Abstract
Background
An effective vaccine response is currently a critical issue in the control of COVID-19. Little is known about humoral and cellular immunity comparing protein-based vaccine with other types of vaccines. The relevance of basal immunity to antibody production is also unknown.
Methods
Seventy-eight individuals were enrolled in the study. The primary outcome were the level of spike-specific antibodies and neutralizing antibodies measured by ELISA. Secondary measures included memory T cells and basal immunity estimated by flow cytometry and ELISA. Correlations for all parameters were calculated using the nonparametric Spearman correlation method.
Results
We observed that two doses of mRNA-based Moderna mRNA-1273 (Moderna) vaccine produced the highest total spike-binding antibody and neutralizing ability against the wild-type (WT), Delta, and Omicron variants. The protein-based MVC-COV1901 (MVC) vaccine developed in Taiwan produced higher spike-binding antibodies against Delta and Omicron variants and neutralizing ability against the WT strain than the adenovirus-based AstraZeneca-Oxford AZD1222 (AZ) vaccine. Moderna and AZ vaccination produced more central memory T cells in PBMC than the MVC vaccine. However, the MVC vaccine had the lowest adverse effects compared to the Moderna and AZ vaccines. Surprisingly, the basal immunity represented by TNF-α, IFN-γ, and IL-2 prior to vaccination was negatively correlated with the production of spike-binding antibodies and neutralizing ability.
Conclusion
This study compared memory T cells, total spike-binding antibody levels, and neutralizing capacity against WT, Delta, and Omicron variants between the MVC vaccine and the widely used Moderna and AZ vaccines, which provides valuable information for future vaccine development strategies.
Keywords: SARS-CoV-2, COVID-19, Vaccine, Memory T cell, Neutralization
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the 2019 coronavirus infectious disease (COVID-19), an ongoing worldwide outbreak of pneumonia-like respiratory disease.1 In addition to the SARS-CoV-2 wild-type (Wuhan strain) virus, several variants have emerged since the start of the COVID-19 pandemic, including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta) and B.1.1.529 (Omicron) variants.2 However, the Omicron variant is the most mutated SARS-CoV-2 variant with high transmissibility and immune evasion. Owing to its enhanced transmissibility, Omicron has rapidly replaced Delta as the dominant variant in several regions and is more difficult to eradicate.3
In addition to the development of therapeutics,4 the immune response to SARS-CoV-2 is critical for disease control; therefore, prophylactic vaccines are sought as an ultimate intervention. SARS-CoV-2 is known to use the receptor-binding domain (RBD) of the spike (S) protein to enter cells after binding to angiotensin-converting enzyme-2 (ACE2).5 Therefore, current vaccine development uses the S protein as a vaccine antigen to induce antibodies that block the binding of SARS-CoV-2 to ACE2. As of April 2022, four COVID-19 vaccines have been approved for administration in Taiwan, including the AZ, Moderna, MVC, and BNT vaccines. Moderna and BNT are mRNA-based vaccines expressing the S protein, and the efficacy rates were 95% for the BNT vaccine and 94.5% for the Moderna vaccine in phase III clinical trials.6 AZ vaccine expresses S protein from adenovirus vector platforms and has achieved an efficacy of 70% in a phase III clinical trial.6 MVC is a protein-based subunit vaccine comprising the S protein, and the seroconversion rate was 99.8% in a phase II clinical trial.7 All of these vaccines have been evaluated in clinical trials and approved by regulatory authorities based on demonstrated safety profiles and acceptable efficacy rates. By April 2022, the coverage rate of the COVID-19 vaccine in Taiwan was 79.8% for the second dose and 58.2% for the third dose.8 However, no studies have compared their ability to produce total and neutralizing antibodies against virus variants before and after administration.
In addition to eliciting cytotoxic T lymphocyte (CTL) responses and B cell/antibody responses to protect against microbial infection, another goal of vaccine development is to induce memory T cell/B cell responses to facilitate rapid viral clearance during reinfection.9, 10, 11 Memory T cells, including CD4 and CD8 memory T cells, have traditionally been divided into two major subpopulations: central memory T (Tcm) cells and effector memory T (Tem) cells.12 Tcm cells are present in secondary lymphoid organs and blood, which require further differentiation signals to make effector cytokines and proliferate substantially after reactivation. In contrast, Tem cells are mainly found in the spleen, blood and peripheral organs, which results in rapid effector responses and less proliferation.13 Moreover, preexisting cross-reactive memory T cells are associated with reduced symptoms of viral shedding following infection with the 2009 pandemic H1N1 IAV strain.14 Furthermore, most S-specific memory CD4+ T cells were central memory cells, whereas most memory CD8+ T cells were of the effector memory phenotype after SARS-CoV infection.15 Although it has been demonstrated that the level of antibodies produced after SARS-CoV-2 mRNA vaccination is strongly correlated with the frequency of antigen-specific memory B cells,10 the levels of induction of Tcm cells and Tem cells after vaccination with different COVID-19 vaccines remain unclear. Additionally, TNF-α, IFN-γ, and IL-2 are known to regulate the activation, growth, and differentiation of a wide variety of cell types, including T cells, B cells, NK cells, and macrophages.16 Previous studies have measured cytokines, such as IL-2, IFN-γ, and TNF-α, using a whole-blood cell culture system to determine the immune status of healthy and cancer patients.17, 18, 19 However, it is currently unknown whether the levels of the production of these cytokines prior to vaccination affect the efficacy of COVID-19 vaccines on antibody production.
In this study, we determined the levels of S-binding antibodies and neutralizing antibodies against the WT, Delta, and Omicron variants of three different forms of the vaccine. Another important result was the determination of the relationship between antibody production, neutralization capacity, adverse effects, age, sex, memory T cell population, and basal immunity after AZ, Moderna, and MVC vaccination. These findings clarify the characteristics, efficacy, and immune associations of COVID-19 vaccines against SARS-CoV-2 variants in a real-world data analysis.
Methods
Recruitment and clinical sample collection
Seventy-eight individuals signed informed consent forms and were enrolled in the study, which was approved by the Research Ethics Committee of China Medical University and Hospital, Taichung, Taiwan (CMUH110-REC2-056). All participants received two doses of homologous AZ, Moderna, MVC, BNT vaccine schedules or a heterologous prime-boost schedule. Ten milliliters of peripheral blood samples were collected at two time points: baseline (before vaccination) and 4 weeks after the second dose. The questionnaires of adverse reactions were collected one week after the first and second doses. Each “adverse reaction” is rated on a five-point scale from 0 to 4, with 0 = “not at all” and 4 = “very much.” Demographic and clinical information is provided in Supplemental Table 1. Due to the small number of people who received two doses of homologous BNT vaccine (n = 4) and heterologous vaccine (n = 6), these two groups of participants were excluded from the statistical analysis.
Sample processing
Venous blood was collected into EDTA tubes and then centrifuged at 3000 rpm for 15 min to separate plasma. Plasma samples were stored at −80 °C for downstream antibody analysis. The remaining whole blood was diluted with PBS, and peripheral blood mononuclear cells (PBMCs) were isolated using Lymphoprep gradient centrifugation (STEMCELL, Oslo, Norway). The collected PBMCs were divided into two parts: one part was used to detect the memory T cell populations, and the other was used to detect the production of cytokines after culture and stimulation.
Detection of SARS-CoV-2-specific antibodies
The presence of S-specific antibodies to the different variants in serum was determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 50 μL of 2 μg/mL S protein of different variants, including WT (Wuhan strain), B.1.617.2 (Delta) and B.1.1.529 (Omicron) strains (kindly provided by Dr. S. J. Liu, National Institute of Infectious Diseases & Vaccinology, National Health Research Institutes, Taiwan) in 0.1 M carbonate buffer (pH 9.5) was coated onto 96-well microplates by overnight incubation at 4 °C. After washing twice with 0.05% Tween 20 in PBS, the microplates were blocked with 3% BSA in PBS at room temperature for 2 h. The plasma samples were diluted using 1% BSA in PBS and incubated for another 2 h at room temperature. Following the addition of HRP-conjugated donkey anti-human IgG (Biolegend, CA, USA), the assay was visualized by 3,3′, 5,5’ tetramethylbenzidine (TMB) dihydrochloride substrate (Millipore, CA, USA). The absorbance was measured by an ELISA reader at 450 nm.
ACE2 competitive ELISA
An ACE2 competitive ELISA was performed using the Anti-SARS-CoV-2 (WT/B.1.617.2 (Delta)/B.1.1.529 (Omicron)) Neutralizing Antibody Titer Serologic Assay Kit (ACRO Biosystems, DE, USA) according to the recommended protocol. Briefly, precoated human ACE2 microplate 96-well plates were provided by the kit. Then, 50 μL of 1:10, 1:40, and 1:80 diluted serum samples and positive and negative controls were added to each well, followed by 50 μL of HRP-SARS-CoV-2 Spike RBD (WT/Delta/Omicron). The plate was washed, and 100 μL substrate solution was added to each well for 20 min at 37 °C. The reaction was stopped with the provided stop solution. The absorbance was measured using an ELISA reader at 450 nm. The competitive activity of serum antibodies was expressed as a percentage of inhibition ((1− OD450 nm of sample/OD450 nm of negative control) × 100%).
Flow cytometry
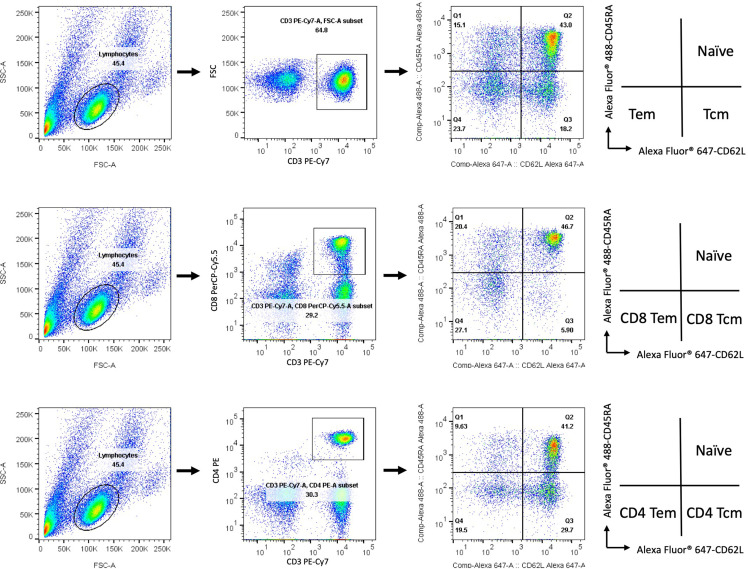
The isolated PBMCs were stained with fluorescently labeled CD3, CD4, CD8, CD45RA, and CD62L monoclonal antibodies (BioLegend, CA, USA). Flow cytometric analysis was performed on a flow cytometer using a FACSVerse instrument (BD Bioscience, CA, USA), and the data were analyzed using FlowJo software (Tree Star Inc., OR, USA). Gating strategies and representative flow cytometry plots of different memory T cell populations are shown in Supplementary Figure 1.
Measurement of cytokine production
Human PBMCs were plated at a density of 1 × 106 cells per milliliter in complete Iscove's Modified Dulbecco's Medium (IMDM) medium (GIBCO, NY, USA). Then, 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma, MO, USA) was added, and the cells were subsequently incubated at 37 °C in a 5% CO2 atmosphere. The supernatants of cells incubated for 24 and 48 h were collected, and human IL-2, TNF-α and IFN-α levels were determined using ELISA kits (BD Bioscience, CA, USA) according to the manufacturer's protocol.
Statistical analysis
Statistical data were generated using GraphPad Prism software. The results are expressed as the means ± SD. Differences between groups were assessed by Student's t test. P values less than 0.05 were considered statistically significant. Correlations were calculated using the nonparametric Spearman correlation method and are shown with linear trend lines. The coefficient of correlation had to be > 0.25 or < −0.25 and p < 0.05 for a significant correlation.
Results
Antibody responses to COVID-19 vaccination
For this study, we recruited 78 participants who received COVID-19 vaccines in Taiwan. Because the number of participants with two doses of BNT (n = 4) and heterologous (n = 6) vaccination was too low, the results of this study only included those in the two-dose AZ (n = 38), Moderna (n = 17) and MVC (n = 13) groups for statistical analysis. Full cohort information is described in Supplemental Table 1. First, we determined circulating S-binding antibodies against WT, Delta, and Omicron (Fig. 1 A–C) strains in serum samples by ELISA. The upper panel of Fig. 1A–C shows that the second doses of AZ, Moderna, and MVC vaccine induced a significant increase in total S-binding antibodies against WT (p < 0.01, < 0.0001, and = 0.065, respectively), Delta (p < 0.01, < 0.0001, and < 0.0001, respectively), and Omicron (p < 0.05, < 0.0001, and < 0.05, respectively) variants. Based on the variation among individuals with different baselines, we calculated the fold enhancement by dividing the level of antibodies after the second dose by the baseline. The fold changes in antibodies against WT, Delta, and Omicron S protein by the Moderna vaccine booster were 84, 14, and 18, respectively, which were highest compared to AZ and MVC vaccines (bottom panel of Fig. 1A–C). Moreover, the fold change in anti-Delta and Omicron S antibodies was significantly higher in the two-dose MVC vaccine group than in the AZ vaccine group (p < 0.01 and < 0.001, respectively) (bottom panel of Fig. 1B and C). Notably, the fold change in anti-Delta and Omicron S antibodies was significantly lower than that in the anti-WT S antibodies produced following Moderna vaccination (p < 0.05). There was no significant difference in antibody production against WT, Delta, and Omicron variants in the AZ and MVC vaccines (Fig. 1D). In addition, we found no differences between males and females in terms of antibody production (against WT, Delta, and Omicron S proteins) following immunization with AZ, Moderna, and MVC vaccines (Supplemental Fig. 1). The association between age and antibody production was also not significant in the immunization with AZ, Moderna, and MVC vaccines (Supplemental Fig. 2).
Fig. 1.
Antibody responses following AZ, Moderna, and MVC vaccination. Antibody titers were measured in serum collected before vaccination (pre vac) and 4 weeks after the second dose (post 2nd). The presence of S-specific antibodies (anti-S IgG) against WT (A), Delta (B), and Omicron (C) variants in serum was determined by ELISA. The upper panels of Figs. A, B, and C show the antibody titers produced by each individual against the three variants following AZ, Moderna, and MVC vaccines. The lower panels of Figs. A, B and C are the folds of antibody calculated by dividing the antibody titer after the second dose by the prevaccination. (D) The folds of antibodies against WT, Delta and Omicron S protein were compared after AZ, Moderna and MVC vaccination. The numbers in each column on the graph are the means for each group. The results are presented as the means ± SD. p < 0.05 (∗), p < 0.01 (∗∗), p < 0.001 (∗∗∗) and p < 0.0001 (∗∗∗∗).
Neutralization responses to COVID-19 vaccination
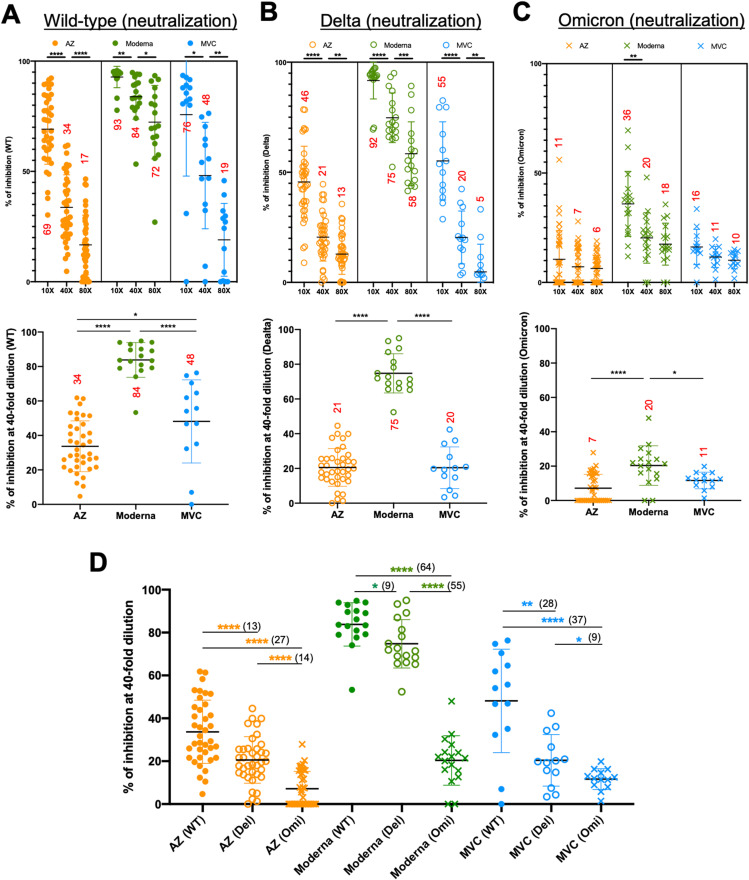
In addition to total S-binding antibodies, we further assessed the neutralizing ability of antibodies produced by two doses of the AZ, Moderna, and MVC vaccines using an ACE2 competitive ELISA. We used 10-, 40-, and 80-fold dilutions of sera to compete with WT, Delta, and Omicron S proteins for binding to ACE2. In the upper panel of Fig. 2 A and B, we found that the sera from the booster of all three vaccines significantly reduced the neutralizing ability of WT and Delta variants with increasing dilutions. However, only the booster of the Moderna vaccine significantly reduced the neutralizing ability (36% down to 18%) of the Omicron variant with increasing dilutions (upper panel of Fig. 2C). However, neutralization of the Omicron variant by the AZ and MVC vaccines was low and did not change with increasing dilutions. To analyze the neutralizing effect of each vaccine in WT, Delta, and Omicron variants, we compared the percentage of inhibition in 40-fold diluted serum from all three vaccines. We demonstrated that Moderna immunization had the best neutralizing effect in the WT (84%), Delta (75%), and Omicron (20%) variants, while the MVC vaccine (48%) had a better neutralizing effect in the WT than the AZ vaccine (34%) (p < 0.05) (lower panel of Fig. 2A–C). The AZ, Moderna, and MVC vaccines had the best neutralizing effects in the WT strain (34, 84, and 48, respectively) and the least in the Omicron variant (7, 20, and 11, respectively). In comparison, neutralization of the Delta and Omicron variants was reduced by 13% and 27% compared to the WT strain in AZ vaccination, reduced by 9% and 64% in Moderna vaccination, and reduced by 28% and 37% in MVC vaccination (Fig. 2D). None of the participants had a neutralizing effect on the WT, Delta, or Omicron variants before vaccination (data not shown). In addition, we found no differences between males and females in neutralizing ability and no association between age and neutralizing ability after immunization with AZ, Moderna, and MVC vaccines (Supplemental Figs. 3 and 4).
Fig. 2.
Antibody responses following AZ, Moderna, and MVC vaccination. Competitive activity in serum samples collected from two doses of vaccine was detected by competitive ELISA. The percent inhibition was measured using 10-, 40-, and 80-fold dilutions of serum to compete with WT (A), Delta (B), and Omicron (C) S proteins for binding to ACE2. The upper panels of Figs. A, B, and C show the percent inhibition following AZ, Moderna, and MVC vaccination. The lower panels of Figs. A, B and C show the percentage of inhibition in 40-fold diluted serum from all three vaccines. The numbers in each column on the graph are the means for each group. (D) The percentage of inhibition in 40-fold diluted serum against WT, Delta and Omicron S protein was compared after AZ, Moderna and MVC vaccination. The numbers in each column in Fig. D are the mean subtractions between groups. The results are presented as the means ± SD. p < 0.05 (∗), p < 0.01 (∗∗), p < 0.001 (∗∗∗) and p < 0.0001 (∗∗∗∗).
Adverse reactions to COVID-19 vaccination
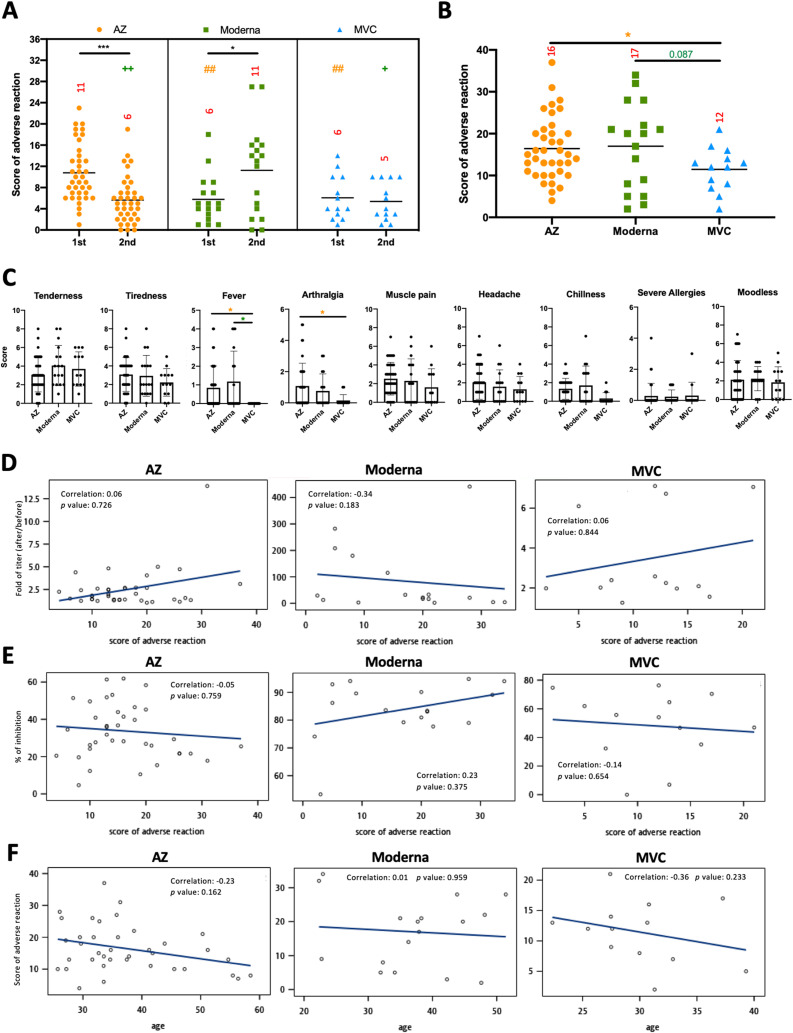
Subsequently, we compared the adverse reactions to the prime-boost AZ, Moderna, and MVC vaccines and investigated the potential relationships between the fold change in antibodies, neutralization capacity, or age and adverse reactions after two doses of vaccines. As in previous studies,20, 21, 22 we confirmed a higher frequency of adverse reactions after AZ primary vaccination compared with AZ booster vaccination (p < 0.001). However, there was a higher frequency of adverse reactions after Moderna booster vaccination than after Moderna primary vaccination (p < 0.05). In addition, there was no difference in the frequency of adverse reactions between MVC primary and booster vaccinations. Moreover, Moderna and MVC had fewer adverse reactions than the AZ vaccine in the primary vaccination (p < 0.05 and p < 0.05, respectively), and AZ and MVC had fewer adverse reactions than Moderna in the booster vaccination (p < 0.01 and p < 0.05, respectively) (Fig. 3 A). Combining the adverse reaction scores for the primary and booster vaccinations, the MVC vaccine had the lowest adverse reactions (11.5) compared to AZ (16.4) and Moderna (17.0) (Fig. 3B). The frequency of fever and arthralgia with the MVC vaccine was significantly lower than that with AZ and Moderna, especially because all participants who received the MVC vaccine had no fever symptoms (Fig. 3C). Furthermore, there were no correlations between adverse reactions and the fold change in antibodies (Fig. 3D), neutralization capacity (Fig. 3E), or age (Fig. 3F) after two doses of AZ, Moderna, and MVC vaccine.
Fig. 3.
Adverse reactions after AZ, Moderna, and MVC vaccination. (A) Scores of adverse reactions after the first (1st) and second (2nd) doses of AZ, Moderna, and MVC vaccines are shown. (B) The sum of the scores for the first and second doses of the vaccines is shown. The numbers in each column on the graph are the means for each group. (C) The scores for each clinical symptom were summed from the scores for the first and second doses of vaccines. Associations between adverse reactions and antibody titer (D), inhibition activity (E), and age (F) were calculated using Spearman rank correlation and are shown with linear trend lines. The results are presented as the means ± SD. p < 0.05 (∗), p < 0.01 (∗∗), and p < 0.001 (∗∗∗).
Memory T cell responses to COVID-19 vaccination
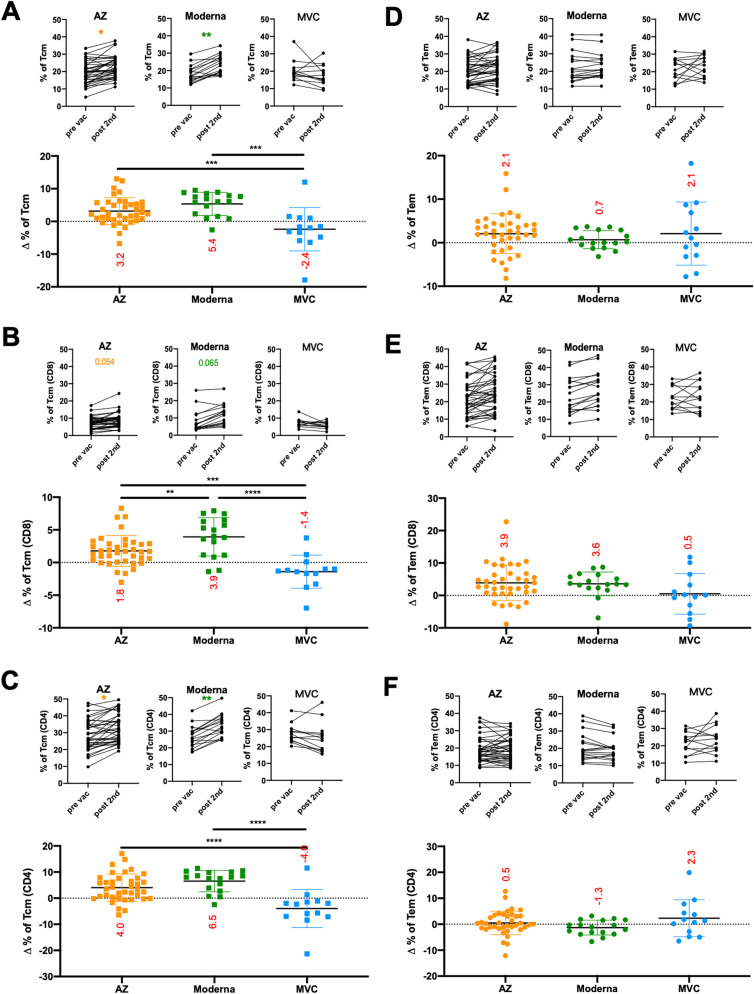
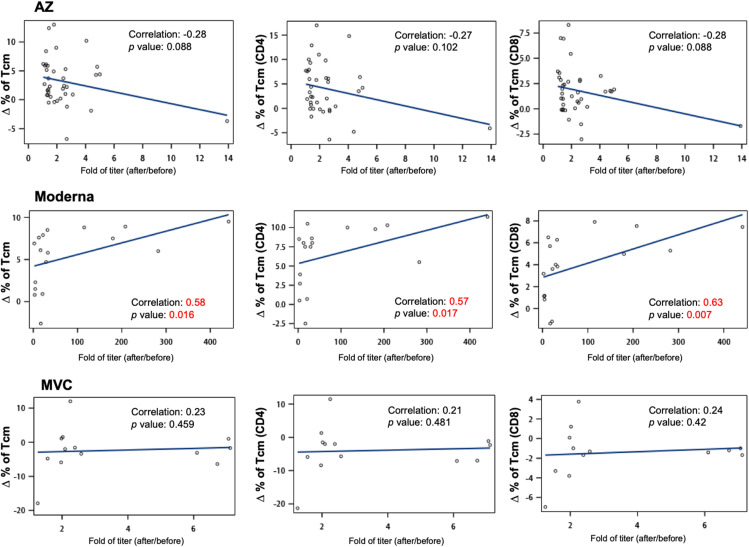
Regarding human memory CD4 and CD8 T cells, multiple phenotypes and broad functions have been observed in different viral infections.15 , 23 We next asked whether AZ, Moderna, and MVC vaccination affected the levels of different memory T cell populations. To address this question, we developed a flow cytometric assay that uses fluorescently labeled markers to track memory T cell populations in PBMC samples (Supplemental Fig. 5). We demonstrated that the percentage of the central memory population (upper panel of Fig. 4 A–C) but not the effector memory population (upper panel of Fig. 4D–F) in total T, CD8 T, and CD4 T cells was significantly enhanced after two doses of AZ and Moderna. To analyze changes in central and effector memory populations in total T, CD8 T, and CD4 T cells, we subtracted the percentage of the cell population prevaccination from the postvaccination (lower panel of Fig. 4A–F). Increased central memory populations in total T, CD8 T, and CD4 T cells were significantly enhanced after two doses of AZ (increased by 5.6%, 3.2%, and 8.0%, respectively) and Moderna (increased by 7.8%, 5.3%, and 10.5%, respectively) vaccination compared to MVC vaccination. Furthermore, Moderna vaccination had a higher level of increased CD8 Tcm cells than AZ vaccination (increased by 2.1%) (bottom panel of Fig. 4B). In addition, there was a significant positive correlation between changes in Tcm cells and fold of antibody production in Moderna vaccination (correlation in total Tcm cells was 0.58, in CD4 Tcm was 0.57, in CD8 Tcm was 0.63) but not in AZ or MVC vaccinations (Fig. 5 ). These results suggested that the adenovirus-based AZ and mRNA-based Moderna vaccines may be better than the protein-based MVC vaccine in generating memory T cells.
Fig. 4.
Memory T cell responses following AZ, Moderna, and MVC vaccination. The frequencies of central memory T cells (Tcm) (A–C) and effector memory T cells (Tem) (D–F) in total T (CD3+) cells (A and D), CD8+ T cells (B and E), and CD4+ T cells (C and F) were detected by flow cytometry. The upper panels of Figs. A–F show the percentage of Tcm/Tem populations before vaccination (pre vac) and 4 weeks after the second dose (post 2nd). The lower panels of Figs. A–F show the changes in Tcm and Tem populations in total T, CD8+ T, and CD4+ T cells. The changes in percentage were subtracted from the memory T cell populations prevaccination from those postvaccination. The numbers in each column on the graph are the means for each group. The results are presented as the means ± SD. p < 0.05 (∗), p < 0.01 (∗∗), p < 0.001 (∗∗∗) and p < 0.0001 (∗∗∗∗).
Fig. 5.
Association between changes in Tcm cells and fold of antibody production. Associations between antibody titers following AZ, Moderna, and MVC vaccination and changes in memory T cell populations were calculated using Spearman rank correlation and are shown with linear trend lines. Groups with significant trends are shown in red.
Relationships between basal immunity and antibody production to COVID-19 vaccination
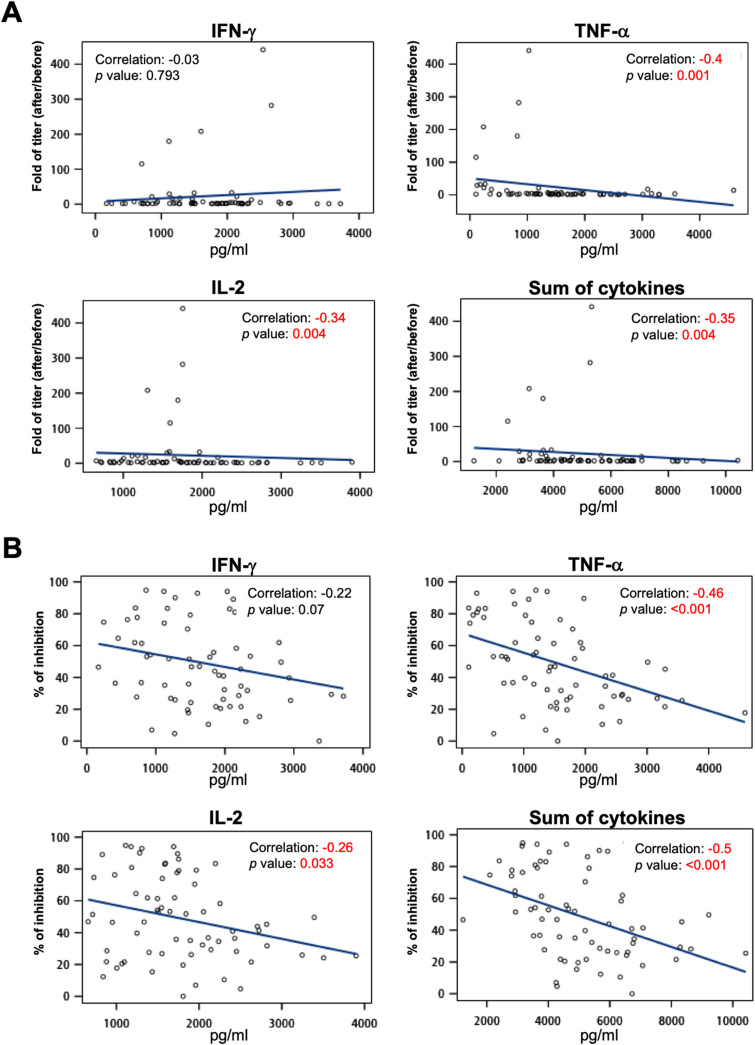
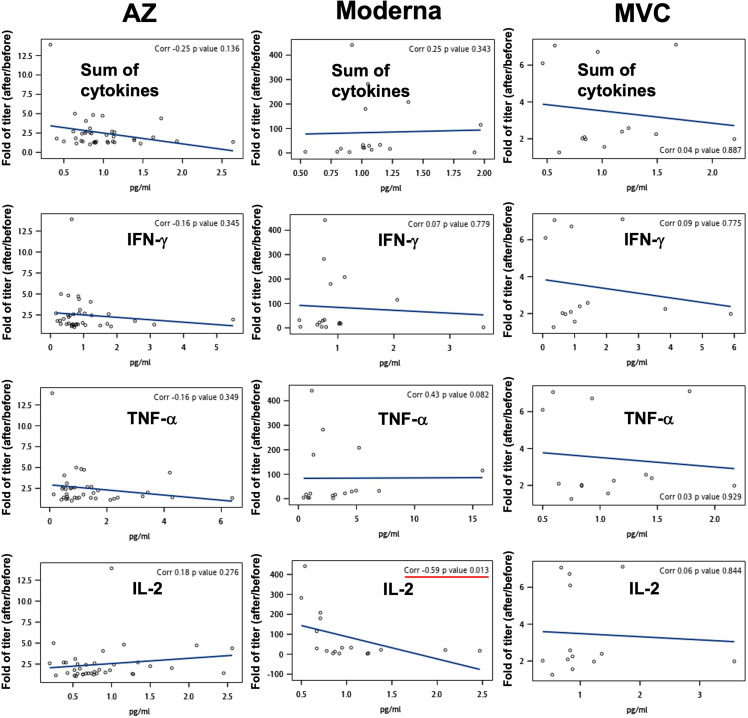
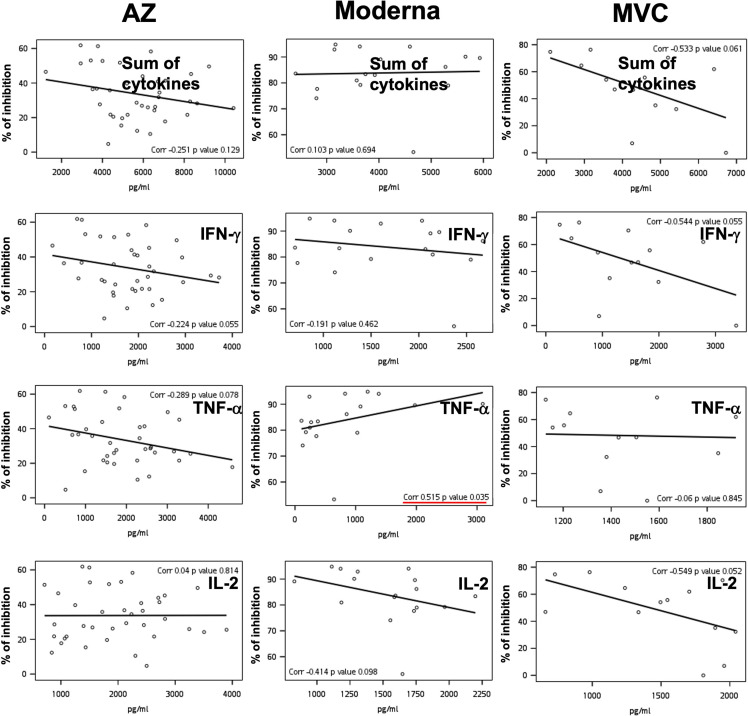
To delineate the correlation between basal immunity and antibody production, we measured the secretion levels of TNF-α, IFN-γ, and IL-2 using a PBMC culture system before vaccination.17, 18, 19 Surprisingly, the levels of TNF-α and IL-2 and the sum of three cytokines before COVID-19 vaccination were negatively correlated with antibody titers produced postvaccination (correlations were −0.4, −0.34, and −0.35, respectively) (Fig. 6 A). In addition, there were also negative correlations between the levels of TNF-α, IL-2, and the sum of the three cytokines before vaccination and the neutralizing capacity after vaccination (correlations were −0.46, −0.26, and −0.5, respectively) (Fig. 6B). Supplemental figure 6 and 7 show individual data analysis of cytokine responses to AZ, Moderna, and MVC vaccines. This result suggested that excessive basal immunity before vaccination does not promote the production of S-binding or neutralizing antibodies.
Fig. 6.
Association of basal immunity and antibody production following vaccination. IFN-γ, TNF-α, and IL-2 secretion from a PBMC culture system was measured by ELISA. Associations between the fold change in antibody titer (A) or neutralization (% of inhibition) (B) following AZ, Moderna, and MVC vaccination and the levels of IFN-γ, TNF-α, IL-2, and the sum of three cytokines before vaccination were calculated using Spearman rank correlation. Lines in the graph are displayed as trend lines. Groups with significant trends are shown in red.
Discussion
The novelty of this study is the comparison of total S-binding antibody levels and neutralizing capacity against WT, Delta, and Omicron variants between the MVC vaccine developed in Taiwan and the widely used Moderna and AZ vaccines in the world. We observed that two doses of Moderna vaccination produced the highest total S antibody and neutralizing ability against the WT, Delta, and Omicron variants compared to the AZ and MVC vaccines. MVC vaccination produced higher S-binding antibodies against Delta and Omicron variants and neutralizing ability against WT SARS-CoV-2 than AZ vaccination. Moreover, the MVC vaccine had the lowest adverse reactions compared to AZ and Moderna. Another important result was that Moderna and AZ vaccination produced more Tcm populations in PBMC than the MVC vaccine. Surprisingly, high levels of cytokines (especially TNF-α and IL-2) before vaccination did not help to enhance the S-binding antibody level or neutralizing ability. The summary and key findings are described in Table 1 .
Table 1.
Research summary and key findings.
| S-binding Ab titer |
Neutralization |
Adverse reactions | Tcm | |||||
|---|---|---|---|---|---|---|---|---|
| WT | Delta | Omicron | WT | Delta | Omicron | |||
| AZ | ++ | + | + | + | ++ | ++ | +++ | ++ |
| Moderna | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| MVC | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
The number of + indicates levels among the three vaccines.
+++ highest, ++ medium, + minimum.
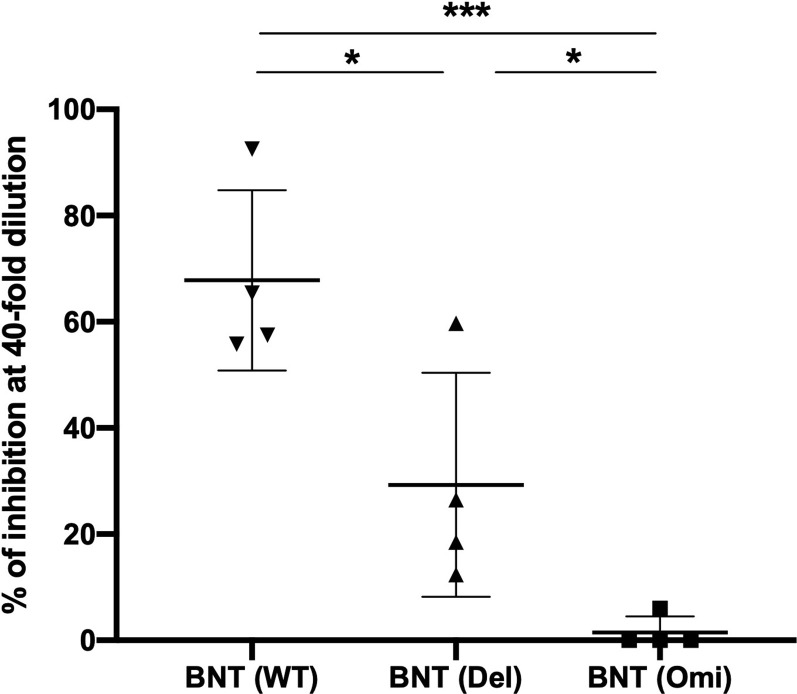
At least 50 mutations were found in the Omicron genome in a study of SARS-CoV-2 gene sequencing, 32 of which affected the spike protein.24 However, there are 11 common mutations in the RBD of Omicron and its subvariants. Since the main effect of the mutation in the RBD of Omicron has a higher positive electrostatic surface potential, this may increase the interaction between RBD and ACE2. Omicron and subvariants have the potential to increase transmission compared to the WT.25 These mutations in the RBD of Omicron may result in the inability of vaccine-induced antibodies to neutralize binding of the S protein to ACE2. Our results confirmed that the AZ, Moderna and MVC vaccines had the best neutralization effect in the WT virus and the least neutralization effect in the Omicron variant. The same results were observed even with the BNT vaccine (n = 4), with 48% neutralizing effect on WT virus, 23% neutralizing effect on the Delta variant, and 0% neutralizing effect on the Omicron variant when serum was diluted forty-fold (Supplemental Fig. 8). On the other hand, our results showed that the production of antibodies against WT, Delta, and Omicron variants had no significant difference in the AZ and MVC vaccines but not the Moderna vaccine. This may be because AZ and MVC vaccine-induced antibodies do not affect the recognition of other parts of the Delta and Omicron S protein.
A previous study found that higher antibody titers and better neutralizing capacity in young adults (<40 years old) than in older adults (>60 years old) were inversely associated with age in AZ and Moderna vaccination in Taiwan.26 However, there were no significant differences in correlation analyses between age and antibody titers, neutralizing ability, or adverse reactions in our study. This may be because the participants were all between the ages of 20 and 60, and there were no participants over 60.
A recent study demonstrated that 100% of individuals who were vaccinated with Moderna mRNA-1273, Pfizer/BioNTech BNT162b2, Janssen Ad26.COV2.S, and Novavax NVX-CoV2373 vaccines generated specific memory CD4+ T cells,27 which is in accordance with our findings in the increased memory T cells after vaccinations. Even though we did not use antigen-specific stimulated T cells to examine the antigen-specific memory T cell population, we could still observe an increase in memory T cells after vaccination. On the other hand, Tcm cells are present in secondary lymphoid organs and blood and Tem cells are mainly found in the spleen, blood, and peripheral organs. Therefore, the migration of memory T cells to other lymphoid organs generated by MVC vaccination cannot be ruled out. In summary, we are the first to show this finding, but the mechanism still needs to be explored in the future. Our findings still provided some clues to re-think how to improve the protein-based vaccine in the future.
Previous studies have shown that chronic inflammatory diseases, such as spondyloarthritis and air pollutant-induced inflammation, may suppress plasma neutralizing antibody production after vaccination.28 , 29 Here, we found that there were negative correlations between basal immunity (IFN-γ, TNF-α, and IL-2) before vaccination and antibody production/neutralizing capacity after vaccination. These results suggested that an excessive immune response prior to vaccination may impair the production of neutralizing antibodies.
Limitations
In Taiwan, AZ was the first COVID-19 vaccine (March 2020) to be administered to the public, followed by the Moderna (June 2020), MVC (August 2020), and BNT (September 2020) vaccines. Therefore, more participants received the AZ vaccine than the Moderna, MVC, and BNT vaccines. Nonetheless, some of the results of this study confirmed previous findings, such as comparing the S-binding antibody levels and side effects of the Moderna vaccine with the AZ vaccine20, 21, 22 , 30 , 31 and the ability of the MVC vaccine to produce antibodies against WT and Omicron variants.7 Therefore, even though the case number was not sufficiently large, the statistical analysis has a certain credibility and new findings. Therefore, we still observed that MVC vaccinations were better than AZ in certain measurements such as anti-S IgG against delta and omicron variants. In addition to the lower number of participants, another limitation of this study is that the age range was not wide enough. Most of the people who were willing to vaccinate in Taiwan in the early stage were between 20 and 60 years old; therefore, there was no significant difference in age-related statistical analysis.
Conclusions
This study found that the Moderna vaccine produced the best effect against the WT, Delta, and Omicron variants of SARS-CoV-2, while the MVC vaccine may be more effective than the AZ vaccine. In addition, the ability of Moderna and AZ to generate memory T cells was higher than that of the MVC vaccine. However, MVC had minimal side effects. The surprising finding was that strong basal immunity before vaccination did not promote antibody production.
Ethics approval and consent to participate
The study involving human subjects followed the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects and was approved by the Research Ethics Committee of China Medical University & Hospital, Taichung, Taiwan (CMUH110-REC2-056).
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was financially supported by grants from China Medical University (CMU111-S-02) and the Ministry of Science and Technology (MOST 110-2320-B-039-036) in Taiwan to YCS, as well as grants from "Chinese Medicine Research Center, China Medical University” from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (CMRC-CHM-1) and China Medical University (CMU107-TU-04) to HRY.
Authors’ contributions
YCS and HRY conceived and supervised the project. YCS and HRY designed the project. SJL, HCL, CTL, HJL, MYW and YCS performed the experiments. HJL and YCS analyzed all of the data. YCS and HRY prepared the manuscript. All authors read the manuscript, provided feedback, and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Experiments and data analysis were performed in part through the use of the Medical Research Core Facilities, Office of Research & Development at China Medical University, Taichung, Taiwan. None of the funders or institutions listed had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2023.03.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
Fig. S5.
Fig. S6.
Fig. S7.
Fig. S8.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22(12):757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu Y.J., Chiang J.H., Fu C.W., Hour M.J., Ha H.A., Kuo S.C., et al. Analysis of COVID-19 prevention and treatment in Taiwan. Biomedicine. 2021;11(1):1–18. doi: 10.37796/2211-8039.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh S.M., Liu M.C., Chen Y.H., Lee W.S., Hwang S.J., Cheng S.H., et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9(12):1396–1406. doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC Taiwan Centers for Disease Control. 2022. https://www.cdc.gov.tw/File/Get/u4tsqZvXT5WR237TuDieIw
- 9.Song Y.C., Huang H.C., Chang C.Y., Lee H.J., Liu C.T., Lo H.Y., et al. A potential herbal adjuvant combined with a peptide-based vaccine acts against HPV-related tumors through enhancing effector and memory T-cell immune responses. Front Immunol. 2020;11:62. doi: 10.3389/fimmu.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58) doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y.C., Chou A.H., Homhuan A., Huang M.H., Chiang S.K., Shen K.Y., et al. Presentation of lipopeptide by dendritic cells induces anti-tumor responses via an endocytosis-independent pathway in vivo. J Leukoc Biol. 2011;90(2):323–332. doi: 10.1189/jlb.0111046. [DOI] [PubMed] [Google Scholar]
- 12.Benichou G., Gonzalez B., Marino J., Ayasoufi K., Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol. 2017;8:170. doi: 10.3389/fimmu.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller S.N., Gebhardt T., Carbone F.R., Heath W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar S., Begom S., Bermingham A., Hoschler K., Adamson W., Carman W., et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19(10):1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 15.Yang L.T., Peng H., Zhu Z.L., Li G., Huang Z.T., Zhao Z.X., et al. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120(2):171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsby R.A., Kindt T.J., Osborne B.A., Kuby J. 5 edn. Vol. 12. W. H. Freeman and Company; 2003. Immunology. [Google Scholar]
- 17.Elsasser-Beile U., von Kleist S., Stahle W., Schurhammer-Fuhrmann C., Monting J.S., Gallati H. Cytokine levels in whole blood cell cultures as parameters of the cellular immunologic activity in patients with malignant melanoma and basal cell carcinoma. Cancer. 1993;71(1):231–236. doi: 10.1002/1097-0142(19930101)71:1<231::aid-cncr2820710136>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Elsasser-Beile U., von Kleist S., Lindenthal A., Birken R., Gallati H., Monting J.S. Cytokine production in whole blood cell cultures of patients undergoing therapy with biological response modifiers or 5-fluorouracil. Cancer Immunol Immunother. 1993;37(3):169–174. doi: 10.1007/BF01525431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischer T., Chang T.T., Chiang J.H., Yen H.R. A controlled trial of sheng-yu-tang for post-hematopoietic stem cell transplantation leukemia patients: a proposed protocol and insights from a preliminary pilot study. Integr Cancer Ther. 2018;17(3):665–673. doi: 10.1177/1534735418756736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts A.D., Ely K.H., Woodland D.L. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202(1):123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S., Karuppanan K., Subramaniam G. Omicron (BA.1) and sub-variants (BA.1.1, BA.2, and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. J Med Virol. 2022 doi: 10.1002/jmv.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang C.M., Lee N.Y., Lin C.H., Hsu Y.S., Chang Y.C., Chung M.Y., et al. Immunogenicity and safety of homologous and heterologous ChAdOx1-S and mRNA-1273 vaccinations in healthy adults in Taiwan. J Clin Virol. 2022;150–151:105156. doi: 10.1016/j.jcv.2022.105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Mateus J., Coelho C.H., Dan J.M., Moderbacher C.R., Gálvez R.I., et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185(14):2434–2451 e17. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casagrande T.Z., Costa-Rocha I.A.D., Gavi M., Miyamoto S.T., Martins P.C., Serrano E.V., et al. Previous biological therapy and impairment of the IFN-gamma/IL-10 axis are associated with low immune response to 17DD-YF vaccination in patients with spondyloarthritis. Vaccine. 2022;40(32):4580–4593. doi: 10.1016/j.vaccine.2022.05.071. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S., Chen S., Xiao G., Zhao M., Li J., Dong W., et al. The associations between air pollutant exposure and neutralizing antibody titers of an inactivated SARS-CoV-2 vaccine. Environ Sci Pollut Res Int. 2022;29(9):13720–13728. doi: 10.1007/s11356-021-16786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.