Abstract
Background and Objectives
There is increasing interest in characterizing the earliest phases of Parkinson disease (PD). However, few studies have investigated prediagnostic trajectories of cognition and function. Our objective was to describe prediagnostic cognitive and functional trajectories in PD in older women and men.
Methods
We studied 9,595 women and 5,795 men from 2 prospective cohort studies of community-dwelling elders followed up to 20 years. In individuals without prevalent PD, we estimated the associations of incident PD diagnosis with rates of change in cognition and function before and after diagnosis compared with healthy older adults using multivariate mixed-effects models.
Results
Over follow-up, 297 individuals developed incident PD. Interactions between the terms in our model and sex were statistically significant for the 3 outcomes (p < 0.001 for all), so we stratified results by sex. Compared with older men without PD, men who developed PD exhibited faster decline in global cognition (0.04 SD more annual change, p < 0.001), executive function (0.05 SD more annual change, p < 0.001), and functional status (0.06 SD more annual change, p < 0.001) in the prediagnostic period. Women who developed PD compared with women without PD displayed faster decline in executive function (0.02 SD more annual change, p = 0.006) and functional status in the prediagnostic period (0.07 SD more annual change, p < 0.001).
Discussion
Individuals with incident PD exhibit cognitive and functional decline during the prediagnostic phase that exceeds rates associated with normal aging. Better understanding heterogeneity in prodromal PD is essential to enable earlier diagnosis and identify impactful nonmotor symptoms in all subgroups.
Parkinson disease (PD) is a currently incurable, progressive disorder that is rising in prevalence.1,2 PD is diagnosed when individuals develop the typical motor symptoms. However, up to half of dopamine-containing neurons are lost before diagnosis3 due to years of preceding neurodegeneration.4 Nonmotor features such as dysautonomia, sleep disruption, and neuropsychiatric changes are increasingly appreciated as an integral part of the disease and typically predate the onset of motor symptoms.5 To enable earlier diagnosis, improve recognition and management of symptoms, and facilitate recruitment of patients into neuroprotective interventions, there is growing interest in further characterizing the earliest, premotor stages of PD. There is also little understanding of heterogeneity of prodromal symptoms in different demographic groups, such as men and women, despite evidence for sex differences in diagnosed PD.6-8
Previously considered a symptom of more advanced disease or an indication of the diagnosis of Lewy body dementia, cognitive changes are now recognized even in newly diagnosed individuals with PD.9-12 Cognitive and functional changes are likely to be an early manifestation of neurodegeneration, characterized by the accumulation of misfolded alpha-synuclein in structures outside of the substantia nigra.13 Cohorts of individuals at risk for PD due to the presence of REM sleep behavior disorder, hyposmia, or genetic risk factors provide evidence for early changes in executive function, global cognition, and attention.14-17 However, there have been few population-based studies evaluating trajectories of prediagnostic function using repeated cognitive and functional measures.18 Women have been underrepresented in PD research19 to date, so heterogeneity in prodromal changes by sex is largely unknown. Better understanding the prodromal phase of PD and its heterogeneity through prospective studies has been identified as a high research priority to identify at-risk individuals as prior therapeutic interventions may have failed due to administration too late in the disease course.20
Therefore, our aim was to determine the prediagnostic trajectories of the cognitive and functional changes among those with incident PD in 2 large, prospective aging cohort studies of community-dwelling men and women.
Methods
Participants
The Study of Osteoporotic Fractures (SOF) is a prospective cohort study of aging that enrolled 9,704 community-dwelling women aged 65 years and older at baseline between 1986 and 1988 from 4 sites in the United States (Minneapolis, MN; Portland, OR; Baltimore, MD; and the Monongahela Valley, PA).21 Participants were identified through mailings to community-based listings (e.g., health maintenance organizations and voter registration lists). Participants underwent 8 evaluations over 20 years of follow-up.
The Osteoporotic Fractures in Men Study (MrOS) is a related prospective cohort study of aging that enrolled 5,994 community-dwelling men aged 65 years and older from 2000 to 2002 from 6 sites in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; the Monongahela Valley, PA; Portland, OR; and San Diego, CA).22 Recruitment methods included mailings using community and provider contact lists, newspaper advertisements, and presentations to elderly individuals.23 Participants underwent 12 study evaluations over 17 years and are currently being followed. From 2003 to 2005, 3,135 men were recruited into an ancillary sleep study and underwent 2 additional visits with cognitive measurements. These 2 studies used comparable eligibility criteria and measures.
Cognitive Assessments
In the SOF study, a modified version of the Mini-Mental Status Examination was performed at baseline and years 5, 8, 10, and 16. This global assessment of cognition includes questions evaluating orientation, concentration, language, praxis, and immediate and delayed memory. Total scores range from 0 (worst cognitive function) to 26 (best cognitive function).24 Trails B was used to measure attention, executive function, sequencing, and visual scanning25 and was administered shortly after baseline and then at years 5, 10, and 16. Trails B is scored in seconds needed to complete the task, with slower times representing worse cognitive function.
All MrOS participants were administered 2 cognitive tests at baseline and years 5, 7, and 14. The Modified Mini-Mental State Examination (3 MS)26 assesses global cognition with questions on orientation, concentration, language, praxis, and immediate and delayed memory and is scored 0 (worst cognition) to 100 (best cognition).26 Trails B was also administered at these time points. The ancillary sleep study participants underwent additional cognitive assessments at 3.5 and 9.5 years.
Functional Assessments
Participants in both studies completed an interviewer administered questionnaire on 3 instrumental activities of daily living (housework, shopping, and meal preparation) and 2 measures of physical function (climbing stairs and walking 2–3 blocks outside on level ground). Participants rated their difficulty on a Likert scale, and this was used to calculate a total score from 0 (least functional difficulty) to 20 (most functional difficulty).27 Women completed the questionnaire at baseline and years 2, 5, 8, 10, and 16. Men completed this at baseline and years 5, 7, 9, and 14 with additional measurements at years 3.5 and 9.5 for the ancillary study.
Ascertainment of Incident PD
In both studies, participants were repeatedly queried about a physician diagnosis of PD at baseline and follow-up visits. At every visit, participants were asked about medications they took in the last 30 days for PD (carbidopa/levodopa, pramipexole, ropinirole, benztropine, selegiline, amantadine, entacapone, rivastigmine, or trihexyphenidyl in both studies, with the addition of rotigotine, and rasagiline in MrOS), which were matched to the Iowa Drug Information Service Drug Vocabulary. Date of PD diagnosis was defined as the first visit where a participant reported a physician diagnosis of PD. For the main analysis, we defined PD diagnosis as those self-reporting a physician diagnosis of PD. In a sensitivity analysis, we defined PD diagnosis as those who self-reported both a physician diagnosis of PD and use of PD medications.
Other Measurements
Age, race, sex, educational level, tobacco and alcohol use, and medical history were self-reported at baseline. Body mass index (kg/m2) was calculated based on direct height and weight measurements at baseline. To assess depression, participants in both studies completed the Geriatric Depression Scale either at baseline or shortly after baseline.28 This well-validated assessment is scored from 0 to 15, with higher scores correlating with more severe symptoms of depression and score ≥6 coded as likely clinical depression. Cardiovascular risk factors and disease (diabetes, stroke, and high blood pressure) were ascertained at baseline in both studies. Participants were considered to have baseline coronary artery disease if they reported a prior diagnosis of congestive heart failure, angina, or myocardial infarction at their first visit.
Statistical Analysis
Differences between participants who did and did not develop incident PD were compared using analysis of variance and χ2 tests. To make cognitive assessments comparable, raw scores were z-transformed to mean 0 and SD 1, and signs were modified so that negative values represent worse cognition and positive values represent better cognition. To investigate associations of PD diagnosis with the rate of changes in cognition and function, we estimated mixed-effects models for repeated measures data, with random intercepts and slopes to account for within-subject correlation of the responses. Model 1 included a term for time in years since study start, the interaction between time and PD status, and time in years after PD diagnosis (set to zero before diagnosis). Model 2 included these terms and additionally adjusted for age, education, race, cardiovascular risk factors and disease, depression, tobacco use, and alcohol use. We also tested for interactions between the terms in our model and sex. Given the statistically significant interactions between the terms in our model and sex (p ≤ 0.0001), we reported all results as stratified by sex. We also plotted outcome trajectories, estimated using 4-knot cubic splines, against time since study entry, as well as against time since PD diagnosis for the incident PD group. Between-group comparisons of the slopes were done using χ2 tests for differences in the first derivative of the cubic spline, evaluated at 5-year intervals. All analyses were performed using STATA statistical software.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided written informed consent. The studies were approved by institutional review boards at each clinical site and the coordinating center at UCSF. These analyses followed reporting guidelines specified by Strengthening in Reporting of Observational Studies in Epidemiology.
Data Availability
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Results
Sample Characteristics
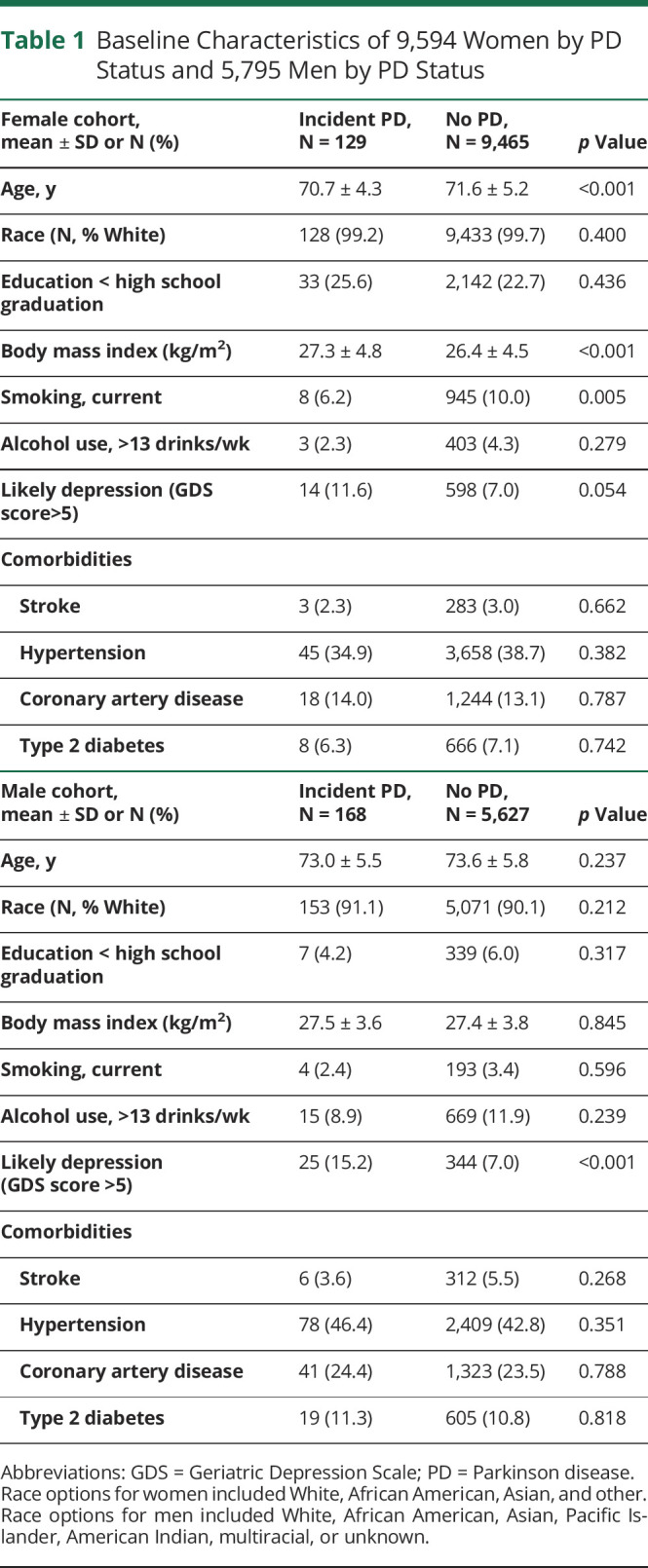
Our analytic sample included 9,594 women from SOF and 5,795 men from MrOS (selection of the sample is summarized in eFigure 1, links.lww.com/WNL/C585). We excluded individuals with prevalent PD or missing cognitive or functional data at baseline. In the SOF study, participants were followed for a maximum of 22 years (mean 6.3 years ±SD 5.4), and 129 women (1.3%) developed incident PD. Participants in MrOS were followed up to 17 years (mean 4.9 years ±SD 4.0), and 168 men (2.8%) developed incident PD. Compared with women without incident PD, women with incident PD were younger (70.7 ± 4.3 compared with 71.6 ± 5.2; p < 0.001), less likely to report current smoking (6.2% vs 10.0%; p = 0.005), and showed a trend toward endorsing more depressive symptoms (11.6% vs 7.0%; p = 0.054) (Table 1) Men with incident PD were more likely to endorse symptoms of depression compared with men without incident PD (15.2% vs 7.0%; p < 0.001) (Table 1).
Table 1.
Baseline Characteristics of 9,594 Women by PD Status and 5,795 Men by PD Status
Longitudinal Effect of Incident PD Before and After Diagnosis on Rate of Change in Cognition
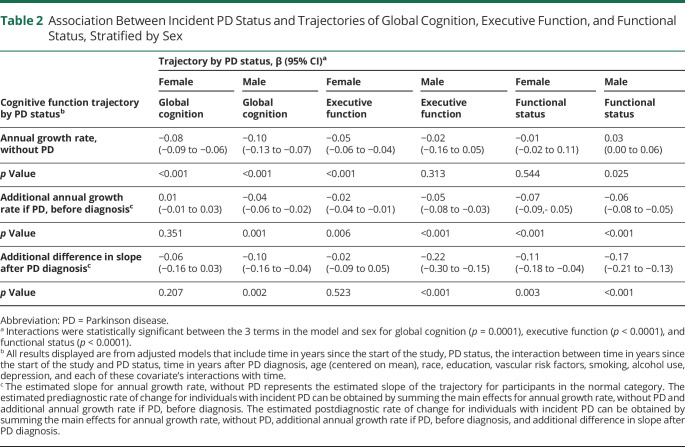
Table 2 summarizes the results from our mixed-effects model analyses. For women, there were no differences in slopes for global cognition. Men with incident PD exhibited greater prediagnostic decline in global cognition compared with men without PD (−0.04 SD more annual change, p < 0.001), which further increased after PD diagnosis (−0.10 SD more annual change, p = 0.002). In other words, men without PD declined in global cognition by 1 SD in 10 years, whereas those with prodromal Parkinson declined by 1 SD in 7 years and those with PD declined by 1 SD in 4 years.
Table 2.
Association Between Incident PD Status and Trajectories of Global Cognition, Executive Function, and Functional Status, Stratified by Sex
Women with prediagnostic PD also exhibited faster decline in executive function before diagnosis (−0.02 more annual change, p = 0.006) compared with women without PD, but no differences were observed between these groups after diagnosis. Men with incident PD had greater rate of decline in executive function compared with men without incident PD in the prediagnostic (−0.05 SD more annual change, p < 0.001) and postdiagnostic period (−0.20 SD more annual change, p < 0.001).
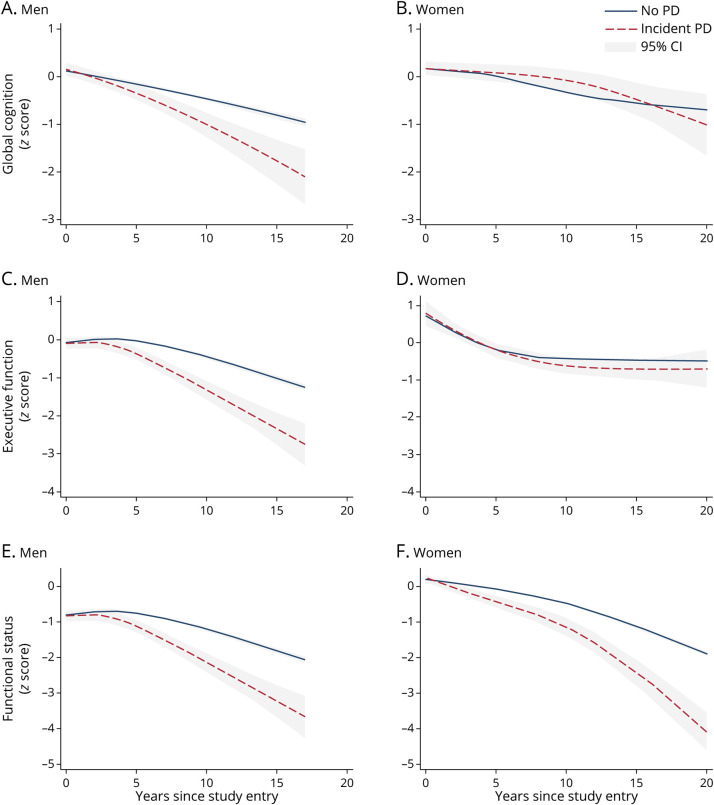
As shown in Figure 1, rates of decline between those with and without incident PD for global cognition diverged at year 5 for men (p < 0.001) and at year 10 for women (p = 0.022). Among both sexes, the level and rate of decline in executive function between those with and without incident PD diverged at year 5 (p < 0.001 for all), although for women, there was no difference at subsequent time points. Sex differences in slopes emerged at 5 years before diagnosis for global cognition (p = 0.006) and 15 years before diagnosis for executive function (p = 0.007) (see Figure 2 for cognitive trajectories of participants who developed incident PD, zeroed at diagnosis).
Figure 1. Trajectories in Global Cognition in Men (A), Global Cognition in Women (B), Executive Function in Men (C), Executive Function in Women (D), Functional Status in Men (E), and Functional Status in Women (F) for Individuals Who Developed Incident Parkinson Disease (PD) During the Course of the Study Compared With Those Without Incident PD*.
*For global cognition, differences in level between those with incident PD and those without became statistically significant at year 5 of the study for men and year 15 for women (p < 0.001 for both). Differences in rates of change of global cognition between those with incident PD and those without became statistically at year 5 of the study for men (p < 0.001) and year 10 for women (p = 0.022). For executive function, level and rate of change in executive function for those with incident PD significantly differed from those without PD at year 5 for both men and women (p < 0.001 for all), although for women, this difference did not remain statistically significant at subsequent time points. For functional status, differences in level of functional status for both men and women (p = 0.003 for men, p < 0.001 for women) and rates of change in functional status (p < 0.001 for men and women) between those with and without PD became statistically significant at year 5. PD = Parkinson disease.
Figure 2. Trajectories of Change in Standardized Global Cognition (A), Executive Function (B), and Functional Status (C) for Men and Women Who Developed Incident Parkinson Disease (PD) During the Course of the Study, Centered at the Time of Diagnosis*.
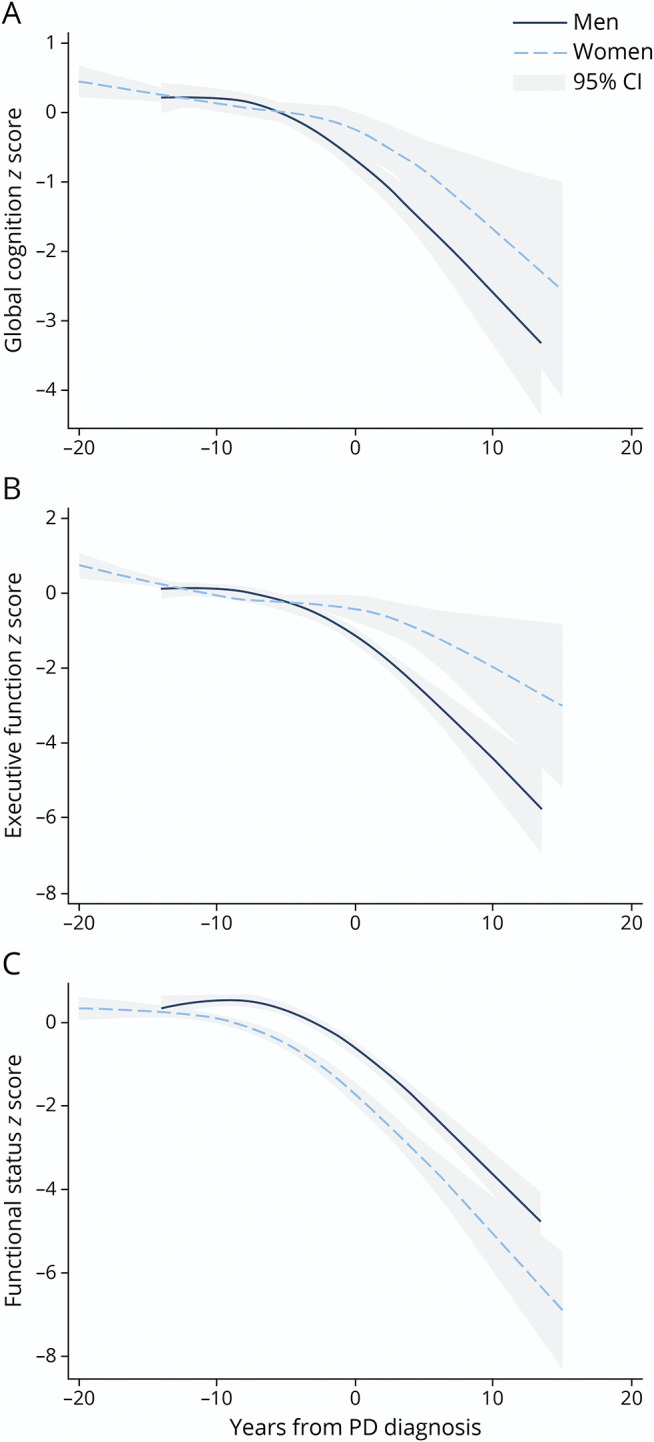
*For global cognition, sex differences in level emerged as statistically significant at the time of diagnosis (p = 0.024), and between-sex differences in slopes were different 5 years before diagnosis (p = 0.006). For executive function, sex differences in level emerged as statistically significant 15 years before PD diagnosis (p = 0.005), and between-sex differences in slopes were different 15 years before diagnosis (p = 0.007). For functional status, sex differences in level became statistically significant 15 years before diagnosis (p < 0.001). Between-sex differences in slopes were different at 15, 10, and 5 years before diagnosis (p = 0.01, p < 0.001, and p = 0.004) and at diagnosis (p = 0.04), but not after diagnosis (p > 0.4 for all time points after diagnosis).
Longitudinal Effect of Incident PD Before and After Diagnosis on Rate of Change in Function
Women showed increased rates of prediagnostic (−0.07 SD more annual change, p < 0.001) and postdiagnostic (−0.11 SD more annual change, p = 0.003) functional decline compared with women without PD. Similarly, men with incident PD similarly displayed increased prediagnostic (−0.06 SD more annual change, p < 0.001) and postdiagnostic decline (−0.17 SD more annual change, p < 0.001) compared with men without PD. For both sexes, differences in rates of functional decline between those with and without PD emerged at study year 5 (p < 0.001 for both) (Figure 1). As shown in Figure 2, sex differences in level and rate of change in functional status emerged 15 years before diagnosis (p < 0.001) (Figure 2).
We conducted a sensitivity analysis defining incident PD as those that reported both patient-reported physician-diagnosed PD and PD medication use (eTable 1, links.lww.com/WNL/C586). Of the 297 incident cases of physician-diagnosed PD, 163 individuals (55%) also reported a PD medication. Of 15,092 individuals who did not report PD, 186 (0.01%) reported the use of a PD medication. The results of our analysis were largely unchanged except prediagnostic decline in global cognition for men became borderline statistically significant (p = 0.06).
Discussion
In 2 community-based cohorts of older adults, both men and women with incident PD exhibited faster prediagnostic decline in cognition and function compared with those without incident PD. There were important sex differences. Men with incident PD had a steeper decline in executive function compared with women. Men but not women with incident PD exhibited detectably faster prediagnostic decline in global cognition. Our study provides novel information on the long-term cognitive and functional trajectories in prediagnostic PD as well as evidence for heterogeneity by sex starting up to 15 years before diagnosis.
There is a growing literature showing that cognitive and functional changes occur earlier in PD than previously appreciated, with measurable impairment at the time of diagnosis.11,29 Cohorts of individuals at risk for PD due to REM sleep behavior disorder, hyposmia, or genetics provide additional evidence for even earlier, prodromal cognitive changes in executive function,30 attention,15 memory,31 and visuoconstructional abilities.32 The few prior studies evaluating prediagnostic cognitive trajectories in population-based cohorts of PD have yielded mixed results, which may be due to a number of factors including unidentified prodromal heterogeneity. In a nested case-control study in the Rotterdam study, there were differences in decline between those with and without PD in functional status 6 years before PD diagnosis, global cognition 6 years before diagnosis, and executive function 3–6 years before diagnosis.33 In smaller studies over 2–3 years, investigators found evidence for changes in psychomotor speed and functional status 2–3 years before PD diagnosis and functional status 2–3 years before diagnosis,34 but no change in global cognition.34 Several population-based cohorts such as the Honolulu Aging Study identified executive impairment as a risk factor for future PD, but often, no trajectory analyses were performed. Another case-control study alternatively found changes in language but not executive function, though in a small sample with 5 incident PD cases.35 Our study provides additional evidence from a large, prospective, community-based US cohort that cognitive changes may occur even earlier before PD diagnosis, exhibit a faster rate of progression over time compared with a control group with age-related cognitive decline, and vary by sex. Our results presented in SDs can provide insight into the rate of decline over years in men and women pre- and post-PD diagnosis, which can be interpreted in the context that mild cognitive impairment is typically diagnosed when an individual tests 1–1.5 SDs below the population mean.36 In our sample, a decline of 1 SD clearly exceeded minimal clinically significant differences for each neuropsychological test (eTable 2, links.lww.com/WNL/C587).
Our finding that participants with incident PD declined faster on the Trails B assessment confirms prior literature showing early changes executive function in diagnosed PD6,7 and extends these findings to the prodromal period. Of interest, the decline in executive function in our study accelerated in men after diagnosis (with men with diagnosed PD declining at 10 times the rate as those without PD), but women did not show increased postdiagnostic decline. Sex differences in the clinical manifestation of PD may be due to neuroprotective effects of estrogen,37 although mechanisms have not been fully elucidated. Our finding that sex differences in the rate of change of executive function were detectable 15 years before diagnosis in our study highlights potential very early differences.
Our finding that global cognition declines in the prediagnostic period in men but not women with incident PD further indicates cognitive heterogeneity in the prodromal period by sex. Prior studies in diagnosed PD showed that men exhibited faster decline in global cognition compared with women,8 and our study shows that sex differences may be detectable as early as 5 years before diagnosis. However, measurement of global cognition differed between the studies, and this finding could be partially due to differences in ascertainment or sample. Of note, men in this study had higher educational attainment but also increased prevalence of vascular risk factors compared with female participants.
Our finding that both men and women with prediagnostic PD exhibit a faster decline in functional status compared with those without PD highlights functional changes as a possible early marker of PD. In both men and women, function declined faster in the prediagnostic period (occurring faster and earlier for women) and accelerated in the postdiagnostic period. Prior studies on rates of functional status change in diagnosed PD have yielded mixed results, with some studies showing slower decline in ADLs in women38 and others finding that women have worse functional status at the time of PD presentation.39 Early functional changes are an important and measurable aspect of prodromal PD.
Strengths of this study include its design with repeated assessments over long duration before and after PD diagnosis and in the comparison group, larger sample size enabling an interaction analysis, and its recruitment from multiple geographic communities in the United States rather than from a specific subpopulation of at-risk individuals that may only represent a subtype of PD. Our study was less subject to the referral bias that occurs in academic settings, though may be influenced by healthy volunteer bias. Limitations include the ascertainment of PD by physician diagnosis reported by the patient, which may not be as sensitive as repeated clinical evaluations. However, our intent was to characterize changes before the typical timing of PD diagnosis in the community. In addition, several prior studies have shown high concordance between clinically determined PD and patient self-report.40-42 Our results were also unchanged in a sensitivity analysis including only those who reported both a physician diagnosis of PD and PD medication use. If individuals were underdiagnosed in our cohort, our results underestimate differences compared with cognitive and functional changes associated with normal aging. Our study was limited by a lack of exact date of symptom onset. Because we used the first visit where PD was reported as the date of diagnosis, there may be some changes classified as prediagnostic that in fact represent very early PD. These studies were conducted in mostly White older adults, and our findings may not be generalizable to those with younger onset PD or other racial and ethnic groups. The Trails B assessment and certain components of the MMSE are dependent on motor speed, so deficits likely reflect both physical and executive function, although physical impairment is likely to be subtle in the prediagnostic phase. Although changes in rates of decline were statistically significant, the magnitude of changes was small and would likely take several years to become clinically significant.
This study contributes novel information about the prodromal phase of PD and its heterogeneity, which has been identified as a high research priority to enable earlier diagnosis43 and clinical priority to appropriately counsel patients about the trajectory of cognitive symptoms. Even dementia-level impairment is underrecognized in current PD clinical practice.44 These findings can inform widely used diagnostic and research criteria. Importantly, the current MDS criteria for prodromal PD do not incorporate functional changes or executive function deficits as risk factors for PD.45 There is no mention of heterogeneity of prodromal symptoms by sex in the guidelines as there is no prior evidence for this, likely because women are underrepresented in prior PD research.19 PD has a low prevalence in the population, and cognitive and functional changes are common in the elderly, so these changes alone are unlikely to be sufficient to identify prodromal PD. However, cognitive and functional changes can be incorporated into a risk score, in which there is growing interest, but prior attempts have had modest predictive value.46-49 Symptom constellations are a particularly promising approach to PD risk assessment, with inclusion of multiple clinical features improving specificity of predictive scores.50 This study additionally contributes to the growing body of work challenging the notion that cognitive impairment occurs later in the disease course of PD compared with Lewy Body dementia and supports a spectrum inclusive of these 2 diagnoses.
Our study illustrates the presence of cognitive and functional changes in PD years before diagnosis, and these changes may differ in important ways by sex. A better understanding of the prodromal phase of PD can facilitate symptom management for the many impactful sequelae of early neurodegeneration and recruitment into neuroprotective trials. Future research is needed to further explore the predictive value of combinations of prodromal symptoms and prodromal heterogeneity in other subpopulations.
Acknowledgment
The authors thank the participants of these studies for their time and contributions. The writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Glossary
- MrOS
Osteoporotic Fractures in Men
- PD
Parkinson disease
- SOF
Study of Osteoporotic Fractures
Appendix. Authors
Study Funding
This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC). This research was supported by R35: NIA R35AG071916. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. The Osteoporotic Fractures in Men (MrOS) Study (mrosonline.ucsf.edu) is supported by NIH funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NCATS, and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR000128. The NHLBI provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Disclosure
M. Bock is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs San Francisco Health Care System and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC) and the American Academy of Neurology Career Development Award; E. Vittinghoff and A. Bahorik report no disclosures relevant to the manuscript; Y. Leng is supported by grant NIA R00AG056598; H. Fink reports no disclosures relevant to the manuscript; K. Yaffe is on the Data Safety and Monitoring Boards for Eli Lilly, DIAN, and for several National Institute on Aging–sponsored trials. She is a board member of Alector, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384-386. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Neurology Collaborators, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(5):2283-2301. [DOI] [PubMed] [Google Scholar]
- 4.Marek K, Innis R, van Dyck C, et al. [123I] -CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology. 2001;57(11):2089-2094. [DOI] [PubMed] [Google Scholar]
- 5.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435-450. [DOI] [PubMed] [Google Scholar]
- 6.Liu R, Umbach DM, Peddada SD, et al. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology. 2015;84(21):2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord. 2014;20(1):99-105. [DOI] [PubMed] [Google Scholar]
- 8.Bakeberg MC, Gorecki AM, Kenna JE, et al. Differential effects of sex on longitudinal patterns of cognitive decline in Parkinson's disease. J Neurol. 2021;268(5):1903-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weintraub D, Simuni T, Caspell-Garcia C, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. 2015;30(7):919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G; For the Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121-1126. [DOI] [PubMed] [Google Scholar]
- 11.Foltynie T, Brayne CEG, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(3):550-560. [DOI] [PubMed] [Google Scholar]
- 12.Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82(4):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197-211. [DOI] [PubMed] [Google Scholar]
- 14.Lerche S, Machetanz G, Roeben B, et al. Deterioration of executive dysfunction in elderly with REM sleep behavior disorder (RBD). Neurobiol Aging. 2018;70:242-246. [DOI] [PubMed] [Google Scholar]
- 15.Génier Marchand D, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83(5):1016-1026. [DOI] [PubMed] [Google Scholar]
- 16.Chahine LM, Weintraub D, Hawkins KA, et al. Cognition in individuals at risk for Parkinson's: Parkinson associated risk syndrome (PARS) study findings. Mov Disord. 2016;31(1):86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chahine LM, Urbe L, Caspell-Garcia C, et al. Cognition among individuals along a spectrum of increased risk for Parkinson's disease. PLoS One. 2018;13(8):e0201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liepelt-Scarfone I, Ophey A, Kalbe E. Cognition in prodromal Parkinson's disease. Prog Brain Res. 2022;269(1):93-111. [DOI] [PubMed] [Google Scholar]
- 19.Tosserams A, Araújo R, Pringsheim T, et al. Underrepresentation of women in Parkinson's disease trials. Mov Disord. 2018;33(11):1825-1826. [DOI] [PubMed] [Google Scholar]
- 20.Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson's disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32(9):1264-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332(12):767-773. [DOI] [PubMed] [Google Scholar]
- 22.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569-585. [DOI] [PubMed] [Google Scholar]
- 23.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26(5):557-568. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 25.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Mot Skills. 1958;8(7):271-276. [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 27.Pincus T, Summey JA, Soraci SA Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346-1353. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Haworth Press; 1986:165-173. [Google Scholar]
- 29.Weintraub D, Caspell-Garcia C, Simuni T, et al. Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease. Ann Clin Transl Neurol. 2020;7(4):449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler A, Mirelman A, Gurevich T, et al. Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology. 2012;79(10):1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weintraub D, Chahine LM, Hawkins KA, et al. Cognition and the course of prodromal Parkinson's disease. Mov Disord. 2017;32(11):1640-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fantini ML, Farini E, Ortelli P, et al. Longitudinal study of cognitive function in idiopathic REM sleep behavior disorder. Sleep. 2011;34(5):619-625. [PMC free article] [PubMed] [Google Scholar]
- 33.Darweesh SKL, Verlinden VJA, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson's disease. Brain. 2017;140(2):429-441. [DOI] [PubMed] [Google Scholar]
- 34.Foubert-Samier A, Helmer C, Le Goff M, et al. Cognitive and functional changes in prediagnostic phase of Parkinson disease: a population-based study. Parkinsonism Relat Disord. 2020;79:40-46. [DOI] [PubMed] [Google Scholar]
- 35.Pausch C, Schomburg R, Wagenpfeil S, et al. Neuropsychological impairment in prodromal Parkinson's disease. J Neurol Sci. 2016;371:117-120. [DOI] [PubMed] [Google Scholar]
- 36.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119-132. [DOI] [PubMed] [Google Scholar]
- 38.Iwaki H, Blauwendraat C, Leonard HL, et al. Differences in the presentation and progression of Parkinson's disease by sex. Mov Disord. 2021;36(1):106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol. 2005;252(10):1201-1205. [DOI] [PubMed] [Google Scholar]
- 40.Winslow AR, Hyde CL, Wilk JB, et al. Self-report data as a tool for subtype identification in genetically-defined Parkinson's disease. Sci Rep. 2018;8(1):12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HM, Leverenz JB, Burdick DJ, et al. Diagnostic validation for participants in the Washington state Parkinson disease registry. Parkinson's Dis. 2018;2018:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers CGT TL, Adams JL, Barbano R, et al. Video-based Parkinson's disease assessments in a nationwide cohort of Fox Insight participants. Clin Parkinsonism Relat Disord. 2021;4:100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011;10(5):377-393. [DOI] [PubMed] [Google Scholar]
- 44.Hu M, Cooper J, Beamish R, et al. How well do we recognise non-motor symptoms in a British Parkinson's disease population?. J Neurol. 2011;258(8):1513-1517. [DOI] [PubMed] [Google Scholar]
- 45.Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2019;34(10):1464-1470. [DOI] [PubMed] [Google Scholar]
- 46.Yuan W, Beaulieu-Jones B, Krolewski R, et al. Accelerating diagnosis of Parkinson's disease through risk prediction. BMC Neurol. 2021;21(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marini K, Mahlknecht P, Tutzer F, et al. Application of a simple Parkinson's disease risk score in a longitudinal population-based cohort. Mov Disord. 2020;35(9):1658-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darweesh SKL, Koudstaal PJ, Stricker BH, Hofman A, Steyerberg EW, Ikram MA. Predicting Parkinson disease in the community using a nonmotor risk score. Eur J Epidemiol. 2016;31(7):679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. 2015;14(10):1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson's disease: the Honolulu-Asia aging study experience. Parkinsonism Relat Disord. 2012;18(suppl 1):S199-S202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author on reasonable request.