Abstract
Background:
Patents with major depression have evidence of a proinflammatory state with consistent elevations in acute phase proteins and in the levels of inflammatory mediators such as Interleukin-6 and Tumor necrosis factor-α. We report here a study of the serum levels of Immunoglobulin A (IgA) in medication-free patients with major depression in the remitted state (ruMDD). Selective IgA deficiency is the most common form of immunoglobulin abnormality, and is often associated with a higher than expected incidence of proinflammatory and autoimmune phenomena.
Methods:
We measured serum IgG, IgM, and IgA in 28 ruMDD patients and 27 healthy subjects (Ctrl) at 0 (pretreatment), 7, and 24 hours following sham depletion and tryptophan (TrpD) depletion conducted at least 8 days apart under balanced, randomized, blinded conditions. Immunoglobulins were measured by automated immunonephelometry. Data were analyzed by repeated measures ANOVA with diagnosis as a fixed effect and drug (TrpD vs. sham), and time as repeated measures factors.
Results:
Serum IgA was consistently lower in ruMDD patients vs. Ctrl at all time points examined (p< 0.04 for main effect of diagnosis). Serum IgG and IgM levels did not show significant differences by diagnosis.
Conclusions:
Medication-free patients with major depression in the remitted state have a significant reduction in serum IgA levels measured on multiple occasions. In the light of the fact that IgA serves many immunomodulatory, anti-inflammatory roles, this finding supports the concept that major depressive illness represents a proinflammatory state.
Keywords: Major depression, Immunoglobulin IgA, Proinflammatory state
Introduction:
Alterations in mood and affect in patients with major depression is only one component of a systemic syndrome associated with premature coronary disease[11], osteoporosis [20], and a doubling of mortality at any age, independent of suicide, smoking, hypertension, or other common risk factors for poor health. One common denominator of relevance to each of these components of major depression is a proinflammatory state comprised predominantly of mediators of the innate immune response [1, 9, 21, 23].
We have previously reported that the female medication-free patients with major depression in the remitted state (ruMDD) in the present study, have increased levels of two key acute phase proteins that are mainstays of innate immunity, C-reactive protein and serum amyloid A (SAA) [16]. The present study contains not only the female studies in this tryptophan depletion (TrpD) study, but the males as well. Since major depression is thought to represent a deficiency of serotonin neurotransmission, the Trp depletion strategy was designed to determine if reduction of central serotonin availability in the CNS for three days precipitated a return of depressive symptoms. There is no evidence linking serotonin function to levels of IgA. To determine if other components of the immune response are altered in these subjects, we examined the levels of the immunoglobulin (Ig) A in the overall group of subjects. Selective IgA deficiency is by far the most common abnormality of immunoglobulin secretion [8, 31], and is not associated with overt pathology in the majority of cases. However, 6-10% of patients with isolated complete or partial IgA deficiency suffer from atopic states or autoimmune disease. Moreover, 40% have abnormal serum antibodies to cells or tissues including antinuclear (ANAs) and anti-thyroglobulin antibodies [8, 14, 31].
The increased incidence of atopic phenomena and autoimmune disease in patients with selective IgA deficiency is compatible with emerging data that in addition to its significant role in mucosal immunity, IgA is also a mediator of the immune system that contributes to maintaining a balance between pro-and-anti-inflammatory states [14]. IgA exerts a multiplicity of anti-inflammatory effects that include interference with the complement system of the innate immune system, down-regulation of cell mediated responses of heterologous receptors such as monocyte chemotactic protein-1 (MCP-1j and tumor necrosis factor- α (TNF-α), and inhibition of processes that include IgG-mediated phagocytosis and proinflammatory cytokine release [12, 14, 25, 29]
Given the immune activation in patients with major depression and the anti-inflammatory effects of IgA, we postulated that patients with major depression were in a state of partial selective IgA deficiency. To determine the extent to which the putative IgA deficiency is selective, we also measured the levels of IgG and IgM.
Material and Methods
Patients with a history of Major Depressive Disorder (MDD) who were off psychotropic medications and in clinical remission (ruMDD) for at least two months prior to the time of study and carefully matched control subjects (Ctrl) were studied, as previously described (Neumeister et al 2004). Patients and controls were recruited from the greater Washington, DC metropolitan area through newspaper and radio advertisements; respondents passing a telephone screen for eligibility were evaluated in the National Institute of Mental Health (NIMH) outpatient clinic at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. All subjects gave written, informed consent for participation in the study, which was conducted under protocols approved by the NIMH Institutional Review Board, after having the study explained to them in detail and having an opportunity to ask questions of the investigator.
Subjects:
Twenty-eight ruMDD patients according to Diagnostic and statistical manual of mental disorders (DSM-IV) criteria (20 women, and 8 men; mean age = 39.5 years [SD = 12.5]; mean baseline 24-item Hamilton depression rating scale (HDRS) total score = 1.0 [SD = 1.2]; age at onset = 23.1 years [SD = 8.3]; number of previous episodes = 3.5 [SD = 2.6]) and 27 Ctrl (18 women; mean age = 33.8 years [SD = 11.2]; mean baseline 24-item HDRS score = 0.4 [SD = 0.7]), all non-smokers, participated in the study. Duration of the depressive illness and number of episodes were estimated from the Past History of MDD addendum of the Structural Clinical Interview for DSM-IV. Information about family history of mental illness (Axis I diagnoses) was obtained from the study participants for all first-degree relatives using the Family Interview for Genetic Studies.
Controls had no personal or first-degree relative history of psychiatric disorders. For ruMDD patients, no lifetime diagnosis other than MDD was allowed. Remission was defined as at least 3 months during which the individual did not take an antidepressant agent and had 24-item HDRS scores in the non-depressed range (< 8) [10]. The depressed patients were in remission for a mean of 40.4 months (SD = 48.4) and had not been taking antidepressant medication for a mean of 37.6 months (SD = 43.9) at the time of the study. No use of medication was allowed during the study. Subjects were medically healthy as determined by history and results of physical examination, electrocardiogram, and laboratory tests, including liver and kidney function tests, hematologic profile, thyroid function tests, urinalysis and toxicology. Premenopausal women were studied during the follicular phase of the menstrual cycle as determined using plasma estradiol and progesterone concentrations, time since onset of last menses, and home urine ovulation kits (Clear Plan Easy; Whitehall Laboratories, Madison, NJ)
In a randomized, placebo-controlled, balanced, double-blind crossover study, participants received capsules containing an amino acid mixture without tryptophan during Trp depletion, and they received identical capsules containing lactose during sham depletion. None of the patients or controls showed any evidence of current or recent (within one week) infectious process at the time of blood sampling.
We measured serum immunoglobulins (IgM, IgG, and IgA) in 28 ruMDD patients and 27 Ctrl at 0 (pretreatment), 7, and 24 hours following sham depletion and TrpD conducted at least 8 days apart under balanced, randomized, blinded conditions.
Serum immunoglobulins were measured by immunonephelometry using a BN II automated analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Our reference intervals are 7.0-16.0 g/L for IgG, 0.7-4.0 g/L for IgA and 0.4-2.3 g/L for IgM.
Data were analyzed using a full factorial linear mixed model with a compound symmetry covariance structure, restricted maximum likelihood estimates, diagnosis as a between subjects factor, and drug (TrpD vs. sham) and time as repeated measures factors. Bonferroni post hoc tests were used to examine significant interactions. Cohen’s d effect size was calculated to show the size of diagnosis effects. Significance was evaluated at p<.05, two-tailed.
Results
Serum IgA:
The linear mixed model revealed a significant main effect of diagnosis on serum IgA levels (F = 4.68, d.f. = 1,53: p = 0.04). Mean serum IgA levels in the ruMDD patients were reduced by approximately 20% relative to controls (estimated marginal means, 1.73 + (sem) 0.14 vs. 2.17 + 0.15 g/L, respectively: d=0.59) (Figure 1).
Figure 1.
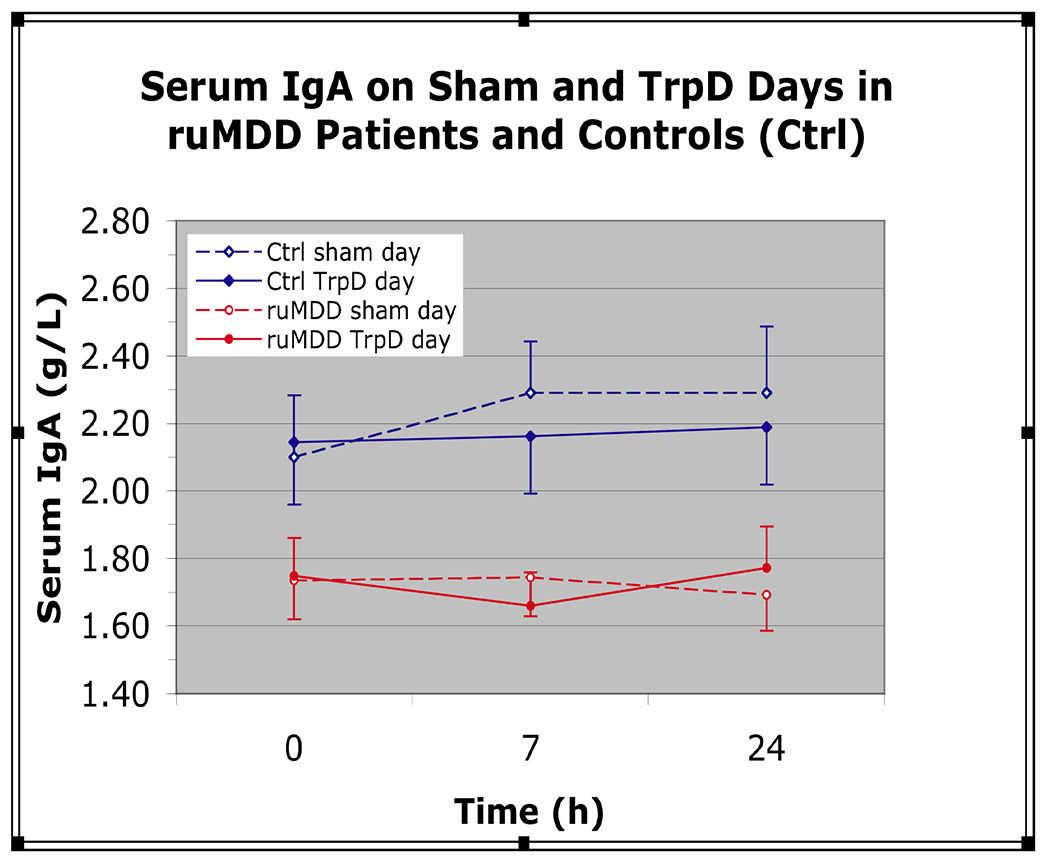
Serum Immunoglobulin A (IgA) on sham and Tryptophan depletion (TrpD) Days in medication-free patients with major depression in the remitted state (ruMDD) and Controls (Ctrl). Figure shows serum IgA in 28 ruMDD (red) and 27 healthy Ctrl subjects (blue) matched closely for age, gender, and body mass index (BMI) at 0 h (pretreatment) and at 7 and 24 hours after oral administration of a tryptophan-depleting amino acid mixture (solid lines) or a sham control mixture (dotted lines). Serum IgA levels were reduced by 20% in ruMDD patients vs. controls
A significant main effect of time on serum IgA was observed (F = 5.47, d.f. = 2,265; p = 0.005). Mean serum IgA levels were 3.6% higher at 24h vs. baseline (1.99 ± 0.10 vs. 1.93 ± 0.10 g/L, respectively) and 2.8% higher than at 7h (1.92 ± 0.10 g/L). A significant diagnosis*drug*time interaction (F = 6.90, d.f. = 2,265; p = 0.001) indicated IgA levels were 4.6% higher in the controls on the sham day vs. the TrpD day (2.29 + 0.15 vs. 2.19 + 0.15 g/L, p < 0.02).
Serum IgG:
No significant main effects of diagnosis (d=0.17), drug condition, or time on serum IgG levels were observed. There was a significant diagnosis*drug*time interaction (F = 3.76, d.f. = 2,265; p = 0.03), which was due to 4.7% higher IgG levels at 24h vs. baseline in the control subjects on the sham day (11.04 ± 0.53 vs. 10.54 ± 0.53 g/L; p = 0.04).
Serum IgM:
No significant main effects of diagnosis (d=0.09), drug condition, or time on serum IgM levels were observed. There was a significant diagnosis*drug*time interaction (F = 4.05, d.f. = 2,265; p = 0.02), which was due to higher IgM levels in the ruMDD patients at 24 h following the TrpD day (1.32 + 0.13 g/L) vs. both the 7 h value (1.21 + 0.13 g/L) and vs. the sham day (1.22 + 0.13 g/L).
Discussion
Patients with major depression who were remitted and unmedicated for at least three months prior to study had significantly reduced mean plasma IgA levels taken over 24 hours at midnight, 7AM and the following midnight on two occasions separated by 8 days. In contrast, plasma IgG and IgM levels were similar to those in controls.
Given the nature of this patient population, these findings are unlikely to be due to transient effects of depressive episodes or to effects of antidepressants. It is also unclear how generalizable these findings are to other patients with MDD, as most patients with recurrent MDD do not remain in remission for extended periods.
No consistent differences were observed between sham and TrpD conditions.
Isolated deficiency of IgA is the most common of the primary immunodeficiencies. The worldwide incidence varies depending on ethnic background, with 1:143 in the Arabian Peninsula and 1:875 in England [8, 31]. Partial deficiency, defined as IgA levels 2 SD below the normal mean, occurs less frequently [4, 31]. In contrast, the patients in this study had mean levels approximately 20% lower than in controls. To our knowledge, there has been no other report of reduced levels in this range in other patient populations.
IgA is the most abundant immunoglobulin isotype produced in mammals [31]. Its half-life is however, five times shorter than that of IgG, so that plasma IgG levels are higher [12]. The rapid half-life is thought to facilitate more tightly controlled levels of IgA in keeping with its role as an immunomodulator helping to maintain the proper balance between pro- and anti-inflammatory states.
IgA is best known as an immunoglobulin found in all mucosal barriers, namely tears, saliva, colostrum, gastrointestinal (GI) tract, prostate, respiratory epithelium, and most notably, gut [8, 31]. It is therefore thought to be important in mucosal immunity. Its actions include preventing attachment to or penetration of body surfaces by microorganisms and protecting against respiratory, gastrointestinal, and genitourinary infections [30]. Mucosal IgA, or secretory IgA (SIgA) is the dimeric form of the immunoglobulin. Secretory IgA cannot be directly quantified by plasma measurements, though it can be estimated via GI biopsy [26, 27]
IgA is also found in plasma in its monomeric form [8, 31]. Its role in promoting immune function has not been definitively established. In the portal circulation, it may serve as a back up to mucosal immunity by surveillance of bacteria that penetrate the intestinal wall [8, 28]. Although monomeric IgA in the circulation does not fix the classical pathway of complement [12], it may have a role in activation of phagocytosis by binding to its Fc receptor of group A streptococci (FcRa) on monocytes and granulocytes [8, 28]. In this way, it may form immune complexes that are cleared from the circulation by the phagocytic system without causing inflammation. Sialated glycan bound IgA in the systemic circulation can also bind to lectins on the surface of bacteria [24], thus also functioning as a component of the innate immune system There have been no definitive studies documenting the relationship between deficiencies in serum or mucosal IgA. One study in which serum levels and jejeunal biopsies were obtained simultaneously showed a deficiency in both, but with no correlation between the defects in the two subtypes [26, 27] Thus, the two systems, though not identical, are nevertheless related [7].
An expanding literature documents a housekeeping role for IgA in helping to maintain a balance between immune surveillance and anti-inflammatory activity and in possessing distinct anti-inflammatory activity [12, 14, 25, 29]. Serum IgA down-regulates cell-mediated responses of heterologous receptors such as MCP-1 and TNF-α and processes that include IgG-mediated phagocytosis, bactericidal activity, oxidative burst activity, and cytokine release [14, 29]. IgA may also play a role in controlling the immune system through inhibition of neutrophil chemotaxis by binding to other inhibitory proteins such as alpha-1 antitrypsin [14].
As noted, IgA does not activate complement under biologic conditions [12]. More importantly, it blocks activation of complement by direct binding of complement components [12]. By blocking specific steps of complement-mediated immune mechanisms, it functions as an anti-inflammatory housekeeper not only directly, but also indirectly by blocking complement activation by IgM and IgG [12].
The anti-inflammatory activity of IgA is postulated to underlie a 6-10% incidence of autoimmunity in patients with selective IgA deficiency, while, as noted, 40% have abnormal serum antibodies to cells or tissues including ANA and antithyroglobulin antibodies [8, 31]. The prevalence of celiac disease is estimated to be 2.6%, a 15-fold increase over the incidence in the general population. [19]. There is also a significant increase in allergic phenomena [15, 17], and about half of the patients with selective IgA deficiency have some elevation in IgE [4]. In the case of celiac disease, some postulate that there is a possible insufficient exclusion of food antigens that contributes to the illness [31].
Surprisingly, approximately 85% of patients with selective IgA deficiency are without significant related illnesses [17, 31]. Those with symptoms have predominantly sinopulmonary [5] or gastrointestinal infections [32], or as indicated, autoimmune or allergic symptomatology. The presence of a modest excess in pulmonary and gastrointestinal disturbances further supports other work indicating that selective IgA deficiency affects both serum and mucosal IgA systems [7, 26]. The paucity of clinical manifestations reflects, in part, the fact that there is a compensatory increase in IgM and IgD in the gut, nasal mucosa, and other mucosal linings [3]. Thus, the principal clinical manifestations seem to be secondary to a loss of the anti-inflammatory effects of the IgA system in selective IgA deficiency [14, 25, 29].
In IgA deficiency, B cells express IgA; however, they are of immature phenotype with the co-expression of IgM and IgD, and they cannot fully develop into IgA-secreting plasma cells [13]. The defect appears to involve the stem cells since IgA deficiency can be transferred [13].
Abnormalities in the regulation of various cytokine species could also contribute to selective IgA deficiency. As an example, interleukin-21 (IL-21) induces immunoglobulin class switching to IgA, and IL-21 administration can partially restore IgA in individuals with selective IgA deficiency [2]. Conversely, interleukin-4 (IL-4) inhibits the switch to IgA, and could, hence, contribute to selective IgA deficiency as well [22].
Conclusions
We do not know the significance of the fact that plasma IgA levels are significantly reduced in ruMDD patients. It is tempting to speculate that these findings and other proinflammatory phenomena in patients with major depression are trait markers. Thus, there may be discordance between demonstrable affective and cognitive symptoms and what are clear systemic manifestations of depressive illness. Alternatively, such findings may reflect the sequellae of somatic changes that occurred during the depressed state and that persist after depressive symptoms have resolved. For instance, during the hypercortisolism of major depression, visceral fat accumulates that may not be lost after resolution of hypercortisolism, analogous to, but more subtle than that seen after recovery in Cushing’s disease [6, 18]. Increased visceral fat mass is associated with a proinflammatory state that includes the hypersecretion of IL-6, TNF-α, and acute phase proteins.
Patients with major depression are in a proinflammatory state, with activation of acute phase protein release that includes SAA and CRP, and increased levels of plasma IL-6 and TNF-α. The extent to which the decreased levels of plasma IgA contribute to these abnormalities cannot be inferred on the basis of available data. None of the patients in this study had overt evidence of autoimmune disease. On the other hand, serotonin itself can be a proinflammatory mediator, but we found no effect of perturbing central serotonergic activity on plasma IgA levels. Further studies should clarify the extent to which patients with major depression have elevated levels of antibodies to tissue or cells or elevations in IgE [4]. Moreover, the extent to which abnormalities in IL-21 or IL-4 contribute to the present decrease in plasma IgA levels is entirely unknown. Prospective studies should clarify the significance of a selective, modest reduction in serum IgA.
Highlights.
> Patents with major depression have evidence of a proinflammatory state > Selective IgA deficiency is the most common form of immunoglobulin abnormality > We measured Immunoglobulin A in medication-free major depressive in remission patients > Serum IgA was lower in remitted patients vs. controls measured on multiple occasions > this finding supports the proinflammatory state of major depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
All authors have contributed to the research and/or article preparation and they have approved the final article.
Dr. Philip W. Gold, Dr. Maria G. Pavlatou, Dr. Paul J. Carlson, Dr. David A. Luckenbaughc, Mr. Rene Costello, Dr. Omer Bonne, Dr. Gyorgy Csako, Dr. Alan Remaley, Dr. Alexander Neumeister, and Dr. Mitchel A. King have no conflicts to disclose. Dr. Wayne C. Drevets has reported that the research support for this project was provided entirely by the NIMH DIRP. Although Dr. Drevets has no financial conflicts that are relevant to the subject matter of the manuscript, during the past two years he has served as a consultant for the following companies: Pfizer, Eisai, Rules Based Medicine, Johnson and Johnson. Dr.Drevets also is named as coinventor for a Use Patent filed by the NIMH for Scopolamine in the Treatment of Depression. Dr. Dennis S. Charney has reported that he and Mount Sinai School of Medicine have been named on a use patent of Ketamine for the treatment of depression. If Ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration (FDA) for this indication, Dr. Charney and Mount Sinai School of Medicine could benefit financially. Dr. Charney has also disclosed that he is not currently engaged in any consulting activity and has had no activity for the past three years.
References
- [1].Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW, Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications, J Clin Endocrinol Metab 90 (2005) 2522–2530. [DOI] [PubMed] [Google Scholar]
- [2].Borte S, Pan-Hammarstrom Q, Liu C, Sack U, Borte M, Wagner U, Graf D, Hammarstrom L, Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency, Blood 114 (2009) 4089–4098. [DOI] [PubMed] [Google Scholar]
- [3].Brandtzaeg P, Karlsson G, Hansson G, Petruson B, Bjorkander J, Hanson LA, The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa, Clin Exp Immunol 67 (1987) 626–636. [PMC free article] [PubMed] [Google Scholar]
- [4].Burgio GR, Duse M, Monafo V, Ascione A, Nespoli L, Selective IgA deficiency: clinical and immunological evaluation of 50 pediatric patients, Eur J Pediatr 133 (1980) 101–106. [DOI] [PubMed] [Google Scholar]
- [5].Chipps BE, Talamo RC, Winkelstein JA, IgA deficiency, recurrent pneumonias, and bronchiectasis, Chest 73 (1978) 519–526. [DOI] [PubMed] [Google Scholar]
- [6].Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, Marzullo P, Cerbone G, Siciliani M, Lombardi G, Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure, J Clin Endocrinol Metab 84 (1999) 2664–2672. [DOI] [PubMed] [Google Scholar]
- [7].Conley ME, Delacroix DL, Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense?, Ann Intern Med 106 (1987) 892–899. [DOI] [PubMed] [Google Scholar]
- [8].Cunningham-Rundles C, Physiology of IgA and IgA deficiency, J Clin Immunol 21 (2001) 303–309. [DOI] [PubMed] [Google Scholar]
- [9].Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, A meta-analysis of cytokines in major depression, Biol Psychiatry 67 (2010) 446–457. [DOI] [PubMed] [Google Scholar]
- [10].Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM, Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence, Arch Gen Psychiatry 48 (1991) 851–855. [DOI] [PubMed] [Google Scholar]
- [11].Glassman AH, Shapiro PA, Depression and the course of coronary artery disease, Am J Psychiatry 155 (1998) 4–11. [DOI] [PubMed] [Google Scholar]
- [12].Griffiss JM, Biologic function of the serum IgA system: modulation of complement-mediated effector mechanisms and conservation of antigenic mass, Ann N Y Acad Sci 409 (1983) 697–707. [DOI] [PubMed] [Google Scholar]
- [13].Hammarstrom L, Lonnqvist B, Ringden O, Smith CI, Wiebe T, Transfer of IgA deficiency to a bone-marrow-grafted patient with aplastic anaemia, Lancet 1 (1985) 778–781. [DOI] [PubMed] [Google Scholar]
- [14].Jacob CM, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC, Autoimmunity in IgA deficiency: revisiting the role of IgA as a silent housekeeper, J Clin Immunol 28 Suppl 1 (2008) S56–61. [DOI] [PubMed] [Google Scholar]
- [15].Janzi M, Kull I, Sjoberg R, Wan J, Melen E, Bayat N, Ostblom E, Pan-Hammarstrom Q, Nilsson P, Hammarstrom L, Selective IgA deficiency in early life: association to infections and allergic diseases during childhood, Clin Immunol 133 (2009) 78–85. [DOI] [PubMed] [Google Scholar]
- [16].Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, Gold PW, Neumeister A, Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A, Biol Psychiatry 62 (2007) 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Latiff AH, Kerr MA, The clinical significance of immunoglobulin A deficiency, Ann Clin Biochem 44 (2007) 131–139. [DOI] [PubMed] [Google Scholar]
- [18].Leong GM, Abad V, Charmandari E, Reynolds JC, Hill S, Chrousos GP, Nieman LK, Effects of child- and adolescent-onset endogenous Cushing syndrome on bone mass, body composition, and growth: a 7-year prospective study into young adulthood, J Bone Miner Res 22 (2007) 110–118. [DOI] [PubMed] [Google Scholar]
- [19].Meini A, Pillan NM, Villanacci V, Monafo V, Ugazio AG, Plebani A, Prevalence and diagnosis of celiac disease in IgA-deficient children, Ann Allergy Asthma Immunol 77 (1996) 333–336. [DOI] [PubMed] [Google Scholar]
- [20].Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P, Bone mineral density in women with depression, N Engl J Med 335 (1996) 1176–1181. [DOI] [PubMed] [Google Scholar]
- [21].Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA, Clinical depression and inflammatory risk markers for coronary heart disease, Am J Cardiol 90 (2002) 1279–1283. [DOI] [PubMed] [Google Scholar]
- [22].Okahashi N, Yamamoto M, Vancott JL, Chatfield SN, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee JR, Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses, Infect Immun 64 (1996) 1516–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M, Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study, Biol Psychiatry 54 (2003) 566–572. [DOI] [PubMed] [Google Scholar]
- [24].Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, Wilson IA, Daha MR, Dwek RA, Rudd PM, Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems, J Biol Chem 278 (2003) 20140–20153. [DOI] [PubMed] [Google Scholar]
- [25].Russell MW, Sibley DA, Nikolova EB, Tomana M, Mestecky J, IgA antibody as a non-inflammatory regulator of immunity, Biochem Soc Trans 25 (1997) 466–470. [DOI] [PubMed] [Google Scholar]
- [26].Savilahti E, IgA deficiency in children. Immunoglobulin-containing cells in the intestinal mucosa, immunoglobulins in secretions and serum IgA levels, Clin Exp Immunol 13 (1973) 395–406. [PMC free article] [PubMed] [Google Scholar]
- [27].Savilahti E, Pelkonen P, Clinical Findings and intestinal immunoglobulins in children with partial IgA deficiency, Acta Paediatrica 68 (2008) 513–519. [DOI] [PubMed] [Google Scholar]
- [28].van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG, IgA and the IgA Fc receptor, Trends Immunol 22 (2001) 205–211. [DOI] [PubMed] [Google Scholar]
- [29].Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM, Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes, Blood 83 (1994) 1278–1288. [PubMed] [Google Scholar]
- [30].Woof JM, Kerr MA, The function of immunoglobulin A in immunity, J Pathol 208 (2006) 270–282. [DOI] [PubMed] [Google Scholar]
- [31].Yel L, Selective IgA deficiency, J Clin Immunol 30 (2010) 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zinneman HH, Kaplan AP, The association of giardiasis with reduced intestinal secretory immunoglobulin A, Am J Dig Dis 17 (1972) 793–797. [DOI] [PubMed] [Google Scholar]


