Abstract
Tracking vaccination coverage is a critical component of monitoring a vaccine program. Three different surveillance systems were used to examine trends in varicella vaccination coverage during the United States vaccination program: National Immunization Survey–Child, National Immunization Survey–Teen, and immunization information systems (IISs). The relationship of these trends to school requirements and disease decline was also examined. Among children aged 19–35 months, ≥1 dose of varicella vaccine increased from 16.0% in 1996 to 89.2% by the end of the 1-dose program in 2006, stabilizing around at least 90.0% thereafter. The uptake of the second dose was rapid after the 2007 recommendation. Two-dose coverage among children aged 7 years at 6 high-performing IIS sites increased from 2.6%–5.5% in 2006 to 86.0%–100.0% in 2020. Among adolescents aged 13–17 years, ≥2-dose coverage increased from 4.1% in 2006 to 91.9% in 2020. The proportion of adolescents with history of varicella disease declined from 69.9% in 2006 to 8.4% in 2020. In 2006, 92% of states and the District of Columbia (DC) had 1-dose daycare or school entry requirements; 88% of states and DC had 2-dose school entry requirements in the 2020–2021 school year. The successes in attaining and maintaining high vaccine coverage were paramount in the dramatic reduction of the varicella burden in the United States over the 25 years of the vaccination program, but opportunities remain to further increase coverage and decrease varicella morbidity and mortality.
Keywords: varicella vaccination, chickenpox, immunization information system, National Immunization Survey, varicella school attendance requirement
Before the introduction of routine vaccination, varicella contributed significantly to the burden of childhood disease in the United States (US), with approximately 4 million cases, 10 500–13 500 hospitalizations, and 100–150 deaths occurring annually; >90% of cases and the majority of severe complications occurred among children and adolescents [1]. The live, attenuated varicella vaccine (VARIVAX™) was licensed for prevention of varicella in the US in 1995. The American Academy of Pediatrics and the Advisory Committee on Immunization Practices recommended 1 dose of VARIVAX™ in 1995 and 1996, respectively, for healthy children aged 12–18 months, catch-up vaccination of susceptible children aged 19 months to 12 years, and 2 doses administered 4–8 weeks apart for susceptible persons aged ≥13 years who had close contact with persons at high risk for serious complications (eg, healthcare workers and family contacts of immunocompromised persons) [2]. The recommendations were expanded in 1999 to facilitate wider population coverage [3]. States were advised to require all children entering childcare facilities and elementary schools to show proof of vaccination or present evidence of varicella immunity. Additionally, the vaccine was recommended for certain human immunodeficiency virus-infected children, for persons at high risk for varicella exposure, and for outbreak control.
Though an impressive reduction in varicella morbidity and mortality was observed following implementation of routine 1-dose childhood vaccination [4–7], smaller outbreaks continued even among highly vaccinated populations [8–10]. Additionally, a combined measles-mumps-rubella-varicella (MMRV) vaccine (ProQuad™) was licensed in 2005 to streamline the routine childhood immunization schedule. In response to outbreaks and to further control varicella burden, the vaccination policy was revised in 2007 to a 2-dose schedule for children, with 1 dose at age 12–15 months followed by a second dose at age 4–6 years [1]. Catch-up vaccination was recommended for persons without evidence of varicella immunity, including children and adolescents who had previously received 1 dose of varicella vaccine.
In this manuscript, trends in varicella vaccination coverage in the United States among young children aged 19–35 months from 1996 to 2020, children aged 7 years from 2006 to 2020, and adolescents aged 13–17 years from 2006 to 2020 using 3 different surveillance systems, and the relationship of these trends to disease decline, were examined. Daycare and school enrollment varicella vaccination requirements in each state and the relationship between middle and high school requirements and adolescent vaccine coverage were assessed.
METHODS
National Immunization Survey–Child, 1996–2020
The National Immunization Survey–Child (NIS-Child) is an annual household telephone survey launched in 1994 to monitor vaccination coverage among children aged 19–35 months. Eligible households are identified via random-digit dialing and parents or guardians are interviewed via telephone [11]. Initially, the survey was conducted via landline telephones. In 2011, because of the increase in the use of cellular telephones, the survey became a dual-frame landline and cellular telephone survey and in 2018, with the decline in households with landline telephones, a single-frame cellular telephone survey.
During the telephone interview, parents or guardians answer questions about household characteristics and all children aged 19–35 months in the household. Permission is requested to follow up with their children’s vaccination provider(s) and, with consent, a questionnaire is mailed to the providers to obtain children’s vaccination history. The provider-reported medical records are the source of all vaccination coverage estimates; interviews without verified provider-reported medical records were excluded from this analysis. Estimates across vaccination providers for each child are synthesized and combined with the demographic data from household interviews to provide coverage estimates overall and by demographic subgroups. NIS-Child began collecting data on ≥1-dose varicella vaccination for children aged 19–35 months in 1996. A valid varicella dose was defined as a dose received at ≥12 months of age.
National Immunization Survey–Teen, 2006–2020
The National Immunization Survey–Teen (NIS-Teen) was launched in 2006 to monitor vaccination coverage among adolescents aged 13–17 years [12]. NIS-Teen interview administration follows the NIS-Child interview in sequence and uses the sampling frame of NIS-Child [11]. For varicella vaccination, the assessment includes coverage with ≥1 dose of varicella vaccine and ≥2 doses of varicella vaccine among adolescents with no history of varicella disease [13, 14]. Vaccination coverage estimates are based on provider-reported immunization records. One-dose varicella-vaccinated adolescents were defined as those who received 1 dose of varicella vaccine at ≥12 months of age; ≥2-dose varicella-vaccinated adolescents were defined as those who received ≥2 doses of varicella vaccine at ≥12 months of age. Varicella disease history was obtained from both providers and parents or guardians who were asked whether the adolescent had a history of chickenpox or varicella. NIS-Teen methodology does not include interval requirements for a valid dose (ie, at least 28 days between the first and second dose) in computing ≥2 dose varicella vaccine coverage. The difference in the valid ≥2 dose varicella vaccine definition and the NIS-Teen operationalized definition without an interval restriction for the 2020 data was assessed; a change in ≥2-dose status for only 6 of 18 513 adolescents was identified. Thus, the difference in ≥2-dose coverage with or without the interval requirement was minimal.
For both NIS-Child and NIS-Teen, data were weighted and analyzed to account for the complex survey design. Analyses were conducted using SAS-callable SUDAAN (version 11, RTI International).
Immunization Information Systems, 2006–2020
Immunization information systems (IISs) were used to estimate implementation of the childhood 2-dose varicella vaccination recommendation at the routinely recommended ages of 4–6 years by examining coverage among children aged 7 years. IISs are computerized, population-based systems that collect and consolidate data from participating vaccine providers, which allows for monitoring vaccine coverage over time [15]. A previous analysis that examined coverage through 2012 in which de-identified, individual record–level data for varicella- and measles-containing vaccines (measles-mumps-rubella [MMR], MMRV, measles-only) were obtained from 6 sites (Michigan, Minnesota, North Dakota, New York City, Oregon, and Wisconsin) with high-performing IISs since 2006 was updated [16] (Supplementary Appendix).
Valid doses of varicella vaccine were defined as dose 1 administered no earlier than 4 days before age 12 months, dose 2 administered at least 28 days after dose 1, and either dose administered on the same day as or ≥4 weeks after any other live vaccine [16]. Two-dose vaccination coverage for varicella- and measles-containing vaccines was calculated for each year of the study period (ie, 1 January 2006–31 December 2020) among children aged 7 years (born during 1 January 1999–31 December 2014). Intercensal population estimates for 2006–2009 and postcensal estimates for 2010–2020 were used for population denominators [17]. Vaccination coverage was calculated by dividing the number of children aged 7 years who received 2 valid doses of varicella vaccine by the population denominators for the children within the same age group. For statistical analysis, the number of doses allowed per jurisdiction was capped at population size minus 1 for a maximum vaccination coverage of 100%. Unweighted average vaccination coverage estimates for each year for each of the 6 sites were also calculated. All analyses were conducted using SAS software (version 9.4, SAS Institute).
Daycare and School Vaccination Requirements, 1996–2020
Information on varicella vaccination requirements for school enrollment was obtained by reviewing state immunization program websites and state administrative codes; data were verified with state health department immunization program managers. Vaccination coverage with ≥2 doses of varicella vaccine among adolescents in 2020 was examined in relation to state school vaccination requirements. The association between daycare and school entry requirements and 1-dose coverage had been previously examined [18]. This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy.
RESULTS
NIS-Child, Coverage With ≥1 Dose Varicella Vaccine
Among children aged 19–35 months, an estimated 16.0% (95% confidence interval [CI], 15.3%–16.7%) had received ≥1 dose of varicella vaccine in 1996. Subsequently, coverage increased gradually, reaching 80.6% in 2002 and 89.2% by the end of the 1-dose program in 2006 (Figure 1 and Supplementary Table 1). During the 2-dose period, 2007–2020, 1-dose varicella vaccine coverage has remained stable at or above 90% (89.6%–92.7%). In 2020, 1-dose varicella vaccination coverage was 92.7% (95% CI, 92.0%–93.4%) nationally and varied by state from 85.6% to 96.9%. Coverage was ≥95% in 5 (10%) states, 90%–94.9% in 37 (74%) states and the District of Columbia (DC), and <90% in 8 (16%) states. Coverage by sociodemographic characteristics is available for children by age 24 months (born in 2017 and 2018) [19]. In this cohort, varicella vaccination coverage was similar for most race/ethnicities, but some disparities remain (P < .05) (Supplementary Figure 1), with Black children and Hispanic children having lower coverage compared with White children (89.3% and 89.2% vs 92.2%). Additionally, coverage was lower for children living below the federal poverty level compared with those living at or above the poverty level (88.0% vs 92.1%) and for children not privately insured (on Medicaid, uninsured, or other insurance) compared to children with private insurance (78.2%–89.8% vs 93.3%); there were no differences in coverage by urban/rural status.
Figure 1.
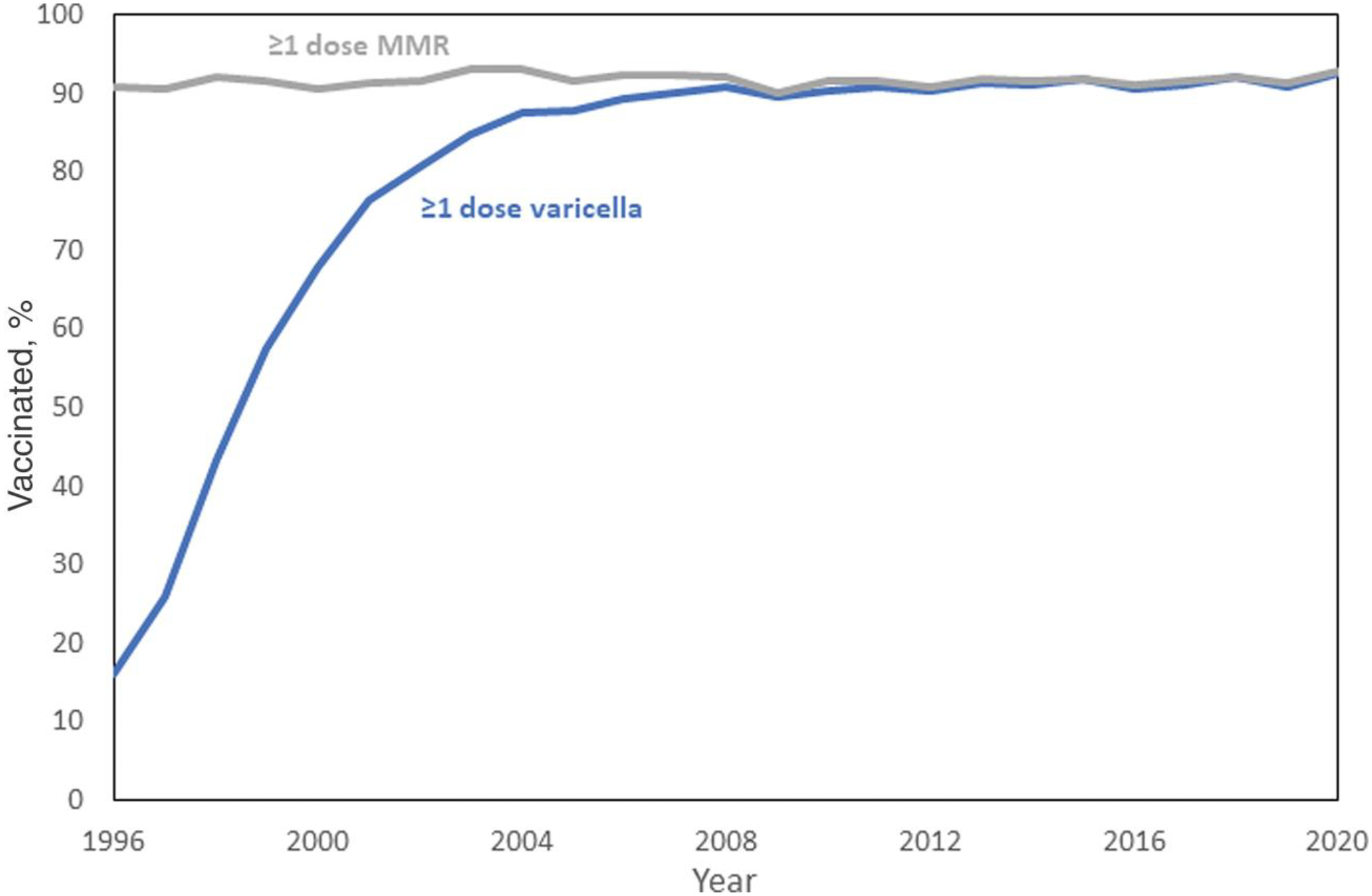
Estimated vaccination coverage for ≥1 dose of varicella and ≥1 dose of measles-mumps-rubella (MMR) vaccine among children aged 19–35 months, National Immunization Survey–Child (NIS-Child), United States, 1996–2020. NIS-Child data collection for ≥1 dose of varicella vaccine began in July 1996. Vaccination may have occurred prior to July 1996, creating uncertainty in the 1996 vaccination coverage estimate.
The first requirements for varicella vaccination were established in 1997 with Pennsylvania implementing a requirement for daycare entry and DC implementing a requirement for day-care, elementary school, and middle school entry. Daycare entry requirements were implemented rapidly after 1999; more than half of the states had 1-dose entry requirements in place by 2001 [18]. Implementation of elementary school entry requirements was slower but by 2002, half the states had elementary school entry requirements in place. This gap continued to narrow and was closed by 2006. By the start of the 2006–2007 school year (end of the 1-dose period), 46 (92%) of states and DC had requirements for 1-dose varicella vaccination at entry and all states and DC had daycare and school entry requirements by 2015 (Supplementary Figure 3). Coverage levels among children aged 19–35 months were higher in states with daycare entry requirements that were implemented earlier: states that implemented entry requirements in 2000 or earlier were more likely to have varicella vaccination coverage levels of ≥90% by 2005, compared with states that implemented entry requirements during 2001–2002 or 2003–2005 [18].
NIS-Teen, Coverage With ≥2 Doses Varicella Vaccine
In 2006, the first year that varicella vaccination coverage was assessed among adolescents, 65.5% (95% CI, 61.4%–69.4%) of adolescents aged 13–17 years (born January 1988 through February 1994) without a history of varicella disease had received ≥1 dose of vaccine. Vaccination coverage with ≥1 dose of varicella vaccine reached 90.5% in 2010 and increased to 95.6% in 2020 (adolescents born January 2002 through February 2008). Vaccination coverage with ≥2 doses of varicella vaccine increased rapidly after the 2007 second dose recommendation, both routine for children and catch-up vaccination for children and adolescents who had received 1 dose, from 4.1% in 2006 to 58.1% in 2010 and 91.9% by 2020 (Figure 2 and Supplementary Table 2). Among adolescents, 2-dose coverage increased more rapidly than coverage with at least 1 dose. In 2020, ≥2-dose varicella vaccine coverage among adolescents was ≥95% (range, 95.2%–98.9%) in 17 (34%) states, 90%–94.9% in 24 (48%) states, and <90% (range, 83.9%–89.9%) in 9 (18%) states and DC (Supplementary Figure 2). Consequently, with the increase in administration of varicella vaccine, the proportion of adolescents with varicella disease history declined from 69.9% in 2006 to 8.4% in 2020 (Figure 2 and Supplementary Table 2). For adolescents, immunity to varicella (history of varicella disease or documentation of receipt of ≥2 doses of varicella vaccine) increased from 73.5% in 2008 to 92.6% in 2020 (Figure 2).
Figure 2.
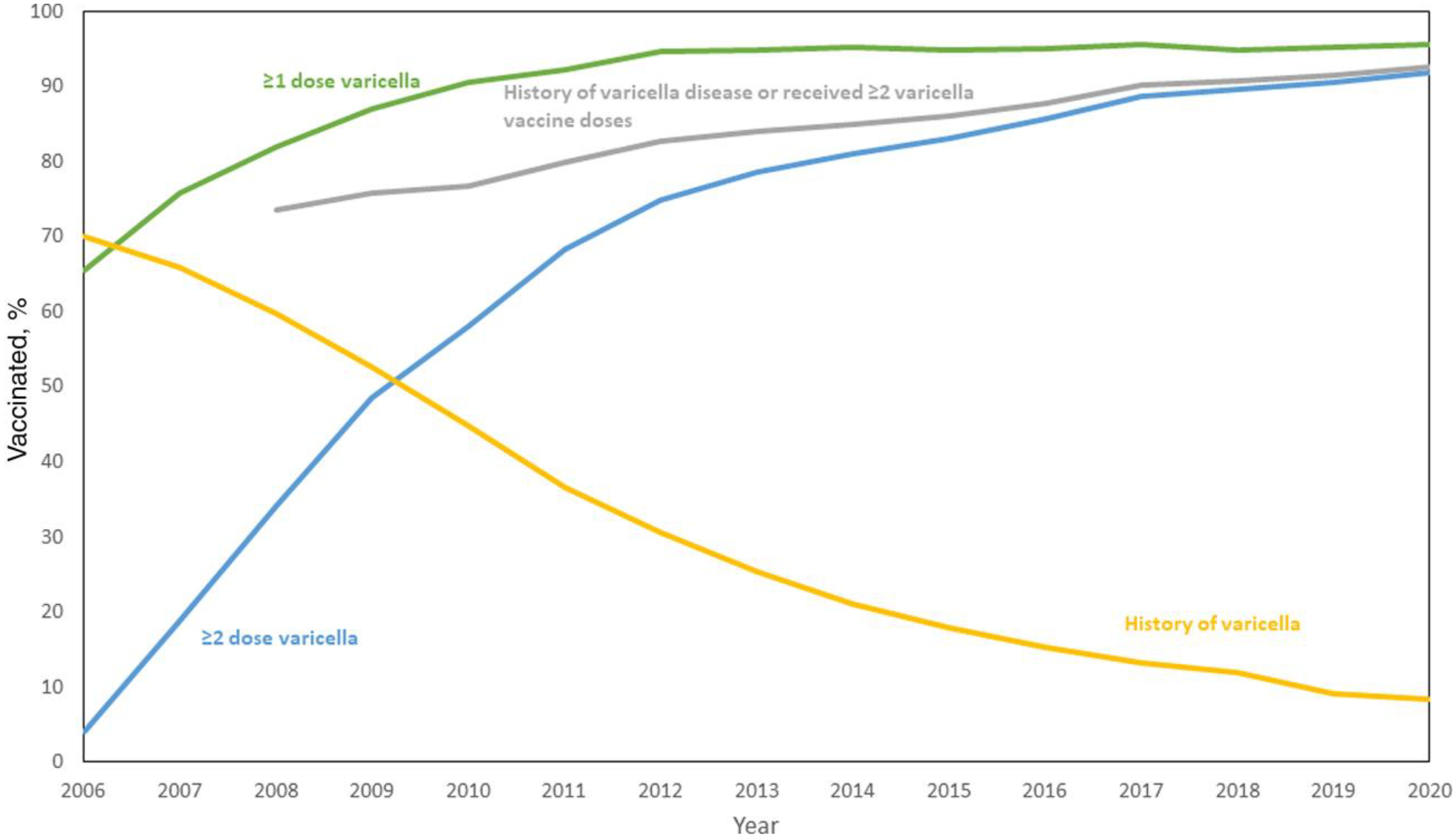
Varicella history and estimated varicella vaccination coverage among adolescents aged 13–17 years, National Immunization Survey–Teen, 2006–2020.
In the 2020–2021 school year, 44 (88%) states and DC required 2 doses of varicella vaccine for school attendance in 1 or more grades (Table 1). The earliest 2-dose requirements were implemented by Colorado, Georgia, and South Dakota in the 2007–2008 school year; Hawaii was the latest state to implement a 2-dose requirement for the 2020–2021 school year. Twenty-five states (50%) and DC required 2 doses of varicella vaccine for enrollment in elementary, middle, and high school (22 states and DC require 2 doses of varicella vaccine for all grades, kindergarten through 12th grade), 11 (22%) had requirements for enrollment in elementary and middle school, and 8 (16%) required proof of vaccination for enrollment in elementary school or at first entrance into the school system only (for students transferring into the school system).
Table 1.
Estimated Vaccination Coverage With ≥2 Doses of Varicella Vaccine Among Adolescents Aged 13–17 Years, National Immunization Survey-Teen, 2020, and 2020–2021 School Vaccination Requirements by State
| State | ≥2 Dose Varicella, % (95% CI) | 2-Dose School Vaccination Requirement | Grades Implemented for 2020–2021 SY | 2-Dose School Vaccination Requirement Implementation Timeline |
|---|---|---|---|---|
| Alabama | 94.9 (92.0–97.8) | Noa | … | … |
| Alaska | 91.2 (87.3–95.1) | Yes | K-6 | 1 Jul 2009 |
| Arizona | 84.2 (79.3–89.1) | Noa | … | … |
| Arkansas | 95.9 (93.0–98.8) | Yes | K-12 | K, 2010; K-12, 1 Sep 2014 |
| California | 85.8 (80.5–91.1) | Yes | K, 7; students new to school system, K-12 | 1 Jul 2019 |
| Colorado | 93.2 (89.9–96.5) | Yes | K-12 | PI beginning with K, 2007–2008 SY |
| Connecticut | 96.7 (94.4–99.0) | Yes | K, 7; students new to school system, K-12 | 2011–2012 SY |
| Delaware | 93.6 (90.2–97.0) | Yes | K-12 | PI beginning with K, 2008–2009 SY; paused in 2015; required K-12 by 2020–21 SY |
| District of Columbia | 86.4 (81.6–91.2) | Yes | K-12 | 2009–2010 SY |
| Florida | 94.8 (91.8–97.8) | Yes | K-12 | PI beginning with K, 2008–2009 SY |
| Georgia | 96.2 (93.7–98.7) | Yes | K, 6; students new to school system | 1 Jul 2007 |
| Hawaii | 91.2 (87.1–95.3) | Yes | K; students new to school system, K-12 | 1 Jul 2020 |
| Idaho | 88.6 (84.2–93.0) | Yes | K-10 | Students born after 1 Sep 2005, implemented 7 Apr 2011 |
| Illinois | 94.0 (91.5–96.5) | Yes | K-12 | PI beginning with K, 6, and 9, 2014–2015 SY |
| Indiana | 97.1 (95.2–99.0) | Yes | K-12 | K, 1 Sep 2010; K-12, 2013–2014 SY |
| Iowa | 96.1 (93.8–96.1) | Yes | K-12 | Students born on or after 15 Sep 2003, implemented 16 Jul 2008 |
| Kansas | 91.5 (87.5–95.5) | Yes | K-12 | K, 2009–2010 SY; phased implementation in subsequent years |
| Kentucky | 96.5 (94.1–98.9) | Yes | K-12 | K and 6; 1 Jul 2011; K-12, 1 Jul 2017 |
| Louisiana | 95.7 (93.3–98.1) | Yes | K, 6; students new to school system, K-12 | 2009–2010 SY |
| Maine | 95.7 (93.0–98.4) | No | … | … |
| Maryland | 93.7 (91.4–96.0) | Yes | K-6 | PI beginning with K, 2014–2015 SY |
| Massachusetts | 96.0 (93.7–98.3) | Yes | K-12 | PI beginning with K, 7, 2011–2012 SY |
| Michigan | 95.2 (92.5–97.9) | Yes | K, 7; students new to school system, aged 7–18 y | K and 6, 2010–2011 SY; change from 6 to 7, 2014–2015 SY |
| Minnesota | 97.0 (94.9–99.1) | Yes | K-12 | K and 7, 2009–2010; K-12, 2014–2015 SY |
| Mississippi | 94.9 (91.5–98.3) | Yes | First entrance into school system, K-12 | 2009–2010 SY |
| Missouri | 91.0 (87.3–94.7) | Yes | K-10 | PI beginning with K, 2010–2011 SY |
| Montana | 93.1 (89.5–96.7) | Yes | K-12 | 1 Oct 2015 |
| Nebraska | 87.7 (83.4–92.0) | Yes | K, 7; students new to school system, K-12 | 1 Jul 2011 |
| Nevada | 83.9 (78.3–89.5) | Yes | K; students new to school system, K-12 | 1 Oct 2008 |
| New Hampshire | 98.9 (97.8–100.0) | Yes | K-12 | PI beginning with K, 1, and 6, 2009–2010 SY |
| New Jersey | 90.4 (86.2–94.6) | No | … | … |
| New Mexico | 93.6 (90.4–96.8) | Yes | K-12 | PI beginning with K, 2008–2009 SY; required K-12, 2019–2020 SY |
| New York | 93.6 (91.1–96.1) | Yes | K-12 | PI beginning with K and 6, 2014–2015 SY |
| North Carolina | 96.4 (93.4–99.4) | Yes | K, students new to school system, K-12 | 1 Jul 2015 |
| North Dakota | 97.0 (94.8–99.2) | Yes | K-12 | PI beginning with K, 2008–2009 SY |
| Ohio | 91.8 (88.1–95.5) | Yes | K-10 | PI beginning with K, 2010–2011 SY |
| Oklahoma | 89.9 (85.9–93.9) | No | … | … |
| Oregon | 93.8 (90.6–97.0) | Noa | … | … |
| Pennsylvania | 94.5 (91.7–97.3) | Yes | K-12 | 2011–2012 SY |
| Rhode Island | 96.1 (93.3–98.9) | Yes | K-12 | PI beginning with K and 7, 1 Aug 2009 |
| South Carolina | 90.1 (85.4–94.8) | Yes | K-6 | PI beginning with K, 2014–2015 SY |
| South Dakota | 95.4 (92.5–98.3) | Yes | K; students new to school system, K-12 | 2007–2008 SY |
| Tennessee | 93.3 (89.9–96.7) | Yes | K; students new to school system, K-12 | K, 7, and first entrance into system, Jul 2010; dropped 7 requirement, 2018 |
| Texas | 86.7 (83.2–90.2) | Yes | K-12 | PI beginning with K and 7, 2009–2010 SY |
| Utah | 88.3 (83.8–92.8) | Yes | K, 7 | 2015–2016 SY |
| Vermont | 95.3 (92.7–97.9) | Yes | K, 7 | 1 Aug 2008 |
| Virginia | 92.5 (89.2–95.8) | Yes | K | 3 Mar 2010 |
| Washington | 92.4 (88.5–96.3) | Yes | K-12 | PI beginning with K, 2008–2009 SY; required 9–12, 2016–2017 SY |
| West Virginia | 88.8 (84.7–92.9) | Yes | First entrance into school system, K-12 | 2008–2009 SY |
| Wisconsin | 93.5 (89.8–97.2) | Yes | K-12 | PI beginning with K, 6, and 12, 2008–2009 SY |
| Wyoming | 92.1 (88.3–95.9) | Yes | K-12 | 2010–2011 SY |
Abbreviations: CI, confidence interval; K, kindergarten; PI, progressive implementation; SY, school year.
Two doses are required in Alabama, Arizona, and Oregon only if the first dose is administered a er 13 years of age.
The 6 states with no 2-dose varicella vaccination requirement for school enrollment in 2020–2021 had an average ≥2-dose vaccination coverage of 91.5% among adolescents aged 13–17 years (median, 92.1% [range, 84.2%–95.7%]). The 44 states and DC with any 2-dose vaccination requirement had an average ≥2-dose vaccination coverage of 93.1% (median, 93.6% [range, 83.9%–98.9%]), although the difference in medians is not statistically significant (P = .4793). The 25 states and DC with 2-dose vaccination requirements for enrollment in high school had an average ≥2-dose vaccination coverage of 93.7% (median, 93.6% [range, 86.4%–98.9%]) compared with an average coverage of 92.4% among the 11 states with a 2-dose vaccination requirement for middle school only (median, 93.7% [range, 85.8%–96.7%]).
Immunization Information Systems
Two-dose varicella vaccination coverage among children 7 years of age increased substantially across all 6 sites over time, with a range in coverage of 2.6%–5.5% (median, 3.9%) in 2006 to 86.0%–100.0% (median, 92.8%) in 2020 (Figure 3). There was a rapid uptake of 2-dose varicella vaccine after the 2-dose recommendation was made in 2007, coverage reaching 84.5%–100.0% within the first 5 years of the recommendation. In 2020, all but 1 of the states had 2-dose school entry requirements; in the state without requirements, 2-dose coverage was 88.3%. Over time, 2-dose vaccination coverage for varicella vaccine at 7 years of age approached that of 2-dose coverage for MMR vaccine (92.5%–94.4% in 2020).
Figure 3.
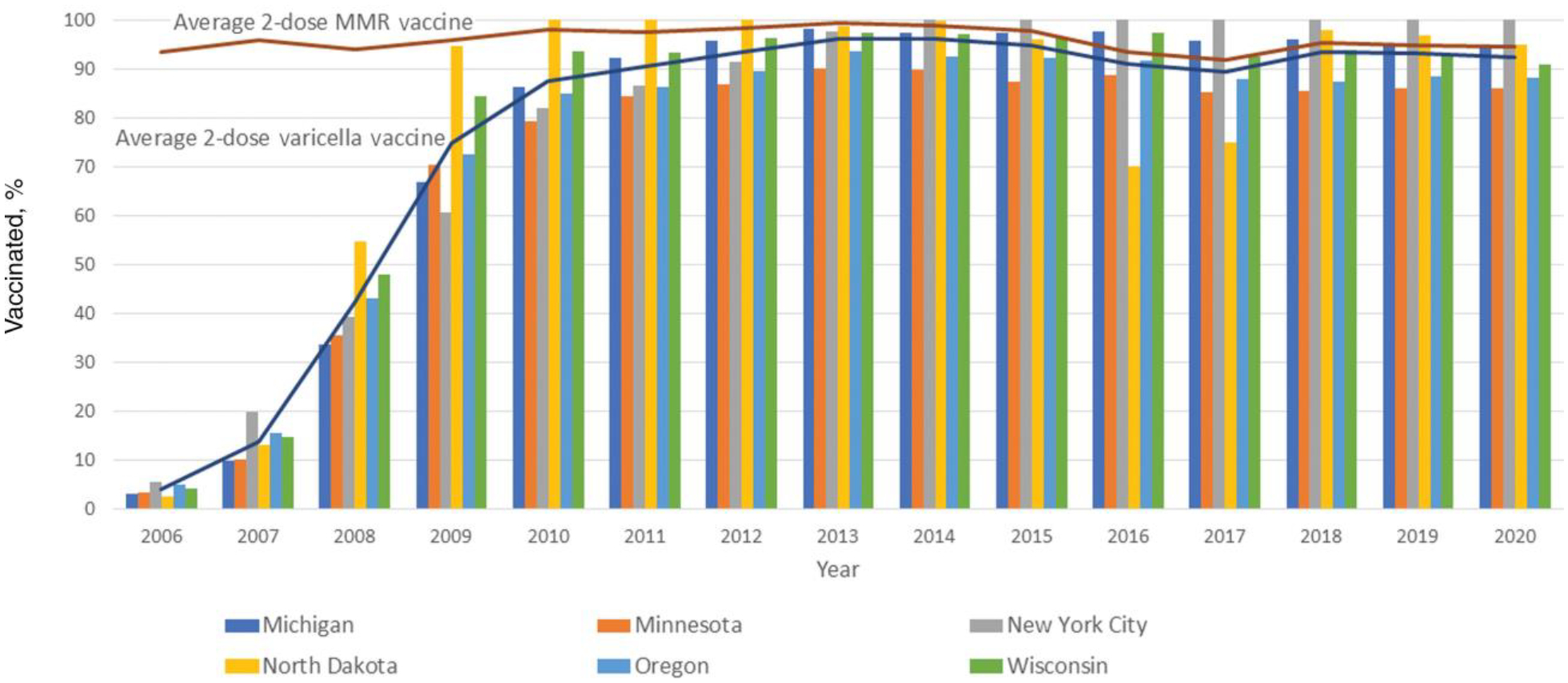
Average percentage of 2-dose varicella vaccination coverage compared with average percentage of 2-dose measles-mumps-rubella (MMR) vaccination coverage and 2-dose varicella vaccination coverage by immunization information system site among children aged 7 years in 6 sites, United States, 2006–2020.
DISCUSSION
Tracking vaccine coverage is a critical component of monitoring a vaccine program. Vaccines can only have impact if they are administered to the populations for whom they are recommended. The US varicella vaccination program has been successful in efforts to introduce a new vaccine and then to increase vaccination coverage among children and adolescents. By the end of the decade of the 1-dose program in 2006, 1-dose coverage had reached 89.2% among children aged 19–35 months, and 92% of states and DC had daycare or school entry requirements in place. Beginning in 2009, 1-dose varicella coverage among children aged 19–35 months was similar to 1-dose MMR vaccine coverage, stabilizing at or above 90%. After the second dose recommendation in 2007, the uptake of the second dose has been rapid. By 2020, ≥86% of children in the 6 high-performing IIS sentinel sites had received the second dose by the routinely recommended age and 88% of states and DC had 2-dose school entry requirements. The high 1- and 2-dose coverage in children is also reflected among adolescents in whom significant progress has been made: among teens without a history of varicella, 95.6% had received 1 dose of varicella vaccine and 91.9% had received ≥2 doses by 2020. The proportion of adolescents with evidence of immunity to varicella (either by 2-dose vaccination or history of varicella) also increased to 92.6%. The successes in attaining and maintaining high vaccine coverage were paramount in the dramatic reduction in the burden of varicella documented in the US during the vaccination program [20, 21].
Increase in coverage has led to declines of >97% in varicella incidence and ≥90% in varicella-related hospitalizations and deaths (94% and 97% among persons age <50 years) over the 25 years of the vaccination program [20, 21]. While national case surveillance data from before or early in the vaccination program are not available due to varicella not being a reportable condition until 2003, in 4 states that continuously reported varicella cases since before vaccination program implementation (Illinois, Michigan, Texas, and West Virginia), a remarkable consistency was observed between declines in incidence (96.5%–99.0% from 1990–1994 to 2018–2019) [20] and increases in vaccine coverage among children aged 19–35 months (88.7%–95.6% in 2019). Similarly, in 2 varicella active surveillance sites, the declines in incidence paralleled increases in varicella vaccination coverage [4, 22, 23]. Within the first 5 years of the program, varicella incidence declined 71% and 79% as coverage among children aged 19–35 months reached 82% and 84%, respectively [4]. By the end of the 1-dose period in 2005, the decline in incidence was 90% in both sites and coverage among children 19–35 months reached 92% and 94% [22]. Four years into the 2-dose program, in 2010, varicella incidence declined 98% with 1-dose coverage among young children reaching 95% in both sites; 2-dose coverage was 84% among kindergarten students (1 site) and 61% among children aged 7–9 years (second site) [23].
Daycare and school entry requirements have been associated with increased 1-dose varicella vaccination rates among preschool and elementary children [18, 24]. A previous study also documented an association between 2-dose school immunization requirements against varicella and greater adolescent vaccination coverage [25]. Our analysis of school immunization requirements for the 2020–2021 school year and 2-dose adolescent vaccination coverage found that states with a 2-dose varicella vaccine requirement did not have a statistically significant higher vaccination coverage than states with no 2-dose vaccination requirements for kindergarten through 12th-grade school attendance. The number of years that requirements had been in place and other state-level factors that may influence vaccination coverage including immunization exemption policies for school attendance or the level of enforcement of the requirements were not examined. However, our data highlight that after more than a decade of implementation of the 2-dose program, there is high public acceptance of the program regardless of school enrollment requirements.
Despite the tremendous successes of the varicella vaccination program [20, 21], there are opportunities for improvement to reach every child with varicella vaccine and further decrease varicella disease and its complications in the US. National coverage of 1 dose of varicella vaccine among children aged 19–35 months has been relatively stable at approximately 90% for the past decade, but in 8 states coverage is still <90%. Data from the early years of the varicella vaccination program suggested no racial and ethnic disparities in varicella vaccine uptake among Black and White children aged 19–35 months, but a disparity between White and Hispanic children existed from 1998 to 2001 and in 2004 [26]. In 2020, some disparities were reported in NIS-Child by race/ethnicity, poverty level, and insurance status, similar to disparities in coverage for most childhood vaccines among young children [19]. In the IIS sites, although coverage was high, 2-dose coverage at age 7 years was <90% in 2 of the 6 sites. While many of these children will be caught up by or during adolescence given the high coverage we report with 1 and 2 doses, approximately 8%–9% of adolescents remained inadequately protected (either unvaccinated or had only received 1 dose of varicella vaccine) in 2020. In the 2020 NIS-Teen data, no disparities in ≥1-dose and ≥2-dose varicella vaccine coverage were present by metropolitan statistical area or poverty level, although lower coverage for Hispanic adolescents and those with any Medicaid insurance compared to private insurance was reported [13]. An earlier analysis of adolescent varicella vaccination found that while catch-up campaigns were successful, missed opportunities persisted and 77% of 1-dose-vaccinated adolescents had at least 1 missed opportunity for receiving their second dose of varicella vaccine [27]. It is important for providers to ensure that adolescents are up to date on their varicella vaccination as varicella can be more severe in adolescents and adults. Additionally, adolescence is one of the last opportunities to screen and vaccinate a large population to ensure protection before adulthood.
There are several limitations to the data we report. The household interview response rate in 2020 was low (22.5% in NIS-Child and 20.7% in NIS-Teen), and adequate provider data were available for approximately half (54.2% in NIS-Child and 45.2% in NIS-Teen) of those with completed interviews [13, 19]. Selection bias could be introduced if study respondents and nonrespondents differed on factors related to vaccination coverage. Weighting adjustment for nonresponse and for households without mobile phones might not completely eliminate bias. Incomplete provider-reported vaccination histories might result in coverage being underestimated. However, these limitations should be interpreted considering that total survey error assessed for the 2020 NIS-Child data found no underestimation for varicella vaccine [19]. Six IIS sites with provider-verified data were included to assess coverage at the age the second dose is routinely recommended. Although almost all states have an IIS, their capacity varies in completeness of vaccination and population coverage. The mechanisms to assess national coverage in this age group need improvement. Two-dose varicella vaccination coverage data are available from surveys of kindergarten-aged children; however, data are voluntarily provided by states, and methodologies vary by state [28]. NIS-Teen could provide estimates by age <7 years but with a substantial lag because data would not be available until these children turn 13 to be included in the NIS-Teen survey.
The US varicella vaccination program, implemented following vaccine policy recommendations in 1995 and 1996, has resulted in declines of >97% in varicella incidence and ≥90% in hospitalizations and deaths [20, 21]. Vaccine impact was evident early during the 1-dose program as vaccine coverage increased [4]; implementation of daycare and school entry requirements for 1-dose varicella vaccination was an important strategy for this achievement [18]. Uptake of the second dose was rapid, indicating high healthcare provider and public acceptance. Sustaining high 1- and 2-dose varicella vaccination coverage will help maintain the gains achieved in controlling the burden of varicella in the US. Addressing disparities in coverage is important to afford program benefits to all communities and to ensure the equitable use of resources for disease control.
Supplementary Material
Footnotes
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention, Atlanta, GA, USA.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56(RR-4):1–40. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1996; 45(RR-11):1–36. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention of varicella. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999; 48(RR-6):1–5. [PubMed] [Google Scholar]
- 4.Seward JF, Watson BM, Peterson CL, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA 2002; 287:606–11. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA 2005; 294:797–802. [DOI] [PubMed] [Google Scholar]
- 6.Davis MM, Patel MS, Gebremariam A. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics 2004; 114:786–92. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005; 352:450–8. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Outbreak of varicella among vaccinated children–Michigan, 2003. MMWR Morb Mortal Wkly Rep 2004; 53:389–92. [PubMed] [Google Scholar]
- 9.Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 2004; 113:455–9. [DOI] [PubMed] [Google Scholar]
- 10.Lopez AS, Guris D, Zimmerman L, et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics 2006; 117:e1070–7. [DOI] [PubMed] [Google Scholar]
- 11.Wolter KK, Smith PJ, Khare M, et al. Statistical methodology of the National Immunization Survey, 2005–2014. Vital Health Stat 2017; 1:1–107. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Immunization Survey–Teen: a user’s guide for the 2019 public-use data file. https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF19-DUG.pdf. Accessed 19 July 2021.
- 13.Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy N, Rodgers L, Pabst L, Fiebelkorn AP, Ng T. Progress in childhood vaccination data in immunization information systems—United States, 2013–2016. MMWR Morb Mortal Wkly Rep 2017; 66:1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez AS, Cardemil C, Pabst LJ, et al. Two-dose varicella vaccination coverage among children aged 7 years—six sentinel sites, United States, 2006–2012. MMWR Morb Mortal Wkly Rep 2014; 63:174–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National vital statistics system: bridged-race population estimates—data files and documentation. https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm. Accessed 15 October 2021.
- 18.Lopez AS, Kolasa MS, Seward JF. Status of school entry requirements for varicella vaccination and vaccination coverage 11 years after implementation of the varicella vaccination program. J Infect Dis 2008; 197(Suppl 2):S76–81. [DOI] [PubMed] [Google Scholar]
- 19.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Sterrett N. Vaccination coverage by age 24 months among children born in 2017 and 2018—National Immunization Survey–Child, United States, 2018–2020. MMWR Morb Mortal Wkly Rep 2021; 70:1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin M, Leung J, Anderson TC, Lopez AS. Monitoring varicella vaccine impact on varicella incidence in the United States: surveillance challenges and changing epidemiology, 1995–2019. J Infect Dis 2022; 226(Suppl 4):S392–9. [DOI] [PubMed] [Google Scholar]
- 21.Marin M, Lopez AS, Melgar M, Dooling K, Curns AT, Leung J. Decline in severe varicella disease during the United States varicella vaccination program: hospitalizations and deaths, 1990–2019. J Infect Dis 2022; 226(Suppl 4):S407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995–2005. J Infect Dis 2008; 197(Suppl 2):S71–5. [DOI] [PubMed] [Google Scholar]
- 23.Bialek SR, Perella D, Zhang J, et al. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics 2013; 132:e1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MM, Gaglia MA. Associations of daycare and school entry vaccination requirements with varicella immunization rates. Vaccine 2005; 23:3053–60. [DOI] [PubMed] [Google Scholar]
- 25.Kawai K, O’Brien MA, Conway JH, Marshall GS, Kuter BJ. Factors associated with receipt of two doses of varicella vaccine among adolescents in the United States. Pediatric Infect Dis J 2013; 32:538–42. [DOI] [PubMed] [Google Scholar]
- 26.Luman ET, Ching PL, Jumaan AO, Seward JF. Uptake of varicella vaccination among young children in the United States: a success story in eliminating racial and ethnic disparities. Pediatrics 2006; 117:999–1008. [DOI] [PubMed] [Google Scholar]
- 27.Leung J, Reagan-Steiner S, Lopez A, Jeyarajah J, Marin M. Varicella vaccination among US adolescents: coverage and missed opportunities, 2007–2014. J Public Health Manag Pract 2019; 25:E19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellerson JL, Street E, Knighton C, Calhoun K, Seither R, Underwood JM. Centers for Disease Control and Prevention’s school vaccination assessment: collaboration with US state, local, and territorial immunization programs, 2012–2018. Am J Public Health 2020; 110:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


