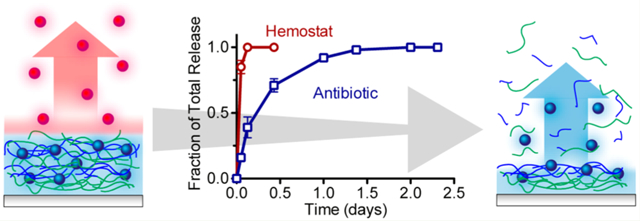
Abstract
Uncontrolled bleeding and infection are the major causes of death and morbidity from traumatic wounds during military conflicts, disasters, and accidents. Because immediate treatment is critical to survival, it is desirable to have a lightweight and rapidly applicable bandage—one capable of delivering a hemostat that can quickly resolve bleeding while addressing infection over short and longer time frames. It is challenging to design thin film coatings capable of multidrug release, particularly when the drugs are quite different in nature (biologic versus small molecule, charged versus neutral) and the desired release profiles are different for each drug. Herein we have adopted a layer-by-layer film assembly technique to create a linear combination of two independently functional films capable of rapidly releasing thrombin within minutes while sustaining vancomycin elution for more than 24 h. By conjugating vancomycin to a hydrolytically degradable polyacid, poly(β-L-malic acid), we were able to create a robust thin film with loading and release kinetics that remain unaffected by the additional deposition of a thrombin-based film, demonstrating the possibility for future multitherapeutic films with independently tunable release kinetics.
Keywords: coagulation, hemostasis, antibiotic, layer-by-layer, controlled release
Graphical Abstract
INTRODUCTION
Uncontrolled bleeding is the most significant cause of mortality on the battlefield, causing more than 85% of the deaths from potentially survivable wounds.1 In civilian populations, it is a factor in more than half of trauma related deaths.2 Once soldiers survive beyond the initial few hours, infection becomes the major cause of morbidity, as pathogens can populate open wounds from environmental contamination (e.g., blast debris and soil) and human transmission.3 To address this medical crisis, it would be ideal to have a multifunctional bandage that is easily applied, lightweight, and immediately active to provide hemostasis and fight infection.4
For this application, it would be desirable to deliver multiple therapeutics directly to the wound, each with individually optimized release kinetics. To stop bleeding, a hemostat would need to be delivered immediately on-contact in a bolus release, whereas killing pathogens would require a sustained release of antibiotics above the critical concentration from one to a few days to eradicate the presence of infection.5 Generating complex multimodal release behavior from biodegradable matrices has traditionally been challenging because of the diverse array of physical and chemical interactions that can affect drug loading and elution. These factors become more complicated when more than one drug is involved.6
We aimed to generate layer-by-layer (LbL) assembled films capable of addressing both hemostasis and infection. We have previously developed films capable of sustained release of antibiotics7 as well as those rapidly achieving hemostasis through thrombin delivery.8 With LbL, nano-to-micron scale thin films can be fabricated through the deposition of compounds with complementary intermolecular interactions (e.g., electrostatic, hydrogen-bonding, covalent, etc.) from aqueous solutions.9 This aqueous-based approach eliminates the potential for protein denaturation from organic solvents that have been traditionally used for controlled release from hydrophobic polyesters like poly(lactic acid-co-glycolic acid) and poly(ε-caprolactone)-based bulk films.6 Since the first demonstration of protein incorporation into LbL films,10 a number of different types of multilayer films incorporating therapeutics, including proteins, peptides, and small molecules, have demonstrated controlled release with a high level of activity retained. It can be challenging to generate distinctly different release behaviors of multiple therapeutics from typical polymeric drug carriers, but recently, using LbL, we have been able to demonstrate sequential release of two polysaccharides,11 and the staged release of an antibiotic and growth factor,12 each of which has relied on a physical or chemically cross-linked barrier layer for controlling release kinetics.
Herein, we address the challenge of maintaining high drug loading and tuned release by using a polymer-drug conjugation strategy that covalently links vancomycin to the hydrophilic and hydrolytically degradable polyanion, poly(β-l-malic acid) (PMLA). We found that the PMLA-Vanco conjugate retains its antibacterial activity and can confer extraordinarily high drug loadings of greater than 56 wt % in (poly-l-lysine/PMLA-Vanco) ultrathin films assembled at the physiological pH of 7.4, and releases 19.5 ± 1.4 μg/cm2 of loaded drug from the film. Such high small molecule loadings in a polymer matrix are unusual, and speaks to the versatility of the LbL loading and release mechanisms. Hydrolytic degradation of the ester backbone in PMLA facilitates film degradation and controlled release of vancomycin. We found that deposition of (thrombin/tannic acid)n on top of these layers generated a composite film that retained a rapid thrombin release over the time frame of several minutes and sustained vancomycin release over a period of 24 h, similar to that achieved with the independent films when studied separately. Notably, despite the known sensitivity of proteins to extracellular environments and process conditions, we found that thrombin fully retained its clotting activity and that the vancomycin also retained its antibacterial potency after release from this composite film.
MATERIALS AND METHODS
All materials were used without further purification unless otherwise noted. Tannic acid, vancomycin HCl (hereafter referred to as vancomycin unless otherwise noted), and poly-l-lysine (PLL, 30–70 kDa) were obtained from Sigma-Aldrich (St. Louis, MO). Poly(β-l-malic acid) (PMLA, 40 kDa) was cultured and purified as previously described from Physarum polycephalum.13 Bovine thrombin (high purity) was obtained from Biopharm Laboratories (Bluffdale, UT). Cation-adjusted Mueller Hinton broth (CaMHB) was obtained from Becton Dickenson (Franklin Lakes, NJ). All other chemicals unless otherwise noted, were obtained from Sigma-Aldrich (St. Louis, MO).
Synthesis of PMLA-Vancomycin.
In a typical strategy for conjugating vancomycin to PMLA, which was adapted from previously described approaches,14 25 mg (216 μmol) of PMLA was dissolved in 2 mL of acetone (dried over molecular sieves) under an argon atmosphere. While stirring on ice, a solution of 2 mL of anhydrous N,N-dimethylformamide (DMF) containing 22 mg (108 μmol) of dicyclohexylcarbodiimide (DCC) and 12 mg (108 μmol) of N-hydroxysuccimide (NHS) was added dropwise, prior to incubation for 4 h at room temperature. The precipitated byproducts were removed by filtration with Celite 545 (EM Science, Gibbstown, NJ) and the filtrate was added dropwise to a stirring solution of 2 mL of anhydrous DMF containing 160 mg (108 μmol) of vancomycin and 302 μL (2.2 mmol) of triethylamine. Use of a 20-fold molar excess of triethylamine has been shown to favor reaction through vancomycin’s primary amine.15 After 2 h of stirring at room temperature, the reaction was quenched with 10 mL of 10 mM sodium phosphate, pH 7.4, on ice for 30 min prior to further dilution to 40 mL with H2O. This solution was syringe filtered (0.45 μm), concentrated by centrifugal filtration (Corning Spin-X UF 20, 10k MWCO; Corning, NJ) then purified with a PD-10 desalting column (GE Healthcare; Little Chalfont, United Kingdom) according to the manufacturer’s instructions and dried by lyophilization yielding 50 mg of PMLA-Vanco (74 wt % vancomycin conjugation) for a 23% reaction efficiency. The degree of vancomycin functionalization to PMLA was determined by HPLC (described below). Other degrees of modification were similarly synthesized using proportionately smaller quantities of DCC, NHS, vancomycin, and triethylamine.
Characterization of PMLA-Vancomycin Conjugates.
Quantification of vancomycin and PMLA-Vanco was performed by HPLC (Agilent 1100 Series) using an injection volume of 50 μL and a C18 column (Supelco Discovery C18; Sigma-Aldrich, St. Louis, MO) with a 1 mL/min mobile phase ramped from 100% PBS to 100% methanol over 20 min. This protocol was found to uniquely distinguish vancomycin and PMLA-vanco from other film components, namely tannic acid. Vancomycin concentrations were quantified by comparison of fluorescence (λex = 280 nm; λem = 355 nm) to standard curves and the degree of conjugation was calculated by comparison of the measured vancomycin in solution to the total mass concentration of PMLA-Vanco in solution.1H NMR spectra were obtained in dimethyl sulfoxide-d6 (Sigma-Aldrich) using a Bruker Avance 400 MHz NMR spectrometer. Vancomycin HCl was converted to its free base to more closely recapitulate the compound for comparison with PMLA-Vanco conjugates by previously described methods16 where vancomycin HCl dissolved in water was precipitated at pH 8, collected by filtration and washed with ethanol and methanol prior to dissolution in water and lyophilization.
Antibacterial Activity.
Antibacterial activity was determined by a microdilution assay as previously described.7 Samples in PBS were initially diluted 2-fold with 2X concentrated CaMHB and then 2-fold serially diluted with 1X CaMHB in a 96-well microplate for final volumes of 90 μL. To these dilutions were added 10 μL of 1 × 106 cells/mL of S. aureus (ATCC, Manassas, VA, #25923), and the samples were then incubated overnight at 37 °C with shaking. After 18–24 h, the most diluted sample concentration that remained optically clear (i.e., no evidence of turbidity) was determined to be the minimum inhibitory concentration.
Layer-by-Layer (LbL) Film Assembly and Characterization.
LbL-assembled films were constructed on silicon wafers precleaned with methanol and water, then irradiated with plasma (Harrick PDC-32G) and coated with a baselayer of (linear polyethylenimine/sodium polystyrenesulfonate)10 as described previously7 using an automated slide stainer (Carl Zeiss) for film assembly. All polymer/protein solutions were formulated in 10 mM phosphate, pH 7.4 while rinses were performed in H2O, pH adjusted to 7—7.5. Films of (PLL/PMLA-Vanco)n were constructed with a repeated n number of cycles of 15 min incubation in 1 mg/mL of PLL followed by rinsing for 10, 20, and 30 s then incubation for 15 min in 1 mg/mL of PMLA-Vanco followed by rinsing for 10, 20, and 30 s. After construction, films were gently dried with N2 and then house vacuum overnight. Films of (thrombin/tannic acid)n were deposited with 5 min incubation in 1 mg/mL of thrombin followed by rinsing for 10, 20, and 30 s, then incubation for 5 min in 2 mg/mL of tannic acid followed by rinsing for 10, 20, and 30 s. Film thicknesses were determined by step-height measurement of razor-scored films with profilometry using a 2.5 μm tip (Dektak 150 Profilometer).
Release profiles of vancomycin and thrombin eluted from films were determined by incubation in 500 μL of phosphate buffered saline (PBS), pH 7.4 or tris-CaCl2−NaCl-Brij35 (TCNB), pH 7.4 at 37 °C. These solutions were periodically collected for analysis and replaced with fresh buffer solutions. TCNB buffer was composed of sterile-filtered 50 mM of Trizma, 150 mM of sodium chloride, 1.1 mM of calcium chloride, 0.05% of Brij-35, and 0.2 mg/mL of BSA at pH 7.4. Vancomycin concentration and activity was determined from these releases as described above. The total vancomycin loading in the films was calculated based on the cumulative amount of drug released per cm2 of film area.
Film degradation during vancomycin elution of (PLL/PMLA-Vanco)40.5 films composed of PMLA-Vanco with 74 wt % functionalization was tracked by incubating films in 500 μL of PBS, pH 7.4 at 37 °C then at predermined times briefly immersed in H2O, drying with N2 and measuring the thickness with spectroscopic ellipsometry (Woollam XLS-100) at 70° from 190 to 1000 nm before continued incubation in fresh PBS solution. Modeling of the measurements used a Cauchy layer on top of SiO2 and Si layers.
Coagulation Activity.
Thrombin activity was determined by a fibrin-clot forming assay that measures the time for clot formation by enzymatic conversion of soluble fibrinogen to an insoluble fibrin. Fibrinogen dilution buffer was composed of 10 mM of sodium citrate, 120 mM of glycine, and 32 mM of tranexamic acid at pH 7.4 and sterile filtered. Fibrinogen solution was composed of 20 mM sodium citrate and 10 mg/mL of human fibrinogen (Sigma-Aldrich, St. Louis, MO) at pH 7.4, sterile filtered, and then stored in aliquots at −20 °C. Prior to testing, coagulation reagent was prepared by combining 9 mL of fibrinogen dilution buffer (with predissolved 10 mg/mL of bovine serum albumin (BSA)) with 1 mL of fibrinogen solution. To assay clotting time, 125 μL of coagulation reagent was added to 50 μL of thrombin solution in TCNB buffer at 37 °C and the time for clotting was quantified with a coagulation analyzer (Diagnostica Stago ST4) that measures the time until a small vibrating bead in solution becomes too entangled with fibrin to continue moving. Comparison of clotting time to a standard curve yielded concentration of thrombin activity.
Characterization of Fibrin Formation.
To morphologically characterize the thrombin activity from the LbL films, we added a 2.9 μL droplet of TCNB buffer to the top of the films and then immediately added 7.1 μL of coagulation reagent. If sufficient thrombin activity is present in the films, the released enzyme will generate a fibrin clot within the droplet. We treated (thrombin/tannic acid)25 films, (PLL/PMLA-Vanco)40.5 films, and (thrombin/tannic acid)25 + (PLL/PMLA-Vanco)40.5 films in this manner as well as an uncoated Si wafer as a negative control, which showed no fibrin formation. For a positive control, 25 IU/mL of thrombin was included in the TCNB buffer, deposited onto an uncoated Si wafer, and then mixed with coagulation reagent. After incubation of each of these samples at 37 °C for ~2 min to promote fibrin formation, they were soaked in a solution of 2.5% glutaraldehyde in PBS at room temperature for 4 h prior to rinsing twice with water and then serially dehydrating with 25, 50, 75, 80, 90, and 100% ethanol. After a second rinse in ethanol, samples were critical-point dried with liquid CO2 (Sorvall critical point drying system).
Films were imaged by scanning electron microscopy with a field-emission gun scanning electron microscope (JEOL-6700F) at 5 kV and an 8 mm working distance for dry films and 6 mm working distance for critical point dried films, both after sputter coating with ~8 nm of Au/Pd.
Cytotoxicity Assay.
To investigate the possibly toxic effects on mammalian cell viability, (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25 films of ~1 cm2 were incubated in 500 μL of Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) at 37 °C for predetermined periods, after which the films were transferred to fresh prewarmed media for continued film elution.
Mammalian fibroblast cells, NIH3T3 cells (ATCC, Manassas, VA) were prepared for cell viability assays by seeding at 10 000 cells/well in 150 μL DMEM with 10% FBS in 96-well plates the night prior. After an overnight incubation, the media was replaced with 150 μL of sterile-filtered DMEM with 10% FBS containing film eluent, as described above and incubated for an additional 24 h. Next, 15 μL of solution in each well was replaced with 15 μL of Alamar Blue (Invitrogen, Carlsbad, CA) and incubated for 4 h, after which the fluorescence (λex = 565 nm; λem = 585 nm) corresponding to metabolic activity, was measured. Relative cell viabilities were determined by comparison to control cells not exposed to film eluent.
RESULTS AND DISCUSSION
Because of the difficulty in combining multiple drugs with independently tunable release kinetics, we sought to use a covalent conjugation strategy to improve vancomycin’s incorporation into the LbL film by tethering it to a linear polyelectrolyte. Vancomycin is a relatively small (~1450 Da) glycopeptide antibiotic with a low charge density that can be directly incorporated into LbL films at pH 5,7 but the labile intermolecular interactions are easily perturbed by slight changes in aqueous conditions or the presence of other compounds17—conditions needed to incorporate other drugs into the film. Therefore, improving the film stability of the vancomycin multilayers was of primary importance for the construction of multifunctional films containing multiple types of drugs. By pendant covalent attachment to a polyacid, significant negative charge could be imparted to vancomycin, anchoring it into the film through multivalent electrostatic cross-linking. This approach has proved fruitful for other LbL films for the incorporation of peptides18 and small molecules.19 In addition to an improved drug loading with greater film stability (potentiating future downstream processing), we aimed to introduce a controlled release mechanism. To this end, we used poly(β-l-malic acid) (PMLA) as both the polyanion to which vancomycin could be covalently conjugated and a hydrolytically degradable component (whose backbone ester cleavage could mediate controlled drug release (Figure 1)). This naturally derived polyacid has been shown to be nontoxic and nonimmunogenic20 with its drug conjugates showing potent therapeutic activity.14,20 We have previously found that use of PMLA in LbL films is capable of generating sustained release profiles of durations from minutes to weeks.21
Figure 1.
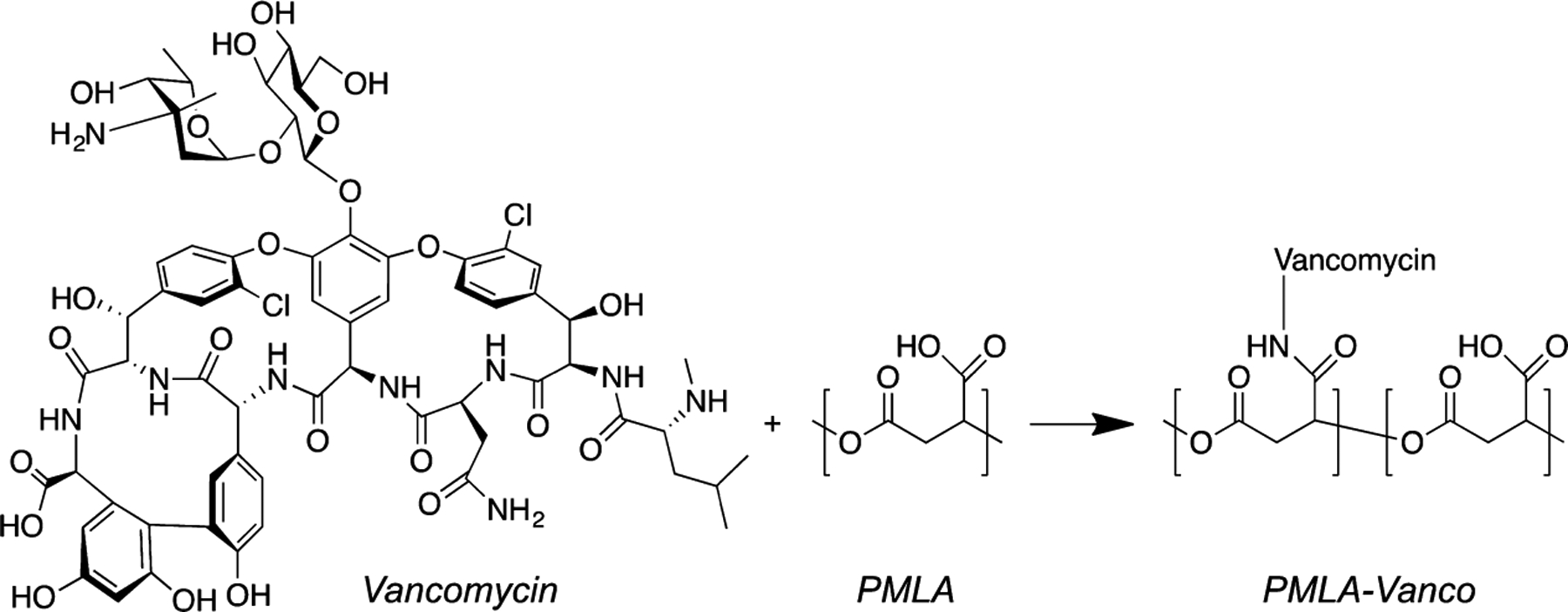
Covalent functionalization of PMLA with vancomycin through DCC mediated amide coupling.
Vancomycin acts to bind to the free termini of bacterial peptidoglycans through five hydrogen-bonds, thereby preventing cross-linking and weakening the cell wall’s integrity.22 Studies have shown that chemical modification of vancomycin can enhance or inhibit its potency depending on the location,23 and covalently linked dimer or trimer variants can have multivalently enhanced potency against drug resistant strains.24 We chose reaction conditions that preferentially conjugated vancomycin’s vancosamine moiety (the primary amine) to PMLA through a DCC-mediated amide coupling15 (Figure 1), allowing the modification to be sterically distant from the cell wall binding region and maximizing the resultant potency. Proton NMR spectra revealed peak shifts for methyl groups adjacent to the vancosamine as well as the secondary amine suggesting that although the reaction conditions were preferential, they were not completely selective (Figure S1). We used a microdilution assay to titrate for the minimum inhibitory concentration (MIC) of PMLA-Vanco compounds with different degrees of functionalization against the Gram-positive pathogen, Staphylococcus aureus, and found that increasing vancomycin loading was not substantially deleterious to antibacterial potency (Table 1). For antibacterial activity against S. aureus, vancomycin MICs of ≤2, 4—8, and ≥16 μg/mL indicate the bacterium is considered susceptible, intermediately resistant, or resistant, respectively.25 Based on these definitions, the derivatized form of vancomycin remains active up to high levels of functionalization. Previous examination of functional modifications to vancomycin at this disaccharide region has shown that the introduction of a negatively charged moiety is not specifically enhancing or deleterious to antibacterial potency on its own,26 which corroborates our observation that malic acid residues do not significantly affect its activity. It is noted that as the functionalization degree increased to weight fractions greater than 10%, there was some increase in MIC observed.
Table 1.
Antibacterial Activity of PMLA-Vanco with Different Degrees of Functionalization Against S. aureusa
| compd | degree of functionalizationb | minimum inhibitory concentration (MIC) (μg/mL) | |
|---|---|---|---|
| molar % | wt % | ||
| free vancomycin | 100 | 1.3 | |
| PMLA-Vanco8.4 | 0.7 | 8.4 | 1.2 ± 0.1 |
| PMLA-Vanco10 | 0.84 | 10.2 | 1.6 ± 0.3 |
| PMLA-Vanco43 | 5.4 | 43.3 | 1.7 ± 0.1 |
| PMLA-Vanco74 | 17.6 | 74.0 | 2.3 ± 0.2 |
MIC values were calculated on the basis of the concentration of vancomycin present in solution.
Degree of functionalization is shown in the percentage of pendant carboxylates of PMLA that are functionalized with vancomycin (molar %) or percentage of total mass constituted by vancomycin (wt %).
Because attachment to the PMLA backbone did not deteriorate vancomycin’s antibacterial potency, we examined its capability for both film assembly and controlled release. Previously, LbL films utilizing Poly 2, a hydrolytically degradable poly(β-amino ester), in alternation with dextran sulfate and vancomycin (Poly 2/dextran sulfate/vancomycin/dextran sulfate)n were assembled at pH 57. This lower pH was used to leverage vancomycin’s net +1 charge for electrostatic incorporation, which was otherwise unattainable at the physiological pH of 7.4 (pI ~7.227). When assembling a composite film (i.e., the stacking of two or more independently stable films), we found that the vancomycin release kinetics were highly dependent on the way the films were assembled, including the components, pH, order of stacking, and method of assembly (i.e., spray- or dip-LbL).17 Furthermore, the deposition of a (thrombin/tannic acid)n film at pH 7.4 directly on top of the tetralayer vancomycin film caused the film to strip away due to the loss of charge of the vancomycin at these incompatible assembly conditions. With thrombin’s activity being highly dependent on pH,28 it is important to find assembly conditions that both maintain its activity and do not destabilize vancomycin loading or release kinetics. To this end, we leveraged the stable negative charge of the acidic groups and increased charge density of the PMLA-Vanco conjugate to assemble a film at pH 7.4, which was otherwise infeasible with the (poly 2/dextran sulfate/vancomycin/dextran sulfate)n film architecture.
To verify that we could indeed construct a desirable vancomycin-loaded film using our PMLA-Vanco conjugate, we studied the release kinetics for (PLL/PMLA-Vanco)40.5 films assembled from 10 mM phosphate, pH 7.4. For PMLA-Vanco having 8.4 and 74 wt % conjugation, we found that both had similar release durations of roughly 1 day (Figure 2A) with film loadings of 10.4 ± 0.3 and 19.5 ± 1.4 μg/cm2 of vancomycin, respectively. As would be expected with the higher degree of conjugation, a greater amount of drug could be incorporated into the film for the same number of layers. In addition, the latter case had a high drug-loading density of 560 μg/mm3, which roughly corresponds to ~56 wt % vancomycin assuming a film density of ~1000 μg/mm3, an approximation for polymer thin films that is also comparable for polyelectrolyte multilayer films.29 In our previously investigated LbL film architectures, we had reached a relatively high ~20 wt % vancomycin loading7 and the improvement we find here is likely due to use of a polymer-drug conjugate and the simplified architecture (i.e., bilayer instead of tetralayer) that reduces the inert components in the film. Interestingly, the duration of release was similar for both conjugates and is comparable to that of PLL released from (PLL/PMLA) films,21 which suggests that vancomycin does not significantly participate in the stabilization or destabilization of these films during drug release. Of the amines present in vancomycin, only two are of sufficiently basic to be ionized under physiological conditions: an amino sugar primary amine (pKa ~8.630) and an N-terminal, secondary amine (pKa ~6.830). The former is predominantly linked to the PMLA backbone through an amide conjugation, while the latter is weakly charged at the assembly condition pH of 7.4. The other ionizable group, a C-terminal carboxylate (pKa ~2.530) confers a negative charge. Because of vancomycin’s poor net charge and the densely polyanionic backbone, it is presumed that the PMLA-Vanco conjugate acts essentially as a polyanion during LbL assembly. Vancomycin’s inability to form strong intermolecular cross-links likely allows it to play a nonstructural role in the ionically cross-linked film. Thus, vancomycin is released after sufficient fragmentation of the PMLA backbone by hydrolysis, allowing it to elute. Our previous studies with other PMLA-based multilayer films revealed that the hydrolysis of this polymer could facilitate controlled film degradation and hence controlled elution of drugs embedded in the film.21 To elucidate this effect in (PLL/PMLA-Vanco)40.5 films, we tracked the fraction of film remaining during vancomycin elution (Figure 2B) and found that drug release was concomitant with film degradation. This further indicates that hydrolysis of the PMLA backbone not only causes film erosion but also liberates the vancomycin conjugated along the backbone. Vancomycin’s lack of influence on how it is released from the film demonstrates an interesting aspect, as the release kinetics may be tuned independently of the pendant drug.
Figure 2.
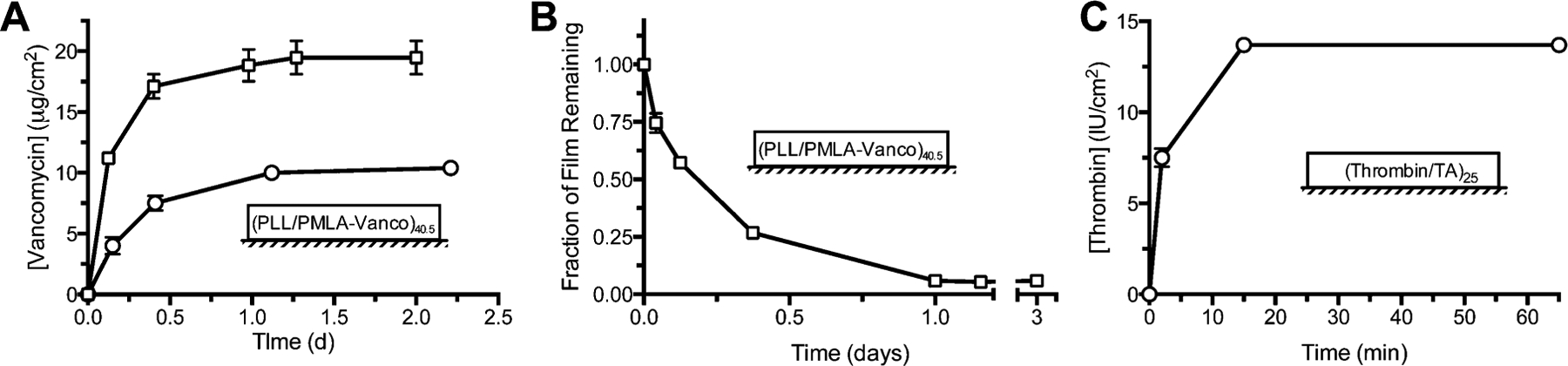
Characteristics of films containing vancomycin and thrombin. (A) Release profiles of (PLL/PMLA-Vanco)40.5 films with 8.4 (circles) and 74 wt % (squares) vancomycin functionalization eluted into PBS, pH 7.4 at 37 °C, (B) the thickness of the latter film during this elution, and (C) the release profile of (thrombin/tannic acid)25 films eluted into TCNB, pH 7.4 at 37 °C. Data points represent the mean ± standard deviation.
With the release of vancomycin being dependent upon the ester linkages along PMLA, the rate of release (i.e., acceleration or prolonging) could benefit from the tuning of backbone hydrolysis. One approach could utilize hydrolytically degradable polycations, poly(β-amino ester)s, which have shown tunable degradations as short as a few hours31 and could accelerate the release of PMLA-Vanco from these films. If perhaps it is desired to have a more prolonged release of vancomycin, barrier layer strategies such as laponite12 shells could significantly extend the duration of release.
In a slight modification to the films we previously reported, we assembled thrombin-containing films from 10 mM phosphate, pH 7.4 (rather than PBS) to match the conditions we used for the PMLA-Vanco films and found a linear growth profile (Figure S2). Our previous studies of (thrombin/tannic acid)n films assembled from and eluted into PBS, pH 7.4 showed a slow film degradation through a diffusional release mechanism, and not the desired bolus thrombin release. Although PBS is good for simulating physiological pH and ionic strength it does not contain serum proteins. Incubation in tris-CaCl2−NaCl-Brij35 buffer (TCNB) containing bovine serum albumin (BSA) was better able to recapitulate in vivo conditions with the presence of serum protein, and showed a rapid elution of active thrombin that corroborated well with in vivo efficacy.8 We similarly found that our (thrombin/tannic acid)25 films did not elute into PBS (Figure S3) but released rapidly into TCNB (Figure 2C), which is likely due to the competitive interactions from proteins and possibly the other components in solution that disrupt the hydrogen-bonds in the film. Because the (PLL/PMLA-Vanco)40.5 films are assembled through more robust electrostatic interactions and drug release is facilitated through hydrolytic degradation, the vancomycin release kinetics were unaltered whether the film was eluted in PBS or TCNB (Figure S4).
Although these independent vancomycin and thrombin containing films demonstrated desirable release profiles, combining them into a composite film may introduce competitive interactions that significantly alter elution kinetics. During LbL film assembly, polyelectrolytes can interdiffuse to create blending throughout the film’s thickness32 and the introduction of a new component can displace existing portions of the film.33 We have similarly seen this effect modulate the release profiles in a composite film comprised of independently validated vancomycin and diclofenac films.17 By covalently conjugating vancomycin to PMLA, the high charge valency and molecular size of the polyanionic backbone should enhance the stability of the film and eliminate displacement and exchange when an additional film is deposited, especially one based on weaker, hydrogen-bonding interactions as is the case for the (thrombin/tannic acid)n films. We studied the film growth for the composite film (Figure 3A) where we first deposited (PLL/PMLA-Vanco)n films (comprising the 74 wt % PMLA-Vanco conjugate) and then a (thrombin/tannic acid)n film on top. The superlinear growth observed for the first portion of the film corresponds to what was observed for (PLL/PMLA)n films21 and is characteristic of some polyelectrolytes, including PLL, which is known to be capable of interdiffusing at higher pH values.32 The (thrombin/tannic acid)n portion of the films showed growth that was commensurate with layers deposited, showing that film assembly could continue on top of another film. The greater degree of uncertainty associated with the growth of the (thrombin/tannic acid)n film is due to the underlying (PLL/PMLA-Vanco)40.5 film, as can be seen in Figure 3A.
Figure 3.
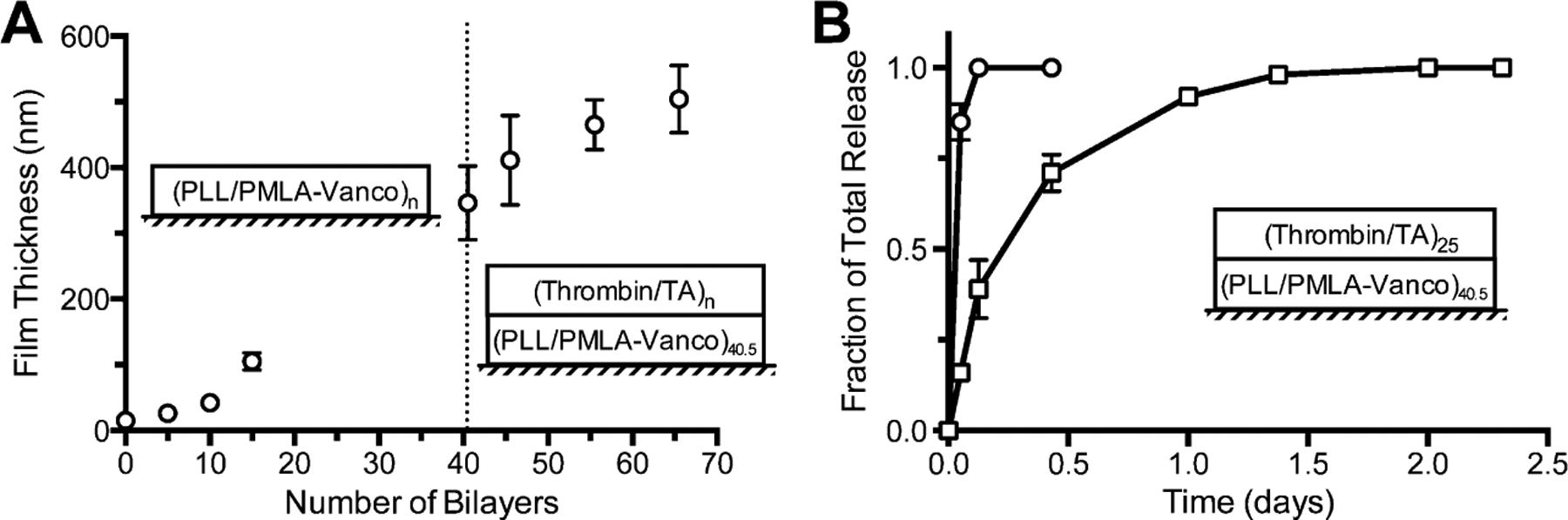
(A) Growth curves of a composite films of (PLL/PMLA-Vanco)n and (thrombin/tannic acid)n and the (B) release profiles for thrombin (circles) and vancomycin (squares) eluted into TCNB, pH 7.4 at 37 °C. Data points represent the mean ± standard deviation.
The uniformity of the film is important for ensuring that precise dosages can be delivered. A feature of using LbL film assembly is the conformal nature of these coatings, which can reproducibly deliver a known quantity of drug per film area.34 For some biomedical applications, it is imperative to have temporally well-defined release kinetics that fit into therapeutic windows,35 and inconsistent nonuniform coatings can lead to poorly defined drug releases. To ascertain their conformal coverage, we examined the surface morphology of these films by scanning electron microscopy (SEM) and found a relatively uniform conformal coating. At 5000× (Figure 4A) and 10 000× (Figure 4B) magnification, the (PLL/PMLA-Vanco)40.5 films are relatively smooth, which is common for polyelectrolyte based films, especially those assembled in pH regimes where both polyelectrolytes are substantially ionized.36 In contrast, after deposition of the hydrogen-bond-based LbL (thrombin/tannic acid)25 films, the SEM micrographs show significant texturing and the appearance of aggregates on the surface at 5000× (Figure 4C) and 10 000× (Figure 4D) magnifications. Such features have been previously observed for protein-containing bilayer LbL films,37 as well as (thrombin/tannic acid)n films8 and demonstrate that there is a distinctly different morphology associated with this film as compared to the film based on charge interactions. Although the aggregates appear to be noncontinuous, their regular distribution suggests a consistent coating, which corresponds with the reproducibility in thrombin loadings we observed between samples.
Figure 4.
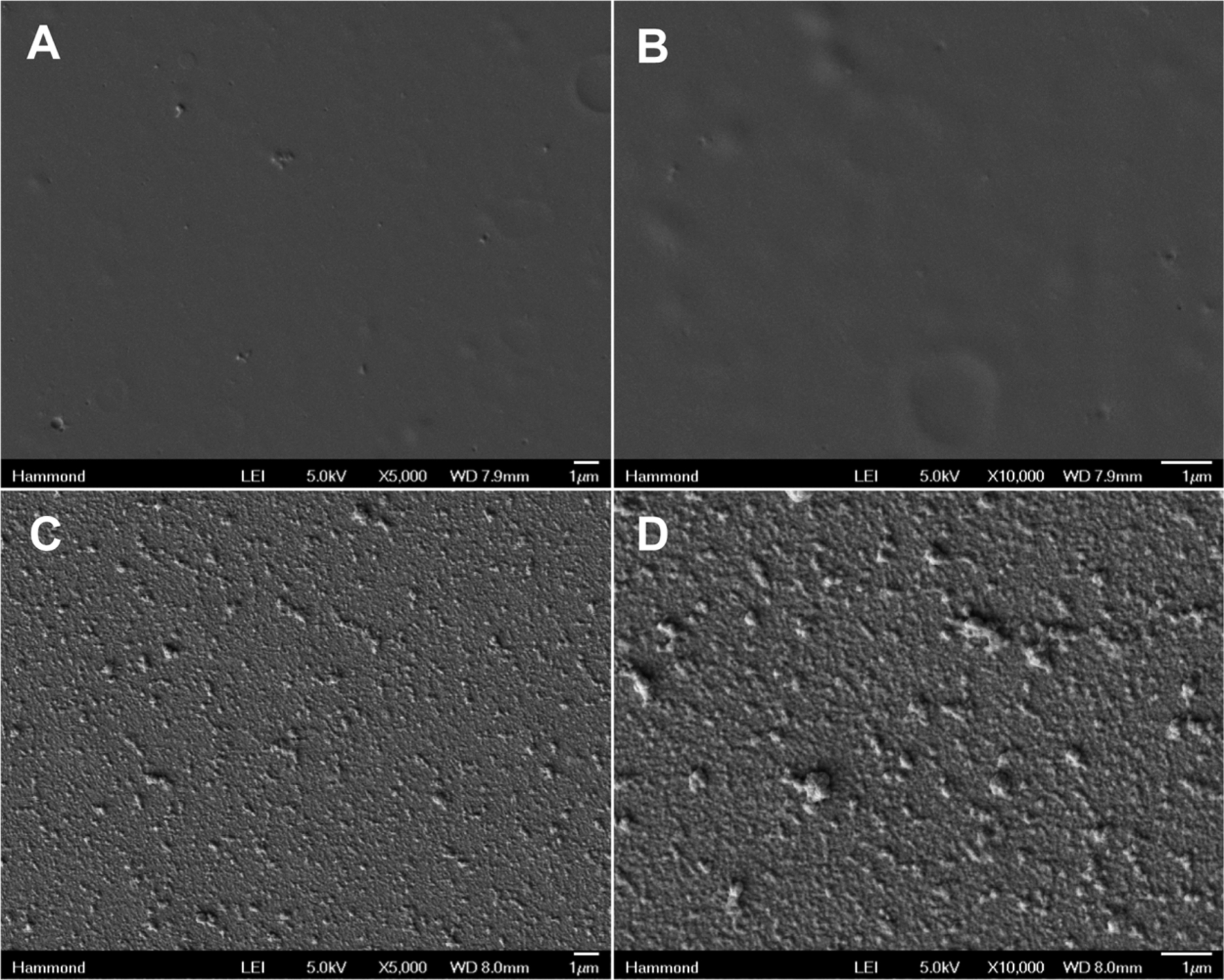
Scanning electron micrographs of (A, B) (PLL/PMLA-Vanco)40.5 films and (C, D) (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25 films at magnifications of (A, C) 5000× and (B, D) 10 000×.
For the biomedical application of the film to rapidly stop bleeding and fight infection, it is imperative to have a large bolus release of thrombin and a sustained release of vancomycin. Therefore, we examined the release kinetics of thrombin and vancomycin from a composite film architecture of (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25. Upon incubation in TCNB at 37 °C, we found thrombin’s rapid release coincided with a sustained release of vancomycin for more than a day (Figure 3B). The release profiles of these drugs appear strikingly similar to those from their individual films, indicating that a linear combination of these two films does not significantly alter their release kinetics. This is further evidenced by the 20.6 ± 0.7 μg/cm2 of vancomycin loaded in the composite film, which is comparable to its loading in the individual film (19.5 ± 1.4 μg/cm2). Furthermore, thrombin release from these films is not impeded by deposition onto the (PLL/PMLA-Vanco)40.5 film as compared to deposition onto silicon. The lack of interference between these two films is likely a consequence of their types of intermolecular interactions and order of deposition. As reported earlier, the introduction of a polyelectrolyte to a hydrogen-bonded film can cause film rearrangement and materials displacement to accommodate the stronger electrostatic cross-links.33 Herein, we deposited a hydrogen-bonded film on top of an electrostatically bonded film and found that the release kinetics for both drugs remained intact. The components in the (thrombin/tannic acid)n film are too weakly charged to undergo exchange with the (PLL/PMLA-Vanco)40.5 film, and similarly, the latter film is electrostatically compensated with no driving force to influence the (thrombin/tannic acid)25 film.
To determine the activity of the compounds upon elution from the film, we examined the antibacterial activity of the film-released vancomycin against S. aureus and found that its potency remained unchanged (2.2 ± 0.2 μg/mL), demonstrating that the film assembly process including the deposition of the (thrombin/tannic acid) film and subsequent drug elution did not affect its potency. The method used for quantification of thrombin is based on activity; we found that 5.7 ± 2.1 IU/cm2 of active thrombin was eluted from the composite films. This loading is significant, as one IU is capable of clotting one milliliter of plasma in 15 s.38 In conjunction with assessing the thrombin activity, we also characterized the morphology of the fibrin clot generated. Instead of eluting thrombin into solution and subsequently testing its enzymatic activity, we placed films in direct contact with a solution containing fibrinogen, which more directly mimics the use of the coated bandage in application. Following chemical fixation and critical point drying, we were able to capture the representative solution-phase microarchitecture and compare the effect of thrombin from the film on fibrin clot morphology. A simple mixture of fibrinogen with thrombin in solution as a control showed the characteristic fibrin matrix with intertwined fibers that give a blood clot its mechanical stability (Figure 5A). Comparison to the fibrin generated from a (thrombin/tannic acid)25 film reveals a very similar morphology (Figure 5B) and suggests that the incorporation of thrombin does not impair or deleteriously affect clot formation. On the other hand, the (PLL/PMLA-Vanco)40.5 films lacking thrombin did not generate fibrin clots and only the surface of the hydrated film (Figure 5C) is visible. The dual component films of (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25 had significant fibrin clot formation (Figure 5D) with similar morphological characteristics to solution-based and film-based thrombin, indicating that the underlying film is a passive participant in this regard.
Figure 5.
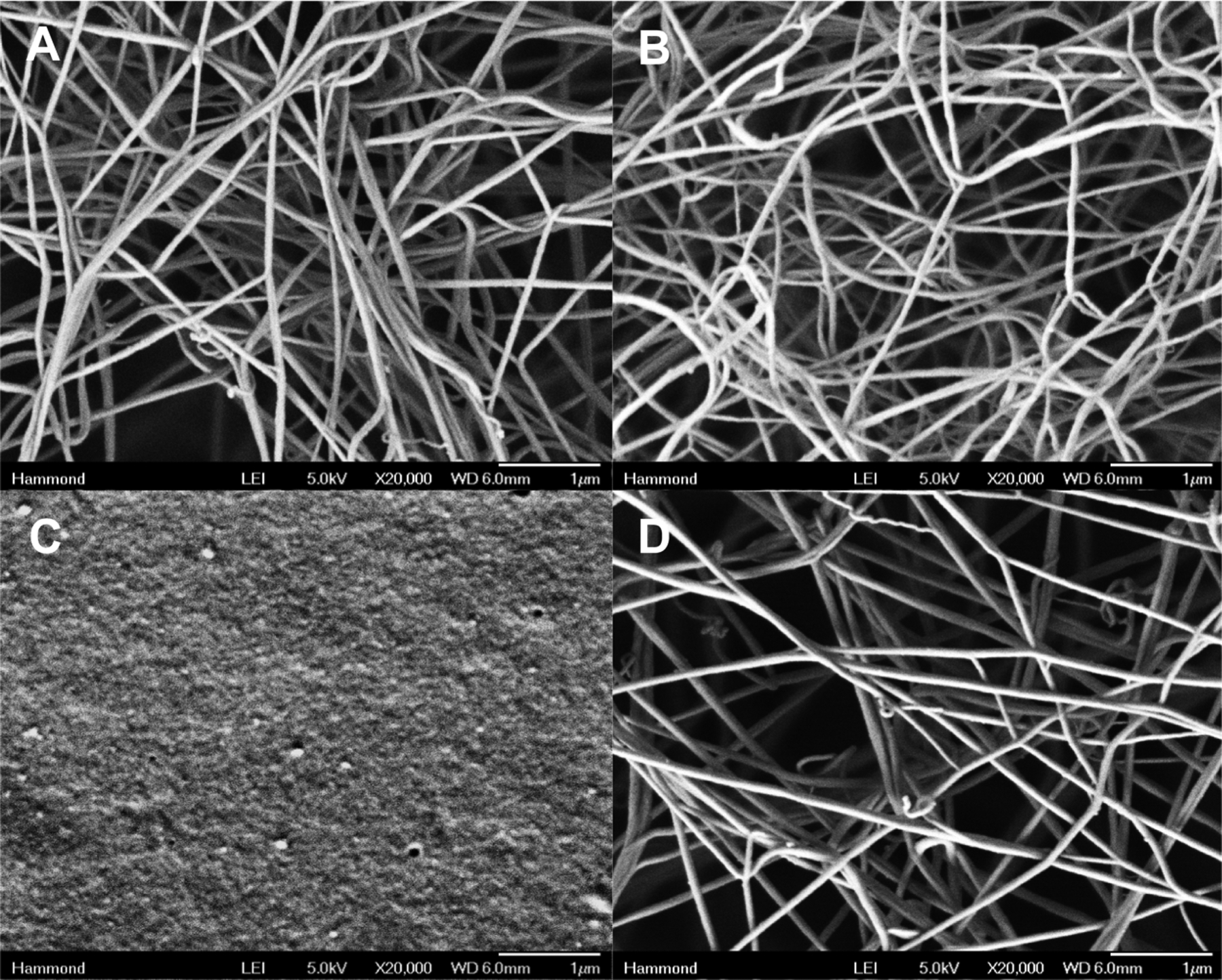
Scanning electron microscopy characterization of fibrin network formation from (A) a simple mixture of thrombin and fibrinogen in solution, and fibrinogen solutions deposited onto (B) (thrombin/tannic acid)25 films, (C) (PLL/PMLA-Vanco)40.5 films, and (D) composite (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25 films. Each sample was imaged at 20 000× magnification.
With application to traumatic wounds, it is important that these biomaterials have minimal toxicity to the surrounding tissues. As a preliminary investigation into the biocompatibility of these thrombin and vancomycin releasing films, we examined the effect that eluted film components had on the viability of NIH3T3 cells in vitro. As revealed in Figure 6, there is no substantial toxicity observed with only a slight decrease in the relative cell viability upon incubation with the eluted film components, which is a promising outcome for possible biomedical applications.
Figure 6.
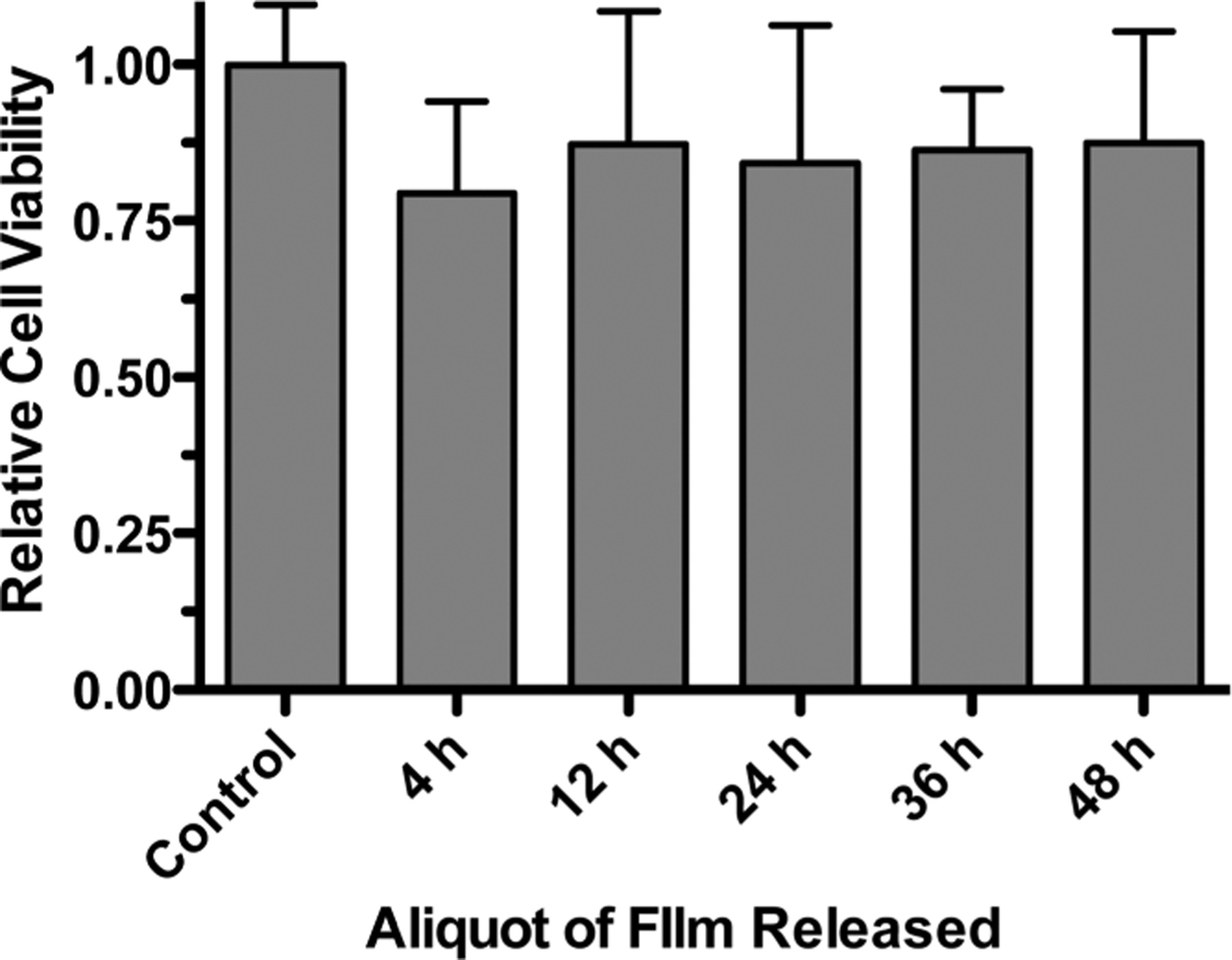
Relative viability of NIH3T3 cells after exposure to solutions containing eluted (PLL/PMLA-Vanco)40.5 + (thrombin/tannic acid)25 films. The control represents solution without eluted film components. Data points represent the mean ± standard deviation.
CONCLUSIONS
Herein, we have developed a biodegradable, composite thin film composed of a linear combination of two independent films that is capable of multitherapeutic release for traumatic wounds. We used a polymer-drug strategy to covalently link vancomycin to a hydrolytically degradable polyanion, PMLA. Release was sustained from (PLL/PMLA-Vanco)40.5 films for more than a day. Upon deposition of rapidly eluting (thrombin/tannic acid)25 films on top, the vancomycin release kinetics and loading were unaffected. Thrombin release is similarly unperturbed whether the film is deposited on top of silicon or on top of this vancomycin-loaded film. We also found that antibacterial activity and hemostatic activity were retained in these films, and that the clotting of fibrin was rapid and effective as determined in vitro tests. The composite film demonstrates the desirable release kinetics for addressing the immediate hemostasis and prolonged antibacterial activity necessary for improving survivability and reducing morbidity from traumatic wounding.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by the U.S. Army Research Office under Contract W911NF-13-D-0001 and the Air Force under Contract W911NF-07-D-0004. The work was also supported by NIH/NCI U01 CA151815 and Martz Translational Breast Cancer Research Discovery Fund 2012 grants to Drs. E. Holler and J.Y. Ljubimova.
Footnotes
Supporting Information
The following file is available free of charge on the ACS Publications website at DOI: 10.1021/ab500050m.
NMR spectra of PMLA-Vanco, growth curve of (thrombin/tannic acid)n films, and the separate release profiles of (thrombin/tannic acid)25 and (PLL/PMLA-Vanco)40.5 films in PBS and TCNB with BSA buffers (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Kelly JF; Ritenour AE; McLaughlin DF; Bagg KA; Apodaca AN; Mallak CT; Pearse L; Lawnick MM; Champion HR; Holcomb JB Injury severity and causes of death from operation Iraqi freedom and operation enduring freedom: 2003–2004 versus 2006. J. Trauma-Injury Infect. Crit. Care 2008, 64 (2), S21–S26. [DOI] [PubMed] [Google Scholar]
- (2).Evans JA; van Wessem KJP; McDougall D; Lee KA; Lyons T; Balogh ZJ Epidemiology of Traumatic Deaths: Comprehensive Population-Based Assessment. World J. Surg 2010, 34 (1), 158–163. [DOI] [PubMed] [Google Scholar]
- (3).(a) Murray CK Infectious disease complications of combat-related injuries. Crit. Care Med 2008, 36 (7), S358–S364. [DOI] [PubMed] [Google Scholar]; (b) Zapor MJ; Moran KA Infectious diseases during wartime. Curr. Opin. Infect. Dis 2005, 18 (5), 395–399. [DOI] [PubMed] [Google Scholar]; (c) Eardley WGP; Brown KV; Bonner TJ; Green AD; Clasper JC Infection in conflict wounded. Philos. Trans. R. Soc., B 2011, 366 (1562), 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Capturing the Full Power of Biomaterials for Military Medicine: Report of a Workshop. The National Academies Press: Washington, D.C., 2004. [PubMed] [Google Scholar]
- (5).Hospenthal DR; Murray CK; Andersen RC; Bell RB; Calhoun JH; Cancio LC; Cho JM; Chung KK; Clasper JC; Colyer MH; Conger NG; Costanzo GP; Crouch HK; Curry TK; D’Avignon LC; Dorlac WC; Dunne JR; Eastridge BJ; Ficke JR; Fleming ME; Forgione MA; Green AD; Hale RG; Hayes DK; Holcomb JB; Hsu JR; Kester KE; Martin GJ; Moores LE; Obremskey WT; Petersen K; Renz EM; Saffle JR; Solomkin JS; Sutter DE; Tribble DR; Wenke JC; Whitman TJ; Wiesen AR; Wortmann GW Guidelines for the Prevention of Infections Associated With Combat-Related Injuries: 2011 Update Endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J. Trauma-Injury Infect. Crit. Care 2011, 71, S210–S234. [DOI] [PubMed] [Google Scholar]
- (6).Siepmann J; Siegel RA; Rathbone MJ Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, 2012. [Google Scholar]
- (7).Shukla A; Avadhany SN; Fang JC; Hammond PT Tunable Vancomycin Releasing Surfaces for Biomedical Applications. Small 2010, 6 (21), 2392–2404. [DOI] [PubMed] [Google Scholar]
- (8).Shukla A; Fang JC; Puranam S; Jensen FR; Hammond PT Hemostatic Multilayer Coatings. Adv. Mater 2012, 24 (4), 492–496. [DOI] [PubMed] [Google Scholar]
- (9).Decher G; Schlenoff JB Multilayer Thin Films, 2nd ed.; Wiley−VCH: Weinheim, Germany, 2012. [Google Scholar]
- (10).Lvov Y; Ariga K; Ichinose I; Kunitake T Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc 1995, 117 (22), 6117–6123. [Google Scholar]
- (11).Wood KC; Chuang HF; Batten RD; Lynn DM; Hammond PT Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc. Natl. Acad. Sci. U.S.A 2006, 103 (27), 10207–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Min J; Braatz RD; Hammond PT Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35 (8), 2507–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lee B-S; Vert M; Holler E Water-Soluble Aliphatic Polyesters: Poly(malic acid)s. In Biopolymers: Polyesters I—Biological Systems and Biotechnological Production; Doi Y, Steinbuchel A, Eds.; Wiley: New York, 2002; Vol. 3a, pp 75–103. [Google Scholar]
- (14).(a) Ding H; Inoue S; Ljubimov AV; Patil R; Portilla-Arias J; Hu J; Konda B; Wawrowsky KA; Fujita M; Karabalin N; Sasaki T; Black KL; Holler E; Ljubimova JY Inhibition of brain tumor growth by intravenous poly(β-l-malic acid) nanobioconjugate with pH-dependent drug release. Proc. Natl. Acad. Sci. U.S.A 2010, 107 (42), 18143–18148. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee B-S; Fujita M; Khazenzon NM; Wawrowsky KA; Wachsmann-Hogiu S; Farkas DL; Black KL; Ljubimova JY; Holler E Polycefin, a New Prototype of a Multifunctional Nanoconjugate Based on Poly(β-l-malic acid) for Drug Delivery. Bioconjugate Chem. 2006, 17 (2), 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Greenwald RB; Zhao H; Xia J; Martinez A Poly(ethylene glycol) transport forms of vancomycin: A long-lived continuous release delivery system. J. Med. Chem 2003, 46 (23), 5021–5030. [DOI] [PubMed] [Google Scholar]
- (16).Li B; Brown KV; Wenke JC; Guelcher SA Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J. Controlled Release 2010, 145 (3), 221–230. [DOI] [PubMed] [Google Scholar]
- (17).Shukla A; Fuller RC; Hammond PT Design of multi-drug release coatings targeting infection and inflammation. J. Controlled Release 2011, 155 (2), 159–166. [DOI] [PubMed] [Google Scholar]
- (18).(a) Chluba J; Voegel J-C; Decher G; Erbacher P; Schaaf P; Ogier J Peptide Hormone Covalently Bound to Polyelectrolytes and Embedded into Multilayer Architectures Conserving Full Biological Activity. Biomacromolecules 2001, 2 (3), 800–805. [DOI] [PubMed] [Google Scholar]; (b) Schultz P; Vautier D; Richert L; Jessel N; Haikel Y; Schaaf P; Voegel J-C; Ogier J; Debry C Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, α-MSH, for coating a tracheal prosthesis. Biomaterials 2005, 26 (15), 2621–2630. [DOI] [PubMed] [Google Scholar]
- (19).Hsu BB; Park M; Hagerman SR; Hammond PT Multi-month controlled small molecule release from biodegradable thin films. Proc. Natl. Acad. Sci. U.S.A 2014, 111 (33), 12175–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ljubimova JY; Portilla-Arias J; Patil R; Ding H; Inoue S; Markman JL; Rekechenetskiy A; Konda B; Gangalum PR; Chesnokova A; Ljubimov AV; Black KL; Holler E Toxicity and efficacy evaluation of multiple targeted polymalic acid conjugates for triple-negative breast cancer treatment. J. Drug Targeting 2013, 21 (10), 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hsu BB; Hagerman SR; Jamieson K; Veselinovic J; O’Neill N; Holler E; Ljubimova JY; Hammond PT Multilayer films assembled from biocompatible, naturally-derived materials for controlled protein release. Biomacromolecules 2014, 15 (6), 2049–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Walsh CT; Fisher SL; Park IS; Prahalad M; Wu Z Bacterial resistance to vancomycin: Five genes and one missing hydrogen bond tell the story. Chem. Biol 1996, 3 (1), 21–28. [DOI] [PubMed] [Google Scholar]
- (23).Griffin JH; Linsell MS; Nodwell MB; Chen QQ; Pace JL; Quast KL; Krause KM; Farrington L; Wu TX; Higgins DL; Jenkins TE; Christensen BG; Judice JK Multivalent drug design. Synthesis and in vitro analysis of an array of vancomycin dimers. J. Am. Chem. Soc 2003, 125 (21), 6517–6531. [DOI] [PubMed] [Google Scholar]
- (24).Li L; Xu B Multivalent vancomycins and related antibiotics against infectious diseases. Curr. Pharm. Des 2005, 11 (24), 3111–3124. [DOI] [PubMed] [Google Scholar]
- (25).Tenover FC; Moellering RC The Rationale for Revising the Clinical and Laboratory Standards Institute Vancomycin Minimal Inhibitory Concentration Interpretive Criteria for Staphylococcus aureus. Clin. Infect. Dis 2007, 44 (9), 1208–1215. [DOI] [PubMed] [Google Scholar]
- (26).Fu X; Albermann C; Zhang C; Thorson JS Diversifying Vancomycin via Chemoenzymatic Strategies. Org. Lett 2005, 7 (8), 1513–1515. [DOI] [PubMed] [Google Scholar]
- (27).Gasper MP; Berthod A; Nair UB; Armstrong DW Comparison and modeling study of vancomycin, ristocetin A, and teicoplanin for CE enantioseparations. Anal. Chem 1996, 68 (15), 2501–2514. [DOI] [PubMed] [Google Scholar]
- (28).Fenton JW; Fasco MJ; Stackrow AB; Aronson DL; Young AM; Finlayson JS Human thrombins—prodiuction, evaluation, and properties of alpha-thrombin. J. Biol. Chem 1977, 252 (11), 3587–3598. [PubMed] [Google Scholar]
- (29).Losche M; Schmitt J; Decher G; Bouwman WG; Kjaer K Detailed structure of molecularly thin polyelectrolyte multilayer films on solid substrates as revealed by neutron reflectometry. Macromolecules 1998, 31 (25), 8893–8906. [Google Scholar]
- (30).Sitrin RD; Chan GW; Dingerdissen JJ; Holl W; Hoover JRE; Valenta JR; Webb L; Snader KM Aridicins, novel glyocopeptide antibiotics 0.2. Isolation and characterization. J. Antibiot 1985, 38 (5), 561–571. [DOI] [PubMed] [Google Scholar]
- (31).Zhang JT; Fredin NJ; Janz JF; Sun B; Lynn DM Structure/property relationships in erodible multilayered films: Influence of polycation structure on erosion profiles and the release of anionic polyelectrolytes. Langmuir 2006, 22 (1), 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Picart C; Mutterer J; Richert L; Luo Y; Prestwich GD; Schaaf P; Voegel JC; Lavalle P Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. U.S.A 2002, 99 (20), 12531–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gilbert JB; Rubner MF; Cohen RE Depth-profiling X-ray photoelectron spectroscopy (XPS) analysis of interlayer diffusion in polyelectrolyte multilayers. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (17), 6651–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hammond PT Building biomedical materials layer-by-layer. Mater. Today 2012, 15 (5), 196–206. [Google Scholar]
- (35).(a) Lee K; Silva EA; Mooney DJ Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc., Interface 2011, 8 (55), 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mehta M; Schmidt-Bleek K; Duda GN; Mooney DJ Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Delivery Rev 2012, 64 (12), 1257–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Shiratori SS; Rubner MF pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33 (11), 4213–4219. [Google Scholar]
- (37).Caruso F; Furlong DN; Ariga K; Ichinose I; Kunitake T Characterization of polyelectrolyte-protein multilayer films by atomic force microscopy, scanning electron microscopy, and Fourier transform infrared reflection-absorption spectroscopy. Langmuir 1998, 14 (16), 4559–4565. [Google Scholar]
- (38).Edgell TA; Gaffney PJ Soluble fibrin—characterization and assay procedures. Thromb. Haemost 1995, 73 (6), 1435–1435. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


