From December 2022 to January 2023, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections caused by BA.5 and BF.7 subvariants of B.1.1.529 (Omicron) swept across mainland China. The infection wave was rapid and widespread, impacting a large proportion of the population.1,2 Previously, we recruited a cohort of individuals that experienced Omicron BA.1, BA.2, and BA.5 infections in Jan–Feb, Mar–Jul, and Aug–Sep 2022 in Beijing and Tianjin, China, confirmed by polymerase chain reaction (PCR) (Supplementary Fig. S1 and Table S1).3, 4, 5 A control cohort with no history of SARS-CoV-2 infection and similar vaccination profiles was also recruited in the same region (Supplementary Table S1). Frequent PCR testing and epidemiological investigations based on the zero-COVID interventions ensured no additional infections prior to the current outbreak.
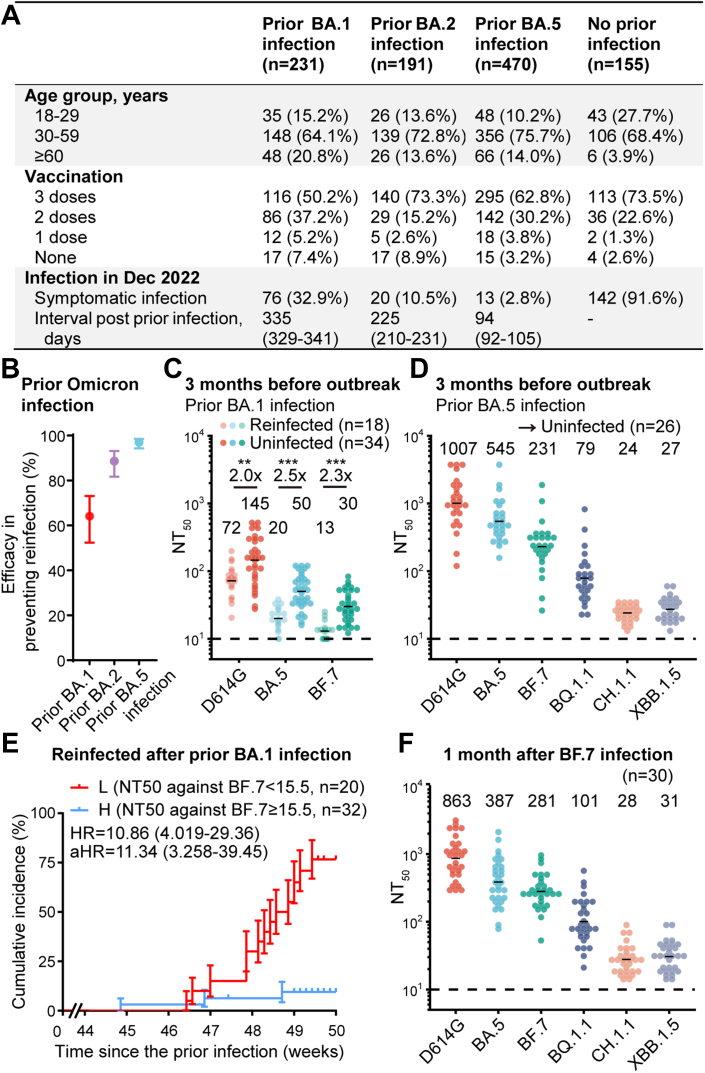
BF.7 was the predominant subvariant in Beijing and Tianjin during the December outbreak, with BF.7/BA.5 accounting for 68%/32% and 58%/42%, respectively.6,7 We found that in the previously uninfected cohort, the total symptomatic infection rate surveyed during December 2022 to January 2023 is 91.6%. Among individuals with prior Omicron BA.1 (n = 231), BA.2 (n = 191) and BA.5 (n = 470) infections, symptomatic infection rates were 32.9%, 10.5% and 2.8%, respectively, and median intervals of infections were 335, 225 and 94 days. Due to the differences in time intervals for different subvariants, it indicated mixed outcomes influenced by both variables (Fig. 1A). Note that the confirmation of infection was based on a survey with at least one of symptoms of fever, cough, pharyngalgia, nasal congestion, nasal discharge, diarrhea, emesis, allotriogeustia/heterosmia, cephalalgia, and fatigue, not by strict screening using PCR or antigen testing, which is a limitation of the study. Compared to previously uninfected individuals, prior Omicron infection was associated with reduced susceptibility to the current BF.7/BA.5 subvariant epidemic, with a relative protection rate of 64.1% (95% CI: 52.4%–73.1%), 88.6% (95% CI: 81.7%–93.1%) and 97.0% (95% CI: 94.3%–98.5%) for previous BA.1, BA.2 and BA.5 infections (Fig. 1B).
Fig. 1.
Protection by humoral immune responses in individuals with prior Omicron infection. (A) Demographic characteristics of individuals previously infected with Omicron BA.1/BA.2/BA.5 and those not infected with SARS-CoV-2, as well as the infection status in December 2022. Continuous variables were shown in median (interquartile ranges) and categorical variables were summarized as counts (percentages). (B) The efficacy of the prior Omicron infections in preventing the ongoing epidemic of BA.5/BF.7 compared to previously uninfected individuals. The efficacy was estimated by the formula 100 × (1 − IRR), where IRR is the calculated ratio of incidence in the prior Omicron infection group to that in the control group with Bayesian beta-binomial model. (C) Plasma 50% neutralization titres (NT50) against SARS-CoV-2 D614G, BA.5, and BF.7 pseudovirus in those infected with prior BA.1 and reinfection (n = 18) and infected with prior BA.1 without reinfection (n = 34). The geometric mean titres are labeled, and dashed lines show the detection limits (NT50 = 10). Statistical significance was determined by a two-tailed Mann–Whitney test. (D) Plasma 50% neutralization titres (NT50) against SARS-CoV-2 D614G, BA.5, BF.7, BQ.1.1, CH.1.1, and XBB.1.5 pseudovirus in those infected with prior BA.5 without reinfection (n = 26). Plasma samples were collected approximately 3 months before the pandemic (1 month after prior infection). The geometric mean titres are labeled, and dashed lines show the detection limits. (E) Cumulative incidence of reinfection in previously BA.1-infected individuals (n = 52), stratified on plasma NT50 against BF.7 subvariant above or below the threshold calculated by the ROC curve in Fig. S4. HRs (95% Confidence Intervals) from Log-rank test and aHR standed for adjusted HR from Cox regression model after adjustment with age, gender and vaccination. (F) Plasma 50% neutralization titres (NT50) against SARS-CoV-2 D614G, BA.5, BF.7, BQ.1.1, CH.1.1, and XBB.1.5 pseudovirus in those infected with BF.7 (n = 30). Plasma samples were collected approximately 1 month after prior infection. The geometric mean titres are labeled, and dashed lines show the detection limits (NT50 = 10).
Samples of infections caused by the same subvariant at different time intervals are lacking in this study, which is difficult to achieve in the real world. Thus, the current data prevented us from evaluating whether the antigenic distance of subvariants or the interval from the initial infection causes the differences in protection rates. However, the effects of both variables were reflected in specific neutralising antibody titres, so we set out to estimate the correlation between the titres and the protection rates. The continuous follow-up of the cohort allowed us to collect plasma samples approximately 3 months prior to the infection wave in Dec 2022 (Supplementary Table S2 and Supplementary Data). Pseudovirus neutralization assays were performed in the plasma from previously Omicron BA.1-infected individuals to estimate the correlation between neutralising antibody titres and the protective effect against symptomatic reinfection. Importantly, the geometric mean of the 50% neutralising titres (NT50) against D614G, BA.5, and BF.7 were 2.0, 2.5, and 2.3-fold higher in individuals without symptomatic reinfection than in those with symptomatic reinfection (p < 0.01, Fig. 1C). And there was no difference in neutralising antibody titres between symptomatic reinfected individuals without confirmed pathogen detection and those with positive PCR/antigen detection results (Supplementary Fig. S2). We also collected plasma samples approximately 3 months prior to the current outbreak from those previously infected with BA.5 subvariant. Plasma NT50 showed high levels of neutralization against Omicron BA.5 subvariants, which accounts for the rare symptomatic reinfection cases (Fig. 1D and Supplementary Fig. S3). Low plasma neutralising antibody titer against BF.7 subvariant (below 15.5, the threshold calculated by the receiver operating characteristic curve, Supplementary Fig. S4) of the BA.1-infected cohort was associated with an enhanced cumulative risk of symptomatic reinfection, with a hazard ratio (HR) of 10.86 (95% CI: 4.019–29.36) (Fig. 1E). The same applies to NT50 against BA.5 subvariant (Supplementary Fig. S5). Multivariate regression suggested high neutralising titres against BF.7 and BA.5 were indicative of a reduced probability of being symptomatically reinfected in the current outbreak, with a relative risk (RR) of 0.063 (95% CI: 0.009–0.422) for BF.7 and a RR of 0.067 (95% CI: 0.009–0.506) for BA.5 by adjusting previous vaccination (Supplementary Table S3). Together, these results showed a robust correlation between the plasma neutralising titres and the protective effect against Omicron symptomatic reinfection. Notably, the reason such a significant correlation was observed here could be owing to the extensive viral exposure of the population during the outbreak, which minimizes the chance that uninfected individuals were due to the lack of viral exposure.
It is crucial to estimate the protective effect of the neutralising antibodies generated by such mass BF.7/BA.5 infections against the next SARS-CoV-2 reinfection wave, potentially driven by the newly-emerged Omicron variants CH.1.1, BQ.1.1, or XBB.1.5. A BF.7 cohort of 30 individuals initially infected with BF.7 subvariant were additionally recruited and plasma was collected at approximately 1 month after the infection (Supplementary Table S2 and Supplementary Data). Plasma samples from BA.5 cohort in Fig. 1D were also collected approximately 1 month post-infection. We measured the neutralising titres against BQ.1.1, CH.1.1, and XBB.1.5 in plasma collected from individuals one month after BF.7 (Fig. 1F) or BA.5 (Fig. 1D) infection. The NT50 values of plasma from BF.7 or BA.5 infection against CH.1.1 and XBB.1.5 (24–31) are similar to that threshold against BF.7 and BA.5 (15.5–29.5) of plasma from individuals with prior BA.1 infection with or without experiencing a symptomatic BF.7/BA.5 reinfection during the December outbreak. Given that the plasma of BA.1 cohort was collected 3 months before the outbreak and plasma from BF.7 or BA.5 patients were collected 1 month after infection, this suggests that the humoral immunity generated by the current BF.7 or BA.5 infection may provide at least 4 months of protection against the CH.1.1 and XBB.1.5 symptomatic reinfection wave, while the protection against a BQ.1.1 may last even longer. Of note, the higher hACE2 binding of XBB.1.5 may reduce the period of protection due to the potential increase of infectivity.8 Also, the lack of measurement of the mucosal and cellular immunity would add uncertainty to the estimation, which is a limitation of the study.
Contributors
Y.C. designed the study. X.C. and Y.C. wrote the manuscript. W.S., A.Y., F.S., and Y.C. performed the pseudovirus neutralization assays and analyzed the neutralization data. X.C., Y. Xu., Y. Xie, and S.Y. accessed and verified the underlying data, and performed the statistical analyses. Y. Xu., Y. Xie., L.G., H.G., S.Z., R.J., and Z.S. recruited and performed the follow-up in prior Omicron-infected cohorts and individuals with no SARS-CoV-2 infection history. X.C., Y.H., Y.W., and Y.S. collected plasma samples from previously Omicron-infected individuals. Y.C. were responsible for the decision to submit the manuscript.
Declaration of interests
Y.C. is a co-founder of Singlomics Biopharmaceuticals and inventor of provisional patents associated with SARS-CoV-2 neutralising antibodies. All other authors declare no competing interests.
Acknowledgments
We thank all the volunteers involved in this study for providing critical information and peripheral blood samples. This project is financially supported by the Ministry of Science and Technology of China and Changping Laboratory (2021A0201; 2021D0102), and the National Natural Science Foundation of China (32222030). The funders of the study had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100758.
Contributor Information
Zhongyang Shen, Email: zhongyangshen@nankai.edu.cn.
Yunlong Cao, Email: yunlongcao@pku.edu.cn.
Appendix A. Supplementary data
References
- 1.Leung K., Lau E.H.Y., Wong C.K.H., Leung G.M., Wu J.T. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after adjustment of the zero-COVID policy in November-December 2022. Nat Med. 2023 doi: 10.1038/s41591-023-02212-y. published online Jan 13. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T., Mi B., Shen M., et al. Exploring the transmission dynamics of the COVID-19 outbreaks after Dec. 2022 in Shaanxi Province, China: analysis of baseline data from a large scale cohort. medRxiv. 2023 doi: 10.1101/2023.01.24.23284952. [DOI] [Google Scholar]
- 3.Zheng H., Cao Y., Chen X., et al. Disease profile and plasma neutralising activity of post-vaccination Omicron BA.1 infection in Tianjin, China: a retrospective study. Cell Res. 2022;32:781–784. doi: 10.1038/s41422-022-00674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y., Jian F., Wang J., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2022;614:521–529. doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Center for Disease Control and Prevention . 2023. COVID-19 clinical and surveillance data.https://weekly.chinacdc.cn/news/covid-surveillance/bfa0d054-d5bf-42bb-b8b4-f7ce34539b74_en.htm [Google Scholar]
- 7.hCov19 variants - GISAID. https://gisaid.org/hcov19-variants/
- 8.Yue C., Song W., Wang L., et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. 2023;23:278–280. doi: 10.1016/S1473-3099(23)00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.