Abstract
Background and Objectives
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause a wide range of neurologic complications; however, its neuropenetrance during the acute phase of the illness is unknown.
Methods
Extracellular vesicles were isolated from brain biopsy tissue from a patient undergoing epilepsy surgery using ultracentrifugation and analyzed by Western blot and qPCR for the presence of virus protein and RNA, respectively. Biopsy tissue was assessed by immunohistochemistry for the presence of microvascular damage and compared with 3 other non-COVID surgical epilepsy brain tissues.
Results
We demonstrate the presence of viral nucleocapsid protein in extracellular vesicles and microvascular disease in the brain of a patient undergoing epilepsy surgery shortly after SARS-CoV-2 infection. Endothelial cell activation was indicated by increased levels of platelet endothelial cell adhesion molecule-1 and was associated with fibrinogen leakage and immune cell infiltration in the biopsy tissue as compared with control non-COVID surgical epilepsy brain tissues.
Discussion
Despite the lack of evidence of viral replication within the brain, the presence of the nucleocapsid protein was associated with disease-specific endothelial cell activation, fibrinogen leakage, and immune cell infiltration.
Reports of neurologic complications of COVID-19 are common and can occur during acute infection or develop postinfection as a chronic illness.1 The mechanism by which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can contribute to neurologic complications is still an active area of investigation with 2 prevailing hypotheses, (1) direct infection of the CNS by the virus or (2) indirect damage mediated by immune responses to peripheral infection. Here, we report the detection of the SARS-CoV-2 nucleocapsid (N) protein isolated from brain tissue from a patient with epilepsy who recovered from COVID-19.
Methods
This 27-year-old woman had an 11-year history of multidrug resistant epilepsy. She had stereotypical focal impaired awareness seizures with a cephalic aura and feeling of anxiety followed by behavioral arrest with oral automatisms, at times progressing to bilateral tonic clonic seizures. She had no seizure risk factors or other medical illnesses aside from depression controlled with escitalopram. EEG and FDG-PET hypometabolism suggested a left temporal seizure focus. Brain MRI showed only mild hippocampal volume loss. Intracranial subdural and depth electrodes showed typical seizures from the left mesial temporal region. Four days after electrode placement, she developed a fever (38.1 °C), pharyngitis, headache, and fatigue. Despite a negative screening test on admission, SARS-CoV-2 PCR as an inpatient was positive. For several days, she experienced fevers and dry cough which resolved. She did not report myalgias, anosmia, ageusia, or difficulty breathing. Seventeen days after symptom onset, she underwent surgical resection of her left anterior temporal lobe. She was discharged 4 days after surgery and has remained seizure-free since surgery (Engel Class-1) with no reported subjective changes in language or memory function during follow-up visits. She was unvaccinated for SARS-CoV-2 because she was admitted in December 2020 before the availability of vaccines. She was most likely infected with the delta variant (see eMethods for detailed clinical history, links.lww.com/WNL/C526). Written informed consent was obtained from the patient.
The brain tissue excised during the temporal lobectomy was fixed in formalin. Standard pathologic examination demonstrated the presence of hippocampal sclerosis. The tissue was also immunostained for SARS-CoV-2 nucleocapsid antigen, platelet endothelial cell adhesion molecule (PECAM-1), CD68, TMEM119, GFAP, CD3, CD4, CD8, and CD20. Extracellular vesicles were isolated from frozen brain tissue and analyzed by PCR and by Western blotting for SARS-CoV-2 nucleocapsid (eMethods in the Supplement). The SARS-CoV-2 N antibody was validated using 293T cells transfected with a SARS-CoV-2 N plasmid as a positive control and a SARS-CoV-2 S plasmid as a negative control. The histopathologic findings were compared with resected tissue from 3 other patients who underwent epilepsy surgery (see eTable 1 for details, links.lww.com/WNL/C526).
Results
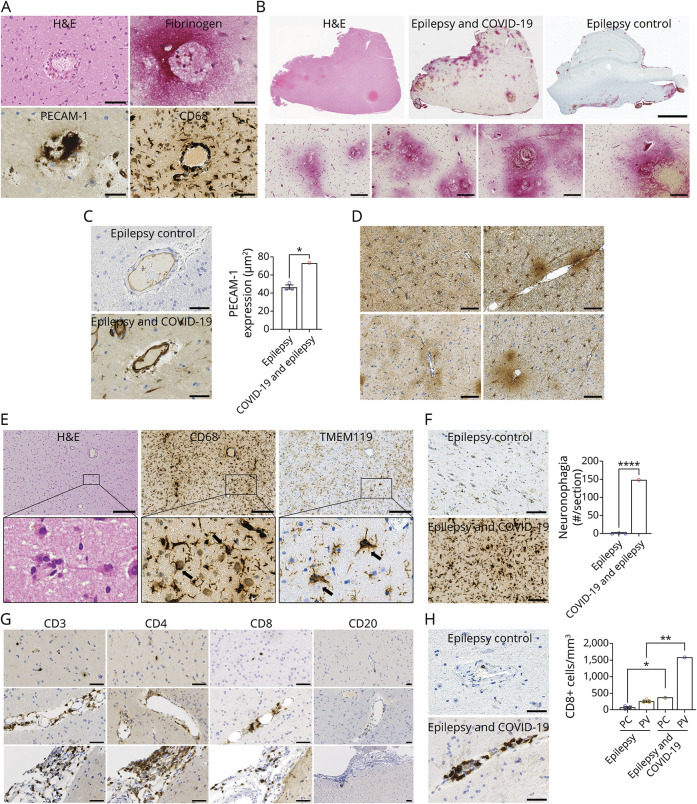
Immunostaining of the temporal cortical tissue from the patient was performed along with tissue from 3 patients with epilepsy without COVID-19 and 2 with similar preoperative subdural and depth electrode placements. The perivascular region from the patient showed immune cell infiltrates, fibrinogen leakage, increased PECAM-1 expression, and CD68 infiltrates (Figure 1A). Strong diffuse fibrinogen immunostaining was localized to the perivascular area. The control cases showed no immunostaining (Panel B). There was a significant increase in PECAM-1 expression in the COVID-19–infected patient compared with control tissues (Panel C). The perivascular region had astrocyte activation (Panel D). Numerous neurons were found to be surrounded by microglial cells in the brain of the patient, considered to represent neuronophagia (Panel E), and the number of the CD68+ microglial nodules was significantly increased compared with control tissues (Panel F). Panel G shows lymphocytic infiltrates (CD3+, CD4+, and CD8+) in the parenchyma (top row), perivascular region (middle row), and meninges (bottom row). CD20+ cells were absent from all locations. Quantitation of CD8+ cells showed a significant increase in the number of positive cells in the parenchyma and perivascular regions as compared with controls (Panel H). A limitation of this study is the lack of control tissue from patients with another cause of viral respiratory illness.
Figure 1. Neuropathology of SARS-CoV-2 Infection in a Patient With Epilepsy.
(A) H&E and immunostaining (fibrinogen, PECAM-1, and CD68) of perivascular region of temporal cortical biopsy from a patient with COVID-19. Scale bars are 50 µm. (B) H&E and fibrinogen immunostaining of the biopsy tissues from the patient and fibrinogen immunostaining of control tissue. Bottom 4 panels are representative images of observed perivascular fibrinogen leakage. Scale bars are 3 mm (upper) 100 µm (lower). (C) PECAM-1 expression was significantly greater in the patient compared with uninfected controls and statistically different (*p = 0.0270). For quantification, PECAM-1 levels were assessed using ImageScope software (Leica Biosystems). One hundred small blood vessels per section were selected. The area of the entire vessel and lumen area were measured by tracing the contours of the entire vessel and lumen within the vessel. PECAM-1 expression area was determined by subtracting luminal area from entire vessel area. To exclude the effect of vessel size, the PECAM-1 area was normalized by dividing it by the blood vessel area. Data represent mean areas of PECAM-1 expression (µm2). Scale bars are 50 µm. (D) GFAP immunostaining of the perivascular regions in the patient with COVID-19. Scale bars are 100 µm. (E) Neurons surrounded by microglia clusters were shown in H&E, CD68, and TMEM119-stained COVID-19 patient's biopsy tissue. Scale bars are 200 µm. (F) Clusters of CD68+ microglia surrounding neurons (neuronophagia) were absent in the brain of controls but present in the brain of patient. The number of foci of neuronophagia was significantly increased in the brain of patient compared with controls (p < 0.0001). Quantification of neuronophagia was performed by counting the number of CD68+ clusters. Values were expressed by the average number of foci of neuronophagia per section (#/section). Scale bars are 100 µm. (G) Immunostaining of the parenchyma (top row), perivascular region (middle row), and meninges (bottom row) for CD3, CD4, CD8, and CD20 in the patient with COVID-19. Scale bars are 50 µm. (H) The CD8+ T cells were present in greater numbers in the brain of patient, predominantly in the perivascular (PV) regions. The number of CD8+ T cells were significantly increased in both parenchymal (*p = 0.0344) and perivascular (**p = 0.0023) regions of patient compared with controls. For quantification of CD8+ cells, 50 regions of each stained brain section were randomly selected, and the number of positive cells was counted. Each area was 0.4 mm2. Values represented the average number of cells per volume (#/mm3). Scale bars are 50 µm. Antibodies used for staining: fibrinogen (Abcam, ab58207), PECAM-1 (Leica Biosystems, CD31-607-L-CE), CD68 (Thermo Fisher Scientific, 14-0688-82), TMEM119 (Novus Biologicals, CL8714), GFAP (Dako, Z0334), CD3 (Leica Biosystems, CD3-565-L-CE), CD4 (Leica Biosystems, CD4-368-L-CE), CD8 (Leica Biosystems, CD8-4B11-L-CE), and CD20 (Leica Biosystems, CD20-L26-L-CE). PECAM = platelet endothelial cell adhesion molecule-1
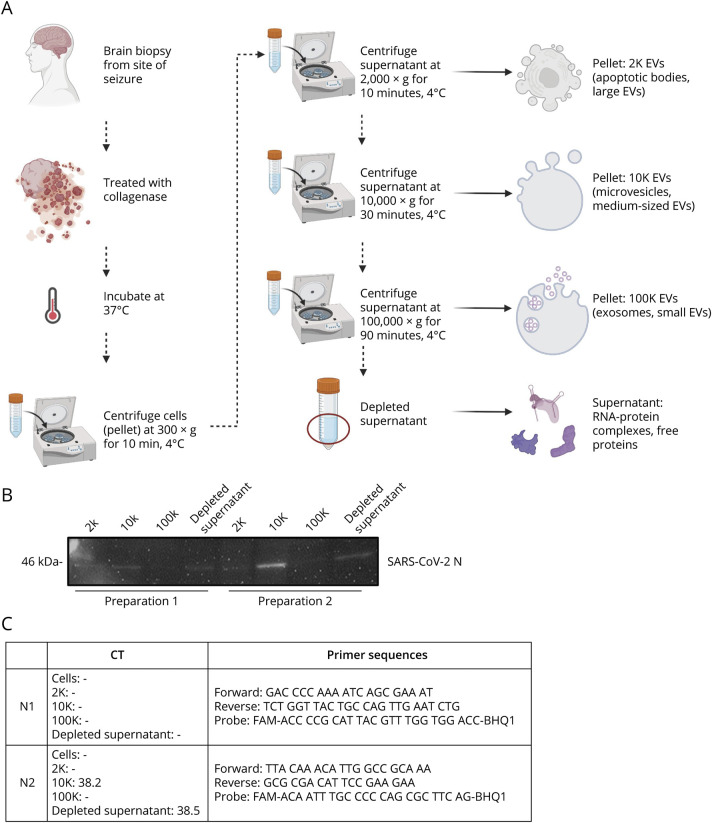
Western blot of extracellular vesicle fractions (Figure 2A) showed the presence of SARS-CoV-2 nucleocapsid protein in the 2K, 10K, and depleted supernatant in 2 independent EV preparations from the same tissue (Panel B). Quantitative PCR analysis demonstrated that the 10K and depleted supernatant fractions were positive for SARS-CoV-2 RNA in 2 independent EV preparations using the N2 primer set. All samples were undetectable by the N1 primer set in both replicates, yielding an inconclusive result (Panel C). Viral RNA was undetectable in the dissociated cell pellet by both primer sets. Immunostaining of the biopsy tissue was negative for cell-associated SARS-CoV-2 nucleocapsid protein. Viral culture from the tissue was not attempted.
Figure 2. SARS-CoV-2 Nucleocapsid Can Be Detected in Extracellular Vesicles Isolated From Brain Tissue.
(A) Isolation of extracellular vesicle subpopulations from fresh frozen brain biopsy. A piece of fresh frozen temporal cortex was gently dissociated, and the supernatant-containing vesicles found in the extracellular space was subjected to differential ultracentrifugation to yield 4 fractions: 2K (2000×g), 10K (10,000×g), 100K (100,000×g), and depleted supernatant. Created with BioRender.com. (B) Western blot analysis of 2 independent vesicle preparations to detect the presence of SARS-CoV-2 nucleocapsid (N) protein. (C) RT-qPCR analysis of dissociated cells and vesicle fractions is shown as average CT values from 2 independent vesicle preparations analyzed in technical triplicate along with corresponding CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel N1 and N2 primer probe sets according to manufacturer's protocol. Samples were considered positive when Ct values were less than 40 and reproduced in 2 different vesicle preparations.
Discussion
This patient provided a unique opportunity to study the CNS viral infection in the very early stages of the illness in the absence of respiratory compromise. The absence of new neurologic symptoms, unusual changes on EEG, and complete recovery after surgery suggests the absence of disseminated viral encephalitis. However, the histopathologic changes of activation of endothelial cell, leakage of fibrinogen in the perivascular space, predominant macrophage infiltration with CD8 lymphocytes, and perivascular glial cell activation with neuronophagia were typical of what has been described in autopsy cases of SARS-CoV-2 infection.2 We and others have been unable to detect viral antigen by immunostaining,2,3 but access to fresh tissue allowed us to detect the viral RNA and protein in extracellular vesicles. The lack of cell-associated N protein and viral RNA indicates the absence of an active infection in the resident CNS cells and suggests the detected viral products originated in the periphery. The detection of nucleocapsid protein and viral RNA together in the 10K and depleted supernatant fractions and their absence from the 100K fraction suggests the lack of an infectious virion in the CNS. This points to the incorporation of viral products into EVs and the presence of nucleocapsid as a free protein or an RNA-protein complex.
We have previously shown that there is deposition of immune complexes on the endothelial cells leading to compromise of vascular integrity.4 This may lead to deposition of circulating exosomes with viral protein and RNA as well as fibrinogen leakage and activation/infiltration of immune cells.5 It is possible that these microvascular changes are common with SARS-CoV-2 infection but are transient in most patients. Persistence of these findings may result in long-term neurologic complications as described in patients with post-COVID syndrome.1
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study Funding
This research was supported by the Division of Intramural Research of the NIH, NINDS (NS3130). The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
All authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Nath A. Neurologic manifestations of severe acute respiratory syndrome coronavirus 2 infection. CONTINUUM: Lifelong Learn Neurol. 2021;27(4):1051-1065. doi: 10.1212/con.0000000000000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with covid-19. N Engl J Med. 2021;384(5):481-483. doi: 10.1056/nejmc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565-571. doi: 10.1038/s41586-021-03710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MH, Perl DP, Steiner J, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145(7):2555-2568. doi: 10.1093/brain/awac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Y, Lei T, Patel PS, et al. Direct activation of endothelial cells by SARS-CoV-2 nucleocapsid protein is blocked by simvastatin. J Virol. 2021;95(23):e0139621. doi: 10.1128/jvi.01396-21 [DOI] [PMC free article] [PubMed] [Google Scholar]