), 4.88 m | 9 |
| Verwer, 2018 [54] | 133 | 71.0 (9.3) | 55 (41%) | 3.0T MRI | WMH grade (Fazekas scale); lacunar infarcts (presence/absence); CMB (presence/absence) | GAIT: SPPB | 7 |
| Wang, 2021 [55] | 579 | 67.6 (7.6) | 339 (58.5%) | 3.0T MRI | WMH volume; CMB number; enlarged PVS (presence/absence) | GAIT: Speed, 6 m | 9 |
| Willey, 2018 [56] | 616 | 74.3 (8.6) | 419 (62.7%) | 1.5T MRI | WMH volume; SI (presence/absence) | GAIT: SPPB | 9 |
| Windham, 2016 [57] | 1960 | 61.2 (10.0) | 1,267 (64.6%) | 1.5T MRI | WMH volume | GAIT: Speed; subjective gait difficulty in walking half a mile, 25 feet | 9 |
ARWMC, age-related white matter changes; ICV, intracranial volume; IQR, interquartile range; oPn, older adults without Parkinson’s disease; PSMD, peak width of skeletonised mean diffusivity; SI, silent infarct; UPDRS, Unified Parkinson’s Disease Rating Scale and yPn, young adults without Parkinson’s disease.
Table 3.
Study characteristics of longitudinal studies
| First author, year | Study duration | Sample size | Age, mean (SD) | Female, number (%) | Neuroimaging modality | CSVD markers | Gait and falls measures | Quality score |
|---|---|---|---|---|---|---|---|---|
| Baloh, 2003 [63] | 8–10 years | 59 | 78.5 (3.7) | 25 (42.4%) | 1.5T MRI | WMH volume and grade (Victoroff scale) | GAIT: Tinetti score | 7 |
| Briley, 2000 [64] | 6–36 months | 221 | 67.6 (10.8) | 3 (1%) | CT | WMH grade (Rotterdam Study scale); infarcts (size, vascular distribution and type [i.e. cortical versus subcortical only]) | GAIT: Gait score | 6 |
| Callisaya, 2013 [66] | 30.6 months, SD 4.9 | 225 | 71.4 (6.8) | 98 (45.6%) | 1.5T MRI | WMH volume; infarcts (number) | GAIT: Speed, step length, cadence, step width, 4.6 m | 10 |
| Callisaya, 2014 [82] | 12 months | 655 | 74.5 (6.7) | 319 (48.7%) | 1.5T MRI | WMH volume; infarcts (number) | FALLS: Number of falls | 8 |
| Callisaya, 2015 [65] | 2.5 years, SD 0.4 | 187 | 70.5 (6.5) | 68 (50.0%) | 1.5T MRI | WMH volume | GAIT: Speed, 4.6 m FALLS: Number of falls over 12 months |
7 |
| Heiland, 2021 [81] | 6.0 years, SD 1.4 | 331 | 68.9 (8.3) | 198 (58.3%) | 1.5T MRI | CSVD score (3-point scale), WMH volume, PVS grade, lacune count | GAIT: Speed, 2.44 m | 9 |
| Kreisel, 2013 [67] | 3 years | 639 | 74.1 (5.0) | 351 (54.93%) | 0.5T and 1.5T MRI | ARWMC grade (Fazekas scale) | GAIT: SPPB | 7 |
| Moscufo, 2012 [68] | 1.9 years, SD 0.4 | 77 | 82 (4) | 46 (60%) | 3.0T MRI | WMH volume, as % ICV | GAIT: Tinetti score; SPPB | 9 |
| Pinter, 2018 [69] | 3 years | 443 | 72.5 (0.7) | 199 (44.9%) | 1.5T MRI | WMH volume | GAIT: Speed, chair stand time, standing balance time, 6 m | 8 |
| Rosano, 2005 [70] | 4 years | 2,450 | 74.4 (4.7) | 1,397 (57%) | 0.35T and 1.5T MRI | WMH (10-point visual rating scale); Small brain infarcts (presence/absence) | GAIT: Motor performance (speed, timed chair stand); self-reported physical impairment (difficulty walking half a mile or with one or more activities of daily living), 15 feet | 8 |
| Rosso, 2017 [71] | 6 years | 2,703 | 74.4 (4.8) | 1,521 (56.3%) | 1.5T and 3.0T MRI | WMH (10-point visual rating scale) | GAIT: Speed, self-reported mobility disability defined as unable to walk 0.8 km, 15 feet | 8 |
| Silbert, 2008 [72] | 9.1 years, SD 4.0 | 104 | 85.1 (5.6) | 64 (61.50%) | 1.5T MRI | WMH volume | GAIT: Tinetti score; time to walk, number of steps, 9 m | 6 |
| Soumare, 2009 [73] | 8 years | 1702 | 72.4 (4.1) | 1,031 (60.6%) | 1.5T MRI | WMH volume; lacunar infarcts (presence/absence) | GAIT: Time to walk, gait speed; Tinetti score, 6 m | 9 |
| Srikanth, 2009 [74] | 12 months | 294 | 73.0 (7.0) | 131 (44.6%) | 1.5T MRI | WMH volume | GAIT: Speed, cadence, step length, step width, double support time, variability of stride, 4.2 m length, time and width FALLS: Incident falls (first fall in 12 months after study onset) |
8 |
| Sullivan, 2021 [75] | 6.51 years | 1859 | median 76.7, IQR 72.0 to 80.0 | 1,115 (60%) | 3.0T MRI | WMH volume; Infarct (presence/absence); CMB (number) | GAIT: Speed, 4 m | 9 |
| van der Holst, 2017 [77] | 5.4 years SD 0.2 | 310 | 63.3 (8.4) | 137 (44.2%) | 1.5T MRI | WMH volume; lacunes (number); CMB (number) | GAIT: Speed, stride length and cadence, 5.6 m | 10 |
| van der Holst, 2018 [76] | 5.4 SD 0.2 | 275 | 62.5 (8.20 | 120 (43.6%) | 1.5T MRI | WMH volume; lacunes (number); CMB (number) | GAIT: Speed, stride length, cadence, 5.6 m | 9 |
| Willey, 2013 [78] | 2 years | 701 | 80.3 (5.6) | 471 (67.2%) | 1.5T MRI | WMH volume; silent brain infarcts (presence/absence) | GAIT: Speed, 4 m | 9 |
| Wolfson, 2005 [79] | 19–22 months | Control: 7; Impaired Mobility: 7 | Control: 81 (1.7); Impaired Mobility: 84 (3.4) | Control: 1 (14.3%); Impaired Mobility: 4 (57.1%) | 1.5T MRI | Volume of WM signal abnormalities normalised to ICV | GAIT: SPPB; single stance time, tandem stance time, functional base of support and gait velocity | 8 |
| Zhang, 2020 [80] | 1 year | Fallers: 16; Non-fallers: 78 | Fallers: 76.6 (7.2); Non-fallers: 68.4 (7.4) | Fallers: 7 (43.8%); Non-Fallers: 43 (55.1%) | 3.0T MRI | WMH grade (Fazekas score) | GAIT: Tinetti score, Berg Balance Scale score and TUG test | 8 |
ARWMC, age-related white matter changes; ICV, intracranial volume.
CSVD measurements
Imaging modalities used in the studies were magnetic resonance imaging (MRI), with the exception of one that used computed tomography (CT) [64]. Strengths of MRI scanners were 0.35T (n = 3) [39, 40, 70], 0.5T (n = 3) [16, 27, 67], 1.0T (n = 1) [49], 1.5T (n = 42) [12–16, 18–23, 27, 29, 30, 33, 39, 40, 43, 44, 47, 48, 53, 56–58, 62, 63, 65–67, 69–74, 76–79, 81, 82] or 3.0T (n = 31) [3, 11, 17, 24–26, 28, 31, 32, 34–38, 41, 42, 46–48, 50–52, 54, 55, 59–61, 68, 71, 75, 80]. One study [45] did not report scanner strength (see Tables 2 and 3).
Measurements of CSVD included WMH volume (n = 48) [3, 11, 15, 17–19, 21–24, 26, 27, 29, 31–33, 35–38, 41, 42, 44, 46–48, 50, 53, 55–57, 59, 62, 63, 65, 66, 68, 69, 72–79, 81, 82] or WMH grade (using visual rating scales such as Fazekas scale [83]; n = 21) [13, 14, 16, 20, 27, 28, 30, 39, 40, 43–45, 49, 52, 60, 63, 64, 67, 70, 71, 80]; infarct presence or count (n = 27) [12, 16, 19, 21–23, 30, 32, 34, 39, 40, 46, 47, 50–52, 54, 56, 64, 70, 73, 75–78, 81, 82]; CMB presence or count (n = 15) [12, 19, 22, 30, 32, 34, 46, 47, 50, 52, 54, 55, 75–77] and enlarged PVSs (n = 5) [12, 34, 52, 55, 81]. Six studies used a composite score to measure total CSVD burden [12, 34, 51, 54, 58, 61] and one study defined CSVD cases based on imaging markers and compared them to controls [51]. One study identified CSVD based on a diagnosis of CADASIL by genetic analysis or skin biopsy [25]. No relevant studies examining cSS were found (see Tables 2 and 3).
Gait modalities
Gait analyses were conducted using the following tools: timed walk of a set length (n = 23) [3, 11, 12, 18, 20, 24, 26, 28, 30, 31, 48, 50, 52, 55, 57, 60, 69, 70, 72, 73, 75, 78, 81], GAITRite electronic walkway (n = 14) [19, 21–23, 25, 36, 38, 53, 58, 65, 66, 74, 76, 77], SPPB (n = 17) [11, 12, 16, 27–29, 35, 46, 48, 51, 54, 56, 61, 67–69, 79], Tinetti test (n = 13) [13–15, 22, 23, 33, 49, 61, 63, 68, 72, 73, 80], GaitMat II electronic walkway (n = 6) [17, 37, 39–42], Timed Up and Go (TUG) test (n = 7) [11, 22, 23, 30, 47, 61, 80], calculation of dual task cost (n = 5) [20, 25, 26, 30, 45], timed chair stands (including Five Times Sit to Stand; n = 4) [18, 24, 50, 52], self-report questionnaires inquiring about subjective gait difficulties (n = 5) [11, 36, 57, 70, 71], other automated walkways (n = 4) [44, 59, 61, 62], motor examination (n = 2) [36, 43]; wearable gait-tracking device (n = 2) [34, 60], Dynamic Gait Index (n = 1) [24], Four Square Step Test (n = 1) [24], EquiTest (n = 1) [29], pyramidal and extrapyramidal scale (n = 1) [32] and RehaGait sensor system (n = 1) [45]. Falls were assessed via self-reported history of falls (n = 6; see Tables 2 and 3) [11, 63–65, 74, 82].
Associations of sporadic, age-related CSVD with gait and falls in sporadic CSVD
CSVD summary scores
Cross-sectional studies
Six studies reported associations between a CSVD summary score and gait abnormalities (see Supplementary Appendix F available in Age and Ageing online). One study found that individuals with CSVD (defined by WMH severity and presence of one or more lacunes) performed worse than healthy controls on single task walks (as measured by gait speed and chair stands scale) [51]. Five studies found associations between greater CSVD burden (as indicated by a score out of 3 or 4, composed of presence or severity of select markers of CSVD) and gait impairment, namely on select gait measures (gait speed, cadence, stride length, stride width, swing velocity and double support time variability) and gait scales (SPPB, Tinetti test, TUG test and chair stands) [12, 34, 54, 58, 61].
No associations between CSVD and falls were reported.
Longitudinal studies
None of the studies reported longitudinal associations between CSVD summary scores and either gait or falls.
WMH
Cross-sectional studies
WMH was measured using either volumetric analysis [3, 11, 15, 17, 18, 21, 23, 24, 26–29, 31–33, 36–38, 41, 42, 44, 46–48, 50, 53, 55–57, 59, 62, 68, 82] or using visual rating scales (Brant-Zawadzki scale [43, 84], Fazekas scale [14, 16, 20, 27, 28, 30, 45, 49, 52, 60, 83], Rosano scale [39, 40], Rotterdam Study scale [85, 86], Scheltens scale [27, 87] and Wahlund’s age-related white matter changes scale [88]).
Most cross-sectional studies (36/47) found that WMH was associated with worse gait performance and history of falls (see Appendices G and H). The majority of studies (30/47) adjusted for age. During single task walks, greater WMH was associated with worse performance on several gait measures (i.e. gait speed, stride length, stride width, stride time, single-leg stance time, and variability of gait speed and stride length) [12, 13, 17, 21, 28–31, 34, 36, 37, 40, 41, 44, 47, 52, 54, 56, 57, 60, 62], gait scales (i.e. SPPB, Tinetti test, TUG test, chair stand, overall gait score, walking score, global gait) [11, 13–15, 27, 29, 30, 32, 35, 47, 52, 53, 56], gait disorders [43] and subjective mobility difficulty [57]. Greater WMH volume was also associated with higher odds of poor gait speed, double support time, step length variability, stance time variability, SPPB, TUG test and chair stand [23, 39, 48, 54]. On dual task walks, greater WMH severity was associated with slower gait speed [20, 26, 38]. One study examining falls found that individuals with a history of falls had greater WMH severity, based on a visual rating scale [16].
Of the 11 studies that did not find associations between WMH and gait, 10 failed to find significant associations between WMH severity and gait [3, 18, 24, 42, 45, 46, 49, 50, 55, 59], and one failed to find differences in WMH volume between individuals with and without risk of falls [33].
Longitudinal studies
Of the 20 longitudinal studies of gait and falls outcomes reporting on WMH, 16 examined associations with changes in gait and 4 examined associations with incident falls (see Supplementary Appendix I available in Age and Ageing online). Most studies (15/20) examined baseline WMH as a predictor of change in gait or new falls, while 5/20 correlated change in WMH with changes in gait. Longitudinal measurements of WMH severity were done volumetrically [65, 66, 68, 69, 72–79, 81] or using a visual rating scale (Fazekas scale [67, 70, 80, 83], Rosano scale [70], Rotterdam Study scale [86] and Victoroff scale [89]).
Of the 16 studies on change in gait over time, 12 measured WMH at baseline only and 4 correlated change in WMH with change in gait over time. Of the 12 studies that measured WMH at baseline, 10 found that higher WMH at baseline was associated with worsening of gait speed (n = 5) [70, 71, 73, 78, 81], greater time to walk a specified distance (n = 1) [72], decreased number of steps to walk a specified distance (n = 1) [72], lower summary scores of gait and gait variability (n = 1) [73], and worse performance on Tinetti test (n = 1) [63], SPPB (n = 1) [67] and chair rise (n = 1) [68]. Two studies failed to find associations between baseline WMH volume and gait impairment when examining gait speed, cadence and stride length [75, 77]. Of the four studies that examined the association of change in WMH over time with change in gait over time, three found that an increase in WMH volume was associated with worse gait (measured by gait speed (n = 2) [66, 69], step length (n = 1) [66] and SPPB score (n = 1) [79]; while one study failed to find an association between change in WMH and change in gait [76].
Four of the 20 longitudinal studies examined relationships between WMH and incident falls, of which three measured WMH at baseline and one measured WMH change over time. The three studies reporting baseline WMH found that baseline WMH grade or volume was predictive of future falls [64, 80, 82]. The study that examined the change in WMH volume over time found that an increase in WMH volume was associated with a greater risk for multiple future falls [65].
Meta-analysis
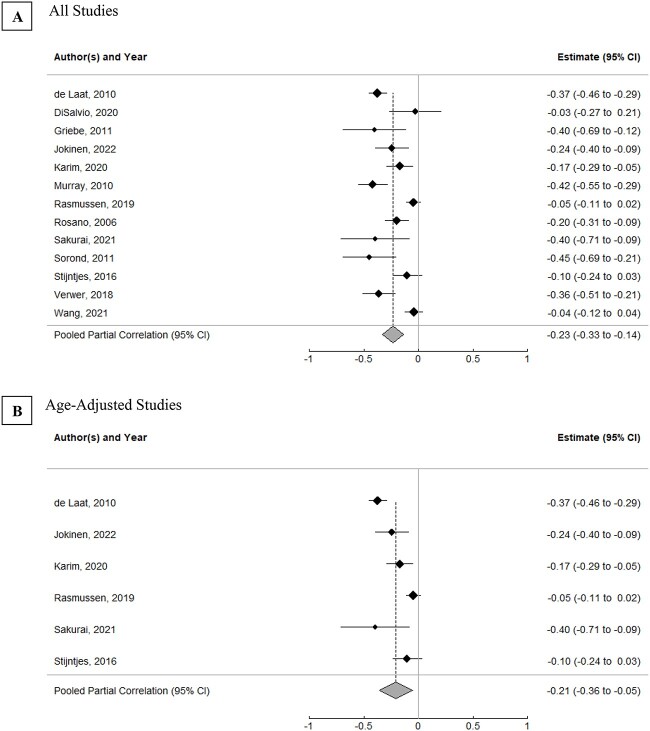
Thirteen cross-sectional studies [11, 23, 24, 28, 31, 36, 38, 39, 44, 48, 50, 54, 55] with sufficiently reported data on gait speed and WMH volume were meta-analysed using a random-effects model to assess the overall relationship between WMH volume and gait speed (see Supplementary Appendix G available in Age and Ageing online), of which six studies controlled for age [11, 23, 31, 39, 44, 50], six controlled for sex [11, 23, 38, 39, 44, 50] and seven controlled for other factors [11, 23, 31, 38, 39, 44, 50] (five of the seven studies controlled for age, sex and other factors [11, 23, 39, 44, 50]; one controlled for age without sex, including other factors [31] and one controlled for sex without age, including other factors [38]). Of note, 24 of the remaining 34 non-meta-analysed cross-sectional studies reporting WMH volume did adjust for age. Nine studies were considered high quality [11, 23, 31, 38, 39, 44, 50, 54, 55] and four were moderate quality [24, 28, 36, 48] based on the Newcastle–Ottawa scale (see Supplementary Appendix D available in Age and Ageing online). The estimated pooled partial correlation between WMH volume and gait speed was −0.23 (95% confidence interval [CI]: −0.33 to −0.14, P < 0.0001; see Figure 2A). When restricted to the studies adjusting for age, the pooled partial correlation was attenuated but still significant (r = −0.21, 95% CI: −0.36 to −0.05, P < 0.05; see Figure 2B).
Figure 2.
Correlations between WMH volume and gait speed. (A) All studies. (B) Age-adjusted studies. Estimates reflect partial correlation coefficients, with the position of the markers denoting the estimate, the horizontal lines representing 95% CI and the size of markers indicative of weight of the corresponding study. Summary scores indicate pooled partial correlation coefficients of included studies. Supplementary Appendix G describes study outcomes for the included studies.
High heterogeneity was observed between studies (I2 = 82.95%; tau2 = 0.02; Q = 79.37, P < 0.0001). The leave-one-out method, wherein each study was removed from the meta-analysis one at a time, found that overall results and heterogeneity were not influenced by any one study. A meta-regression found that heterogeneity was not explained by whether a study was age-adjusted (R2 = 0.00%, Q = 0.33, P = 0.56), participants’ age (R2 = 12.03%, Q = 2.19, P = 0.14), sex (R2 = 0.00%, Q = 0.14, P = 0.71) or study quality (age: R2 = 15.94%, Q = 2.25, P = 0.13). Heterogeneity was also not explained by subgroup analysis of studies that did adjust for age versus those that did not adjust for age (age-adjusted: I2 = 83.10%; tau2 = 0.02; Q = 41.43, P < 0.0001; not age-adjusted: I2 = 82.24%; tau2 = 0.02; Q = 37.52, P < 0.0001). No evidence of publication bias was detected through visual inspection of funnel plot (see Supplementary Appendix Q available in Age and Ageing online) or Egger’s linear regression test (z = −1.57, P = 0.15).
Lacunar infarcts
Cross-sectional studies
Studies examining lacunar infarcts had mixed results (see Supplementary Appendix J available in Age and Ageing online), with 8 of 14 studies reporting some associations between lacunar infarcts and gait and falls. Of these eight studies, four found that infarct presence was associated with impaired gait measures (gait speed, cadence, step and stride length, step and stride width, double support, and variability of step length, stride time and stance time) [23, 39, 40, 50], three studies found that infarct presence was associated with gait scales (Tinetti test, TUG test and chair stands) [15, 47, 54] and one study found that silent lacunar infarcts were associated with greater falls risk [19].
However, 6 of 14 studies found no significant links between lacunar infarcts and gait when examining several gait measures (gait speed, cadence, stride time, stride length, stance phase time and maximum swing velocity) and gait scales (chair stands, summary gait scores and overall assessments of gait impairment on single and dual task walks) [12, 30, 32, 34, 46, 52].
Longitudinal studies
Seven longitudinal studies of gait and falls outcomes examined the presence of lacunar infarcts, of which six studies reported associations with changes in gait and one study reported associations with incident falls (see Supplementary Appendix K available in Age and Ageing online). Only one study examined the association with incident lacunar infarcts.
Of the six studies examining changes in gait, five studies assessed lacunar infarct presence at baseline and one study measured change in lacunar infarct presence over time. Of the five studies reporting baseline lacunar infarcts, two studies found that baseline presence of lacunes was associated with risk of incident walking speed limitation (gait speed <0.8 m/s; n = 1) [81] and decline in gait speed (n = 1) [70]. Three studies found that baseline infarct presence was not associated with declines in gait speed (n = 3) [73, 75, 77], cadence (n = 1) [77] or stride length (n = 1) [77]. The one study that examined the association of new lacunes (defines as the appearance of one or more lacunes at follow-up) with change in gait failed to find an association with changes in gait speed, cadence or stride length; however, the number of patients with incident lacunes was small (17/61 [27.9%] with lacunes at follow-up) [76].
The one study, out of seven longitudinal studies, that reported associations of baseline lacunar infarcts with incident multiple falls found a linear trend for higher risk of falls with increasing number of baseline infarcts (categorised as none, one, two, or three or more) with a significant association between three or more infarcts at baseline and incident multiple falls [82].
CMB
Cross-sectional studies
There were mixed findings with respect to CMBs and gait (see Supplementary Appendix L available in Age and Ageing online). Six of 12 studies found associations between the presence of CMB and gait measures (i.e. gait speed, cadence, stride length, stride width, double support time and stance phase time percent) or Tinetti test [19, 22, 34, 46, 50, 55]. However, six of 12 studies found no associations between CMB presence and gait speed, SPPB, TUG test, chair stand or summary gait scores [12, 30, 32, 47, 52, 54, 55].
No studies reported associations between CMBs and falls.
Longitudinal studies
Three studies examined CMBs in relation to gait performance over time (see Supplementary Appendix M available in Age and Ageing online), of which two studies measured baseline CMB count and one study measured change in CMB count over time. The two studies that examined baseline CMB count failed to find associations with change in gait speed (n = 2) [75, 77], cadence (n = 1) [77] or stride length (n = 1) [77]. One study examined the impact of change in CMB count on change in gait and also did not find associations with changes in speed, stride length or cadence [76]; however, the number of patients with incident CMBs was small (17/56 [30.4%] with CMBs at follow-up).
Falls were not assessed in relation to CMBs.
PVS
Cross-sectional studies
Four studies reviewed the relationship between enlarged PVS and gait (see Supplementary Appendix N available in Age and Ageing online), all of which found no associations when measuring gait with gait speed, cadence, stride length, stride time, stance phase time percentage, maximum swing velocity and chair stands [12, 34, 52, 55].
None of the studies examined associations between PVSs and falls.
Longitudinal studies
One longitudinal study (see Supplementary Appendix O available in Age and Ageing online) found that greater baseline enlarged PVS presence was associated with a greater risk of incident walking speed limitation (gait speed <0.8 m/s) [81].
This study did not report findings relating to PVS and falls.
CADASIL
Cross-sectional studies
One study compared patients with the CSVD subtype, CADASIL, to healthy controls (see Supplementary Appendix P available in Age and Ageing online) and found that individuals with CADASIL had worse double support time and swing time during single task walks and worse velocity, cadence, swing time, stride length and double support time during dual task walks [25].
There were no reported associations between CADASIL and falls.
Longitudinal studies
None of the studies reported longitudinal associations.
Discussion
In this systematic review and meta-analysis, associations between measures of CSVD and gait and falls were analysed. Compared with prior reviews [4, 90], many more studies have been published recently including longitudinal studies of WMH progression and gait impairment. Overall, the data suggest that CSVD is adversely associated with gait and falls in the general population. The most data were available for WMH, where meta-analysis of cross-sectional studies showed that WMH volume was correlated with a mild, but highly statistically significant, decrease in gait speed. For other lesion types, such as infarcts and CMBs, the results were less consistent, and meta-analysis was not possible due to heterogeneity in CSVD assessment and gait measurement.
Previous systematic reviews have described the importance of examining gait and falls in older adults. One such review demonstrated associations between gait problems and increased frailty and falls, decreased cognition and overall lower life satisfaction [4], and another suggested that poor gait performance may predict the onset of dementia [91]. Similar to our findings in the general population, other reviews have found relationships between WMH and gait impairment in adults [90] and patients with Alzheimer’s disease [92].
Gait impairment in CSVD is likely caused by interrupted frontal cortical–subcortical circuits [93]. Decreased connectivity of cerebral white matter tracts in older adults, as seen in CSVD, has been linked to gait impairment [5]. Further, slowed gait was linked to atrophy of the frontal cortex, basal ganglia, hippocampus and cerebellum and to damage of white matter circuits in frontal cortical regions and basal ganglia [5]. More studies are needed to comprehensively understand how the effects of different CSVD lesions on brain networks may contribute to gait decline.
One limitation of this review is that we searched for ‘risk of falls’ and synonyms, but we chose not to search for ‘falls’ as a keyword; this strategy probably increased the specificity of the returned results but may have missed some relevant papers. While we initially hoped to include multiple meta-analyses, we were limited by a lack of meta-analysable data for other CSVD lesion types and diagnoses. Further, there were much fewer studies on lacunes, CMBs and enlarged PVSs than WMH, and none on cSS. There were also fewer longitudinal studies than cross-sectional studies. These limitations, along with the heterogeneity in quantifying the amount of CSVD and harmonising methods across publications, made it difficult to synthesise information, ultimately restricting meta-analyses to just WMH volume and gait speed, the most commonly reported measures of CSVD and gait, respectively. The results, however, must be interpreted with caution as they showed significant heterogeneity that we were unable to explain statistically. In the future, pooling results across studies would be facilitated by greater consensus on standardised gait assessments such as that proposed by the Canadian Consortium on Neurodegeneration in Aging [94].
The applicability of the results must also be considered. The studies included were mostly done in the general population, where most participants would have had only mild covert age-related CSVD. Our review does not include the effects of more severe CSVD on gait and falls. Clinically, it is recognised that gait impairment is frequent in patients with severe symptomatic CSVD.
Although the effect of CSVD on gait and falls, as reflected in our meta-analysis of WMH and gait speed, is mild in strength, the overall public health impact of CSVD may be large because CSVD is so common with ageing. More studies are needed on the effect of CSVD on mobility-related quality of life, falls risk and injurious falls. Clinical trials of strategies to prevent CSVD are needed, and these trials should include the assessment of gait as an outcome. Prevention of CSVD should be part of a comprehensive strategy to improve mobility and reduce risk of falls in later life.
Supplementary Material
Contributor Information
Breni Sharma, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada; Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada; Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada.
Meng Wang, Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada; Department of Community Health Sciences, University of Calgary, Calgary, AB, Canada; O'Brien Institute of Public Health, University of Calgary, Calgary, AB, Canada.
Cheryl R McCreary, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada; Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada; Seaman Family MR Research Centre, University of Calgary, Calgary, AB, Canada.
Richard Camicioli, Department of Medicine (Neurology), University of Alberta, Edmonton, AB, Canada; Neuroscience and Mental Health Institute, University of Alberta, Edmonton, AB, Canada.
Eric E Smith, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada; Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada; Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada; Department of Community Health Sciences, University of Calgary, Calgary, AB, Canada; Seaman Family MR Research Centre, University of Calgary, Calgary, AB, Canada.
Declaration of Conflicts of Interest
Dr Smith reports consulting for Alnylam and Biogen.
Declaration of Sources of Funding
This study was supported by the Katthy Taylor Chair in Vascular Dementia at the University of Calgary.
Data Availability
Data not provided in the article may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
References
- 1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Smith EE, Biessels GJet al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debette S, Schilling S, Duperron MG et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2018; 76: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng JJJ, Delbaere K, Close JCT, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke 2011; 42: 2086–90. [DOI] [PubMed] [Google Scholar]
- 5. Wilson J, Allcock L, Mc Ardle R, Taylor JP, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci Biobehav Rev 2019; 100: 344–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aloe AM. An empirical investigation of partial effect sizes in meta-analysis of correlational data. J Gen Psychol 2014; 141: 47–64. [DOI] [PubMed] [Google Scholar]
- 7. Ambrosius WT. Topics in Biostatistics. Totowa, New Jersey: Humana Press, 2007. [Google Scholar]
- 8. Borenstein M, Hedges LV. Introduction to Meta-analysis. Wiley, 2009. [Google Scholar]
- 9. Kim MJ, Moon S, Oh BC, Jung D, Choi K, Park YJ. Association between diethylhexyl phthalate exposure and thyroid function: a meta-analysis. Thyroid 2019; 29: 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez-Barranco M, Tobías A, Redondo D, Molina-Portillo E, Sánchez MJ. Standardizing effect size from linear regression models with log-transformed variables for meta-analysis. BMC Med Res Methodol 2017; 17: 44. 10.1186/s12874-017-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jokinen H, Laakso HM, Ahlström Met al. Synergistic associations of cognitive and motor impairments with functional outcome in covert cerebral small vessel disease. Eur J Neurol 2022; 29: 158–67. [DOI] [PubMed] [Google Scholar]
- 12. Pinter D, Ritchie SJ, Doubal Fet al. Impact of small vessel disease in the brain on gait and balance. Sci Rep 2017; 7: 41637. 10.1038/srep41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol 1995; 52: 970–4. [DOI] [PubMed] [Google Scholar]
- 14. Ben Salem D, Walker PM, Aho Set al. Brain flexibility and balance and gait performances mark morphological and metabolic abnormalities in the elderly. J Clin Neurosci 2008; 15: 1360–5. [DOI] [PubMed] [Google Scholar]
- 15. Bhadelia RA, Price LL, Tedesco KLet al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke 2009; 40: 3816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blahak C, Baezner H, Pantoni Let al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry 2009; 80: 608–13. [DOI] [PubMed] [Google Scholar]
- 17. Bolandzadeh N, Liu-Ambrose T, Aizenstein Het al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage 2014; 99: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carmelli D, DeCarli C, Swan GEet al. The joint effect of apolipoprotein E epsilon4 and MRI findings on lower-extremity function and decline in cognitive function. J Gerontol A Biol Sci Med Sci 2000; 55: M103–9. [DOI] [PubMed] [Google Scholar]
- 19. Choi P, Ren M, Phan TGet al. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke 2012; 43: 1505–10. [DOI] [PubMed] [Google Scholar]
- 20. David J-P, Ferrat E, Parisot Jet al. White matter lesions: prevalence and clinical phenotype in asymptomatic individuals aged >=50 years. Dement Geriatr Cogn Disord 2016; 42: 159–68. [DOI] [PubMed] [Google Scholar]
- 21. De Laat KF, Tuladhar AM, Van Norden AGW, Norris DG, Zwiers MP, De Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2011; 134: 73–83. [DOI] [PubMed] [Google Scholar]
- 22. De Laat KF, Van Den Berg HAC, Van Norden AGW, Gons RAR, Olde Rikkert MGM, De Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke 2011; 42: 494–7. [DOI] [PubMed] [Google Scholar]
- 23. De Laat KF, Van Norden AGW, Gons RARet al. Gait in elderly with cerebral small vessel disease. Stroke 2010; 41: 1652–8. [DOI] [PubMed] [Google Scholar]
- 24. DiSalvio NL, Rosano C, Aizenstein HJet al. Gray matter regions associated with functional mobility in community-dwelling older adults. J Am Geriatr Soc 2020; 68: 1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finsterwalder S, Wuehr M, Gesierich Bet al. Minor gait impairment despite white matter damage in pure small vessel disease. Ann Clin Transl Neurol 2019; 6: 2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghanavati T, Smitt MS, Lord SRet al. Deep white matter hyperintensities, microstructural integrity and dual task walking in older people. Brain Imaging Behav 2018; 12: 1488–96. [DOI] [PubMed] [Google Scholar]
- 27. Gouw AA, Van der Flier WM, Straaten ECWet al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol 2006; 253: 1189–96. [DOI] [PubMed] [Google Scholar]
- 28. Griebe M, Forster A, Wessa Met al. Loss of callosal fibre integrity in healthy elderly with age-related white matter changes. J Neurol 2011; 258: 1451–9. [DOI] [PubMed] [Google Scholar]
- 29. Guttmann CR, Benson R, Warfield SKet al. White matter abnormalities in mobility-impaired older persons. Neurology 2000; 54: 1277–83. [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto M, Takashima Y, Uchino A, Yuzuriha T, Yao H. Dual task walking reveals cognitive dysfunction in community-dwelling elderly subjects: the Sefuri brain MRI study. J Stroke and Cerebrovasc Dis 2014; 23: 1770–5. [DOI] [PubMed] [Google Scholar]
- 31. Karim HT, Rosso A, Aizenstein HJ, Bohnen NI, Studenski S, Rosano C. Resting state connectivity within the basal ganglia and gait speed in older adults with cerebral small vessel disease and locomotor risk factors. NeuroImage Clin 2020; 28: 102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YJ, Kwon HK, Lee JMet al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 2016; 86: 1199–207. [DOI] [PubMed] [Google Scholar]
- 33. Koo BB, Bergethon P, Qiu WQet al. Clinical prediction of fall risk and white matter abnormalities: a diffusion tensor imaging study. Arch Neurol 2012; 69: 733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li P, Wang Y, Jiang Yet al. Cerebral small vessel disease is associated with gait disturbance among community-dwelling elderly individuals: the Taizhou imaging study. Aging 2020; 12: 2814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moscufo N, Guttmann CRG, Meier Det al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging 2011; 32: 646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray ME, Senjem ML, Petersen RCet al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol 2010; 67: 1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nadkarni NK, Boudreau RM, Studenski SAet al. Slow gait, white matter characteristics, and prior 10-year interleukin-6 levels in older adults. Neurology 2016; 87: 1993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasmussen LJH, Caspi A, Ambler Aet al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw Open 2019; 2: e1913123. 10.1001/jamanetworkopen.2019.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosano C, Brach J, Longstreth WT Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 2005; 26: 52–60. [DOI] [PubMed] [Google Scholar]
- 40. Rosano C, Brach J, Studenski S, Longstreth WT Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology 2007; 29: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosario BL, Rosso AL, Aizenstein HJet al. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci 2016; 71: 968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosso AL, Olson Hunt MJ, Yang Met al. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture 2014; 40: 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry 1999; 67: 658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sakurai R, Inagaki H, Tokumaru AMet al. Differences in the association between white matter hyperintensities and gait performance among older adults with and without cognitive impairment. Geriatrics and Gerontology International 2021; 21: 313–20. [DOI] [PubMed] [Google Scholar]
- 45. Sartor J, Bettecken K, Bernhard FPet al. White matter changes-related gait and executive function deficits: associations with age and Parkinson's disease. Front Aging Neurosci 2017; 9: 213. 10.3389/fnagi.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seiler S, Pirpamer L, Gesierich Bet al. Lower magnetization transfer ratio in the forceps minor is associated with poorer gait velocity in older adults. AJNR Am J Neuroradiol 2017; 38: 500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith EE, O'Donnell M, Dagenais Get al. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol 2015; 77: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorond FA, Kiely DK, Galica Aet al. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol 2011; 70: 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Starr JM, Leaper SA, Murray ADet al. Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry 2003; 74: 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stijntjes M, Craen AJM, Grond J, Meskers CGM, Slagboom PE, Maier AB. Cerebral microbleeds and lacunar infarcts are associated with walking speed independent of cognitive performance in middle-aged to older adults. Gerontology 2016; 62: 500–7. [DOI] [PubMed] [Google Scholar]
- 51. Su N, Liang X, Zhai F-Fet al. The consequence of cerebral small vessel disease: linking brain atrophy to motor impairment in the elderly. Hum Brain Mapp 2018; 39: 4452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Su N, Zhai FF, Zhou LXet al. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front Aging Neurosci 2017; 9: 313. 10.3389/fnagi.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verlinden VJA, Groot M, Cremers LGMet al. Tract-specific white matter microstructure and gait in humans. Neurobiol Aging 2016; 43: 164–73. [DOI] [PubMed] [Google Scholar]
- 54. Verwer JH, Biessels GJ, Heinen R, Exalto LG, Emmelot-Vonk MH, Koek HL. Occurrence of impaired physical performance in memory clinic patients with cerebral small vessel disease. Alzheimer Dis Assoc Disord 2018; 32: 214–9. [DOI] [PubMed] [Google Scholar]
- 55. Wang L, Lin H, Peng Yet al. Incidental brain magnetic resonance imaging findings and the cognitive and motor performance in the elderly: the Shanghai Changfeng Study. Front Neurosci 2021; 15: 631087. 10.3389/fnins.2021.631087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willey JZ, Moon YP, Dhamoon MSet al. Regional subclinical cerebrovascular disease is associated with balance in an elderly multi-ethnic population. Neuroepidemiology 2018; 51: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Windham BG, Wilkening SR, Lirette STet al. Associations between inflammation and physical function in African Americans and European Americans with prevalent cardiovascular risk factors. J Am Geriatr Soc 2016; 64: 1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jayakody O, Breslin M, Beare Ret al. The association between simple reaction time variability and gait variability: the Tasmanian study of cognition and gait. Gait Posture 2021; 89: 206–10. [DOI] [PubMed] [Google Scholar]
- 59. Jenkins LM, Garner CR, Kurian Set al. Cumulative blood pressure exposure, basal ganglia, and thalamic morphology in midlife. Hypertension 2020; 75: 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma R, Zhào H, Wei W, Liu Y, Huang Y. Gait characteristics under single−/dual-task walking conditions in elderly patients with cerebral small vessel disease: analysis of gait variability, gait asymmetry and bilateral coordination of gait. Gait Posture 2022; 92: 65–70. 10.1016/j.gaitpost.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 61. Hou Y, Li Y, Yang S, Qin W, Yang L, Hu W. Gait impairment and upper extremity disturbance are associated with Total magnetic resonance imaging cerebral small vessel disease burden. Front Aging Neurosci 2021; 13: 640844. 10.3389/fnagi.2021.640844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ogama N, Endo H, Satake S, Niida S, Arai H, Sakurai T. Impact of regional white matter hyperintensities on specific gait function in Alzheimer's disease and mild cognitive impairment. J Cachexia Sarcopenia Muscle 2021; 12: 2045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol 2003; 60: 835–9. [DOI] [PubMed] [Google Scholar]
- 64. Briley DP, Haroon S, Sergent SM, Thomas S. Does leukoaraiosis predict morbidity and mortality? Neurology 2000; 54: 90–4. [DOI] [PubMed] [Google Scholar]
- 65. Callisaya ML, Beare R, Phan Tet al. Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J Gerontol A Biol Sci Med Sci 2015; 70: 360–6. [DOI] [PubMed] [Google Scholar]
- 66. Callisaya ML, Beare R, Phan TGet al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc 2013; 61: 1074–9. [DOI] [PubMed] [Google Scholar]
- 67. Kreisel SH, Blahak C, Bazner Het al. Deterioration of gait and balance over time: the effects of age-related white matter change - the LADIS study. Cerebrovasc Dis 2013; 35: 544–53. [DOI] [PubMed] [Google Scholar]
- 68. Moscufo N, Wolfson L, Meier Det al. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr) 2012; 34: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pinter D, Ritchie SJ, Gattringer Tet al. Predictors of gait speed and its change over three years in community-dwelling older people. Aging 2018; 10: 144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005; 53: 649–54. [DOI] [PubMed] [Google Scholar]
- 71. Rosso AL, Studenski SA, Longstreth WT, Brach JS, Boudreau RM, Rosano C. Contributors to poor mobility in older adults: integrating white matter hyperintensities and conditions affecting other systems. J Gerontol A Biol Sci Med Sci 2017; 72: 1246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008; 71: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soumare A, Elbaz A, Zhu Yet al. White matter lesions volume and motor performances in the elderly. Ann Neurol 2009; 65: 706–15. [DOI] [PubMed] [Google Scholar]
- 74. Srikanth V, Beare R, Blizzard Let al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke 2009; 40: 175–80. [DOI] [PubMed] [Google Scholar]
- 75. Sullivan KJ, Ranadive R, Su Det al. Imaging-based indices of neuropathology and gait speed decline in older adults: the atherosclerosis risk in communities study. Brain Imaging Behav 2021; 15: 2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Holst HM, Tuladhar AM, Zerbi Vet al. White matter changes and gait decline in cerebral small vessel disease. NeuroImage: Clinical 2018; 17: 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Holst HM, Uden IWM, Laat KFet al. Baseline cerebral small vessel disease is not associated with gait decline after five years. Mov Disord Clin Pract 2017; 4: 374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R, Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J Neurol 2013; 260: 884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wolfson L, Wei X, Hall CBet al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci 2005; 232: 23–7. [DOI] [PubMed] [Google Scholar]
- 80. Zhang W, Shen H, Yao Xet al. Clinical and diffusion tensor imaging to evaluate falls, balance and gait dysfunction in leukoaraiosis: an observational, prospective cohort study. J Geriatr Psychiatry Neurol 2020; 33: 223–30. [DOI] [PubMed] [Google Scholar]
- 81. Heiland EG, Welmer AK, Kalpouzos G, Laveskog A, Wang R, Qiu C. Cerebral small vessel disease, cardiovascular risk factors, and future walking speed in old age: a population-based cohort study. BMC Neurol 2021; 21: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Callisaya ML, Srikanth VK, Lord SRet al. Sub-cortical infarcts and the risk of falls in older people: combined results of TASCOG and Sydney MAS studies. Int J Stroke 2014; 9 Suppl A100: 55–60. [DOI] [PubMed] [Google Scholar]
- 83. Fazekas F, Kleinert R, Offenbacher Het al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43: 1683–9. [DOI] [PubMed] [Google Scholar]
- 84. Brant-Zawadzki M, Fein G, Van Dyke C, Kiernan R, Davenport L, Groot J. MR imaging of the aging brain: patchy white-matter lesions and dementia. AJNR Am J Neuroradiol 1985; 6: 675–82. [PMC free article] [PubMed] [Google Scholar]
- 85. Breteler MM, Swieten JC, Bots MLet al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 1994; 44: 1246–52. [DOI] [PubMed] [Google Scholar]
- 86. Swieten JC, Hijdra A, Koudstaal PJ, Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatry 1990; 53: 1080–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scheltens P, Barkhof F, Leys Det al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993; 114: 7–12. [DOI] [PubMed] [Google Scholar]
- 88. Wahlund LO, Barkhof F, Fazekas Fet al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–22. [DOI] [PubMed] [Google Scholar]
- 89. Victoroff J, Mack WJ, Grafton ST, Schreiber SS, Chui HC. A method to improve interrater reliability of visual inspection of brain MRI scans in dementia. Neurology 1994; 44: 2267–76. [DOI] [PubMed] [Google Scholar]
- 90. Kilgour AH, Todd OM, Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr 2014; 14: 85. 10.1186/1471-2318-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Beauchet O, Annweiler C, Callisaya MLet al. Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc 2016; 17: 482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Annweiler C, Beauchet O, Celle Set al. Contribution of brain imaging to the understanding of gait disorders in Alzheimer's disease: a systematic review. Am J Alzheimers Dis Other Demen 2012; 27: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Parihar R, Mahoney JR, Verghese J. Relationship of gait and cognition in the elderly. Curr Transl Geriatr Exp Gerontol Rep 2013; 2: 167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Montero-Odasso M, Almeida QJ, Bherer Let al. Consensus on shared measures of mobility and cognition: from the Canadian Consortium on neurodegeneration in aging (CCNA). J Gerontol A Biol Sci Med Sci 2019; 74: 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not provided in the article may be shared at the request of any qualified investigator for purposes of replicating procedures and results.