Abstract
A wide variety of analytical techniques have been employed for monitoring chemical reactions, with online instrumentation providing additional benefits compared to offline analysis. A challenge in the past for online monitoring has been placement of the monitoring instrumentation as close as possible to the reaction vessel to maximize sampling temporal resolution and preserve sample composition integrity. Furthermore, the ability to sample very small volumes from bench-scale reactions allows the use of small reaction vessels and conservation of expensive reagents. In this study, a compact capillary LC instrument was used for online monitoring of as small as 1 mL total volume of a chemical reaction mixture, with automated sampling of nL-scale volumes directly from the reaction vessel used for analysis. Analyses to demonstrate short term (~2 h) and long term (~ 50 h) reactions were conducted using tandem on-capillary ultraviolet absorbance followed by in-line MS detection or ultraviolet absorbance detection alone, respectively. For both short term and long term reactions (10 and 250 injections, respectively), sampling approaches using syringe pumps minimized the overall sample loss to ~0.2% of the total reaction volume.
Keywords: Capillary Liquid Chromatography, Compact, Portable, Reaction Kinetics, Reaction Monitoring
1. Introduction
Bench-scale reaction monitoring is a common practice in both academic and industrial settings. By tracking reactant consumption, product formation, and potential presence of impurities over time, optimization of reaction kinetics can be achieved prior to scaling up a selected synthetic route [1][2]. Generally, reaction monitoring is achieved through manual sampling and offline analysis of a reaction using techniques such as optical spectroscopy [3], GC [4], LC [5], MS [6], and/or NMR [7]. More recently, online reaction monitoring has been adopted to further increase the number of data points gathered in a given reaction as well as reduce the overall volume needed for an individual sample [8][9][10]. To further reduce the amount of sample taken from the reaction vessel for analysis, techniques that require minimal volume such as capillary LC can be implemented [11]. The automated nature of online monitoring provides for more representative sampling of the reaction, as offline approaches can lead to the loss of volatile compounds and/or unstable reaction intermediates, especially if there are long delays between sampling and analysis [9][12].
One challenge in performing on-line reaction monitoring of bench-scale reactions is the size of the instrumentation relative to the fume hood in which the reaction is performed, as lengthy tubing connections can reduce the temporal resolution of sampling due to Taylor dispersion [13]. To accommodate instrumentation within the hood adjacent to the reaction vessel, compact formats are preferred. Recently, a number of different compact LC instruments have been reported [14][15][16][17][18], with some coupled to small footprint MS instruments for detection as well [19][20][21]. For reaction monitoring purposes, a small LC system using columns in a microfluidic format was coupled to several organic reactions for on-line analysis [22]. More recently, a compact LC-UV-MS instrument was coupled to commercial reaction vessels and larger reaction workstations, primarily focused on on-line process monitoring in a pharmaceutical industry setting [11]. The same compact MS has also been coupled directly to a reaction vessel for sampling, using an in-line membrane-based phase separator rather than a chromatographic separation [23]. In this study, an integrated compact LC platform using capillary column cartridges was implemented for on-line monitoring of smaller reaction volumes (on the order of 10–30 mL) with a standard syringe pump for sampling, representing a common strategy that may be used in academic and small-scale R&D environments as an alternative to some larger-scale automated samplers [24]. As a demonstration of this approach, imine formation and acid hydrolysis reactions were monitored using compact on-line LC-UV-MS and LC-UV instruments, respectively.
2. Materials & Methods
2.1. Imine Formation Reaction Monitoring
2.1.1. Chemicals for Imine Formation
Acetonitrile and methanol used as reaction solvents and mobile phases were both OmniSolv LC-MS grade (EMD Millipore, Billerica, MA, USA). Chromasolv LC-MS grade water was acquired from Honeywell (Muskegon, MI, USA). Formic acid (98%+ purity) and 4-dimethylaminobenzaldehyde (DMABA, 98%+ purity) were obtained from TCI America (Portland, OR, USA). Isopropylamine (99% purity) was purchased from Beantown Chemical (Hudson, NH, USA).
2.1.2. Reaction Conditions for Imine Formation
The reaction between DMABA and isopropylamine is shown in Figure 1A. The approach was based on previous studies that explored various conditions for this type of reaction monitored by off-line HPLC [25]. The reaction was initiated when 30, 100, or 300 μL of isopropylamine were added to 30 mL of 0.2 mM DMABA in 1:1 acetonitrile/methanol. The reaction mixture was stirred and heated to 45°C during the entire 2–3 h reaction period, and samples were taken for analysis every 13 min.
Figure 1.
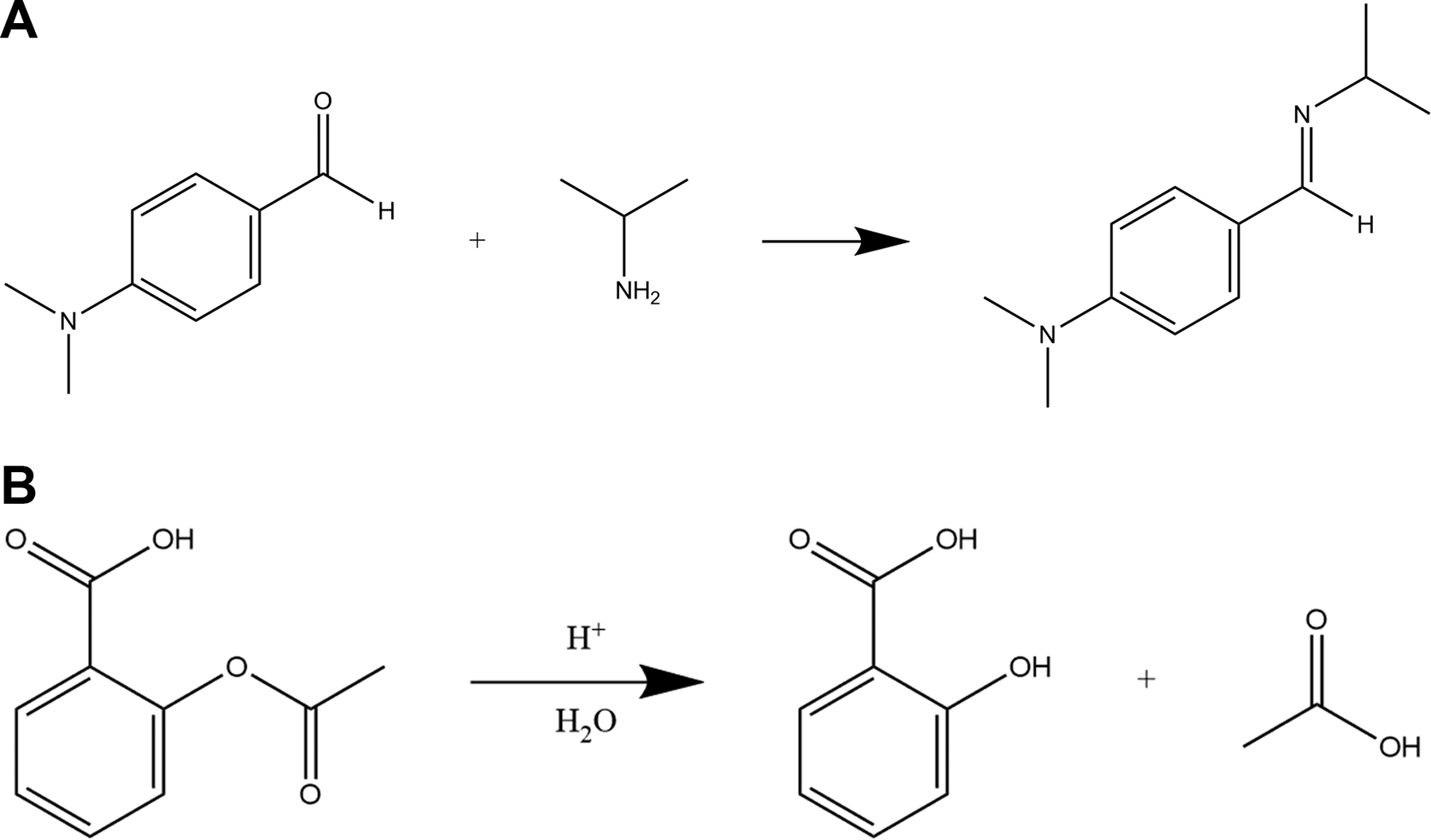
Formation reaction of N,N-Dimethyl-4-[(isopropylimino)methyl]aniline from 4-DMABA and isopropylamine is shown in (A). Acid hydrolysis reaction of acetylsalicylic acid to salicylic acid and acetic acid is shown in (B).
2.1.3. Instrument Set-Up for Imine Formation
Sample was continuously drawn from the reaction vessel and through an Axcend Focus LC (Axcend, Provo, UT, USA) 40 nL internal injection valve loop using a Fusion 200 syringe pump (Chemyx, Stafford, TX, USA), which was operated in the withdraw mode at a rate of 0.5 μL/min over the course of the reaction. Connections from the vessel to the injection valve and from the injection valve to the syringe pump were made using 30 μm inner diameter (i.d.) polyimide-coated fused silica capillaries (Polymicro Technologies, Phoenix, AZ, USA), with a 1 μm microfilter assembly (Idex, Oak Harbor, WA) placed in-line between the reaction vessel and injection valve to prevent particulate material from entering the injection loop and separation column. Separation of the reaction mixture was performed using a UV transparent (i.e., Teflon outer coating) 100 mm × 0.150 mm i.d. fused silica capillary column (Polymicro Technologies) packed with 1.7 μm C18 fully porous particles (CoAnn Technologies, Richland, WA, USA). Mobile phase A was 97:3 water/acetonitrile and mobile phase B was 3:97 water/acetonitrile, both modified with 0.1% formic acid. The mobile phase flow rate was 1.5 μL/min and the gradient program was 10%−75% B over 7 min, back to 10% B over 1 min, and then held for 1 min at 10% B. The pressure range across the gradient was 3,300 psi (high organic content) to 4,000 psi (maximum mobile phase viscosity), with an overall system pressure limit of 10,000 psi. The total cycle time was ~13 min including the syringe fill and pressurization sequence. Both on-capillary UV absorbance detection (255 nm) and single quadrupole MS detection in selected-ion-monitoring mode (MiD-4500, Microsaic, Woking, UK) at m/z values of 150 (DMABA) and 190 (imine product) were used. After the on-capillary absorbance detector, a 20 cm segment of 50 μm i.d. PEEKsil tubing (1/32” outer diameter, Trajan, Ringwood, Victoria, AU) was used to connect the column outlet to the MS source. The ESI source was set at 850 V, with a nitrogen flow rate of 2500 mL/min and a vacuum interface voltage of 70 V. A short capillary was connected to the in-source flow splitter to reduce the flow directed to the MS to 0.6 μL/min. A general schematic of this set-up is shown in Figure 2 and a photograph is shown in Figure S1.
Figure 2.
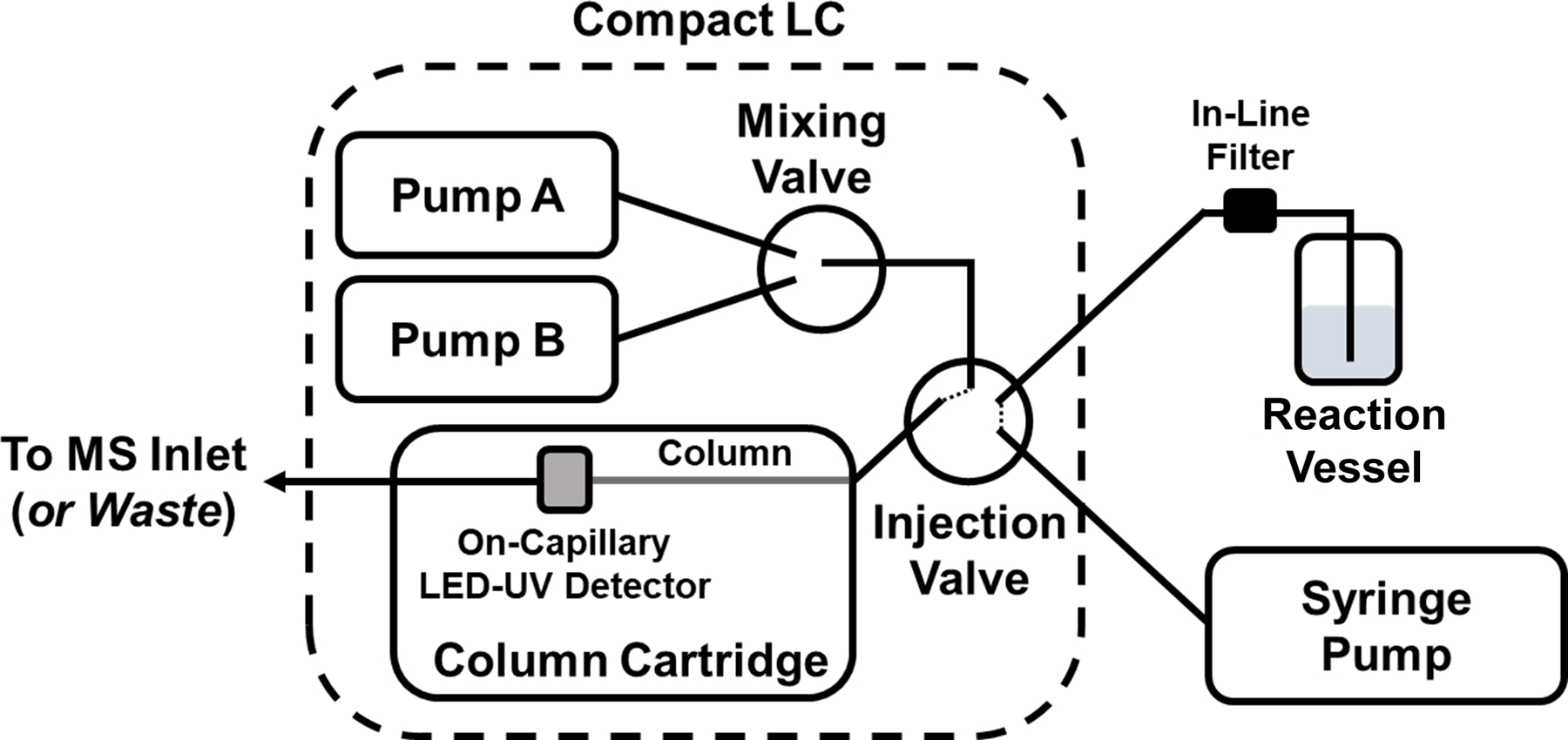
Instrument schematic for on-line sampling from a small reaction vessel into a compact LC injection loop using a syringe pump in the withdrawal mode. The capillary LC column is contained within a column cartridge that contains an on-capillary UV absorbance detector, with the column outlet connected to either the MS inlet (for on-line LC-UV-MS) or a waste line.
2.2. Aspirin Hydrolysis in Acidic Conditions
2.2.1. Chemicals for Aspirin Hydrolysis
Water, acetonitrile, phosphoric acid (all HPLC grade) and acetylsalicylic acid (aspirin) were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2.2. Reaction Conditions for Aspirin Hydrolysis
Acid hydrolysis of 10 mg of aspirin (Scheme 2) was conducted in a solution containing 0.3 mL concentrated phosphoric acid and 0.5 mL 50:50 water/acetonitrile in a 3.7 mL (one-dram) vial (i.e. the reaction vessel with stirring over an approximately 50 h period. Samples were automatically withdrawn from the reaction mixture and analyzed every 12 min.
2.2.3. Instrument Set-Up for Aspirin Hydrolysis
A similar set-up as shown in Figure 2 was used for on-line monitoring of the acid hydrolysis of aspirin (Figure 1B), the main difference being that the reaction was conducted in a smaller volume and over a longer time period compared to imine formation reaction monitoring. A prototype nano/micro LC syringe pump (ePrep, Oakleigh, AU) was used to withdraw approximately 6 μL of solution through the sampling tubing and a 4 nL internal injection loop prior to analysis, and push the remainder of the volume back into the reaction vessel immediately after injection. In this experiment, 150 μm i.d. fused silica capillaries were used for the connections between the vessel, loop, and syringe pump. The column, mobile phases, and on-column detector wavelength were the same, although the gradient program was changed to 5–95% B in 5 min at a flow rate of 2 μL/min. The pressure range across the gradient was 3,200 psi (high organic content) to 4,400 psi (maximum mobile phase viscosity). A photograph of this set-up is shown in Figure S2.
3. Results & Discussion
3.1. On-Line Reaction Monitoring of Imine Formation with Compact LC-UV-MS Instrumentation
In on-line reaction monitoring, maintaining the reaction within a chemical fume hood and bringing the instrumentation closer to the reaction vessel ensures safety and allows for use of existing reaction set-ups. To achieve this within a standard size chemical fume hood, minimizing the size of the instrument(s) is critical, e.g., LC and/or MS. Here, an integrated compact LC instrument was operated directly next to a small reaction vessel, with a small syringe pump for sample withdrawal to simplify the process compared to larger, more expensive commercial reaction samplers. The formation of an imine product through the reaction of a benzaldehyde compound and an amine-containing compound (Figure 1A) was selected as a reaction to explore because the benzaldehyde reactant and imine product could both be monitored by UV and MS detectors. Additionally, a similar reaction has previously been monitored with an on-line LC-based analysis using standard benchtop instrumentation [26].
In Figure 3A, the separation of the two species is shown as monitored by the on-column UV absorbance detector, with the peak area of the DMABA reactant (elution time of ~5.7 min) decreasing over time and the peak area of the imine product (elution time of ~3.7 min) increasing as the reaction proceeds. The extracted ion chromatograms (XICs) for each of these compounds with MS detection are shown in Figure 3B, which depict identical trends in reactant consumption and product formation. The benefit of adding MS to this on-line monitoring approach is the capability for selective mass-based detection. However, an analyte’s response to a detector cannot be assumed to remain identical across detection methods [26]. With soft MS ionization techniques such as ESI, samples with readily ionized groups are overrepresented compared to more neutral compounds [27][28], while differences in molar absorptivity can lead to similar issues with UV detection [29]. In order to account for this discrepancy, a relative response factor (RRF) can be calculated through use of an internal standard [30][31]. This poses an issue when applying internal standards to RRF calculations for on-line reaction monitoring, as the introduction of additional reagents could potentially result in the formation of side products [32][33]. Here, an alternative approach using signals from two different instruments, avoiding the need for an internal standard, was applied to the calculations of RRF for potential differences between UV absorbance and MS detection [26][33]:
| (1) |
| (2) |
To normalize the dataset across the entire reaction period, the RRF was averaged from the calculated value of each collected chromatogram. Once the RRF was established, the MS dataset was adjusted by dividing the area percent of each compound (which is defined as the ratio of the area of an individual peak to the sum of the areas of all peaks in the chromatogram) by its respective RRF. This accounted for potential discrepancies in analyte response without the need for the introduction of an internal standard. Using this approach, the reaction kinetic curves based on the chromatograms obtained with both detectors are shown in Figure 3C. The effects of increasing the isopropylamine volume on the reaction are shown in Figure S3, with increasing volumes found to increase the overall rate of reaction. Although the general trend of the curves is very similar, there are slight differences between the detectors. This can be attributed to the improved detection limits of the MS; i.e., as the reactant peak area decreases over time, it eventually reaches a point where it is nearly undetectable in the UV absorbance chromatogram, but can still be clearly observed in the mass chromatogram. Thus, for reactions performed at low concentrations and/or with compounds with low molar absorptivities at a given wavelength, the use of LC-MS can provide advantages over LC-UV.
Figure 3.
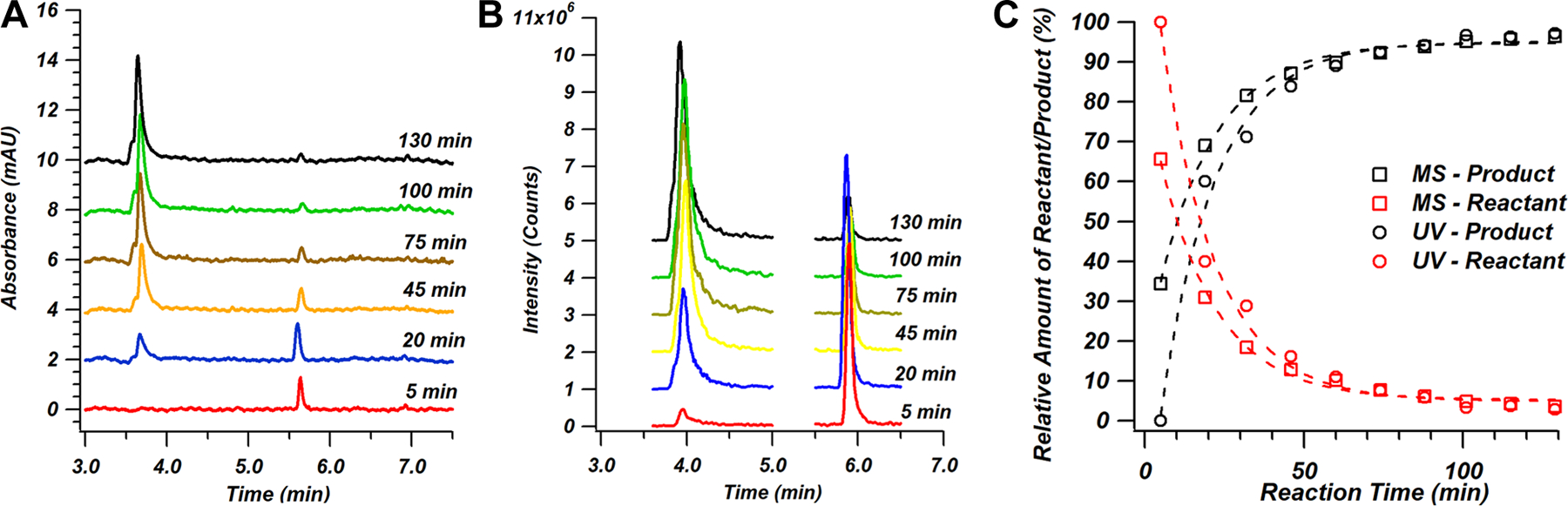
(A) Chromatographic separations of reactants and products in an imine formation reaction mixture over time monitored using UV absorbance detection at 255 nm. The peak eluting at ~3.7 min represents the imine product and the peak at ~5.7 min represents the DMABA reactant. (B) In-line secondary detection by MS in the selected-ion-monitoring mode with m/z values of 150 and 190 selected for the DMABA reactant and imine product, respectively. (C) Reaction kinetic plot showing progression of product formation for both UV and MS detection. Area percentages were calculated as described in the text and MS data were corrected using the RRF approach described in Equations 1 and 2.
The use of miniaturized instrumentation for reaction monitoring not only provides benefits in terms of instrumentation size, but also in sample size. Given the small sample loop volume (40 nL), as well as the low withdrawal rate of the syringe pump (0.5 μL/min), the volume of reaction mixture lost during sampling is negligible. For this ~2 h reaction (10 sample injections), a total sample volume of approximately 60 μL was withdrawn from a 30 mL reaction volume, resulting in a minimal loss of ~0.2% due to sampling. A longer (27 h) on-line reaction monitoring approach for imine formation using analytical-scale LC sampled ~100 μL from a 85 mL reaction vessel every 18 min, leading to an overall sample volume of ~9 mL and ~10% loss due to sampling [26]. In the following section, a variation in the sampling approach was developed to minimize sample loss for longer reaction monitoring times where solely withdrawing the sample could lead to higher volume losses.
3.2. On-Line Dissolution & Hydrolysis Monitoring of Aspirin with Compact LC-UV Instrumentation
Acid hydrolysis reactions are commonly monitored in the pharmaceutical industry [34]. In this study, a simple one-dram reaction vial was used as a reaction vessel to contain a ~1 mL volume for hydrolysis of acetylsalicylic acid to form salicylic acid based on the presence of phosphoric acid in the solution (Figure 1B). In Figure 4A, chromatograms selected at 8 h time points are shown over a period of two days. A slow increase in the peak area of the salicylic acid product is demonstrated in Figure 4B across nearly 250 chromatographic injections from the on-line vessel, with a total time of approximately 50 h. Performance of the system throughout this time period was consistent, with retention time repeatability of 0.5% RSD and 0.6% RSD for acetylsalicylic acid and salicylic acid, respectively.
Figure 4.
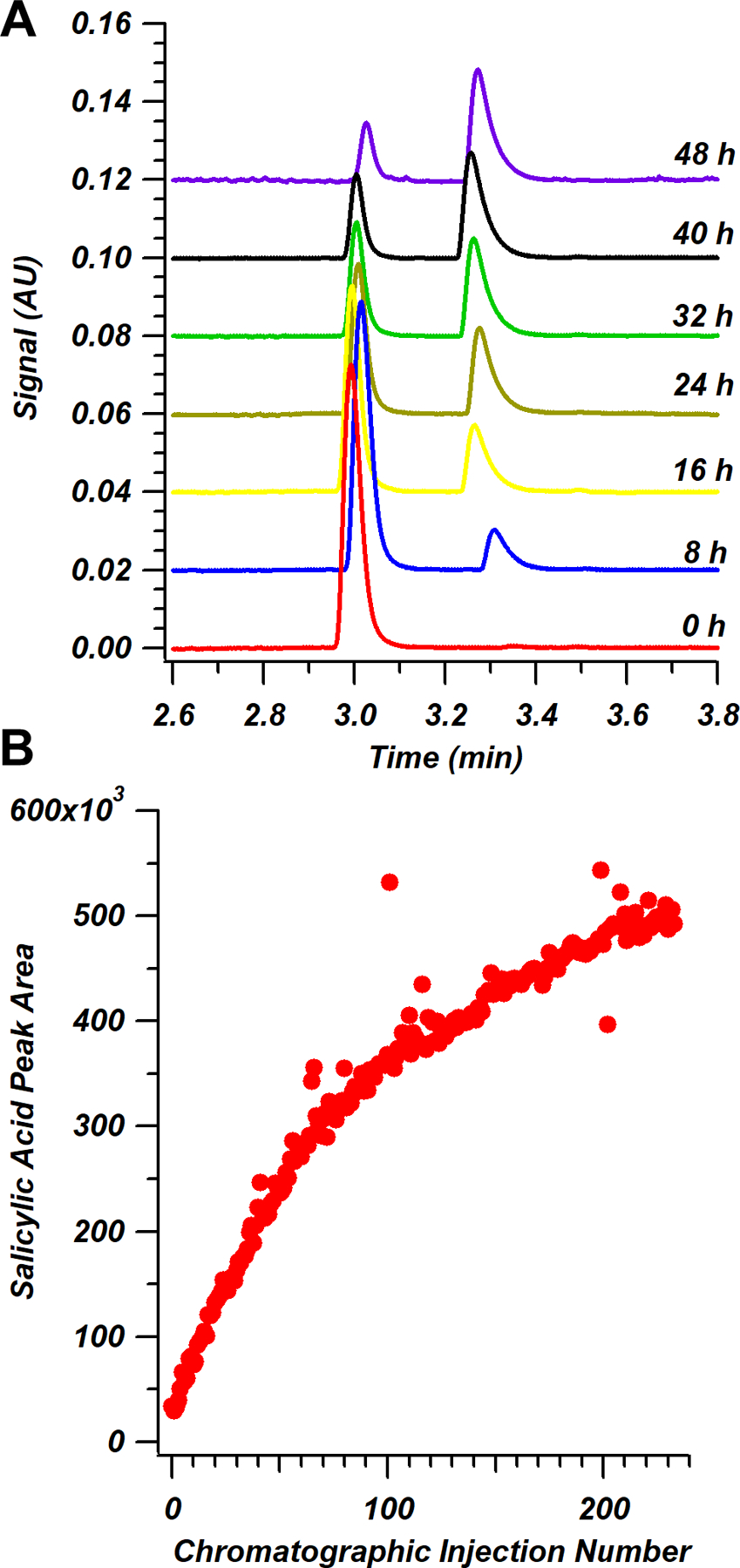
(A) Chromatographic separation of aspirin acid hydrolysis reactants and products collected via UV absorbance at 235 nm. The peak eluting at ~3.0 min represents acetylsalicylic acid and the peak at ~3.3 min represents salicylic acid. (B) Reaction kinetic plot showing salicylic acid formation as a function of time.
In this set-up, a smaller vessel volume (~1 mL) and longer monitoring time (~50 h) require a different approach to on-line sampling than constant withdrawal. Here, the syringe was operated in a cyclic fashion where ~6 μL of sample volume were drawn through the connecting tubes prior to each injection to ensure that the injection loop was filled with fresh solution. Once the injection valve was actuated and the loaded 4 nL of sample was injected into the column, the remainder of the volume in the connecting tubes was pushed back into the vessel by switching the syringe pump to operate in the forward direction. This kept the total volume removed from the vessel across the entire monitoring period to ~1 μL, which is only an overall loss of 0.1% of the total reaction volume. Using this automated procedure not only simplifies the process for long experiments, but also ensures that the total loss due to sampling is negligible, especially for very low volume reactions.
The use of capillary LC is critical to the use of these sampling approaches for low volume reaction vessels, as small injection volumes <100 nL are most compatible with small i.d. columns. In this example, a 5 min gradient analysis at 2 μL/min consumes 5 μL of each solvent per run. The actual volume per run is slightly greater due to the equilibration time between each chromatogram in the sequence, with total solvent consumption of ~18 μL of mobile phase A and ~6 μL of mobile phase B for each full cycle time. Thus, on-line monitoring for an entire week (168 h) would only consume ~20 mL of total mobile phase and <3.4 μL of sample (~0.4% of the reaction volume). This approach is not only beneficial in terms of minimizing the sample loss due to analysis throughout the course of a reaction, but also in terms of overall method “greenness” through a substantial reduction in mobile phase consumption [35].
4. Conclusions
The use of on-line analysis for bench-scale reaction monitoring provides multiple benefits compared to off-line strategies. Compact designs permit the necessary instrumentation to be coupled close to the reaction vessel, which can be advantageous in confined spaces such as chemical fume hoods. In this study, imine formation by condensation and acid hydrolysis of aspirin were monitored using a compact, on-line, integrated LC platform. Both on-capillary UV absorbance and in-line MS detection approaches were demonstrated, with the MS providing mass-selective detection, albeit at higher cost and larger total instrument size. Because this platform utilizes capillary-scale LC columns, only nL-scale sample volumes was required for each sample injection, reducing the total volume loss due to sampling to less than ~0.2% for both reactions. This workflow is well-suited to chromatographic-based on-line reaction monitoring for volumes in the 10 – 50 mL range, common in a variety of synthesis-focused laboratory settings.
Supplementary Material
Acknowledgements
Funding for this project was provided by the National Institutes of Health through award R44 GM137649. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Matthew Morse and Greg Ward (Axcend Corporation) are acknowledged for technical assistance.
Footnotes
Conflict of Interest Statement
XX, WRW, and MLL are associated with Axcend Corporation, which is developing and commercializing technology described in this manuscript.
References
- [1].Fitzpatrick DE, Battilocchio C, Ley SV, A Novel Internet-Based Reaction Monitoring, Control and Autonomous Self-Optimization Platform for Chemical Synthesis. Org. Process Res. Dev 2016, 20, 386–394. [Google Scholar]
- [2].Chanda A, Daly AM, Foley DA, Lapack MA, Mukherjee S, Orr JD, Reid GL, Thompson DR, Ward HW, Industry perspectives on process analytical technology: Tools and applications in API development. Org. Process Res. Dev 2015, 19, 63–83. [Google Scholar]
- [3].Gurden SP, Westerhuis JA, Smilde AK, Monitoring of batch processes using spectroscopy. AIChE J. 2002, 48, 2283–2297. [Google Scholar]
- [4].Cortés-Borda D, Kutonova KV, Jamet C, Trusova ME, Zammattio F, Truchet C, Rodriguez-Zubiri M, Felpin FX, Optimizing the Heck-Matsuda Reaction in Flow with a Constraint-Adapted Direct Search Algorithm. Org. Process Res. Dev 2016, 20, 1979–1987. [Google Scholar]
- [5].Quinn AC, Gemperline PJ, Baker B, Zhu M, Walker DS, Fiber-optic UV/visible composition monitoring for process control of batch reactions. Chemom. Intell. Lab. Syst 1999, 45, 199–214. [Google Scholar]
- [6].Sun J, Yin Y, Li W, Jin O, Na N, Chemical Reaction Monitoring By Ambient Mass Spectrometry. Mass Spectrom. Rev 2022, 41, 70–99. [DOI] [PubMed] [Google Scholar]
- [7].Foley DA, Dunn AL, Zell MT, Reaction monitoring using online vs tube NMR spectroscopy: Seriously different results. Magn. Reson. Chem 2016, 54, 451–456. [DOI] [PubMed] [Google Scholar]
- [8].Gomez MV, De La Hoz A, NMR reaction monitoring in flow synthesis. Beilstein J. Org. Chem 2017, 13, 285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ray A, Bristow T, Whitmore C, Mosely J, On-line reaction monitoring by mass spectrometry, modern approaches for the analysis of chemical reactions. Mass Spectrom. Rev 2018, 37, 565–579. [DOI] [PubMed] [Google Scholar]
- [10].Malig TC, Koenig JDB, Situ H, Chehal NK, Hultin PG, Hein JE, Real-time HPLC-MS reaction progress monitoring using an automated analytical platform. React. Chem. Eng 2017, 2, 309–314. [Google Scholar]
- [11].Hemida M, Haddad PR, Lam SC, Coates LJ, Riley F, Diaz A, Gooley AA, Wirth HJ, Guinness S, Sekulic S, Paull B, Small footprint liquid chromatography-mass spectrometry for pharmaceutical reaction monitoring and automated process analysis. J. Chromatogr. A 2021, 1656, 462545. [DOI] [PubMed] [Google Scholar]
- [12].Fabris D, Mass spectrometric approaches for the investigation of dynamic processes in condensed phase. Mass Spectrom. Rev 2005, 24, 30–54. [DOI] [PubMed] [Google Scholar]
- [13].Norton LW, Yuan F, Reichert WM, Glucose recovery with bare and hydrogel-coated microdialysis probes: Experiment and simulation of temporal effects. Anal. Chem 2007, 79, 445–452. [DOI] [PubMed] [Google Scholar]
- [14].Sharma S, Tolley LT, Tolley HD, Plistil A, Stearns SD, Lee ML, Hand-portable liquid chromatographic instrumentation. J. Chromatogr. A 2015, 1421, 38–47. [DOI] [PubMed] [Google Scholar]
- [15].Rahimi F, Chatzimichail S, Saifuddin A, Surman AJ, Taylor-Robinson SD, Salehi-Reyhani A, A Review of Portable High-Performance Liquid Chromatography: the Future of the Field? Chromatographia 2020, 83, 1165–1195. [Google Scholar]
- [16].Foster SW, Xie X, Pham M, Peaden PA, Patil LM, Tolley LT, Farnsworth PB, Tolley HD, Lee ML, Grinias JP, Portable capillary liquid chromatography for pharmaceutical and illicit drug analysis. J. Sep. Sci 2020, 43, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coates LJ, Lam SC, Gooley AA, Haddad PR, Paull B, Wirth HJ, Modular, cost-effective, and portable capillary gradient liquid chromatography system for on-site analysis. J. Chromatogr. A 2020, 1626, 461374. [DOI] [PubMed] [Google Scholar]
- [18].Chatzimichail S, Rahimi F, Saifuddin A, Surman AJ, Taylor-Robinson SD, Salehi-Reyhani A, Hand-portable HPLC with broadband spectral detection enables analysis of complex polycyclic aromatic hydrocarbon mixtures. Commun. Chem 2021, 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vargas Medina DA, Maciel EVS, de Toffoli AL, Lanças FM, Miniaturization of liquid chromatography coupled to mass spectrometry.: 2. Achievements on modern instrumentation for miniaturized liquid chromatography coupled to mass spectrometry. TrAC - Trends Anal. Chem 2020, 128, 115910. [Google Scholar]
- [20].Lam SC, Coates LJ, Hemida M, Gupta V, Haddad PR, Macka M, Paull B, Miniature and fully portable gradient capillary liquid chromatograph. Anal. Chim. Acta 2020, 1101, 199–210. [DOI] [PubMed] [Google Scholar]
- [21].Hemida M, Ghiasvand A, Gupta V, Coates LJ, Gooley AA, Wirth HJ, Haddad PR, Paull B, Small-Footprint, Field-Deployable LC/MS System for On-Site Analysis of Per- And Polyfluoroalkyl Substances in Soil. Anal. Chem 2021, 93, 12032–12040. [DOI] [PubMed] [Google Scholar]
- [22].Schafer WA, Hobbs S, Rehm J, Rakestraw DA, Orella C, McLaughlin M, Ge Z, Welch CJ, Mobile tool for HPLC reaction monitoring. Org. Process Res. Dev 2007, 11, 870–876. [Google Scholar]
- [23].Sheng H, Corcoran EB, Dance ZEX, Smith JP, Lin Z, Ordsmith V, Hamilton S, Zhuang P, Quantitative perspective on online flow reaction profiling using a miniature mass spectrometer. Org. Process Res. Dev 2020, 24, 2611–2618. [Google Scholar]
- [24].Patel DC, Lyu YF, Gandarilla J, Doherty S, Unattended reaction monitoring using an automated microfluidic sampler and on-line liquid chromatography. Anal. Chim. Acta 2018, 1004, 32–39. [DOI] [PubMed] [Google Scholar]
- [25].Zameo S, Vauzeilles B, Beau JM, Direct composition analysis of a dynamic library of imines in an aqueous medium. European J. Org. Chem 2006, 2006, 5441–5444. [Google Scholar]
- [26].Foley DA, Wang J, Maranzano B, Zell MT, Marquez BL, Xiang Y, Reid GL, Online NMR and HPLC as a reaction monitoring platform for pharmaceutical process development. Anal. Chem 2013, 85, 8928–8932. [DOI] [PubMed] [Google Scholar]
- [27].Antignac JP, De Wasch K, Monteau F, De Brabander H, Andre F, Le Bizec B, The ion suppression phenomenon in liquid chromatography-mass spectrometry and its consequences in the field of residue analysis. Anal. Chim. Acta 2005, 529, 129–136. [Google Scholar]
- [28].Chetwynd AJ, David A, A review of nanoscale LC-ESI for metabolomics and its potential to enhance the metabolome coverage. Talanta 2018, 182, 380–390. [DOI] [PubMed] [Google Scholar]
- [29].Olsen BA, Argentine MD, Investigation of response factor ruggedness for the determination of drug impurities using high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. A 1997, 762, 227–233. [DOI] [PubMed] [Google Scholar]
- [30].Gabelica V, Rosu F, De Pauw E, A simple method to determine electrospray response factors of noncovalent complexes. Anal. Chem 2009, 81, 6708–6715. [DOI] [PubMed] [Google Scholar]
- [31].Wang M, Wang C, Han X, Selection of internal standards for accurate quantification of complex lipid species in biological extracts by electrospray ionization mass spectrometry—What, how and why? Mass Spectrom. Rev 2017, 36, 693–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nussbaum MA, Baertschi SW, Jansen PJ, Determination of relative UV response factors for HPLC by use of a chemiluminescent nitrogen-specific detector. J. Pharm. Biomed. Anal 2002, 27, 983–993. [DOI] [PubMed] [Google Scholar]
- [33].Webster GK, Marsden I, Pommerening CA, Tyrakowski CM, Tobias B, Determination of relative response factors for chromatographic investigations using NMR spectrometry. J. Pharm. Biomed. Anal 2009, 49, 1261–1265. [DOI] [PubMed] [Google Scholar]
- [34].Gilpin RK, Pachla LA, Pharmaceuticals and related drugs. Anal. Chem 1999, 71, 217–234. [DOI] [PubMed] [Google Scholar]
- [35].Płotka J, Tobiszewski M, Sulej AM, Kupska M, Górecki T, Namieśnik J, Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


