Abstract
End-stage liver disease is a rare but fatal complication of non-alcoholic fatty liver disease (NAFLD). In NAFLD, insulin resistance, which is clinically defined as the impairment of insulin’s ability to maintain glucose homeostasis, is associated with perturbations in insulin action that promote triglyceride accumulation, such as increasing de novo lipogenesis. However, the key step in the development of end-stage liver disease is not the accumulation of triglycerides, but hepatocyte injury. Whether and how triglycerides promote hepatocyte injury remains unclear. Consequently, it is difficult to predict whether drugs designed to reduce hepatic triglycerides will prevent the most important complications of NAFLD.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, insulin resistance, lipid metabolism
The Role of Triglycerides in Non-alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD), a disorder of insulin resistance, has increased to epidemic proportions, posing an urgent need for new therapies [1]. The minimal criterion for the diagnosis of NAFLD is the accumulation of triglycerides in >5% of hepatocytes [2]. Thus, triglyceride accumulation is central to NAFLD development, and strategies to lower triglycerides are being pursued. Here, we will discuss how insulin resistance promotes triglyceride accumulation, the mechanisms by which triglycerides may injure the hepatocyte, and the complexities of targeting triglycerides for NAFLD treatment.
NAFLD Therapy: Goals and Challenges
A reasonable question to ask, before considering how to treat NAFLD, is why, or even whether, to treat NAFLD. Because NAFLD is associated with an increased risk of developing diabetes, cardiovascular disease, and chronic kidney disease, it has been suggested that NAFLD treatment could have beneficial effects on cardiometabolic disease [3, 4]. However, whether the underlying associations are causative versus correlative is not yet known, and even the associations themselves have been challenged [5]. Moreover, an extensive armamentarium of proven therapeutics already exists, at least for cardiovascular disease and diabetes, mitigating the need for new, NAFLD-based therapies for these disorders.
Another, more compelling, reason to treat NAFLD is to prevent its hepatic complications, end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC). Admittedly, these complications are rare: a recent meta-analysis estimated a liver-specific mortality rate of 0.8 per thousand person years for NAFLD, and 11.8 for non-alcoholic steatohepatitis (NASH), a more aggressive subset of NAFLD, marked by hepatocyte ballooning, inflammation, and fibrosis [6]. However, ESLD and HCC are often fatal and therapeutic options are limited largely to liver transplantation. Given the significant risks of surgery and immunosuppression as well as the scarcity of donor livers, a liver transplant is not ideal, or even feasible, in many patients. Thus, in contrast to cardiovascular disease and diabetes, options for patients with ESLD and HCC are limited and often ineffective. Furthermore, though the hepatic complications of NAFLD are rare, the number of individuals with NAFLD is staggering. NAFLD affects 25% of the population worldwide [1]. Even 7.6% of children and 18.8% of lean individuals are affected [7, 8]. Moreover, the prevalence of NAFLD is continuing to rise, and current models predict that the proportion of individuals with NAFLD will increase to 33% by 2030 [9].
The challenge in developing NAFLD therapies has not been a shortage of candidate targets or even drugs, but difficulties in testing these drugs. Because NAFLD is diagnosed histologically, testing requires a biopsy--a piece of liver collected by a large bore needle and scored by a pathologist. Biopsies pose several challenges. First, there is a risk of bleeding, occurring in approximately 10% of subjects, and even death, though exceedingly rare [10]. Consequently, serial biopsies are not often possible, making it difficult to closely monitor drug effects. Second, the biopsy represents only 1/50,000 of the liver, and because NAFLD is not a homogenous disease, this introduces sampling error [11]. In one study, two simultaneous biopsies from the same individual were discordant for inflammation in 47% of cases, and Mallory bodies, a feature of ballooned hepatocytes, in 84% of cases [12]. In another study, the amount of hepatocyte injury present in two simultaneous biopsies taken from the same individual showed almost no correlation at all [13]. Third, scoring is subjective, particularly with regard to inflammation and injury: the level of agreement between two pathologists scoring the same slide for inflammation and injury, though dependent upon the expertise of the pathologists, is weak, and even the agreement of scores between the same pathologist scoring the same slide on two different occasions is only moderate [12, 13].
Another difficulty in testing NAFLD drugs is that NAFLD does not progress via a steady, linear course. Steatosis can develop and resolve quickly. A single day of fasting can increase hepatic triglycerides by 10-fold in mice [14]. In parallel, new data in humans show that a shift to an isocaloric, but low carbohydrate diet, for even one day, is sufficient to significantly reduce liver fat, while a shift for two weeks can reduce liver fat by 44% [15]. Similarly, modest insults to the liver just before a biopsy could potentially produce transient damage or inflammation in the setting of obesity and steatosis: for example, 2 g of acetaminophen increased liver transaminase levels within 24 hours in morbidly obese individuals but not lean controls [16]. Thus, short term, modest changes in diets and medications before a biopsy could complicate NAFLD drug testing.
However, the greatest challenge in testing NAFLD drugs is simply the protracted time course of the disease. It can take decades for individuals with NAFLD to develop cirrhosis, ESLD, or HCC. Therefore, drugs are being provisionally approved based on their ability to improve NAFLD histology. However, the extent to which NAFLD histology predicts outcomes, particularly liver outcomes, is not clear. Indeed, the best predictor of NAFLD progression appears to be fibrosis, but even fibrosis can wax and wane [17].
These challenges highlight the need for a clear mechanistic understanding of NAFLD development and its progression to ESLD and HCC. Given how many biopsies and years are required to determine whether a given drug can actually prevent the hepatic complications of NAFLD, picking the right drug target is essential for success.
Insulin Resistance and the Accumulation of Triglycerides in NAFLD
Insulin resistance is one of the few factors consistently associated with the development and progression of NAFLD [18]. To understand how insulin resistance promotes NAFLD, it is first necessary to consider normal insulin signaling, and to disentangle two conflated concepts, molecular and clinical insulin resistance.
Insulin is best understood as the orchestrator of the feeding response: it transitions the body from the fasted, nutrient-scarce state that consumes endogenous energy stores, to the fed, nutrient-abundant state that generates endogenous energy stores. During fasting, triglycerides from the adipose tissue are lipolyzed to free fatty acids, which are released to the liver. The liver uses these free fatty acids to fuel gluconeogenesis; it also re-esterifies them into triglycerides which are complexed with apolipoprotein B (ApoB) and secreted as very low-density lipoprotein (VLDL) particles; VLDL triglycerides can be used for energy by other tissues in the body [19].
Upon feeding, insulin is secreted. Insulin suppresses adipose tissue lipolysis, inhibits gluconeogenesis, and promotes ApoB degradation. In parallel, it stimulates the storage of energy as glycogen and lipids. Finally, consistent with its role in signaling nutrient abundance, insulin stimulates cell growth and proliferation [20–22].
Insulin exerts its effects by binding its receptor and activating a complex signaling cascade which exists in virtually every cell. A key node in this cascade is the kinase, AKT. AKT has multiple targets, including mammalian target of rapamycin complex 1 (mTORC1), which it activates, and forkhead box O1 (FOXO1), which it suppresses. In the liver, mTORC1 promotes lipogenic gene expression and cell growth but suppresses fatty acid oxidation and ApoB secretion [23–29]. On the other hand, FOXO1 promotes gluconeogenesis but suppresses tumorigenesis [30].
Insulin resistance in the molecular sense is any defect in normal insulin signaling, in any tissue. Defects can occur at the level of the insulin receptor, affecting all insulin targets, or downstream of the receptor, affecting only some insulin targets. If insulin resistance occurs downstream of the insulin receptor, the phenotype produced will depend on which signaling molecule is impaired. For example, in the liver, knockout of FoxO1 disrupts gluconeogenic rather than lipogenic gene expression, whereas knockout of AKT disrupts both gluconeogenic and lipogenic gene expression [24, 31, 32].
In addition, it is important to recognize that insulin is not the sole factor that controls metabolism. For example, the metabolite acetyl-CoA can increase hepatic glucose production independently of insulin [26]. Acetyl-CoA inhibits the enzyme pyruvate dehydrogenase, which oxidizes pyruvate, and activates pyruvate carboxylase, which converts pyruvate to a gluconeogenic precursor, thereby shunting pyruvate towards gluconeogenesis. Similarly, carbohydrates have some ability to induce lipogenic gene expression in the liver, even in the complete absence of insulin signaling [14].
Insulin resistance in the clinical sense is much more narrowly defined. It is measured as the ability of insulin to maintain glucose homeostasis. The gold standard for measuring insulin resistance is the hyperinsulinemic-euglycemic clamp, in which insulin is infused at a high, constant rate, and glucose is infused at variable rate to prevent plasma glucose levels from falling. The amount of glucose required to maintain normoglycemia can be used to infer insulin sensitivity. Surrogate markers of insulin resistance, such as plasma glucose, plasma insulin or some combination of these, like the homeostatic model assessment of insulin resistance (HOMA-IR), are also used [33–35]. However, all of these clinical measurements only assess insulin’s effects on glucose metabolism; they do not assess insulin’s effects on lipid metabolism, or other processes, such as proliferation, which may be critical for the development of HCC.
It is clear now that the different actions of insulin are not uniformly impaired with the development of NAFLD [36, 37]. Because assessing insulin signaling and lipid metabolism, particularly in the liver, is difficult in humans, our models of insulin resistance are largely built on in vitro and mouse studies of insulin action, and correlated, when possible, with deep phenotyping of small numbers of individuals. In current NAFLD models, insulin fails to suppress the release of fatty acids from the adipocyte, leading to an increase in free fatty acid flux to the liver, which has two effects. First, free fatty acids, which are a substrate for triglyceride synthesis, promote triglyceride accumulation, independently of hepatic insulin signaling [38]. Second, free fatty acids promote glucose production by increasing levels of acetyl-CoA [26]. At the same time, mTORC1 remains activated in the liver, promoting lipogenesis while suppressing fatty acid oxidation. Thus, insulin resistance in the adipose tissue coupled with the activation of mTORC1 and potentially other insulin-stimulated pathways in the liver, are thought to act together to increase both gluconeogenesis and lipogenesis in NAFLD.
The current model is supported by human studies showing that insulin loses its ability to suppress adipose tissue lipolysis [39, 40] in NAFLD and that de novo lipogenesis is increased [41, 42]. In addition, fatty acid oxidation and VLDL secretion tend to be elevated, but these changes are variable between studies [43].
The Role of Triglycerides in Hepatocyte Injury
When viewed through the lens of preventing ESLD, the key pathophysiological event in NAFLD progression is not the accumulation of triglycerides, but the injury to the hepatocyte, raising the question of how triglycerides cause hepatocyte damage. The most straightforward possibility is that triglycerides themselves are toxic to the hepatocyte, but it is difficult to imagine how these inert lipid species promote injury. It is also inconsistent with the finding that many individuals with NAFLD develop significant triglyceride accumulation without signs of hepatocyte injury.
A second possibility is that triglycerides indirectly promote hepatocyte injury by fueling the production of “lipotoxins”. In this model, with origins in the two-hit hypothesis proposed by James and Day [44], triglycerides provide substrates for the generation of lipotoxic molecules such as free fatty acids, lysophosphatidic acid, lysophosphatidylcholine, lysophosphatidylinositol, diglycerides (also known as diacylglycerols or DAGs), and ceramides (reviewed in [45]). However, which of these lipids, if any, causes hepatocyte damage is not clear. For example, diglycerides can perturb insulin signaling and promote insulin resistance; however, their ability to drive hepatocyte injury and ballooning are untested [46, 47].
A third possibility is that triglycerides accumulate in parallel with the true toxins that drive hepatocyte injury. For example, those individuals who develop insulin resistance and steatosis may have greater exposure to endotoxins such as lipopolysaccharides, due to defects in the epithelial barrier of the intestine [48, 49]. In this case, lipopolysaccharides, which trigger inflammation and cell death, may be the true drivers of hepatocyte injury, and triglycerides may simply be a marker of lipopolysaccharide exposure.
Finally, and potentially most importantly, it should be noted that triglyceride accumulation is also a non-specific response of the hepatocyte to a range of injuries. Many hepatotoxic drugs, like amiodarone, perhexiline, valproate, and tamoxifen can cause steatosis, as can infection with Hepatitis C [50–54]. Surprisingly, kwashiorkor, a disease of severe malnutrition, at the opposite end of the nutritional spectrum as NAFLD, is also associated with steatosis [55]. The propensity of the injured hepatocyte to accumulate triglycerides is perhaps explained by the fact that hepatocytes metabolize a large amount of fatty acids: any disruption in hepatocyte function could potentially produce a secondary increase in triglycerides. For example, injury to the mitochondria and a reduction in fatty acid oxidation could underlie the steatosis induced by hepatotoxic drugs, whereas limiting amino acids, dysfunction of the cellular secretory apparatus, and a defect in VLDL-triglyceride secretion could underlie the steatosis of kwashiorkor [50, 51, 56–59].
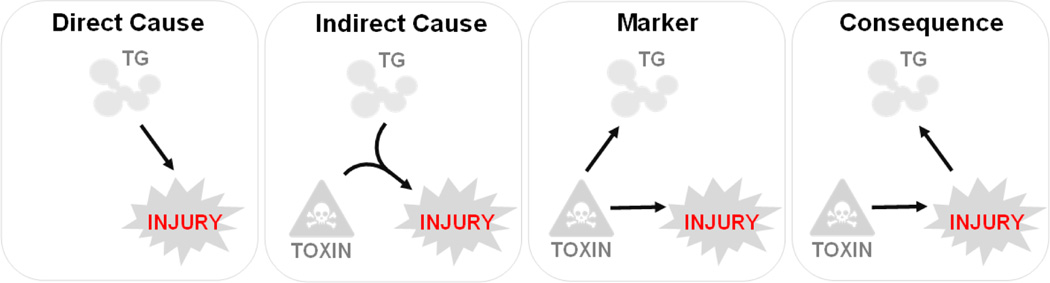
Thus, triglycerides could be a cause, consequence or mere marker of hepatocyte injury (Figure 1). Currently, the lipotoxicity model is favored. This model is supported by several lines of evidence. First, most genetic (for example, deletion of NADPH oxidase) and environmental (for example, excess dietary cholesterol) interventions in mice that produce hepatocyte injury do so only in the presence of high fat feeding [60, 61]. Second, in interventional studies to date, the resolution of NAFLD has been associated with a reduction in steatosis [61]. Finally, Mendelian randomization studies support a causative role for triglyceride accumulation in the development of NASH [62].
FIGURE 1. Triglycerides and hepatocyte injury in NAFLD.
There are four potential relationships between triglyceride accumulation and hepatocyte injury. First, triglyceride accumulation could drive hepatocyte injury (Direct Cause). Second, triglycerides, triggered by a “second hit”, could be metabolized into a lipotoxin that drives injury (Indirect Cause). Third, a triglyceride accumulation could be secondary to hepatotoxin-driven injury (Consequence). TG, triglycerides.
Reducing Triglycerides to Treat NAFLD: Promises and Perils
Since triglyceride metabolism has been studied for decades, there is a long list of enzymes that could be targeted to reduce hepatic triglycerides. Consequently, triglyceride lowering has become a popular approach to NAFLD treatment. However, several caveats exist.
First, such a strategy is contingent upon triglycerides being a cause rather than a marker or consequence of hepatocyte injury. Though the data, in general, support the notion that triglycerides indirectly promote hepatocyte injury, this may not be true in all cases and at all times in the course of the disease. Indeed, in the later stages of NAFLD, fibrosis is inversely associated with steatosis, making it unlikely that triglycerides are the major driver of hepatocyte injury at that point [63]. An important but unanswered question, therefore, is whether triglycerides are important drivers of injury in the subset of NAFLD patients that go on to develop ESLD. It seems plausible that the subset of patients who develop ESLD may have some occult toxin exposure that drives injury, and that triglyceride accumulation is a consequence of that injury.
Second, despite an extensive knowledge of triglyceride metabolism, intervention studies continue to reveal surprising gaps in our knowledge. For example, acetyl-CoA carboxylase (ACC) enzymes generate malonyl-CoA, which serves both as a substrate for lipogenesis and an inhibitor of fatty acid oxidation. Thus, inhibitors of the ACC enzymes would be predicted to reduce lipogenesis, increase fatty acid oxidation, and lower hepatic triglycerides. Indeed, ACC inhibition reduced hepatic triglycerides by 36% in humans. ACC inhibition also had the unexpected beneficial effect of reducing hepatic stellate cell activation and reducing fibrosis in mice [64]. Unfortunately, it also had the unexpected detrimental effect of increasing VLDL secretion and plasma triglycerides in mice and humans [65].
The targeting of diacylglycerol O-acyltransferase 2 (DGAT2) also held surprises. DGAT2 converts diglycerides, which promote insulin resistance, to triglycerides. Deletion of hepatic DGAT2 was expected to reduce triglycerides but increase diglycerides and therefore insulin resistance. Indeed, knockdown, knockout, and inhibition of DGAT2 reduced liver triglycerides [46, 66, 67]. However, they surprisingly reduced diglyceride levels [46, 66], pointing to complex feedback loops that remain incompletely understood, with unpredicted results.
Third, it is possible that individuals may vary in the defects in triglyceride metabolism that they manifest. For example, individuals with NAFLD due to a polymorphism in Patatin-like phospholipase domain-containing protein 3 (PNPLA3), which is associated with increased risk of cirrhosis, do not have an increase in de novo lipogenesis [68]. Thus, these individuals might have a blunted response to inhibitors of lipogenesis.
Finally, any drug used to treat NAFLD must be exceedingly safe. If less than 10% of individuals with NAFLD ultimately develop liver-related morbidity, more than 90% of NAFLD patients will be treated unnecessarily. Moreover, NAFLD progresses over many years, and could require treatment over decades. This is particularly relevant for obeticholic acid, which is one of the most effective NAFLD drugs thus far. Obeticholic acid activates the nuclear receptor FXR, which induces Fibroblast Growth Factor 19 (FGF19) [69]. While FGF19 appears to have beneficial effects on glucose and lipid metabolism, it also has a proliferative effect. Indeed, FGF19 signaling is a driver of HCC, and treatments to inhibit FGF19 signaling are among the most promising therapies for HCC [70–72]. Though the risk-to-benefit ratio of obeticholic acid must ultimately be determined empirically, it remains conceivable that this therapy may promote the very disease it is meant to prevent.
Concluding Remarks
Triglyceride lowering seems to be a reasonable approach, but much work remains ahead (see Outstanding Questions). To definitively prove the lipotoxic hypothesis—that triglycerides promote NAFLD via the production of a lipotoxin--we must identify the lipotoxin, show it can cause hepatocyte damage (ballooning and inflammation) at the levels found in NASH livers, and show that reducing it can prevent or reverse NASH. Such work could also lead to specific therapies targeting the enzymatic processes that synthesize the lipotoxin. It will also be important to determine how triglyceride metabolism differs between individuals and through the course of NAFLD and NASH, as this could enable us to define the specific subsets of patients in whom a particular drug would be most successful.
Outstanding Questions
How does insulin’s effects on hepatocyte growth and proliferation change with the development of insulin resistance?
Do triglycerides promote hepatocyte injury in the subset of patients who ultimately develop end-stage liver disease?
What are the key lipotoxic species that promote hepatocyte injury?
How does triglyceride metabolism change through the course of NAFLD?
Can diabetes drugs that reduce steatosis prevent the hepatic complications of NAFLD?
Highlights
New therapies are necessary to prevent hepatocellular carcinoma and end-stage liver disease, the infrequent but devastating complications of NAFLD.
Insulin resistance in NAFLD is not uniform. Insulin appears to lose its ability to suppress hepatic glucose production and adipose tissue lipolysis, but maintains its ability to stimulate hepatic lipogenesis.
The key pathophysiological event in NAFLD is hepatocyte injury, which can be either the cause or the consequence of triglyceride accumulation.
Though triglyceride reduction is a logical approach to NAFLD treatment, a basic, mechanistic understanding of NAFLD pathogenesis is essential for identifying the best targets and strategies.
In the interim, drugs to treat diabetes may prove an alternative approach to reducing hepatic triglycerides. The advantage of these drugs is that they are already in the clinic, and could more rapidly provide proof-of-concept that triglyceride lowering reduces the hepatic complications of NAFLD. Indeed, the thiazolidinedione pioglitazone lowers hepatic triglycerides, ballooning, and inflammation, while improving liver function, and the glucagon-like peptide 1 (GLP-1) analogue liraglutide reduces steatosis and fibrosis [73–75]. In this case, it would be important to determine how these drugs impact insulin effects on not only glucose metabolism, but lipid metabolism, hepatocyte growth and proliferation. That is, all diabetes drugs lower glucose levels, but how well they reverse the molecular insulin resistance associated with NAFLD is not clear. The ideal drug, in terms of triglyceride metabolism, will promote the suppression of adipose tissue lipolysis by insulin, but not the activation of lipogenesis or inhibition of fatty acid oxidation by insulin.
In summary, the alarming increase in NAFLD prevalence has forced us to develop treatments without the benefit of a clear understanding of the pathophysiology of this disease (see Outstanding Questions). A similar predicament occurred in the field of cardiovascular disease: cardiovascular disease is associated with both high levels of LDL cholesterol and low levels of HDL cholesterol; though drugs to normalize LDL were wildly successful, drugs to normalize HDL were a great disappointment [76–78]. The study of NAFLD is complicated by our reliance on liver biopsies, the complex nature of its pathogenesis, and its protracted course. Nonetheless, continued effort to define the pathophysiology of NAFLD is essential for developing effective therapies for this widespread disorder.
Acknowledgements
We apologize that space limitations precluded our ability to properly recognize our colleagues and their important contributions to this field. We are grateful to Stuart Adamson for discussion. Dr. Semova is supported by the American Diabetes Association (grant 9-18-CVD1-003); Dr. Biddinger is supported by the NIH National Heart, Lung, and Blood Institute (HL109650).
Footnotes
Disclaimer Statement
The opinions presented here are those of the authors. No official support or endorsement of any treatment is intended, nor should it be inferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younossi ZM, et al. , Epidemiology of chronic liver diseases in the USA in the past three decades. Gut, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM, et al. , Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol, 1999. 94(9): p. 2467–74. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, et al. , Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology, 2006. 44(4): p. 865–73. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, et al. , Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism, 2020: p. 154170. [DOI] [PubMed] [Google Scholar]
- 5.Alexander M, et al. , Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. Bmj, 2019. 367: p. l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, et al. , Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology, 2016. 64(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, et al. , Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore), 2012. 91(6): p. 319–27. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z, et al. , Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology, 2019. 69(6): p. 2672–2682. [DOI] [PubMed] [Google Scholar]
- 9.Estes C, et al. , Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology, 2018. 67(1): p. 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Midia M, et al. , Predictors of bleeding complications following percutaneous image-guided liver biopsy: a scoping review. Diagn Interv Radiol, 2019. 25(1): p. 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbantoglu IL and Brunt EM, Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol, 2014. 20(27): p. 9026–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratziu V, et al. , Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology, 2005. 128(7): p. 1898–906. [DOI] [PubMed] [Google Scholar]
- 13.Merriman RB, et al. , Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology, 2006. 44(4): p. 874–80. [DOI] [PubMed] [Google Scholar]
- 14.Haas JT, et al. , Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab, 2012. 15(6): p. 873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen VS, et al. , Variation in diagnostic NAFLD/NASH read-outs in paired liver samples from rodent models. J Pharmacol Toxicol Methods, 2020. 101: p. 106651. [DOI] [PubMed] [Google Scholar]
- 16.van Rongen A, et al. , Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin Pharmacokinet, 2016. 55(7): p. 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiner DE, et al. , Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open, 2019. 2(10): p. e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchesini G, et al. , Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes, 2001. 50(8): p. 1844–1850. [DOI] [PubMed] [Google Scholar]
- 19.Haas ME, Attie AD, and Biddinger SB, The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab, 2013. 24(8): p. 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaya MJ, et al. , The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology, 2014. 59(1): p. 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Dusseault J, and Larose L, Nck1 depletion induces activation of the PI3K/Akt pathway by attenuating PTP1B protein expression. Cell Commun Signal, 2014. 12: p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam R, et al. , Insulin induces phosphorylation of pyruvate dehydrogenase through RhoA activation pathway in HepG2 cells. FASEB J, 2019. 33(2): p. 2072–2083. [DOI] [PubMed] [Google Scholar]
- 23.Peterson TR, et al. , mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell, 2011. 146(3): p. 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Titchenell PM, et al. , Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab, 2016. 23(6): p. 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titchenell PM, et al. , Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun, 2015. 6: p. 7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry RJ, et al. , Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell, 2015. 160(4): p. 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Brown MS, and Goldstein JL, Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc.Natl.Acad.Sci.U.S.A, 2010. 107(8): p. 3441–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta S, et al. , mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature, 2010. 468(7327): p. 1100–1104. [DOI] [PubMed] [Google Scholar]
- 29.Sidiropoulos KG, et al. , Insulin inhibition of apolipoprotein B mRNA translation is mediated via the PI-3 kinase/mTOR signaling cascade but does not involve internal ribosomal entry site (IRES) initiation. Arch Biochem Biophys, 2007. 465(2): p. 380–8. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, et al. , Polydatin inhibits hepatocellular carcinoma via the AKT/STAT3-FOXO1 signaling pathway. Oncol Lett, 2019. 17(5): p. 4505–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan M, et al. , Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab, 2011. 14(4): p. 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling AV, et al. , FoxO1 Is Required for Most of the Metabolic and Hormonal Perturbations Produced by Hepatic Insulin Receptor Deletion in Male Mice. Endocrinology, 2018. 159(3): p. 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye FZ, et al. , Homeostatic model assessment of insulin resistance closely related to lobular inflammation in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol, 2020. 32(1): p. 80–86. [DOI] [PubMed] [Google Scholar]
- 34.Van de Velde F, et al. , Insulin resistance associates with hepatic lobular inflammation in subjects with obesity. Endocr Connect, 2019. 8(9): p. 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii H, et al. , HOMA-IR: An independent predictor of advanced liver fibrosis in nondiabetic non-alcoholic fatty liver disease. J Gastroenterol Hepatol, 2019. 34(8): p. 1390–1395. [DOI] [PubMed] [Google Scholar]
- 36.Biddinger SB, et al. , Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab, 2008. 7(2): p. 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MS and Goldstein JL, Selective versus total insulin resistance: a pathogenic paradox. Cell Metab, 2008. 7(2): p. 95–96. [DOI] [PubMed] [Google Scholar]
- 38.Vatner DF, et al. , Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci U S A, 2015. 112(4): p. 1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanyal AJ, et al. , Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology, 2001. 120(5): p. 1183–92. [DOI] [PubMed] [Google Scholar]
- 40.Bril F, et al. , Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology, 2017. 65(4): p. 1132–1144. [DOI] [PubMed] [Google Scholar]
- 41.Lambert JE, et al. , Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology, 2014. 146(3): p. 726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith GI, et al. , Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest, 2020. 130(3): p. 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCullough A, Previs S, and Kasumov T, Stable isotope-based flux studies in nonalcoholic fatty liver disease. Pharmacol Ther, 2018. 181: p. 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day CP and James OF, Steatohepatitis: a tale of two “hits”? Gastroenterology, 1998. 114(4): p. 842–5. [DOI] [PubMed] [Google Scholar]
- 45.Musso G, et al. , Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology, 2018. 155(2): p. 282–302 e8. [DOI] [PubMed] [Google Scholar]
- 46.Choi CS, et al. , Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem, 2007. 282(31): p. 22678–88. [DOI] [PubMed] [Google Scholar]
- 47.Kumashiro N, et al. , Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A, 2011. 108(39): p. 16381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thuy S, et al. , Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr, 2008. 138(8): p. 1452–5. [DOI] [PubMed] [Google Scholar]
- 49.Zampino R, et al. , Endotoxinemia contributes to steatosis, insulin resistance and atherosclerosis in chronic hepatitis C: the role of pro-inflammatory cytokines and oxidative stress. Infection, 2018. 46(6): p. 793–799. [DOI] [PubMed] [Google Scholar]
- 50.Fromenty B, et al. , Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J Pharmacol Exp Ther, 1990. 255(3): p. 1371–6. [PubMed] [Google Scholar]
- 51.Deschamps D, et al. , Inhibition by perhexiline of oxidative phosphorylation and the beta-oxidation of fatty acids: possible role in pseudoalcoholic liver lesions. Hepatology, 1994. 19(4): p. 948–61. [PubMed] [Google Scholar]
- 52.Zhao F, et al. , The effect and mechanism of tamoxifen-induced hepatocyte steatosis in vitro. Int J Mol Sci, 2014. 15(3): p. 4019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai X, et al. , Valproate induced hepatic steatosis by enhanced fatty acid uptake and triglyceride synthesis. Toxicol Appl Pharmacol, 2017. 324: p. 12–25. [DOI] [PubMed] [Google Scholar]
- 54.Moriya K, et al. , Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol, 1997. 78 ( Pt 7): p. 1527–31. [DOI] [PubMed] [Google Scholar]
- 55.van Zutphen T, et al. , Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol, 2016. 65(6): p. 1198–1208. [DOI] [PubMed] [Google Scholar]
- 56.Kaufmann P, et al. , Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology, 2005. 41(4): p. 925–35. [DOI] [PubMed] [Google Scholar]
- 57.Flores H, et al. , Lipid transport in kwashiorkor. Br J Nutr, 1970. 24(4): p. 1005–11. [DOI] [PubMed] [Google Scholar]
- 58.Seakins A and Waterlow JC, Effect of a low-protein diet on the incorporation of amino acids into rat serum lipoproteins. Biochem J, 1972. 129(3): p. 793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishi H, et al. , Importance of Serum Amino Acid Profile for Induction of Hepatic Steatosis under Protein Malnutrition. Sci Rep, 2018. 8(1): p. 5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mari M, et al. , Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab, 2006. 4(3): p. 185–198. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Ruiz I, et al. , NADPH oxidase is implicated in the pathogenesis of oxidative phosphorylation dysfunction in mice fed a high-fat diet. Sci Rep, 2016. 6: p. 23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dongiovanni P, et al. , Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med, 2018. 283(4): p. 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rastogi A, et al. , Non-alcoholic fatty liver disease - histological scoring systems: a large cohort single-center, evaluation study. APMIS, 2017. 125(11): p. 962–973. [DOI] [PubMed] [Google Scholar]
- 64.Bates J, et al. , Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J Hepatol, 2020. [DOI] [PubMed] [Google Scholar]
- 65.Kim CW, et al. , Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab, 2017. 26(2): p. 394–406 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gluchowski NL, et al. , Hepatocyte Deletion of Triglyceride-Synthesis Enzyme Acyl CoA: Diacylglycerol Acyltransferase 2 Reduces Steatosis Without Increasing Inflammation or Fibrosis in Mice. Hepatology, 2019. 70(6): p. 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amin NB, et al. , Targeting diacylglycerol acyltransferase 2 for the treatment of nonalcoholic steatohepatitis. Sci Transl Med, 2019. 11(520). [DOI] [PubMed] [Google Scholar]
- 68.Mancina RM, et al. , Paradoxical dissociation between hepatic fat content and de novo lipogenesis due to PNPLA3 sequence variant. J Clin Endocrinol Metab, 2015. 100(5): p. E821–5. [DOI] [PubMed] [Google Scholar]
- 69.Inagaki T, et al. , Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab, 2005. 2(4): p. 217–225. [DOI] [PubMed] [Google Scholar]
- 70.Miura S, et al. , Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer, 2012. 12: p. 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joshi JJ, et al. , H3B-6527 Is a Potent and Selective Inhibitor of FGFR4 in FGF19-Driven Hepatocellular Carcinoma. Cancer Res, 2017. 77(24): p. 6999–7013. [DOI] [PubMed] [Google Scholar]
- 72.Weiss A, et al. , FGF401, A First-In-Class Highly Selective and Potent FGFR4 Inhibitor for the Treatment of FGF19-Driven Hepatocellular Cancer. Mol Cancer Ther, 2019. 18(12): p. 2194–2206. [DOI] [PubMed] [Google Scholar]
- 73.Petit JM, et al. , Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab, 2017. 102(2): p. 407–415. [DOI] [PubMed] [Google Scholar]
- 74.Belfort R, et al. , A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med, 2006. 355(22): p. 2297–307. [DOI] [PubMed] [Google Scholar]
- 75.Armstrong MJ, et al. , Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet, 2016. 387(10019): p. 679–90. [DOI] [PubMed] [Google Scholar]
- 76.Goldstein JL and Brown MS, The LDL receptor. Arterioscler.Thromb.Vasc.Biol, 2009. 29(4): p. 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taheri H, et al. , Cholesteryl Ester Transfer Protein Inhibitors and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cardiology, 2020. 145(4): p. 236–250. [DOI] [PubMed] [Google Scholar]
- 78.Barter PJ, et al. , Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007. 357(21): p. 2109–22. [DOI] [PubMed] [Google Scholar]