Abstract
Powered air-purifying respirators (PAPRs) that offer protection from particulates are deployed in different workplace environments. Usage of PAPRs by healthcare workers is rapidly increasing; these respirators are often considered the best option in healthcare settings, particularly during public health emergency situations, such as outbreaks of pandemic diseases. At the same time, lack of user training and certain vigorous work activities may lead to a decrease in a respirator’s performance. There is a critical need for real-time performance monitoring of respiratory protective devices, including PAPRs. In this effort, a new robust and low-cost real-time performance monitor (RePM) capable of evaluating the protection offered by a PAPR against aerosol particles at a workplace was developed. The new device was evaluated on a manikin and on human subjects against a pair of condensation nuclei counters (P-Trak) used as the reference protection measurement system. The outcome was expressed as a manikin-based protection factor (mPF) and a Simulated Workplace Protection Factor (SWPF) determined while testing on subjects. For the manikin-based testing, the data points collected by the two methods were plotted against each other; a near-perfect correlation was observed with a correlation coefficient of 0.997. This high correlation is particularly remarkable since RePM and condensation particle counter (CPC) measure in different particle size ranges. The data variability increased with increasing mPF. The evaluation on human subjects demonstrated that RePM prototype provided an excellent Sensitivity (96.3% measured on human subjects at a response time of 60 sec) and a Specificity of 100%. The device is believed to be the first of its kind to quantitatively monitor PAPR performance while the wearer is working; it is small, lightweight, and does not interfere with job functions.
Keywords: PAPR, real-time monitoring, respiratory protection, sensor
Introduction
Powered air-purifying respirators (PAPRs) designed to reduce exposure of workers to aerosol particulates are deployed in different workplace environments, especially when a high level of protection is required. For instance, there is a high risk of occupational exposure for healthcare workers (HCWs) to various viruses and bacterial pathogens as well as other aerosol hazards. Recent outbreaks of emerging diseases, including, but not limited to, severe acute respiratory syndrome (SARS) in 2003, H1N1 influenza in 2009, and, of course, the ongoing COVID-19 pandemic highlighted the risk of disease transmission among HCWs. During the first SARS epidemic, HCWs accounted for 20% of critically ill SARS cases (Nicas et al. 2004).The recent Ebola and current COVID-19 outbreaks have boosted the concerns of HCWs about personal protective equipment (PPE), including respirators. Findings that HCWs were infected with SARS-CoV, H1N1, and SARS-CoV-2 despite the use of N95 filtering facepiece respirators (FFRs) have lead health and safety professionals to promote the use of PAPRs when highly contagious or novel pathogens pose a major occupational risk for HCWs (Loeb et al. 2004; CDC 2020).
A PAPRs utilizes a blower to draw ambient air through air-purifying elements and deliver the purified air to a respiratory inlet covering. PAPRs with loose-fitting respiratory inlet coverings are reported to be a more attractive option than N95 FFRs for HCWs, primarily because fit testing is not required for the wearer (Liverman et al. 2014). Loose-fitting PAPRs do not require a tight face seal, and thus, usually can be worn by individuals with facial hair and various face shapes/sizes, who otherwise could not wear FFRs. Additionally, loose-fitting PAPR hood designs often provide protection to the wearer’s head, neck, and shoulders. PAPRs are also appreciated in the healthcare community for longer term wearing comfort. In spite of limitations such as cost, interference with hearing, and the need of decontamination, usage of PAPRs by HCWs is rapidly increasing (Wizner et al. 2016), and PAPRs may be the best option in healthcare settings, particularly during public health emergency situations, such as outbreaks of pandemic diseases.
PAPRs provide high levels of protection relative to FFRs. The Occupational Safety and Health Administration (OSHA) defines assigned protection factor (APF) as the “level of expected protection that a respirator or class of respirators is expected to provide” (OSHA 2006). For PAPRs, OSHA specifies an APF of 25 for loose-fitting facepieces and APF of 25 or 1,000 for helmets/hoods, whereas FFRs have an APF of 10. For helmets/hoods, APF of 25 is designated as the default unless the manufacturer demonstrates performance at the level of 1,000 or greater. The level of performance has been demonstrated by measuring the workplace protection factor (WPF) (Myers et al. 1984, 1986) or simulated workplace protection factor (SWPF) (Cohen et al. 2001; Vo et al. 2015). Cohen et al. performed an SWPF study which showed that PAPRs can greatly outperform their designated APFs of 25 or 1,000, with some median SWPF values exceeding 250,000, indicating the protection provided by PAPRs may by far be better than expected. However, subjects in the quoted study were substantially assisted by test personnel in donning the respirators and properly trained on respirator usage; thus, no major decrease in the respirators’ performance was anticipated due to human factors in donning or operation of the PAPR.
In reality, incorrect usage is possible when considering the complexity of PAPRs, lack of user training on PAPRs, and possible inhibition of work activities (Liverman et al. 2014). All these issues may lead to a decrease in performance. For example, many PAPR models have hose attachments which may get tangled, caught on foreign objects, or improperly connected to the blower motor, thereby obstructing airflow and compromising performance. Additional sources of PAPR failure include, but are not limited to, the following:
“over-breathing” (Mackey et al. 2005), when the user’s inspiration rate exceeds the rate of airflow provided to the PAPR;
flaws, caused either by poor design, a manufacturing defect, or lack of proper maintenance (Gao et al. 2016); and
improperly attached PAPR components, such as an untightened hose or untucked bib.
When considering the hazards of the healthcare work environment, which may include highly communicable and lethal diseases, workers’ respiratory protection cannot be compromised. One way to ensure maintenance of expected protection may be through continuous monitoring.
Some commercially available PAPRs have features such as a low-flow or low-battery alarms that alert the user when the airflow is obstructed or the battery-life is low, respectively. The low-flow alarm is activated when the blower motor senses resistance against the airflow it is providing. This is intended to alert the user to possible hose entanglement, airflow obstruction due to excessive hood material, or hose constriction. Although helpful, it is not directly indicative of PAPR performance and does not detect filter penetration or overall respirator leakage.
A wearable tool that directly measures the real-time performance of PAPRs would be useful for not only evaluating PAPR performance, but also for alerting the wearer to a compromise in performance. Such a device must quickly and accurately identify compromised protection resulting from insufficient PAPR airflow rate, design defects, excessive filter penetration, or any other source of respirator failure. Additionally, the device should be compact, lightweight, and comfortable to encourage widespread adoption.
The PAPR performance assessment (e.g., via WPF, SWPF) in environments with low or moderate aerosol concentration levels typically involves a condensation particle counter (CPC) measuring inside and outside of the respirator. This approach was materialized in a well-recognized fit testing instrument, PortaCount (TSI, Inc., Shoreview, MN, USA) as well as other recently developed particle measurement systems deployed to determine the performance of respirators before their usage. These systems are expensive, complex, bulky, and heavy. Most importantly, they were not designed to provide a continuous monitoring of the respirator performance during work activities. A new concept integrated in a novel Respirator Seal Integrity Monitor (ReSIM) allowing a real-time monitoring of the respirator performance was recently developed and validated by Grinshpun and his University of Cincinnati team (Leppänen et al. 2018; Liu et al. 2018; Wu et al. 2018). However, this monitor utilizes a qualitative approach and was tested for FFRs only, not for PAPRs.
The present paper introduces a new robust and low-cost (approx. $100–$200) real-time performance monitor (RePM) for PAPRs and provides the data on its evaluation. The device is believed to be the first of its kind to quantitatively monitor PAPR performance while the wearer is working; it is small, lightweight, and does not interfere with job functions. Through improving upon existing CPC-based approach and test methods for assessing PAPR performance, this development has a far-reaching impact due to its potential to dramatically improve the state of respiratory protection of healthcare workers and other PAPR users.
The objective of this study was to assess the efficacy of the newly-developed RePM for measuring PAPR performance and compare it to the current CPC methods. Both manikin-based and human subject-based evaluation approaches were used. The protection factor (PF) was determined in the form of mPF (manikin studies) and SWPF (human subject studies).
Methods
RePM – concept and design
The RePM developed in this effort is an improved version of the ReSIM, which was introduced and validated earlier (Leppäen et al. 2018; Wu et al. 2018). Both the ReSIM and RePM deploy the optical lightscattering principle and are capable of counting particles of ~0.3 μm and larger. The RePM utilizes a Sensirion SPS30 sensor (Sensirion AG, Staefa, ZH, Switzerland) while the ReSIM was designed and built with a Shinyei PPD60PV-T2 sensor (Shinyei, Kobe, Japan). The former has a lower counting threshold and a faster response as compared to its predecessor. Additionally, RePM is smaller and can be conveniently incorporated inside the PAPR hood to perform a real-time measurement of the in-mask aerosol concentration Cin. Unlike the ReSIM’s sensor that was designed to measure solely Cin, the new design allows deploying a pair of the RePM sensor units—located inside and outside of the PAPR hood. By measuring Cin as well as the outside aerosol concentration Cout, a respirator Protection Factor (PF) is determined as
Thus, the RePM concept represents a quantitative approach, in contrast to the qualitative identification of the “compromised respirator seal” utilized in the ReSIM concept (Liu et al. 2018). The two sensors of the RePM assembly used neodymium magnets to fix the locations of sensors inside and outside the PAPR hood in close proximity.
The Sensirion SPS30 incorporates an internal controller that calculates factors such as the detected mass concentration as well as binning detections into different particle size fractions with raw particle number and mass concentrations. Of particular note for RePM development, the Sensirion SPS30 also provides an average particle size output, which is used as surrogate for concentration for the purposes of detecting PAPR leaks. Since the inside concentrations were expected to be low compared to the SPS30’s particle sensitivity range, the average particle sizes for the internal and external sensors are integrated over several seconds to address sensor noise, trading immediacy of reaction for reductions in false positive and false negative readings. This trade off, from which the time of leak detection derives, is examined in this study as described below.
The sensors were connected to a Programmable Micro Controller (PMC) located in the Control Board (Teensy 3.5, PJRC.com, Sherwood, OR, USA). The Arduino programming environment was used to develop the code for the PMC. The PMC board was equipped with a USB-port and an 8-digit LED display for the real-time PF monitoring. A 3-LED color code output was built-in with the following code system: Red – PF ranges from 10 to 100; Yellow – PF ranges from 100 up to 1,000; and Green – PF is equal or in excess of 1,000. The latter feature was particularly valuable when testing the RePM on respirator-wearing human subjects.
Several design modifications and simplifications made in the course of the RePM development intended to improve its field-compatibility and user-friendliness, thus enhancing the feasibility of commercialization of the new device. At the same time, the current RePM prototype was designed specifically for this evaluation and as such differs from the intended commercial product. For example, while the 3-LED color coding was used in this evaluation study, the utilization of RePM in the field is expected to deploy only a 2-LED color coding: Red at PF < 1,000 and Green at PF ≥ 1,000. Another area is the power supply; the prototype tested in this effort utilized independent power, while production units can be engineered with the capability of drawing power from the main PAPR battery pack as the microcontroller, sensors and LEDs can be designed to minimize power draw. If the controller is integrated into the air pump unit, cabling may be integrated into the air hose assembly, further simplifying the system. In summary, with an inexpensive commercially available sensor and low-cost peripheral parts, the new RePM is believed to have a great potential.
PAPR
The PAPR used in this study (Versaflow, TR-300+, 3 M Co., St. Paul, MN, USA) featured a relatively high Assigned Protection Factor, APF, of 1,000 (OSHA 2006). The TR-300+ unit operates in two motor/blower modes: standard flow (185 L min−1) and high flow (205 L min−1). For conservative testing of the RePM, the lower of the two flow rates was chosen. The TR-300+ unit operates with replaceable headcovers. In this study, we used a headcover hood model S403 (3 M Co., St. Paul, MN, USA) for the manikin-based testing and much cheaper disposable hood model S605 (3 M Co., St. Paul, MN, USA) for the evaluation of RePM on human subjects. Both headcovers had a standard size and similar design that allowed for covering head, face, and shoulders.
RePM evaluation
The RePM evaluation was designed as a two-fold study. The first (preliminary) phase utilized a breathing manikin with a PAPR donned on it. The aerosol concentration was measured inside and outside of the respirator using a reference CPC-based method involving two P-Traks (Model 8025, TSI Inc., Shoreview, MN, USA). Periodically, the PAPR performance was compromised (by a simulated leak; see below). The second (main) phase involved PAPR-wearing human subjects equipped with the RePM sensors that measured the aerosol concentration inside and outside of the respirator. The subjects performed various exercises mimicking the activities of healthcare workers and the PAPR performance was periodically compromised via the leakage simulation. In both the manikin-based and the human subject-based studies, the inside samples were taken in the mouth area inside the hood and the outside ones were taken within 30 cm from the mouth outside the hood.
Both phases of the study were performed in the test chamber, volume = 23.5 m3, with NaCl serving as the challenge aerosol. The particles were generated with a salt particle generator (Model 8026, TSI Inc., St. Paul, MN). The total particle concentration was in the range of 6,000–8,000 cm−3 (for the particle sizes measured approximately from 0.02 to 1 μm). The above ambient particle concentration was chosen as a compromise between “too low,” which would make detection in the optical size range challenging, and “too high,” which would be counterproductive for establishing a low measurement threshold.
While manipulating with the leakage (opening and closing it), the following outcome was determined: the time interval, ΔtON, between the moment the leak leading to PF < 1,000 was established and the moment when it was positively identified (detected either by the CPCs or by the RePM). Additionally, we determined the time interval ΔtOFF between the leak closure and the moment at which the PF exceeded APF = 1,000. The latter enabled us to choose an appropriate time between the replicate tests.
Simulation of a scenario when the PAPR performance is compromised
In this study, performance failure was defined as the occurrence of the respirator PF falling below the OSHA-established APF-value (1,000). Initially, it was considered that turning the motor/blower unit OFF would be a feasible example of a PAPR malfunctioning that could lead to a substantial decrease of PF. However, when exercising this option on a respirator-wearing, breathing manikin (the first phase of this effort), we found that the system response time (the period needed to reach the desired low PF) was too long, likely due to large volume inside the PAPR. Another option considered for compromising the PAPR performance was an improper respirator donning. However, because the airflow from the motor/blower unit was too high to achieve any manikin’s over-breathing, even if the respirator was incorrectly positioned, the concentration Cin did not significantly change as measured with a P-Trak. Additionally, improper donning is not easily reproducible on human subjects, which is a major shortcoming. All the above make this simulation approach unfeasible for testing RePM. These observations were confirmed at different mean inspiratory flow (MIF) rates ranging from 30 to 135 L min−1.
An alternative way of simulating a respirator performance failure was to create a controllable leak inside the hose, which connects the motor/blower unit to the hood. Our preliminary tests showed that this simulation method produces satisfactory testing conditions, namely the desirable drop in PF was achieved within the time interval as low as 30 sec after the leak was established, and the initial PF value was restored within 60 sec after the leak was closed. The results were 100% reproducible (n = 6). The leakage was created by a remotely operated electromagnetic valve. The set-up is schematically presented in Figure 1.
Figure 1.
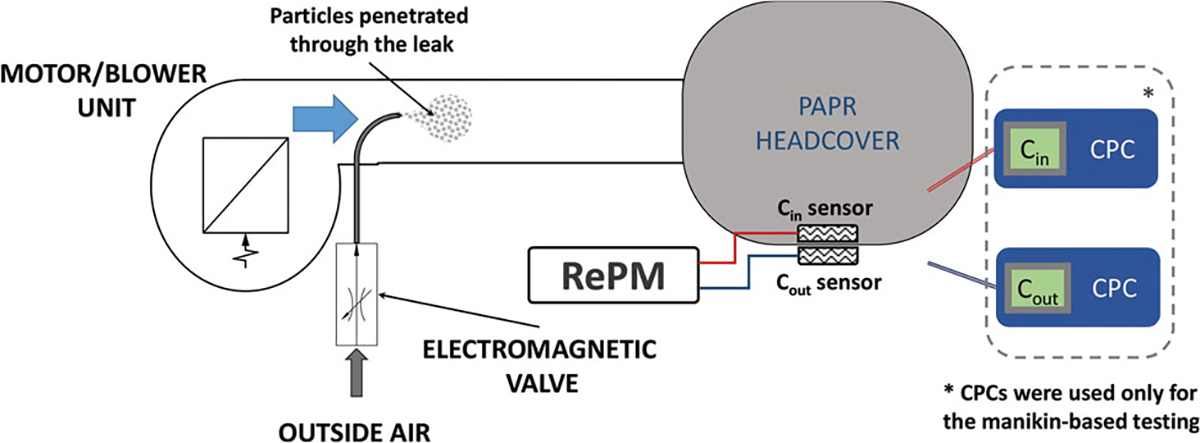
Schematic diagram of the leakage simulation. The reference CPC instruments were used in the manikin-based testing only, while the RePM was used with both manikin and human subject testing.
Manikin-based testing
A full-body standing manikin with the head connected to a breathing simulator (BRSS, Koken Ltd., Tokyo, Japan) was used in the first phase of the study. The connection line was equipped with a HEPA filter. It prevented return of the inhaled particles during the breathing cycle; it is also intended to “block” particles that could potentially be generated by the BRSS motor. The tests were conducted with the breathing simulator producing two sinusoidal breathing patterns with the following flow parameters:
“Low” – MIF = 30L min−1, frequency (f) = 0.25Hz, tidal volume (VT) = 1l) and
“High” – MIF = 135L min−1, f =.42Hz, VT = 2.7l.
The RePM sensor and the P-Trak pair were operating in parallel, and the protection factor was determined for each method. Subsequently, a relationship between mPFs measured with the RePM and P-Traks was established.
The mPF values were calculated in 6-sec time increments. The leak was established and the time intervals ΔtON and ΔtOFF were recorded based on the CPC data. Each test was repeated six times. The time between replicates was set to exceed the highest ΔtOFF measured.
The total number of runs in the manikin-based evaluation phase was 5 donnings × 6 replicates = 30. The entire 30-point ΔtON database was divided into three groups: ≤10 sec, ≤20 sec, ≤30 sec (no time interval obtained in the manikin-based tests exceeded 30 sec). For each ΔtON, we examined the response of P-Traks to the PAPR performance failure, as defined above (PF < APF), and quantified the number of True Positives (TP, occurred failure is correctly identified) and False Negatives (FN, occurred failure is missing). The P-Trak Sensitivity was calculated as
Additionally, the P-Trak response to the “no leakage” situation over a 60-sec interval was examined in 30 replicate tests and quantified by the number of True Negatives (TN) and False Positives (FP). The P-Trak Specificity was calculated as
The manikin-based study allowed us to develop the protocol for measuring the SWPF for human subjects with the reference (CPC-based) method. It also helped addressing the question whether the breathing regime affects the PF measured with the reference method.
Finally, working with the manikin provided us with the opportunity to make adjustments for operating the valve used to introduce the leakage for a PAPR donned on a human subject.
Human subject testing
Ten human subjects were recruited, consented, and medically cleared under an IRB-approved protocol.
In the course of the test, each subject wore a disposable headcover connected to the continuously operating modified PAPR motor/blower unit. During each exercise performed by a given subject, the leak was established and the time intervals ΔtON and ΔtOFF were recorded based on the RePM data. Each subject performed the set of five exercises as specified in Table 1 (the duration of each exercise was up to 6 min depending on ΔtON and ΔtOFF). Each test was repeated six times. By reviewing the ΔtOFF database, we verified our finding from the manikin-based tests regarding the time interval to be established between replicates. The total number of data points in the human subject evaluation phase was 10 subjects × 5 exercises × 6 replicates = 300.
Table 1.
Exercises used for human subject testing.
| Exercise | Description | Work Rate |
|---|---|---|
|
| ||
| 1. Breathing | Normal breathing while standing | Light |
| 2. Talking | Reading the “rainbow passage” while standing | Light |
| 3. Reaching up | Bending the left knee slightly while reaching up both arms overhead and looking up; stepping the right leg out to the side, then repeat with the other leg, at a rate of 12–15 sets min−1 | Moderate |
| 4. Walking with weight | Bending to pick up two weighted bags equipped with a handle (5 lb bag/each hand) and carrying them from side-to-side of the testing chamber at a moderate walking pace, then bending to put down weights | Hard |
| 5. Cardiopulmonary resuscitation (CPR) | Perform CPR on a training manikin by compressing chest cavity ~1.5 – 2 inches at a rate of 80–100 compressions min−1 | Hard |
The entire 300-point ΔtON database was divided into six groups: ≤10 sec, ≤20 sec, ≤30 sec, ≤40 sec, ≤50 sec, and ≤60 sec (only few time intervals exceeded 60 sec). Similar to the manikin-based tests conducted with CPCs, we examined the response of RePM to the PAPR performance failure for each ΔtON and quantified the number of True Positives (TP) and False Negatives (FN) to calculate the RePM’s Sensitivity. We also examined the RePM response to the “no leakage” situation over a 60-sec interval in 300 replicates and quantified by TN and FP to calculate the RePM’s Specificity.
Results
Manikin-based testing
The mPF values measured with the P-Trak units operating on the manikin with no leak established were mostly in a range of 5,000–10,000 for both “Low” and “High” breathing regimes. Once the leak was established, the PAPR’s mPF showed a slow decrease that later accelerates so that mPF eventually dropped below APF = 1,000. The P-Trak measurement correctly identified the leak in 23.5% of observations within ΔtON =10 sec, but the positive identification level increased to 64.7% for ΔtON = 20 s and reached 100% for ΔtON = 30 sec (Figure 2). There was no significant difference in the observations made under “Low” and “High” breathing regimes.
Figure 2.
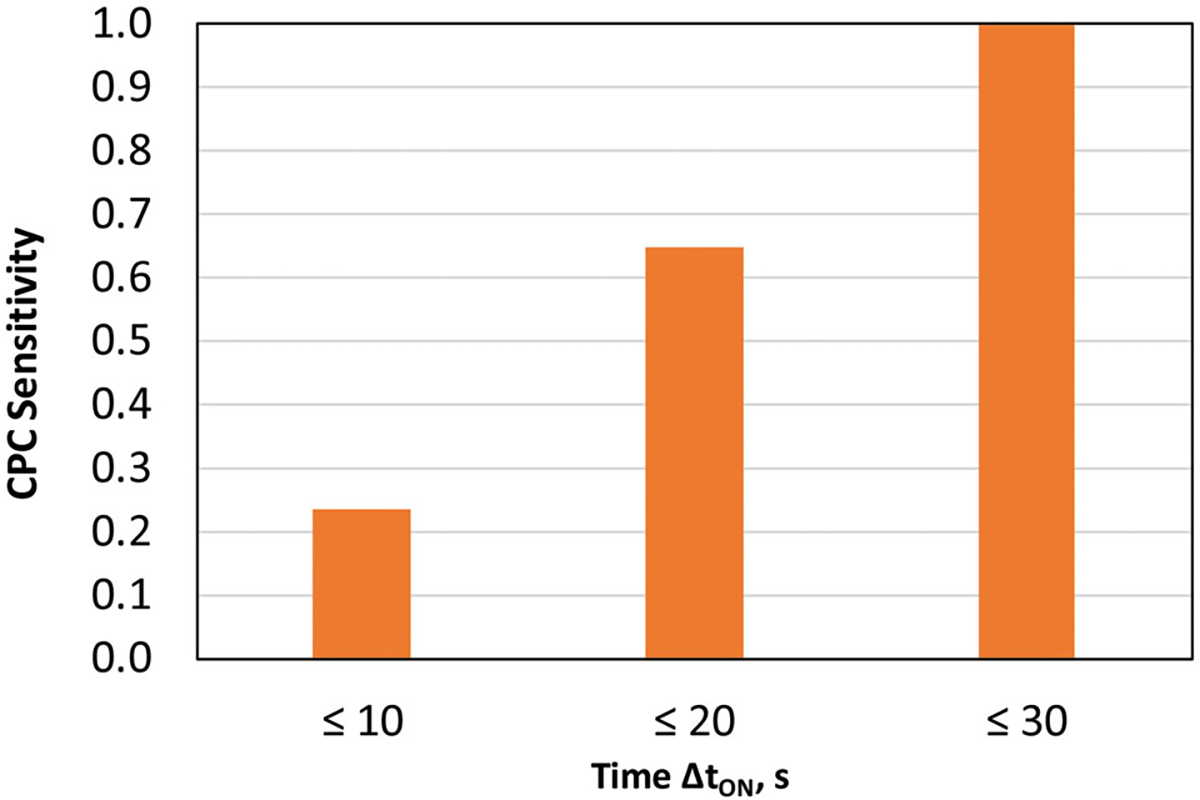
CPC leak identification sensitivity as a function of time ΔtON.
The mPFs measured with RePM vs. those determined with the two reference P-Traks at ΔtON = 30 sec are presented in Figure 3 as the geometric means and 95% confidence intervals (shown as error bars). A near-perfect correlation (with correlation coefficient of 0.99) was observed between the two data sets generated by the tested RePM and the reference CPCs. The correlation coefficient was 0.997. This high correlation is particularly remarkable since RePM and P-Trak measure in different particle size ranges. The data variability increased with increasing mPF because fewer particles were counted inside the respirator offering higher protection.
Figure 3.
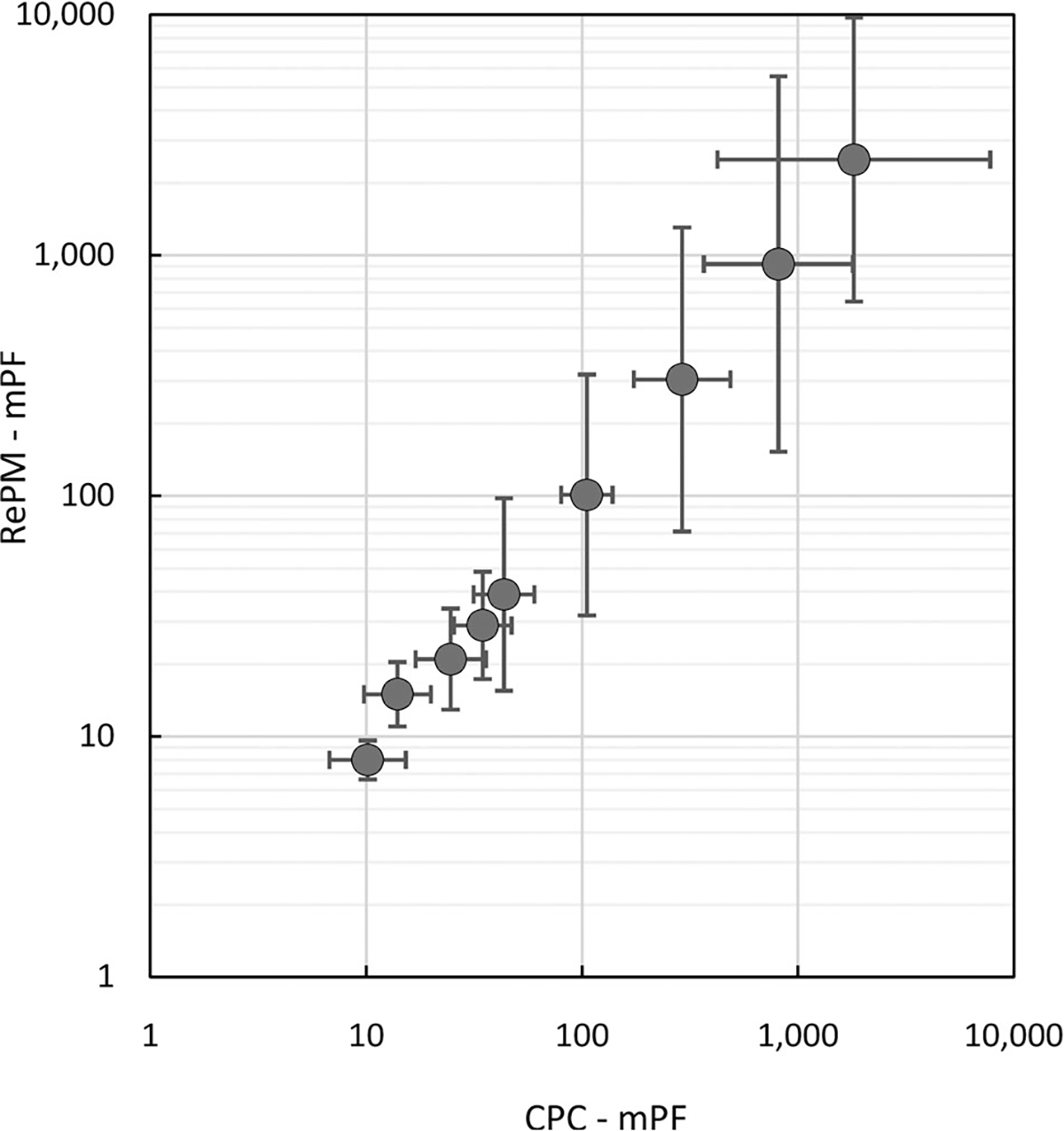
Manikin-based protection factors (mPF) measured with RePM vs. the mPFs determined with the two CPCs (reference) at ΔtON= 30 sec. Each point represents the geometric mean and the error bars represent a 95% confidence interval.
Finally, ΔtOFF was found to always be below 60 sec (100% of observations). This allowed us to conclude that the maximum time needed for the system restoration is 60 sec. Consequently, this interval appeared appropriate to be set between replicate tests.
Human subject testing
The PAPR unit operating normally on a human subject (with no leak established) provided SWPF values ranging approximately from 3,000 to > 10,000 as measured with the RePM. Once the leak was established and maintained over the time interval of ΔtON, the RePM color-code system produced the yellow signal constituting that the PAPR’s PF dropped below 1,000. With the aerosol concentrations measured using the RePM, the positive identification of the leakage (which is defined as the RePM Sensitivity) was found to occur in 32.7% of observations within ΔtON = 10 sec, 60.7% within 20 sec, 72.7% within 30 sec, 81.7% within 40 sec, 91.3% within 50 sec, and 96.3% of observations within 60 sec (Figure 4). As expected, the monitor’s Sensitivity increases as a longer time is allowed for the leak identification. Thus, the time interval can be selected depending on the preferred Sensitivity level. For instance, if a Sensitivity of 60.7% is deemed satisfactory, the RePM can provide it with a very short response time of 20 sec; however, to achieve the Sensitivity as high as 0.963, one should set a 60 s response for the monitor.
Figure 4.
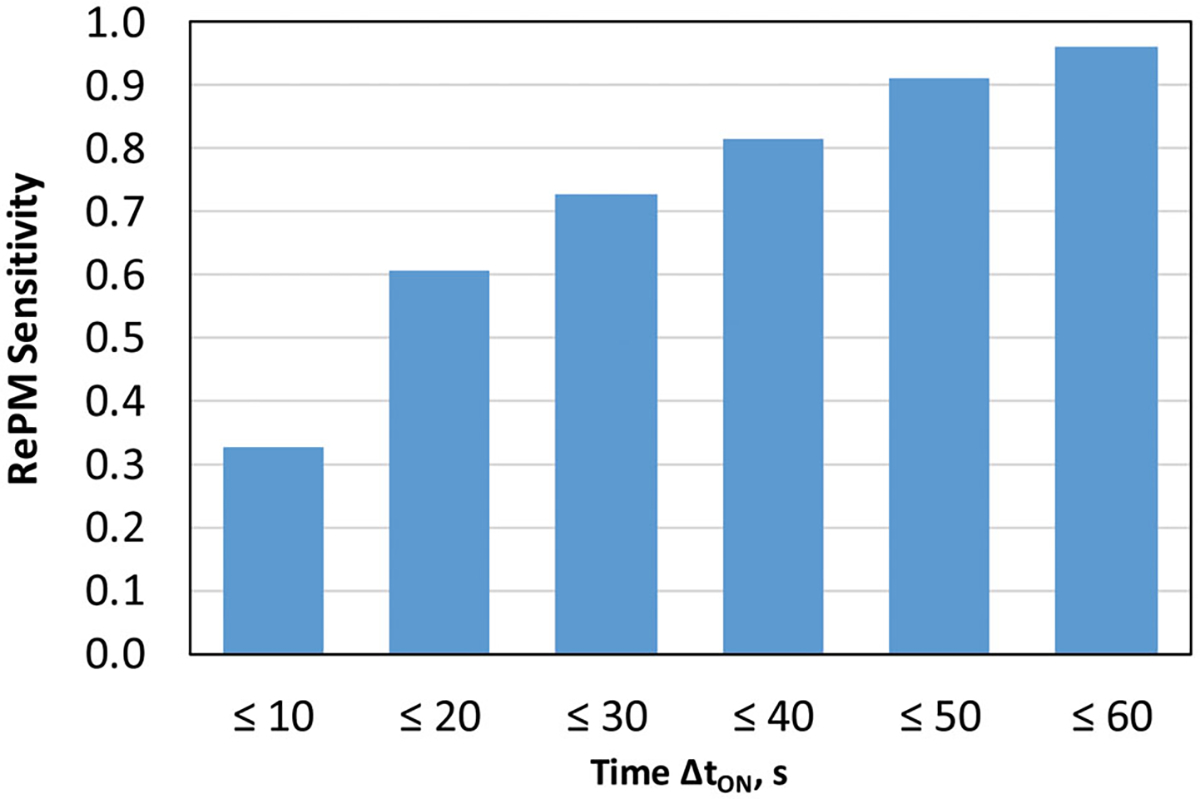
RePM leak identification sensitivity as a function of time ΔtON.
None of the 300 runs performed in absence of leak produced a false positive identification. Thus, the RePM was found to demonstrate a perfect Specificity (100%).
Discussion
Some quantitative differences in the leak identification characteristics between the P-Trak CPC and RePM obtained in this study can, at least partially, be attributed to the different detectible particle size ranges of these instruments, approximately 0.02 μm to 1 μm and ≥ 0.3 μm, respectively. The challenge aerosol used in this study is predominantly composed of particles below the optical size range. This suggests that a P-Trak is capable of providing a more rapid detection than the optical RePM since the latter detects only the fraction of larger particles counted by the former and there are fewer larger particles in the challenge aerosol. Thus, RePM is expected to require a longer time to achieve a countable particle number.
The data show that to achieve a perfect leak identification, the P-Trak CPC should be set at a response time of 30 sec while the actual response time of the instrument is as low as 1 sec and the data are recorded in 6-sec increments. Considering that the P-Trak used as the reference instrument in this study requires ΔtON=30 s to assure a 100% positive identification of the simulated PAPR failures, we believe that any device being tested against this instrument (such as RePM) should be allowed to operate with a response time of at least 30 sec. Therefore, it is feasible to calibrate an RePM for a response time between 30 and 60 sec. From the practical standpoint, the 60-sec interval appears a reasonable option to reach a rather high Sensitivity of 0.963 (<4% false negative identifications). This said, the acceptable Sensitivity threshold depends on the toxicity or infectious risk of the aerosol hazard being controlled.
The delay in detection by both systems compared to their nominal sampling rates can be attributed to a considerable “inertia” in the system. Indeed, the inner volume, Vin, for the PAPR is approximately 2.5–4 l (it varies due to between-subject and between-donning variabilities), which is an order of magnitude greater than that of a half-face elastomeric respirator. The greater Vin increases marginally the travel time from the leak’s location within the hose to the hood. Additionally, with the PAPR flow in the hose (large cross-section) being turbulent while the leak flow (much smaller cross-section) being laminar, a sizeable mixing takes place. At the point where the tube enters the cover, the flow mixing rate decreases. Lastly, the sampling location away from the path can help to further explain the observed delay. In short, it takes longer for the particles penetrating through a small leak to spread throughout the volume and the particle concentration gradient further slows down due to the positive pressure and continuous clean air supply inside the PAPR.
There is a need to account for noise in the individual sensors to prevent individual reading variations from generating false PF ratings. Averaging across multiple sensor readings reduces the impact of random fluctuations. This is done, however, at the expense of increased time between leak event and detection. The latter contributes to the delays observed in RePM detection compared to that of the two P-Trak CPCs.
When applied to human subject testing, setting the RePM response time to 60 sec resulted in a near 100% Sensitivity due to a small fraction of observations (11 out of 300 cases) that required more than 60 sec to assure a positive response. These observations appeared to be outliers. The potential causes for this behavior are varied and worth considering in future experimentation and design efforts.
Future investigations may also address the choice of challenge aerosol. The present study was conducted with a generated salt aerosol within a certain concentration range, and it is yet to be determined whether the nature and concentration of ambient aerosol affect the Sensitivity and Specificity of RePM. The other factor that can affect the RePM performance is the ambient airflow in the subject’s vicinity. The wind speed was shown to impact the fit of early PAPR designs (Cecala et al. 1981). Additionally, considering that different PAPR models utilize different flowrate options, it would be useful to examine if the RePM performance depends on the PAPR flowrate. Finally, the novel concept evaluated in this study should be further validated in the field under real workplace conditions.
Conclusion
Overall, based on the data collected in this study, we concluded that the novel RePM can be successfully utilized for a quantitative real-time performance evaluation of PAPRs in workplace settings. It provides Sensitivity of 0.963 (if a 60-sec response time is chosen) and Specificity of 1.
Funding
This effort was funded by: the National Institute for Occupational Safety and Health (Contract No. 75D30119P04260).
References
- CDC. 2020. Considerations for optimaizing the supply of powered air-purifying respirators (PAPRs). Coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention; [accessed on 2020 April 25]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/powered-air-purifying-respirators-strategy.html. [Google Scholar]
- Cecala AB, Volkwein JC, Thimons ED, Urban CW. 1981. Protection factors of the airstream helmet. Bureau of Mines Report No. 8591; p. 10. https://www.cdc.gov/niosh/nioshtic-2/10002165.html.
- Cohen HJ, Hecker LH, Mattheis DK, Johnson JS, Biermann AH, Foote KL. 2001. Simulated workplace protection factor study of powered air-purifying and supplied air respirators. AIHAJ. 62(5):595–604. doi: 10.1080/15298660108984658 [DOI] [PubMed] [Google Scholar]
- Gao S, McKay RT, Yermakov M, Kim J, Reponen T, He X, Kimura K, Grinshpun SA. 2016. Performance of an improperly sized and stretched-out loose-fitting powered air-purifying respirator: manikin-based study. J Occup Environ Hyg. 13(3):169–176. doi: 10.1080/15459624.2015.1098780 [DOI] [PubMed] [Google Scholar]
- Leppäanen M, Wu B, Corey J, Yermakov M, Grinshpun SA. 2018. Performance of a novel real-time respirator seal integrity monitor on firefighters: simulated workplace pilot study. J Occup Environ Hyg. 15(8):607–615. doi: 10.1080/15459624.2018.1479065 [DOI] [PubMed] [Google Scholar]
- Liu Y, Corey J, Yermakov M, Wu B, Grinshpun SA. 2018. Preliminary development of a real-time respirator seal integrity monitor with low-cost particle sensor. IEEE Trans Ind Appl. 54(4):3928–3933. doi: 10.1109/TIA.2018.2816907 [DOI] [Google Scholar]
- Liverman C, Domnitz S, McCoy M. 2014. The use and effectiveness of powered air purifying respirators (PAPRs) in health care. Washington, DC: Institute of Medicine of the National Academies of Science. [Google Scholar]
- Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T, Levie J, McQueen J, Smith S, Moss L, et al. 2004. SARS among critical care nurses, Toronto. Emerging Infect Dis. 10(2):251–255. doi: 10.3201/eid1002.030838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey KR, Johnson AT, Scott WH, Koh FC. 2005. Over breathing a loose-fitting PAPR. J Int Soc Respir Protect. 22:1–10. [Google Scholar]
- Myers W, Peach MJ 3rd, Cutright K, Iskander W. 1984. Workplace protection factor measurements on powered air-purifying respirators at a secondary lead smelter: results and discussion. Am Ind Hyg Assoc J. 45(10): 681–688. doi: 10.1080/15298668491400449 [DOI] [PubMed] [Google Scholar]
- Myers WR, Peach MJ 3rd, Cutright K, Iskander W. 1986. Field test of powered air purifying respirators at a battery manufacturing facility. J Int Soc Respir Protect. 4(1):62–89. [Google Scholar]
- Nicas M, Harrison R, Charney W, Borwegan B. 2004. Respiratory protection and severe acute respiratory syndrome. J Occup Environ Med. 46(3):195–197. doi: 10.1097/01.jom.0000116808.46760.06 [DOI] [PubMed] [Google Scholar]
- OSHA. 2006. Assigned protection factors: final rule, 29 CFR parts 1910, 1915 and 1926. Federal Register, 71 FR 50122–50192. August 24. [Google Scholar]
- Vo E, Zhuang Z, Horvatin M, Liu Y, He X, Rengasamy S. 2015. Respirator performance against nanoparticles under simulated workplace activities. ANNHYG. 59(8): 1012–1021. doi: 10.1093/annhyg/mev042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizner K, Stradtman L, Novak D, Shaffer R. 2016. Prevalence of respiratory protective devices in U.S. health care facilities: implications for emergency preparedness. Workplace Health Saf. 64(8):359–368. doi: 10.1177/2165079916657108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Corey J, Yermakov M, Liu Y, Grinshpun SA. 2018. Laboratory evaluation of a novel real-time respirator seal integrity monitor. Ann Work Expo Health. 62(6): 742–753. doi: 10.1093/annweh/wxy026 [DOI] [PubMed] [Google Scholar]


