Abstract
Wastewater-based epidemiology (WBE) has been demonstrated for its great potential in tracking of coronavirus disease 2019 (COVID-19) transmission among populations despite some inherent methodological limitations. These include non-optimized sampling approaches and analytical methods; stability of viruses in sewer systems; partitioning/retention in biofilms; and the singular and inaccurate back-calculation step to predict the number of infected individuals in the community. Future research is expected to (1) standardize best practices in wastewater sampling, analysis and data reporting protocols for the sensitive and reproducible detection of viruses in wastewater; (2) understand the in-sewer viral stability and partitioning under the impacts of dynamic wastewater flow, properties, chemicals, biofilms and sediments; and (3) achieve smart wastewater surveillance with artificial intelligence and big data models. Further specific research is essential in the monitoring of other viral pathogens with pandemic potential and subcatchment applications to maximize the benefits of WBE beyond COVID-19.
Keywords: COVID-19, SARS-CoV-2, Wastewater-based epidemiology, Sewer, Artificial intelligence, Influenza
Introduction
Wastewater-based epidemiology (WBE), also known as wastewater surveillance, is a low-cost, objective, and near real-time approach for monitoring disease causing pathogens that complements clinical testing and epidemiological survey. By measuring viral genetic fragments in untreated wastewater over time, public health officials can determine whether infections are increasing or decreasing in a sewer catchment [1, 2∗, 3, 4, 5, 6]. Unlike conventional epidemiological surveillance techniques, WBE does not depend on access to clinical testing [7]. It can be implemented in many communities with a reticulated sewerage system, or even in developing countries with fragmented or open sewers [8] or even by sampling river/surface water instead [9].
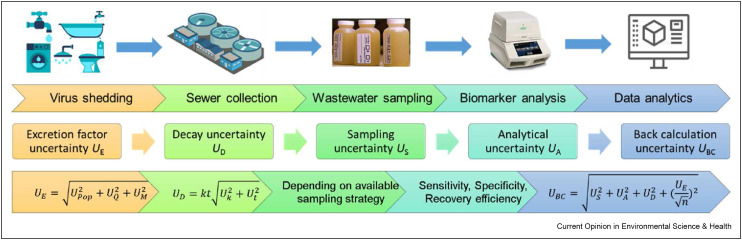
However, the potential of WBE is limited by its several methodological uncertainties (Figure 1 ) in virus shedding rates [10,11], in-sewer decay [12,13], sampling [14, 15, 16], virus concentration and detection [20], and back-calculations to predict community prevalence [17]. As a wastewater-based approach, WBE inherently neglects environmental factors (e.g., weather), epidemic intervention (e.g., masking or social distancing), and pharmaceutical treatment (e.g., vaccination) [2]. Our recent systematic review found that the correlation between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) concentration in wastewater and clinically confirmed cases ranged from −0.38 to 0.99 [18]. These findings highlight the limitations of current WBE for viral diseases, including low accuracy and irreproducible results, and the lack of advanced data analytics to make the best use of WBE data. Moving forward with coronavirus disease 2019 (COVID-19), it is essential to further improve WBE methodology as a more useful tool in managing infectious diseases caused by human coronaviruses and other viral pathogens.
Figure 1.
Workflow and methodological uncertainties for wastewater-based epidemiology of viral infectious diseases [17].
An online scientific workshop was held at the International Water Association ICA Conference 2022 on 16 October 2022 (https://www.iwa-ica2022.com/En/Menu/D7E7E20C-7FBA-4098-92EC-FC820A6EA14A/a6a15b12-3241-4f37-bd22-165476d79194) to bring together scientific researchers, public health officials and decision makers with different research backgrounds in the field of WBE to discuss the limitations and issues of routine monitoring of SARS-CoV-2 in sewage systems for early warning, tracking case numbers, variant detection, transmission dynamics, and the efficacy of prevention and control of major epidemics. In addition to the exchange and learning of current WBE advances, involving WBE experts and scholars, a survey was conducted to identify future research gaps on both WBE methodology and applications. This paper summarizes the discussions and findings from the workshop, and it is aimed to provide a synthesized expert opinion on the prospect of not only how to improve the WBE methodologies but also how to maximize the benefits of using WBE for COVID-19 and other significant pathogens beyond the pandemic.
Future research to improve the WBE methodologies
Despite of many advantages and wide deployments, many WBE programs were unable to provide accurate case numbers or transmission trends due to several associated factors. Current wastewater surveillance suffers from some inherent limitations.
-
(1)
Different protocols have been used in different jurisdictions/programs for the wastewater sampling, virus concentration, RNA extraction, RT-qPCR analysis, genome sequencing, and data analysis. Unfortunately, all these protocols led to varying recoveries of viruses depending on the protocol itself and operational personnel. Thus, measurements of viral concentrations using different protocols, instruments, and personnel are difficult to compare and reproduce.
-
(2)
There is a lack of understanding on the stability of viral nucleic acids and partitioning behaviors of viruses in wastewater during the transport in sewers immediately after shedding. Although it is known that physicochemical properties of wastewater such as temperature play an important role in viral decay and partitioning of coronaviruses and other viruses [13,19,20], the impacts of other factors such as sewer biofilms, sediments, chemical dosing and disposal to sewers, inflow/infiltration (dilution of wastewater) have not yet been investigated systematically. These in-sewer processes can lead to underestimation or overestimation of virus concentrations in wastewater in different sewer catchments.
-
(3)
The conventional back-calculation step of most previous applications of WBE was designed to estimate one single parameter such as the chemical use/exposure or prevalence of infectious disease. There is a lack of research to enhance the data analytics for a range of outputs related to viral infectious diseases such as incidence rate, prevalence rate, transmission rate, hospital and ICU admissions, and early warning. Also, relatively few studies have tried to translate wastewater surveillance data into actionable information to support pandemic management.
The following three future research prospects are thus proposed to address these research gaps related to WBE methodologies.
To standardize wastewater sampling, analysis, and data reporting protocols
Many different protocols were developed for sampling (grab, composite, passive sampling, etc. [21,22]) and virus concentration, RNA extraction and RT-qPCR assays (e.g., 36 methods evaluated by BM Pecson et al. [23]). Also, different organizations, institutions, and government departments have been providing various guidelines, standards, and protocols for wastewater surveillance. Although polymerase chain reaction (PCR)-based detection is the mostly used method and a de facto gold standard, researchers are continuously developing more sensitive and higher throughput analytical methods such as COPMAN and EPISENS-S [24, 25, 26, 27]. This leads to the generation of inconsistent data from different testing facilities such that results may not be comparable within or among different programs or jurisdictions. A recent literature survey and meta-analysis indicated highly varying performances and very low comparability of different wastewater surveillance studies in estimating the COVID-19 cases [18]. Diverse sampling and analytical methods were identified as contributing factors for low accuracy and reproducibility for virus quantification in wastewater [17]. Current analytical approaches recover about 10–80% of the SARS-CoV-2 RNA from wastewater [17,28,29]. It is thus critical to improve the reproducibility and comparability of results by standardizing wastewater sampling, analysis, and data reporting protocols. Through systematic review, meta-analysis, interlaboratory validation, consensus review (a best practice panel), and other standardization work, it is hoped that a de facto or published standard will be formed to provide guidance and best-practice information to the wastewater surveillance of viral infectious diseases.
To understand the in-sewer viral stability and partitioning
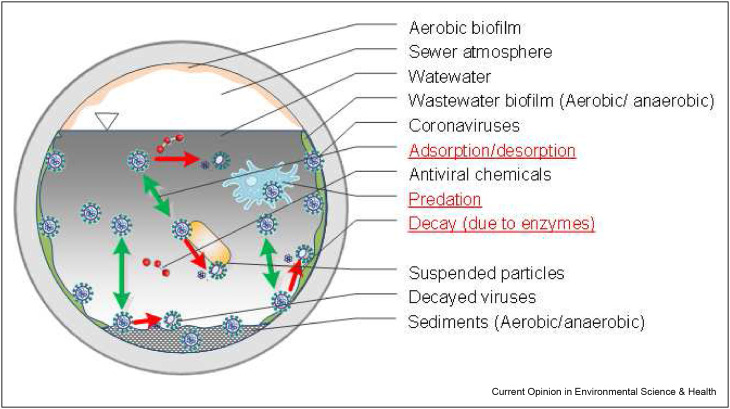
Since 2020, many reports of SARS-CoV-2 detection in wastewater emerged although only a few investigated the fate of coronaviruses in sewers [12,30,31]. Most previous studies on the fate of viruses in wastewater have focused on nonenveloped enteric viruses such as polioviruses and noroviruses [32]. Human coronaviruses are enveloped viruses, in comparison, which showed higher decay [20] and different partitioning behavior [33] in wastewater due to the different functional groups on the outer surfaces. The decay of viruses in wastewater is due to proteolytic enzymes on the lipid envelope, RNA degradation by the RNases, predation by non-host organisms, denaturation of proteins and nucleic acids by high temperature or acid, and the presence of antiviral chemicals [34,35] (Figure 2 ). The influence of different wastewater properties, chemical dosing and sewer operations on viral decay, and partitioning are largely unknown [13,20,36]. A handful of studies investigated the decay of coronaviruses in wastewater and mainly reported the impacts of temperature [13]. The epidemic recurrent potential of coronaviruses and the potential role of wastewater surveillance merit systematic research on their stability and partitioning during the transport in sewers. Such knowledge about the viruses in sewers can be utilized together with the sewer system parameters (e.g., area to volume ratio, wastewater dilution, flow dynamics) to greatly improve the WBE back-calculation accuracy.
Figure 2.
In-sewer factors (black font) and processes (red font, underlined) affecting the stability and partitioning of viral biomarkers in wastewater. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To empower WBE with artificial intelligence and big data
The back-calculation of infected cases by wastewater surveillance can be done using Eq. (1).
| (Eq. 1) |
where I catchment = the case number in the sewer catchment (−); C RNA = the viral concentration in wastewater (gc/L); F = the wastewater flow rate (L/d); D = the decay ratio in sewers (−); P = the population (−); and E = the viral excretion rate (gc/d per person) [17].
This back-calculation is usually impractical due to the dynamic viral shedding, unknown decay/partitioning in sewers, population mobility, and a single output with high uncertainty. Many WBE studies struggled to achieve accurate and reliable back-calculation [18]. Some recent studies explored the potential of machine learning models by integrating WBE data with sewer catchment, weather, clinical testing, and vaccination data [2,37]. The developed artificial neural network model provided accurate estimations of COVID-19 incidence, prevalence, and effective reproduction rate in Utah and Wisconsin, USA [2]. In addition, other similar studies [38,39] indicate the great potential of advanced data analytics in managing COVID-19 by harnessing artificial intelligence (AI) and big data. The wastewater surveillance data can be fed into various actionable strategies like nonpharmaceutical interventions, instead of simply providing infection case numbers. The AI and big data supported smart wastewater surveillance can lead to novel data-driven and evidence-based pandemic management that will minimize viral transmission and societal disruption during an outbreak.
Beyond COVID-19: maximize WBE benefits in applications
Pathogenic viruses with RNA as their genetic material can mutate because of high error rates of the viral genome replicating enzymes (polymerases). History has shown that RNA viral infectious diseases emerge and re-emerge from time to time. Coronaviruses, as a class of enveloped, positive-sense single-stranded RNA virus, cause various diseases in humans, including three devastating coronavirus pandemics within the last 20 years: severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the current COVID-19. Influenza viruses with segmented, single-stranded RNA genomes have caused more than 13 worldwide outbreaks (pandemics), including the Spanish influenza pandemic that killed more than 40 million people [40]. Recurrent coronavirus and influenza epidemics and their pandemic potential are significant risks to global health.
The last three years have witnessed the intensive research, public recognition, and implementation of WBE to detect SARS-CoV-2 RNA in wastewater. Indeed, wastewater contains more than 100 pathogenic viruses, which are primarily RNA viruses that potentially mutate and cause multiple outbreaks [35]. However, limited attention has been paid to other viruses such as influenza. Little research has been done for influenza viruses in wastewater, except for a few very recent wastewater surveillance reports about influenza subtypes A and B viruses [41,42]. Although many studies have investigated the decay of influenza viruses in fresh or marine waters, no wastewater studies have been reported yet [20]. To maximize the benefits of WBE and its applications, wastewater surveillance should be adopted in the monitoring of SARS-CoV-2 variants [43,44], simultaneous monitoring of multiple viruses (e.g., influenza virus and SARS-CoV-2) [45], and emerging viruses such as Monkeypox virus (MPX) [46].
Some research has also been done on the use of WBE in subcatchment levels, such as for university campuses, prisons, high-rise buildings, aircrafts, and cruise ships [47, 48∗, 49, 50]. However, the purpose of such applications and the implications to the WBE methodology can vary a lot from the conventional WBE application within large catchments, that is, wastewater sampling from the inlet of wastewater treatment plants. Much smaller catchments lead to very short hydraulic retention time in sewers, which has implications for the presence of infectious viral particles and potential infection risks [12]. The sporadic shedding patterns and highly fluctuating wastewater flow make it harder for the wastewater sampling to capture viruses, and thus, passive samplers might be more appropriate in these small-catchment or low-prevalence applications [51,52]. Further research needs to investigate application specific WBE methodology to maximize the benefits.
Conclusions
This paper identified the following future research prospects of WBE while the international community is moving forward with COVID-19.
-
•
For the sensitive and reproducible detection of viruses in wastewater, future WBE research should be conducted to standardize the best-practice wastewater sampling, analysis, and data reporting protocols.
-
•
The WBE accuracy can be greatly increased with the understanding of the in-sewer viral stability and partitioning under the impacts of dynamic wastewater flow, properties, chemicals, biofilms, and sediments.
-
•
Smart wastewater surveillance offers a new era for WBE due to the involvement of artificial intelligence and big data for diversified results, actionable data, and measurable outcomes.
-
•
To maximize the benefits of WBE, further research and development are needed for the management of other viral pathogens beyond COVID-19, and specific applications at various catchment and subcatchment levels.
Editorial disclosure statement
Given their role as Guest Editor, Manish Kumar and Ryo Honda had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Payal Mazumder.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Guangming Jiang reports financial support was provided by Australian Research Council. Yanchen Liu reports financial support was provided by National Natural Science Foundation of China. Yanchen Liu reports financial support was provided by Tsinghua University. Guangming Jiang reports financial support was provided by Australian Academy of Science.
Acknowledgments
This research was supported by the Australian Research Council Discovery project (DP190100385) and a COVID-19 Digital Grant funded by Australian Academy of Science and the Department of Industry, Science, Energy and Resources through the Regional Collaborations Programme. This research was also supported by the Major Program of National Natural Science Foundation of China (No. 52091543), Tsinghua University Spring Breeze Fund (No. 20213080026), and the Key Project of the Capital′s Funds for Health Improvement and Research, China (No. 2022-1G-4231).
This review comes from a themed issue on Occupational Safety and Health 2022: COVID-19 in environment: Treatment, Infectivity, Monitoring, Estimation
Edited by Manish Kumar, Ryo Honda, Prosun Bhattacharya, Dan Snow and Payal Mazumder
Data availability
No data was used for the research described in the article.
References
- 1.Kumar M., Jiang G., Kumar Thakur A., Chatterjee S., Bhattacharya T., Mohapatra S., Chaminda T., Kumar Tyagi V., Vithanage M., Bhattacharya P., et al. Lead time of early warning by wastewater surveillance for COVID-19: geographical variations and impacting factors. Chem Eng J. 2022;441:135936. doi: 10.1016/j.cej.2022.135936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Wu J., Weidhaas J., Li X., Chen Y., Mueller J., Li J., Kumar M., Zhou X., Arora S., et al. Artificial neural network-based estimation of COVID-19 case numbers and effective reproduction rate using wastewater-based epidemiology. Water Res. 2022;218:118451. doi: 10.1016/j.watres.2022.118451. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper for the first time developed and demonstrated the smart wastewater surveillance based on artificial neural network for the tracking of COVID-19 prevalence and transmission in communities. The study was based on a large and year long dataset from Wisconsin and Utah sewage surveillance programs. It has been transferred to Wisconsin Department of Health for their test and implementation shortly after the publication.
- 3.Larsen D.A., Wigginton K.R. Tracking COVID-19 with wastewater. Nat Biotechnol. 2020;38:1151–1153. doi: 10.1038/s41587-020-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. e00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahan C.S., Self S., Rennert L., Kalbaugh C., Kriebel D., Graves D., Colby C., Deaver J.A., Popat S.C., Karanfil T., et al. COVID-19 wastewater epidemiology: a model to estimate infected populations. Lancet Planet Health. 2021;5:e874–e881. doi: 10.1016/S2542-5196(21)00230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., Clarke L., Dwyer J., Edson J., Nguyen T.M.H., et al. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci Total Environ. 2021;761:144216. doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ Int. 2020;139:105689. doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora S., Nag A., Kalra A., Sinha V., Meena E., Saxena S., Sutaria D., Kaur M., Pamnani T., Sharma K., et al. Successful application of wastewater-based epidemiology in prediction and monitoring of the second wave of COVID-19 with fragmented sewerage systems–a case study of Jaipur (India) Environ Monit Assess. 2022;194:342. doi: 10.1007/s10661-022-09942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Zhang L., Wang Y., Zhang M., Zhou J., Liu X., Wang Y., Qu C., Han W., Hou M., et al. Detection of the SARS-CoV-2 delta variant in the transboundary rivers of Yunnan, China. ACS ES&T Water. 2022;2:2367–2377. doi: 10.1021/acsestwater.2c00224. [DOI] [PubMed] [Google Scholar]
- Li X., Kulandaivelu J., Guo Y., Zhang S., Shi J., O'Brien J., Arora S., Kumar M., Sherchan S.P., Honda R., et al. SARS-CoV-2 shedding sources in wastewater and implications for wastewater-based epidemiology. J Hazard Mater. 2022;432:128667. doi: 10.1016/j.jhazmat.2022.128667. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed that in addition to feces, sputum can be another major shedding source of SARS-CoV-2 in wastewater. This revolutionary finding fundamentally changed how wastewater surveillance is used to back-calculate the infections in the sampled communities. It also identify a potential reason for low accuracy of previous studies on using wastewater surveillance for the tracking of COVID-19 prevalence.
- 11.Miura F., Kitajima M., Omori R. Duration of SARS-CoV-2 viral shedding in faeces as a parameter for wastewater-based epidemiology: Re-analysis of patient data using a shedding dynamics model. Sci Total Environ. 2021;769:144549. doi: 10.1016/j.scitotenv.2020.144549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Li X., Zhang S., Sharma E., Sivakumar M., Sherchan S.P., Jiang G. Enhanced decay of coronaviruses in sewers with domestic wastewater. Sci Total Environ. 2022;813:151919. doi: 10.1016/j.scitotenv.2021.151919. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported the infectivity and its decay of two surrogate coronaviruses, HCoV-229E and FIPV in the presence of sewer biofilms. This is the first of such studies about the fate of infective coronaviruses in sewers.
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigated the decay of RNA from SARS-CoV-2 and a potential surrogate, murine hepatitis virus (MHV) in wastewater, supporting the use of RNA for wastewater-based epidemiology due to its relative stability in wastewater.
- 14.Ahmed W., Bivins A., Bertsch P.M., Bibby K., Gyawali P., Sherchan S.P., Simpson S.L., Thomas K.V., Verhagen R., Kitajima M., et al. Intraday variability of indicator and pathogenic viruses in 1-h and 24-h composite wastewater samples: implications for wastewater-based epidemiology. Environ Res. 2021;193:110531. doi: 10.1016/j.envres.2020.110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertels X., Demeyer P., Van den Bogaert S., Boogaerts T., van Nuijs A.L.N., Delputte P., Lahousse L. Factors influencing SARS-CoV-2 RNA concentrations in wastewater up to the sampling stage: a systematic review. Sci Total Environ. 2022;820:153290. doi: 10.1016/j.scitotenv.2022.153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res X. 2021;10:100086. doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem Eng J. 2021;415:129039. doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study divided the wastewater surveillance (wastewater-based epidemiology) approach into five steps (i.e., virus shedding; in-sewer transportation; sampling and storage; analysis of SARS-CoV-2 RNA concentration in wastewater; back-estimation) and further quantified the uncertainties associated with each step. The results supports the application of wastewater surveillance as a complementary strategy for monitoring COVID-19 prevalence and provides methodological improvements to enhance the reliability.
- 18.Li X., Zhang S., Sherchan S., Orive G., Lertxundi U., Haramoto E., Honda R., Kumar M., Arora S., Kitajima M., et al. Correlation between SARS-CoV-2 RNA concentration in wastewater and COVID-19 cases in community: a systematic review and meta-analysis. J Hazard Mater. 2023;441:129848. doi: 10.1016/j.jhazmat.2022.129848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence of enveloped viruses in environmental waters and wastewater in the absence of disinfectants. Environ Sci Technol. 2021;55:14480–14493. doi: 10.1021/acs.est.1c03977. [DOI] [PubMed] [Google Scholar]
- 21.Ort C. Quality assurance & quality control of environmental field sampling. 2014. Quality assurance/quality control in wastewater sampling; pp. 146–168. [Google Scholar]
- 22.O'Reilly K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. The Lancet Microbe. 2020;1:e189–e190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pecson B.M., Darby E., Haas C.N., Amha Y.M., Bartolo M., Danielson R., Dearborn Y., Di Giovanni G., Ferguson C., Fevig S., et al. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the U.S. Environ Sci Water Res Technol. 2021;7:504–520. doi: 10.1039/d0ew00946f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi Katayama Y., Hayase S., Ando Y., Kuroita T., Okada K., Iwamoto R., Yanagimoto T., Kitajima M., Masago Y. COPMAN: a novel high-throughput and highly sensitive method to detect viral nucleic acids including SARS-CoV-2 RNA in wastewater. Sci Total Environ. 2023;856:158966. doi: 10.1016/j.scitotenv.2022.158966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ando H., Iwamoto R., Kobayashi H., Okabe S., Kitajima M. The Efficient and Practical virus Identification System with ENhanced Sensitivity for Solids (EPISENS-S): a rapid and cost-effective SARS-CoV-2 RNA detection method for routine wastewater surveillance. Sci Total Environ. 2022;843:157101. doi: 10.1016/j.scitotenv.2022.157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed W., Bivins A., Korajkic A., Metcalfe S., Smith W.J.M., Simpson S.L. Comparative analysis of Adsorption-Extraction (AE) and Nanotrap® Magnetic Virus Particles (NMVP) workflows for the recovery of endogenous enveloped and non-enveloped viruses in wastewater. Sci Total Environ. 2023;859:160072. doi: 10.1016/j.scitotenv.2022.160072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari A., Ahmed W., Oikarinen S., Sherchan S.P., Heikinheimo A., Jiang G., Simpson S.L., Greaves J., Bivins A. Application of digital PCR for public health-related water quality monitoring. Sci Total Environ. 2022;837:155663. doi: 10.1016/j.scitotenv.2022.155663. [DOI] [PubMed] [Google Scholar]
- 28.Kantor R.S., Nelson K.L., Greenwald H.D., Kennedy L.C. Challenges in measuring the recovery of SARS-CoV-2 from wastewater. Environ Sci Technol. 2021;55:3514–3519. doi: 10.1021/acs.est.0c08210. [DOI] [PubMed] [Google Scholar]
- 29.Kumblathan T., Liu Y., Qiu Y., Pang L., Hrudey S.E., Le X.C., Li X.-F. An efficient method to enhance recovery and detection of SARS-CoV-2 RNA in wastewater. J Environ Sci. 2023;130:139–148. doi: 10.1016/j.jes.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morales Medina W.R., D’Elia S., Fahrenfeld N.L. Accumulation of SARS-CoV-2 RNA in sewer biofilms. ACS ES&T Water. 2022;2:1844–1851. [Google Scholar]
- 31.Guo Y., Li X., Luby S., Jiang G. Vertical outbreak of COVID-19 in high-rise buildings: the role of sewer stacks and prevention measures. Curr Opin Environ Sci Health. 2022;29:100379. doi: 10.1016/j.coesh.2022.100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 34.Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ Int. 2020;143:105962. doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ Sci: Water Res Technol. 2015;1:735–746. [Google Scholar]
- 36.Anand U., Li X., Sunita K., Lokhandwala S., Gautam P., Suresh S., Sarma H., Vellingiri B., Dey A., Bontempi E., et al. SARS-CoV-2 and other pathogens in municipal wastewater, landfill leachate, and solid waste: a review about virus surveillance, infectivity, and inactivation. Environ Res. 2022;203:111839. doi: 10.1016/j.envres.2021.111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Kulandaivelu J., Zhang S., Shi J., Sivakumar M., Mueller J., Luby S., Ahmed W., Coin L., Jiang G. Data-driven estimation of COVID-19 community prevalence through wastewater-based epidemiology. Sci Total Environ. 2021;789:147947. doi: 10.1016/j.scitotenv.2021.147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S., Pierson E., Koh P.W., Gerardin J., Redbird B., Grusky D., Leskovec J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2021;589:82–87. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- 39.Ruktanonchai N.W., Floyd J.R., Lai S., Ruktanonchai C.W., Sadilek A., Rente-Lourenco P., Ben X., Carioli A., Gwinn J., Steele J.E., et al. Assessing the impact of coordinated COVID-19 exit strategies across Europe. Science. 2020;369:1465–1470. doi: 10.1126/science.abc5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taubenberger J.K., Morens D.M. Pandemic influenza--including a risk assessment of H5N1. Rev Sci Tech. 2009;28:187–202. doi: 10.20506/rst.28.1.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercier E., D'Aoust P.M., Thakali O., Hegazy N., Jia J.-J., Zhang Z., Eid W., Plaza-Diaz J., Kabir M.P., Fang W., et al. Municipal and neighbourhood level wastewater surveillance and subtyping of an influenza virus outbreak. Sci Rep. 2022;12:15777. doi: 10.1038/s41598-022-20076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe M.K., Duong D., Bakker K.M., Ammerman M., Mortenson L., Hughes B., Arts P., Lauring A.S., Fitzsimmons W.J., Bendall E., et al. Wastewater-based detection of two influenza outbreaks. Environ Sci Technol Lett. 2022;9:687–692. [Google Scholar]
- Amman F., Markt R., Endler L., Hupfauf S., Agerer B., Schedl A., Richter L., Zechmeister M., Bicher M., Heiler G., et al. Viral variant-resolved wastewater surveillance of SARS-CoV-2 at national scale. Nat Biotechnol. 2022;40:1814–1822. doi: 10.1038/s41587-022-01387-y. [DOI] [PubMed] [Google Scholar]; This study reported the SARS-CoV-2 variants and spatiotemporal dyanamics at national scales, which demonstrates the power of national-scale WBE to support public health and promises particular value for countries without extensive individual monitoring.
- 44.Karthikeyan S., Levy J.I., De Hoff P., Humphrey G., Birmingham A., Jepsen K., Farmer S., Tubb H.M., Valles T., Tribelhorn C.E., et al. Wastewater sequencing reveals early cryptic SARS-CoV-2 variant transmission. Nature. 2022;609:101–108. doi: 10.1038/s41586-022-05049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malla B., Thakali O., Shrestha S., Segawa T., Kitajima M., Haramoto E. Application of a high-throughput quantitative PCR system for simultaneous monitoring of SARS-CoV-2 variants and other pathogenic viruses in wastewater. Sci Total Environ. 2022;853:158659. doi: 10.1016/j.scitotenv.2022.158659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Jonge E.F., Peterse C.M., Koelewijn J.M., van der Drift A.-M.R., van der Beek R.F.H.J., Nagelkerke E., Lodder W.J. The detection of monkeypox virus DNA in wastewater samples in The Netherlands. Sci Total Environ. 2022;852:158265. doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karthikeyan S., Nguyen A., McDonald D., Zong Y., Ronquillo N., Ren J., Zou J., Farmer S., Humphrey G., Henderson D., et al. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. mSystems. 2021;6 doi: 10.1128/mSystems.00793-21. e00793-e00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J Trav Med. 2020;27:taaa116. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study successfully demonstrated that surveillance of wastewater from large transport vessels such as aircrafts and cruise ships with their own sanitation systems has potential as a complementary data source to prioritize clinical testing and contact tracing among disembarking passengers.
- 49.Hassard F., Smith T.R., Boehm A.B., Nolan S., O'Mara O., Di Cesare M., Graham D. Wastewater surveillance for rapid identification of infectious diseases in prisons. The Lancet Microbe. 2022;3:e556–e557. doi: 10.1016/S2666-5247(22)00154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S., Dong Q., Li S., Cheng Z., Kang X., Ren D., Xu C., Zhou X., Liang P., Sun L., et al. Persistence of SARS-CoV-2 RNA in wastewater after the end of the COVID-19 epidemics. J Hazard Mater. 2022;429:128358. doi: 10.1016/j.jhazmat.2022.128358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., Verhagen R., Ahmed W., Metcalfe S., Thai P.K., Kaserzon S.L., Smith W.J.M., Schang C., Simpson S.L., Thomas K.V., et al. In situ calibration of passive samplers for viruses in wastewater. ACS ES&T Water. 2022;2:1881–1890. [Google Scholar]
- 52.Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., John N., Baker L., Scales P., Schmidt J., et al. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ Sci Technol. 2021;55:10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.