Abstract
Objectives
To investigate the dose–response association of aerobic physical activity (PA) and muscle-strengthening exercise (MSE) with all-cause mortality.
Methods
National Health Interview Survey data (1997–2014) were linked to the National Death Index through 2015, which produced a cohort of 416 420 US adults. Cox proportional-hazard models were used to estimate HRs and 95% CIs for the associations of moderate aerobic PA (MPA), vigorous aerobic PA (VPA) and MSE with mortality risk. Models controlled for age, sex, race- ethnicity, income, education, marital status, survey year, smoking status, body mass index and chronic conditions.
Results
Relative to those who engaged in no aerobic PA, substantial mortality risk reduction was associated with 1 hour/week of aerobic PA (HR: 0.85, 95% CI: 0.83 to 0.86) and levelled off at 3 hours/week of aerobic PA (0.73, 0.71 to 0.75). Similar results were observed for men and women and for individuals younger and older than 60 years. MSE conferred additional mortality risk reduction at 1 time/week (0.89, 0.81 to 0.97) and appeared no longer beneficial at 7 times/week (0.99, 0.94 to 1.04).
Conclusion
The minimum effective dose of aerobic PA for significant mortality risk reduction was 1 hour/week of MPA or VPA, with additional mortality risk reduction observed up to 3 hours/week. For older adults, only small decreases in mortality risk were observed beyond this duration. Completing MSE in combination with aerobic PA conferred additional mortality risk reduction, with a minimum effective dose of 1–2 times/week.
INTRODUCTION
Regular physical activity (PA) participation lowers non-communicable disease incidence (eg, cardiovascular disease, type 2 diabetes) and confers several other physiological and psychosocial health benefits.1–3 The WHO recommends adults accumulate ≥150 min/week of moderate- intensity aerobic PA (MPA), 75 min/week of vigorous- intensity aerobic PA (VPA) or an equivalent combination of the two.3 Despite the known health benefits, >1.4 billion adults do not meet these recommendations.4 Physical inactivity is thus a major public health concern,5 with the WHO ranking physical inactivity as the fourth leading risk factor for mortality over the past decade.6 Indeed, annual physical inactivity-related deaths and health care costs exceed 5 million1 and $67.5 billion,7 8 respectively. These burdens are particularly prevalent in high-income countries, such as the USA, where technological advancements have reduced daily PA engagement.1 7 9 Thus, identifying minimum effective doses of MPA and/or VPA for producing clinically meaningful mortality risk reduction can crucially inform PA recommendations.
Muscle-strengthening exercise (MSE) can confer health benefits independent of aerobic PA (eg, improved bone mineral density and insulin sensitivity, attenuation of sarcopenia).10 While often overlooked in public health policy,11 WHO guidelines recommend ≥2 days/week of MSE targeting all major muscle groups in addition to aerobic PA recommendations.3 Yet, surveillance data note 70% Of US adults fail to meet this MSE recommendation, with 58% reporting no MSE engagement.12 Individuals of higher weight status and/or lower cardiorespiratory fitness can, at times, find aerobic PA intolerable given the musculoskeletal discomfort generated from the often cyclical and repetitive skeletal impacts of activities like walking and jogging.13 Therefore, MSE promotion, a more movement-varied PA mode, may be especially important for morbidity and subsequent mortality risk reduction considering the high prevalence of US adult overweight and obesity (~75%).14 Notably, prospective cohort studies15 16 and recent meta-analyses 17 18 have demonstrated that MSE decreased all-cause and non-communicable disease-specific mortality (eg, cardiovascular disease, cancer, diabetes, lung cancer) among adults, suggesting it to be as important for mortality risk reduction versus meeting the aerobic PA guidelines. However, the additive benefit and interaction effect of performing MSE in addition to aerobic PA on mortality have yet to be elucidated.
Recent analyses19 20 have examined the association between meeting PA recommendations, as a dichotomous exposure, and mortality. We sought to extend these analyses by examining the association of MPA, VPA and MSE, when completed in various combinations and doses, with all-cause mortality risk. We also sought to examine differences in these associations by stratifying by age and sex. Identifying the minimum effective doses of aerobic PA and MSE associated with lower mortality risk may improve public health efforts to increase adherence to the PA guidelines by providing adults realistic and achievable PA targets.
METHODS
Study population
We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the drafting this manuscript.21
The National Health Interview Survey (NHIS) began in 1957 and uses a geographically clustered probability sampling design to produce a representative sample of the civilian non-institutionalised US population.22 23 In 1997, the NHIS began collecting data on MPA, VPA and MSE. We therefore used NHIS PA data from 1997 to 2014 to complete these analyses,23 with these data linked to National Death Index data through 2015.24
Exclusion criteria were persons without a permanent US residence, persons in correctional facilities, active-duty military, persons in long-term care facilities and US citizens living in foreign countries.22 The analytical dataset contained individuals aged 18–84 years who were surveyed in the contiguous US and for whom data on age, sex, race-ethnicity, leisure-time MPA, VPA and MSE, smoking status, body mass index (BMI), income, marital status, educational attainment, survey date, chronic conditions and date and cause of death (if deceased before 31 December 2015) were available. Survey data were generally consistent across years due to standardised data collection procedures, but household income was reported as inflation-adjusted household income using the Consumer Price Index (base-year 2014). The privacy of the participants surveyed by the National Center for Health Statistics (NCHS) is protected by the Privacy Act of 1974. No efforts were made to identify participants. Participants surveyed by the NCHS were not involved in the study’s analytic design or conduct.
MPA, VPA and MSE assessment
Leisure-time MPA, VPA and MSE data were collected by the NCHS starting in 1997 using a standardised questionnaire (online supplemental table S1).23 Participants reported MPA and VPA over any period (day, week) or length of time (min, hours). All reported times were converted to min/week. MSE was reported in number of times over any length of time and was converted to times/week.
Statistical analyses
Cox proportional- hazard (CPH) models in SAS V.9.4 were used to estimate adjusted HRs and 95% CIs for the association of MPA, VPA and MSE with all-cause mortality (hereafter, ‘mortality’). Confounders of the causal relationship between PA and all-cause mortality were evaluated using a causal directed acyclic graph (see diagram S1 in the online supplemental materials). Models allowed for combinations of age (1 year buckets) at baseline, sex (male and female) and race- ethnicity (black non-Hispanic, Hispanic, other/unknown and white non-Hispanic) to have their own baseline hazard (using the STRATA statement in the PHREG procedure).
We included the following variables in the models as categorical covariates: marital status (divorced, separated, never married, widowed, married), smoking status (never, former, current), inflation- adjusted household income ($0–35 000, $35 000–50 000, $50 000–75 000, >$75 000), educational attainment (<high school graduate, high school graduate, some college, college graduate, >college graduate) and survey year (1 year buckets). Additionally, indicator variables for chronic medical conditions related to the respiratory system, circulatory system, non-diabetes endocrine system, digestive system, genitourinary system, the heart, or blood, as well as hypertension, diabetes and cancer were included in the models. BMI was included in the model as natural (restricted) cubic spline terms with k=3 knots of equal window sizes. We completed sensitivity analyses during which models were estimated with and without inclusion of categorical covariates indicating standard ranges of BMI as well as chronic conditions.
Censored survival times were calculated as the difference in years between the survey date and death, end of follow-up (31 December 2015) or loss to follow-up. To account for the clustered probability sampling of the NCHS, complex CPH models with eligibility-adjusted sample weights, primary sampling units and sampling strata were evaluated.22 Because HRs and 95% CIs were stable between the basic and complex CPH models, we used the basic CPH model. To evaluate for potential reverse causality and allow for comparison with previous literature,19 20 the online supplemental materials contain models estimated using a restricted cohort comprised of only ‘never smokers’, those with no chronic conditions at baseline and those surviving 3 years after baseline (ie, 3-year survivors). Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used for goodness of fit comparisons across models, with BIC generally preferred over AIC in the case of disagreement. The proportional hazard assumption of the Cox model was visually confirmed with log of negative log of estimated survivor functions.
Basic exposure model
The basic exposure model included MPA, VPA and MSE together as exposure variables. The reference group was participants participating in <30 min/week of VPA, <30 min/week of MPA and 0 MSE. MPA and VPA were included in the model as 1-hour indicator variables (30 min/week and <90 min/week, 90 min/week and <150 min/week, 150 min/week and <210 min/week and so on) up to 8+ hours (450 min/week). MSE was included in the model as indicator variables for times/week up to 8+.
US Department of Health and Human Services (HHS) exposure model
In accordance with HHS statements, the HHS exposure model treated VPA as twice as beneficial as MPA.25 As such, an aerobic activity index was calculated as VPA+½ MPA, and participants were categorised by 1-hour intervals of this index (<30 min/week of VPA+½ MPA, 30 min/week of VPA+½ MPA and <90 min/week of VPA+½ MPA and so on) up to 8+ hours (450 min/week of VPA+½ MPA). Groups were further divided by participants participating in no MSE and some MSE. These models were estimated against a reference group of participants participating in <30 min/week of VPA+½ MPA and no MSE.
Modified HHS exposure model
The modified HHS model was identical to the HHS model, except that VPA and MPA benefits were weighted equally. The aerobic activity index was calculated as VPA+MPA, with participants categorised by 1-hour intervals of this index up to 8+ hours and groups further divided by participants participating in no MSE and some MSE. The reference group was participants participating in <30 min/week of VPA+MPA and no MSE.
K-means-informed exposure model
The K-means-informed exposure model used a machine learning model to assign participants to one of nine groups based on their MPA and VPA, with a 10th group of participants reporting engagement in <30 min/week of total aerobic PA (MPA+VPA) and no MSE included as the reference group. K-means clusters were created and used to inform the main aerobic PA categories (ie, ‘k-means-informed’ cluster categories) used in this study (see online supplemental figures S10 and S11 to compare k-means clusters and k-means-informed cluster categories). In the K-means-informed exposure model, each ‘cluster category’ was further divided by participants who participated in no MSE and some MSE.
Cubic spline-modified HHS exposure model
The cubic spline-modified HHS exposure model included natural cubic spline terms (ie, restricted cubic splines) of MPA+VPA with the a priori selection of k=3 knots of equal window sizes. MSE was included in the model as an exposure variable in times/week up to 8+.
RESULTS
Descriptive statistics are displayed in table 1 for the full and restricted cohorts. Of the 416 420 participants in the full cohort, 45 344 deaths were observed by 31 December 2015. The restricted cohort was younger and participated in slightly more VPA and MSE but less MPA.
Table 1.
Summary statistics at baseline for participants aged 18–84 in the full and restricted NHIS cohorts surveyed 1997–2014 with mortality follow- up through 2015
| Full cohort | Never smokers, no conditions and 3 year survivors | |
|---|---|---|
| # of participants | 416 420 | 184 033 |
| # of deaths | 45 344 | 11 297 |
| Cardiovascular | 7737 | 1746 |
| Chronic lower respiratory | 2489 | 196 |
| Cancer | 11 094 | 2456 |
| Survival time in years (median±IQR) | 7.0±10.0 | 9.0±9.0 |
| Aerobic physical activity | ||
| None | 36.7% | 33.7% |
| Moderate only | 22.8% | 21.8% |
| Vigorous only | 8.9% | 10.0% |
| Moderate and vigorous | 31.6% | 34.5% |
| No muscle- strengthening activity | 76.0% | 73.4% |
| MPA in min/week (mean±SD) | 106.9±200.7 | 104.5±192.0 |
| VPA in min/week (mean±SD) | 81.6±177.3 | 87.1±176.7 |
| MSE in times/week (mean±SD) | 0.9±2.3 | 1.0±2.3 |
| Age in years (mean±SD) | 46.8±17.8 | 43.1±17.1 |
| Female | 55.2% | 60.4% |
| Race-ethnicity | ||
| Black non- Hispanic | 14.6% | 15.6% |
| Hispanic | 17.2% | 21.3% |
| Other/unknown | 5.4% | 6.5% |
| White non- Hispanic | 62.9% | 56.7% |
| Household income | ||
| $75 000 and over | 27.9% | 31.9% |
| $50 000–$75 000 | 17.9% | 18.8% |
| $35 000–$50 000 | 14.7% | 14.5% |
| $0–$35 000 | 39.5% | 34.8% |
| Education | ||
| High-school graduate | 27.1% | 23.9% |
| Some college | 29.6% | 29.3% |
| College graduate | 16.5% | 20.1% |
| Post-college grad | 8.8% | 10.9% |
| <High-school grad | 18.0% | 15.8% |
| Marital status | ||
| Divorced | 15.0% | 11.2% |
| Separated | 3.6% | 3.2% |
| Never married | 25.8% | 29.1% |
| Widowed | 9.3% | 7.3% |
| Married | 46.2% | 49.2% |
| BMI in kg/m2 (mean±SD) | 27.4±6.0 | 27.1±5.8 |
| <20 | 6.0% | 6.1% |
| 20–25 | 32.8% | 34.5% |
| 25–30 | 34.8% | 34.7% |
| 30–35 | 16.4% | 15.6% |
| >35 | 10.0% | 9.1% |
| Smoking status | ||
| Current | 21.4% | 0.0% |
| Former | 22.0% | 0.0% |
| Never | 56.6% | 100.0% |
| No chronic conditions | 91.3% | 100.0% |
| 3-year survivors | 82.9% | 100.0% |
BMI, body mass index; MPA, moderate- intensity physical activity; MSE, muscle-strengthening exercise; VPA, vigorous-intensity physical activity.
AIC and BIC
Online supplemental table S2 displays the AIC and BIC from each exposure model. The K-means-informed exposure model demonstrated the best fit according to AIC. The cubic spline-modified HHS exposure model demonstrated the best fit according to BIC. The basic exposure model produced the second-lowest AIC, with the K-means-informed exposure model and the modified HHS exposure model producing the second-lowest and third-lowest BIC, respectively. Results from the restricted cohort are included in the online supplemental figures S1–S5. Results from the HHS (online supplemental figures S6–S81) and K-means-informed exposure models (online supplemental figures S11–S13), as well as secondary results from the inverse association between MPA or VPA and mortality risk was apparent at 1 hour/week, with this observed lower mortality risk largely plateauing at 3 hours/week. A significant inverse association between MSE and mortality risk was observed at 1 time/week for this PA modality, but little additional benefit was found beyond this frequency.
Modified HHS exposure model
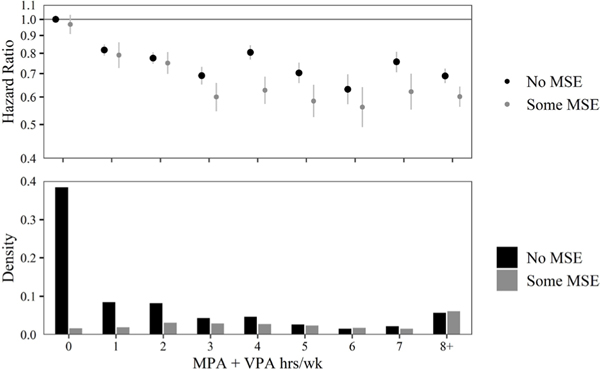
Figure 2 displays HRs, 95% CIs, and densities for each 1- hour interval of MPA+VPA. Almost 40% of participants performed <30 min of aerobic PA and no MSE. Similar to the basic exposure model, the significant inverse association between mortality risk and aerobic PA levelled off at 3 hours/week of MPA+VPA. Regardless of MPA+VPA level, MSE appeared to be associated with at least some additional mortality risk reduction, although this difference was rarely significant.
Figure 2.
Estimated HRs and 95% CIs for all-cause mortality associated with levels of moderate aerobic physical activity (MPA)+vigorous aerobic physical activity (VPA) relative to less than 30 min of MPA+VPA. Results are shown stratified by muscle-strengthening activity. Density of individuals in each group of exercise are shown in bars on the lower part of the plot. CPH models allow for combinations of age, sex and race to have their own baseline hazard. CPH models control for income, education, marital status, smoking status, BMI, chronic conditions and survey year. HRs and 95% CIs are plotted on a natural log scale. BMI, body mass index; CPH, Cox proportional hazard; MSE, muscle- strengthening exercise.
Cubic spline modified HHS exposure model
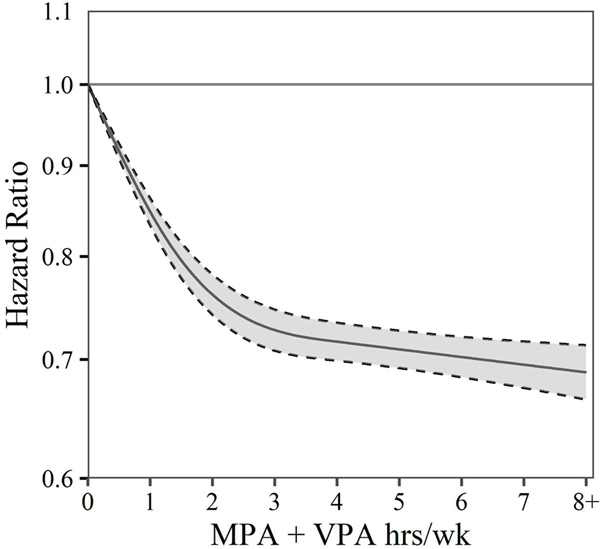
Figure 3 shows a cubic spline smoothing of the HRs and 95% CIs for the association between MPA+VPA and mortality. A marked inverse association between MPA+VPA and mortality risk was present up to 3 hours/week, with only slight evidence of additional benefit beyond 3 hours/week.
Figure 3.
Estimated HRs (solid line) and 95% CIs (dashed lines) for all-cause mortality associated with levels of total aerobic physical activity (MPA+VPA) estimated using cubic splines with three knots. CPH models allow for combinations of age, sex and race to have their own baseline hazard. CPH models control for income, education, marital status, smoking status, BMI, chronic conditions and survey year. HRs and 95% CIs are plotted on a natural log scale. BMI, body mass index; CPH, Cox proportional hazard; MPA, moderate physical activity; VPA, vigorous physical activity.
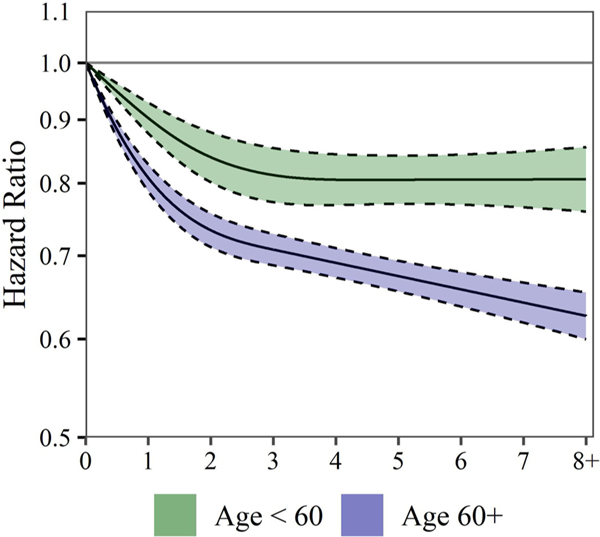
Figure 4 displays the cubic spline smoothing stratified by age (<60 years and 60+ years). Results indicated that the inverse association between aerobic PA and mortality risk may be stronger in older individuals than younger individuals. Specifically, for individuals aged <60 years, there was little evidence of additional mortality risk reduction beyond 3 hours/week of aerobic PA. However, it appears those aged 60+years yielded some additional mortality risk reduction beyond 3 hours/week.
Figure 4.
Estimated HRs (solid lines) and 95% CIs (dashed lines) for all-cause mortality associated with levels of moderate aerobic physical activity (MPA)+vigorous aerobic physical activity (VPA). MPA+VPA associations are estimated using cubic splines with three knots. Results are shown stratified by age (<60 years and 60+ years). CPH models allow for combinations of age, sex and race to have their own baseline hazard. CPH models control for income, education, marital status, smoking status, BMI, chronic conditions and survey year. HRs and 95% CIs are plotted on a natural log scale. BMI, body mass index; CPH, Cox proportional hazard.
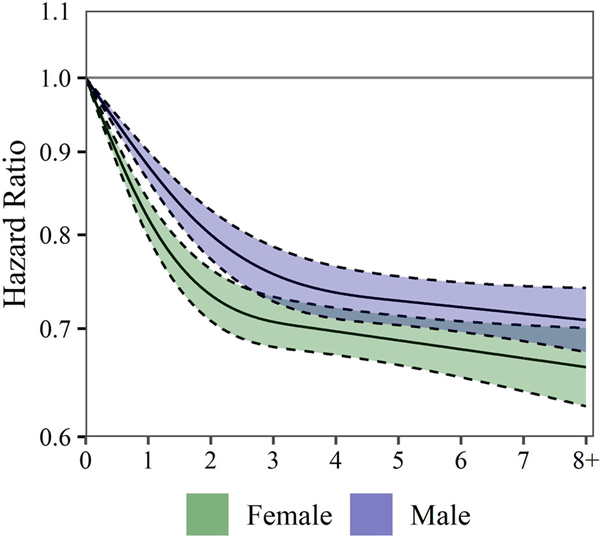
Figure 5 displays the cubic spline smoothing stratified by sex. Some evidence was present that the inverse relationship between other models, are included in online supplemental figures S9–S14. The following results discuss the basic, modified HHS and cubic spline-modified HHS exposure models in the full cohort.
Figure 5.
Estimated HRs (solid lines) and 95% CIs (dashed lines) for all-cause mortality associated with levels of moderate aerobic physical activity (MPA)+vigorous aerobic physical activity (VPA). MPA +VPA associations are estimated using cubic splines with three knots. Results are shown stratified by sex. CPH models allow for combinations of age and race to have their own baseline hazard. CPH models control for income, education, marital status, smoking status, BMI, chronic conditions and survey year. HRs and 95% CIs are plotted on a natural log scale. BMI, body mass index; CPH, Cox proportional hazard.
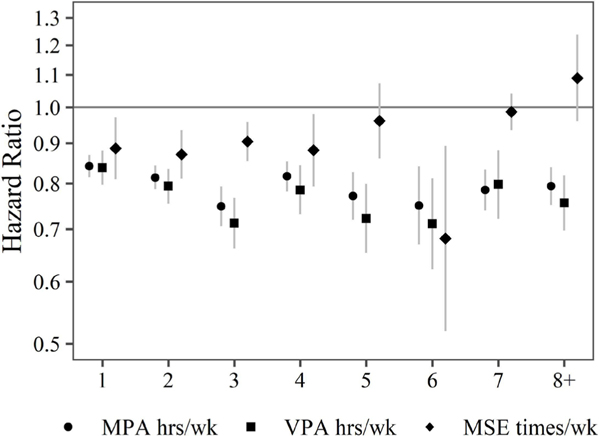
Basic exposure model
Figure 1 shows HRs and 95% CIs for the basic exposure model. Compared with the reference group, a significant aerobic PA and mortality risk was stronger in women than men up to 3 hours/week, but the difference was slight. Furthermore, the full cohort showed some additional mortality risk reduction beyond 3 hours/week.
Figure 1.
Estimated HRs and 95% CIs for all-cause mortality associated with levels of moderate aerobic physical activity (MPA), vigorous aerobic physical activity (VPA) and muscle- strengthening exercise (MSE) relative to less than 30 min of MPA, less than 30 min of VPA and 0 times of MSE per week. HRs are estimated using CPH models including indicator variables for different levels of all forms of physical activity. CPH models allow for combinations of age, sex and race to have their own baseline hazard. CPH models control for income, education, marital status, smoking status, BMI, chronic conditions and survey year. HRs and 95% CIs are plotted on a natural log scale. BMI, body mass index; CPH, Cox proportional hazard.
DISCUSSION
The basic, HHS, modified HHS, K-means-informed and cubic spline-modified HHS exposure models each investigated a unique relationship between PA, MSE and mortality. Although only three models and one cohort were presented given slightly better model fit and space limitations, we note that all five models and both cohorts (see online supplemental materials) drew similar conclusions: (1) higher aerobic PA duration is robustly associated with lower mortality risk and was optimised at ~3 hours/week, largely independent of age and sex and (2) MSE, while having a marked inverse association with mortality risk when completed exclusively, can result in additional mortality risk reduction when completed in combination with aerobic PA. Sensitivity analyses excluding BMI and chronic conditions as covariates within our models yielded highly similar results, demonstrating the robustness of the investigated relationships.
Aerobic PA
Across all models, we observed a significant inverse association of aerobic PA with mortality risk with a dose as short as ~1 hour/week, with results suggesting little additional mortality risk reduction beyond 3 hours/week. Further, little difference was observed for this association when MPA and VPA were examined separately (figure 1). The WHO’s PA guidelines3 state that 2.5 hours/week of moderate-to-vigorous PA (MVPA) is recommended for optimal physical health. Our observations support these recommendations, but also suggested aerobic PA below the recommended guidelines, accumulated as either MPA or VPA, may be sufficient to substantively improve health and lower mortality risk.
There are two nuanced, but practical implications of these observations. First, the similarity of the inverse association of MPA and VPA, when examined separately, with mortality risk suggests that health professionals seeking to promote long- term PA engagement should identify enjoyable aerobic PAs that can be completed for 3 hours/week. Because research notes MPA and VPA induce similar beneficial physiological adaptations, including improved cardiorespiratory26 27 and cardiometabolic28 29 health indices and body composition,29 30 MPA should likely be prioritised over VPA for long- term PA promotion. Indeed, only scant evidence has suggested VPA to elicit greater enjoyment compared with MPA.31 32 Second, although not directly studied, the duration of the aerobic PA bouts may be less important than total accumulated aerobic PA duration. Updated WHO guidelines3 acknowledge that, based on the available literature, aerobic PA in bouts <10 min can confer health benefits.
When analyses were stratified by sex and age using full cohort data, minimal differences were seen between men and women, but results suggested the inverse association between aerobic PA and mortality risk may be stronger in older individuals than younger individuals. Additionally, there was some evidence that mortality risk reduction continues beyond 3 hours/week for older individuals. Older adults have a greater prevalence of chronic diseases, such as cardiovascular disease and/or type 2 diabetes, among others, that heighten mortality risk.33 Yet, many of these diseases have physiological intermediates (eg, blood lipid and glucose levels) that are beneficially impacted by greater aerobic PA engagement.22–26 Aerobic PA engagement may also assist in more robust immune responses to infectious diseases,34 35 such as influenza, that are common in older adult populations.36 As infectious diseases like influenza are among the largest contributors to mortality in older adults,36 this additional benefit of aerobic PA cannot be ignored when examining the association between aerobic PA and all-cause mortality.
Muscle-strengthening exercise
Our findings regarding the association of aerobic PA and mortality are consistent with a large body of research.19 20 37 38 However, research investigating the association of MSE with mortality risk is sparse, with our analyses among the first to investigate the minimum effective dose. We observed that a marked inverse association between MSE and mortality risk with as little as 1 time/week of this PA modality in our basic exposure model, with additional mortality risk reduction present when MSE was performed in combination with MPA and/or VPA during analyses using our modified HHS exposure model. Observations generally suggested that 1–2 times/week of MSE is likely sufficient to reduce mortality risk, with no remarkable additional benefit seen beyond this frequency.
The effects of MSE on physiological and psychological health are well documented.10 39 40 Our observations in this large cohort followed longitudinally suggested that that MSE was associated with a significant mortality risk reduction independent of aerobic PA. MSE provides a unique multisystem effect on health39 and can facilitate significant improvements in cardiometabolic indices (eg, blood lipid and lipoprotein profiles, blood glucose regulation),41 sleep quality,42 depressive symptoms40 and bone mineral density,10 among others. Not only are these benefits important when discussing how to improve physiological intermediates that lower morality risk due to non- communicable diseases as noted in the above discussion of aerobic PA, but they are also salient when discussing healthy ageing. With advanced age, sarcopenia and subsequent dynapenia result in increased frailty and decreased functional independence.43 MSE ≥3 days/ week has shown to reverse muscle loss and improve physical functioning in older adults.44 Likewise, the mechanical loading provided by MSE may improve tendon,45 cartilage46 and skeletal muscle47–49 health and improve musculoskeletal pain management.50 As improved physical functioning into older adulthood is key to maintaining an active lifestyle, continued advocacy for MSE with advancing age is critical—particularly among those who are overweight or obese and may be better able to adhere to the varied PA modes that MSE provides.13
Strengths and limitations
This analysis has several strengths. First, it is a longitudinal analysis of data from a large, nationally representative sample of US adults. Second, using multiple models allowed us to fully vet and better understand the PA/MSE–mortality relationship, with models arriving at similar, clinically relevant conclusions. Additionally, the statistical analyses and presentation of this study are consistent with the checklist for statistical assessment of medical papers (CHAMP statement).51 Finally, because data on several potential confounders (eg, chronic conditions, lifestyle behaviours) were available at each survey year and collected using standardised methodologies,22 24 we were able to robustly adjust for confounders in our models as well as complete stratified analyses by age and sex.
This analysis also has some limitations. First, although we adjusted for several confounders at baseline, residual confounding by other factors (eg, diet and alcohol) may be present. This also includes built-in selection bias from the calculation of HRs.52 Second, PA data were self-reported and potentially susceptible to recall and/or social desirability bias. Relatedly, because the data were self-reported, the analysis may have been susceptible to regression dilution bias given that changes in PA (MPA, VPA and MSE) and important confounders (smoking status and income in particular) could not be assessed over time. Third, only leisure-time aerobic PA was assessed in the NHIS resulting in the potential of underestimating benefits of total daily PA. Fourth, MSE was assessed as frequency and not duration or number of sets and repetitions (ie, volume) per muscle group. Thus, more informative MSE recommendations to reduce mortality risk cannot be drawn. Nevertheless, MSE is recommended as frequency/week in the WHO’s PA guidelines,3 with our MSE observations valuable and contributing to this literature base. Fifth, PA was assessed as the number of times participants completed aerobic PA in ≥10 min bouts. As noted, updated guidelines3 acknowledge that aerobic PA in bouts <10 min confers health benefits and, thus, we may have underestimated the true association of aerobic PA on mortality risk reduction.
Conclusion
Regardless of sex or age, our observations suggested that: (1) significant mortality risk reduction may result from aerobic PA performed 1 hour/week, with minimal additional benefits beyond 3 hours/week and (2) MSE performed in combination with aerobic PA may further decrease mortality risk, with MSE performed exclusively 1–2 times/week resulting in significant morality risk reductions.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
To optimise health, the US Department of Health and Human Services recommends ≥2.5–5 hours/week of moderate-intensity aerobic physical activity (MPA), 1.25–2.5 hours/week of vigorous-intensity aerobic physical activity (VPA) or an equivalent combination of the two, in addition to ≥2 times/week of muscle-strengthening exercise (MSE).
WHAT THIS STUDY ADDS
Using a nationally representative, prospective cohort study of 416 420 US adults, we identified the dose–response association and minimum effective doses of aerobic physical activity (PA) and MSE necessary to result in clinically significant lower all-cause mortality risk.
Total aerobic physical activity (MPA+VPA) durations of 3 hours/week and MSE completed ~1–2 times/week is sufficient to substantively reduce the risk of all-cause mortality. MSE completed in combination with aerobic PA confers additional mortality risk reduction beyond aerobic PA alone.
There is minimal evidence of additional mortality risk reduction beyond 3 hours/week of aerobic PA or 2 times/week of MSE.
HOW MIGHT IT IMPACT CLINICAL PRACTICE IN THE FUTURE
US healthcare providers may inform adults that they may substantially reduce their risk of mortality by performing about 3 hours/week of aerobic PA at their preferred intensity- level and 1–2 times/week of MSE targeting all major muscle groups.
Acknowledgements
The authors express appreciation to the National Center for Health Statistics for the creation of the cohort used in this study.
Funding
This report was supported in part by the Center for Air Climate and Energy Solutions (CACES), which was supported under Assistance Agreement No. R835873 awarded by the US Environmental Protection Agency. CAP was funded by the Mary Lou Fulton Professorship, Brigham Young University.
Footnotes
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable.
Ethics approval This study involves human participants and was approved by Research Ethics Review Board of the National Center for Health Statistics and the US Office of Management and Budget. ID: OMB 0920– 0214. Participants gave informed consent to participate in the study before taking part.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer- reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. De-identified National Health Interview Survey data are publicly available on the NCHS website (For example, data for 2014 is found at the following URL: https://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm).
REFERENCES
- 1.Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ISPAH International Society for Physical Activity and Health. The Bangkok Declaration on physical activity for global health and sustainable development. Br J Sports Med 2017;51:1389–91. [DOI] [PubMed] [Google Scholar]
- 3.Bull FC, Al-Ansari SS, Biddle S, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077–86. [DOI] [PubMed] [Google Scholar]
- 5.Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet 2012;380:294–305. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Global health risks: mortality and burden of disease attributable to selected major risks World Health Organization; 2009. https://apps.who.int/handle/10665/44203 [Accessed 24 Nov 2021].
- 7.Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 2016;388:1311–24. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Kolbe-Alexander T, Nguyen B, et al. The economic burden of physical inactivity: a systematic review and critical appraisal. Br J Sports Med 2017;51:1392–409. [DOI] [PubMed] [Google Scholar]
- 9.Katzmarzyk PT, Friedenreich C, Shiroma EJ, et al. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med 2022;56:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012;11:209–16. [DOI] [PubMed] [Google Scholar]
- 11.Strain T, Fitzsimons C, Kelly P, et al. The forgotten guidelines: cross-sectional analysis of participation in muscle strengthening and balance & co-ordination activities by adults and older adults in Scotland. BMC Public Health 2016;16:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennie JA, Lee D-C, Khan A, et al. Muscle-strengthening exercise among 397,423 U.S. adults: prevalence, correlates, and associations with health conditions. Am J Prev Med 2018;55:864–74. [DOI] [PubMed] [Google Scholar]
- 13.Zdziarski LA, Wasser JG, Vincent HK. Chronic pain management in the obese patient: a focused review of key challenges and potential exercise solutions. J Pain Res 2015;8:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017– 2018. NCHS Data Brief 2020;360:1–8. [PubMed] [Google Scholar]
- 15.Kamada M, Shiroma EJ, Buring JE, et al. Strength training and all-cause, cardiovascular disease, and cancer mortality in older women: a cohort study. J Am Heart Assoc 2017;6. doi: 10.1161/JAHA.117.007677. [Epub ahead of print: 31 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grøntved A, Rimm EB, Willett WC, et al. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med 2012;172:1306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis E, Lee I-M, Bennie J, et al. Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol 2018;187:1102–12. [DOI] [PubMed] [Google Scholar]
- 18.Momma H, Kawakami R, Honda T, et al. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med 2022;56:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Nie J, Ferrari G, et al. Association of physical activity intensity with mortality: a national cohort study of 403 681 US Adults. JAMA Intern Med 2021;181:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Veeranki SP, Magnussen CG, et al. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ 2020;370:m2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 22.National center for health statistics. National health interview survey, 1986– 2014. NHIS data, questionnaires, and related documentation. Available: https://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm [Accessed 16 Dec 2021].
- 23.National center for health statistics. Adult physical activity questions: list of questionnaires. Available: https://www.cdc.gov/nchs/nhis/physical_activity/pa_questions.htm [Accessed 16 Dec 2021].
- 24.NCHS Data Linked to NDI Mortality Files. Available: https://www.cdc.gov/nchs/data-linkage/mortality.htm [Accessed 20 Jue 2022].
- 25.HHS. Physical activity guidelines for Americans. 2nd ed, 2018. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf [Google Scholar]
- 26.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol 2017;595:2915–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung ME, Locke SR, Bourne JE, et al. Cardiorespiratory fitness and accelerometer-determined physical activity following one year of free-living high-intensity interval training and moderate-intensity continuous training: a randomized trial. Int J Behav Nutr Phys Act 2020;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su L, Fu J, Sun S, et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS One 019;14:e0210644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan BJ, Schleh MW, Ahn C. Moderate- Intensity exercise and high- intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab 2020;105. doi: 10.1210/clinem/dgaa345. [Epub ahead of print: 01 08 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sultana RN, Sabag A, Keating SE, et al. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med 2019;49:1687–721. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira BRR, Santos TM, Kilpatrick M, et al. Affective and enjoyment responses in high intensity interval training and continuous training: a systematic review and meta-analysis. PLoS One 2018;13:e0197124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thum JS, Parsons G, Whittle T, et al. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS One 2017;12:e0166299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett JE, Stevens GA, Mathers CD, et al. NCD countdown 2030: worldwide trends in non- communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018;392:1072–88. [DOI] [PubMed] [Google Scholar]
- 34.Brolinson PG, Elliott D. Exercise and the immune system. Clin Sports Med 2007;26:311–9. [DOI] [PubMed] [Google Scholar]
- 35.Scartoni FR, Sant’Ana LdeO, Murillo-Rodriguez E, et al. Physical exercise and immune system in the elderly: implications and importance in COVID-19 pandemic period. Front Psychol 2020;11:593903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC. Estimated Flu-Related illnesses, medical visits, hospitalizations and deaths in the United States — 2019–2020 flu season 2019 https://www.cdc.gov/flu/about/burden/2019-2020.html
- 37.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekelund U, Tarp J, Steene- Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maestroni L, Read P, Bishop C, et al. The benefits of strength training on musculoskeletal system health: practical applications for interdisciplinary care. Sports Med 2020;50:1431–50. [DOI] [PubMed] [Google Scholar]
- 40.Gordon BR, McDowell CP, Hallgren M, et al. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry 2018;75:566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med 2009;48:9–19. [DOI] [PubMed] [Google Scholar]
- 42.Kovacevic A, Mavros Y, Heisz JJ, et al. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Med Rev 2018;39:52–68. [DOI] [PubMed] [Google Scholar]
- 43.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–46. [DOI] [PubMed] [Google Scholar]
- 44.Westcott WL, Winett RA, Annesi JJ, et al. Prescribing physical activity: applying the ACSM protocols for exercise type, intensity, and duration across 3 training frequencies. Phys Sportsmed 2009;37:51–8. [DOI] [PubMed] [Google Scholar]
- 45.Bohm S, Mersmann F, Arampatzis A. Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med Open 2015;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med 2019;53:940–7. [DOI] [PubMed] [Google Scholar]
- 47.Suchomel TJ, Nimphius S, Bellon CR, et al. The importance of muscular strength: training considerations. Sports Med 2018;48:765–85. [DOI] [PubMed] [Google Scholar]
- 48.Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power: Part 1--biological basis of maximal power production. Sports Med 2011;41:17–38. [DOI] [PubMed] [Google Scholar]
- 49.Cormie P, McGuigan MR, Newton RU. Adaptations in athletic performance after ballistic power versus strength training. Med Sci Sports Exerc 2010;42:1582–98. [DOI] [PubMed] [Google Scholar]
- 50.Lin I, Wiles L, Waller R, et al. What does best practice care for musculoskeletal pain look like? eleven consistent recommendations from high-quality clinical practice guidelines: systematic review. Br J Sports Med 2020;54:79–86. [DOI] [PubMed] [Google Scholar]
- 51.Mansournia MA, Collins GS, Nielsen RO, et al. A checklist for statistical assessment of medical papers (the CHAMP statement): explanation and elaboration. Br J Sports Med 2021;55:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernán MA. The hazards of hazard ratios. Epidemiology 2010;21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. De-identified National Health Interview Survey data are publicly available on the NCHS website (For example, data for 2014 is found at the following URL: https://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm).