Abstract
Unacceptably high rates of severe maternal morbidity and mortality in the United States, as well as stark racial disparities in outcomes, are generating efforts to improve both research capacity and quality improvement in obstetrics care. Comprehensive, high-quality datasets on which to build these efforts are critical to the success of obstetric quality improvement efforts. Existing data sources in obstetrics, however, have notable limitations. Other medical and surgical specialties have addressed similar challenges through the creation of national registries, and we argue that obstetrics must take the same approach to improve outcomes. In this article, we summarize the current availability and limitations of large-scale data in obstetrics research, and contrast these with registries developed in other specialties. We then outline guiding principles for the development of a national obstetrics registry and propose future directions.
Keywords: Pregnancy, Quality Indicators, Health Care, United States, Obstetrics / standards
INTRODUCTION
Maternal morbidity and mortality in the United States exceed that of most nations with similar wealth.1 Moreover, adverse outcomes are more common among pregnant people from marginalized racial and ethnic groups, creating a moral imperative to improve care quality and address racial and ethnic disparities.2 Recognition of these unacceptable and disparate outcomes has generated a renewed focus on improving the quality of pregnancy care in the United States. Driven by what is dubbed the “maternal mortality crisis,” efforts to improve maternal outcomes are capturing the attention of the professional and lay press as well as policymakers at institutional, state, and federal levels.3, 4
Improving quality of care and reducing disparities requires the ability to critically examine obstetric practices and ask focused research questions that can generate evidence-based guidelines. Unfortunately, there is a paucity of national data to inform care in maternal and fetal health in the United States. Interventional and prospective studies provide critical insights but are resource-intensive and impractical to conduct for uncommon events. The data to answer many questions in our field exist, but we lack a unified system to access and analyze these data. Following the lead of other specialties, the creation of a standardized national obstetrics registry with comprehensive data on antepartum, intrapartum, and postpartum care and outcomes could fill this gap. Building a national registry would allow us to harness vast amounts of existing information to design and measure improvements in patient care. In this paper, we aim to persuade clinicians, researchers, administrators, and policy makers that the creation of a national obstetrics registry is imperative to improve maternal and fetal health by outlining the: 1) problems a comprehensive registry would address, 2) data resources that currently exist in obstetrics, 3) lessons learned from national registries in other fields, 4) agenda and guiding principles for the creation of a national obstetrics registry.
WHY WE NEED A NATIONAL OBSTETRICS REGISTRY
Quality assessment and improvement
Pettker and Grobman previously outlined the importance of standardized quality metrics to facilitate intervention to improve quality and safety.5 Differences in the quality of care provided at hospitals contribute to excess rates of, and disparities in, maternal morbidity and mortality.2 For this reason, non-profit, governmental, and private groups now utilize performance metrics and value hospital rankings based on quality of obstetric care.6 Hospitals may also benefit from inter-hospital comparisons using performance and outcome metrics to identify specific opportunities for improvement and measure that improvement. Making these comparisons, however, is fraught with methodological challenges, due to inconsistent assessment of outcomes and patient population differences between hospitals.5, 7 Developing the infrastructure and analytic framework to assess performance and quality of care in a robust way is critical to facilitate the broad and individual quality assessment that is essential to improving outcomes.
Identifying and addressing inequities in care
Variation in practice can inadvertently worsen disparities because, without structured data collection and analysis, differing protocols are challenging to compare, examine, and interpret with regards to how they affect marginalized populations. For common problems in obstetric medicine such as postpartum hemorrhage management, implementation of a standardized “bundle” of services has been shown to both improve outcomes, and reduce or eliminate disparities in care.8, 9 Organizations such as the National Committee for Quality Assurance have highlighted the importance of data-driven strategies to address racial and ethnic disparities in healthcare through quality metrics.10, 11 Creation of a national registry will allow hospitals to both identify areas for improvement and measure their facilities adherence to quality metrics.
Expanding the evidence base
There is a troubling lack of evidence with which to inform care decisions in obstetric patients. For example, postpartum pharmacologic venous thromboembolism (VTE) prophylaxis for patients at moderate or low risk remains intensely controversial. American College of Obstetrics and Gynecology guidance recommends adoption of any structured protocol for selecting patients for such prophylaxis.12 However, the choice of guideline adopted alters the percentage of patients receiving anticoagulation for thromboprophylaxis from <1% to >50% - variation that would alter the care of millions of pregnant persons annually.13 While many diseases we manage have been studied extensively in the general population, data in pregnant patients are often limited. In these scenarios, providers rely on case reports and data extrapolated from non-pregnant patients to guide clinical decisions. However, making inferences from uncontrolled case series without generalizability can lead to erroneous conclusions, and currently there is no systematic way to compare outcomes and experiences.
While there is no shortage of prolific researchers in our field, there are several barriers to conducting research in obstetrics. While maternal morbidity and mortality are unacceptably high, especially when compared to other developed nations, the absolute number of adverse events is low. An estimated 754 maternal mortalities were reported in 2019, or 0.026% of American deaths.14 Similarly, rates of postpartum VTE, a major cause of severe maternal morbidity, is 10- to 100-fold less likely in an obstetric patient than the general hospital population.15 Thus, for many obstetrics questions, clinical trials are not practical due to the sample size required. In addition, institutional review boards often set a higher bar for approving interventional research in pregnant populations.16 Observational studies are frequently inadequately powered using single center data, and funding for multicenter research in obstetrics has been historically more limited than our peer specialties, limited in concert by a lack of corporate support for many obstetric interventions and the challenges of performing research on the pregnant population.
However, the data needed to answer many of the critical questions in obstetric care already exist, if only they could be accessed and analyzed in a unified manner. While the number of adverse events and rare patient scenarios may be small in any one center, across the greater than 3.5 million pregnancies that occur annually in the United States, there are comparable cases and experiences from which we could learn. Creation of a national registry of antenatal, delivery, and postpartum risk factors and outcomes would allow us to use this vast amount of existing information to rapidly expand our knowledge base and improve patient care.
CURRENT DATA SOURCES IN OBSTETRICS
Administrative Data
There are a variety of administrative data sources in obstetrics research. The United States Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP) provides a series of all-payor datasets, weighted with the intention of representing most inpatient, short-stay hospitalizations within the United States.17 Many states have similar databases with universal coverage of all hospitalizations (e.g., the State Inpatient Databases from HCUP); in some (e.g., California) it is possible to link administrative data from the patient’s delivery hospitalization to birth certificates.18 Data from the Medicaid program are available, both at the national and state level, as are data from some individual private insurers and consortia of insurers (e.g., Marketscan and Optum) and hospitals (e.g., Premier Healthcare Database); some incorporate data from the electronic medical record for increased granularity (e.g., laboratory values).
Administrative data is advantageous because large numbers of patients are captured in a cost-efficient manner, facilitating the study of rare conditions. The primary limitation is that these databases are designed for healthcare system operations and reimbursement rather than quality improvement or research.19 As such, these datasets lack both clinical granularity and accuracy, presenting methodologic challenges to both research and quality assessment. There can be vast differences in “capture rate” of codes depending on reimbursement practices, and many codes do not provide the detail required for robust risk adjustment for quality comparisons or research purposes. For example, significant differences in the rate of SMM are noted when measured using administrative data based on specific criteria chosen, with poor concordance between metrics.20
Electronic Medical Record Data
The transition from paper chart to electronic medical records (EMR) has created a novel data source. EMR data are typically more granular than administrative data, including laboratory tests, vital signs, and labor features. In obstetrics research, the Consortium on Safe Labor was a landmark multicenter collaboration with record collection from >200,000 deliveries which, despite completion >10 years ago, continues to be used for impactful research.21 Modern EMR platforms increasingly allow amalgamation of data from multiple sites into networks for clinical research. For example, Epic Cosmos is a platform to combine Epic EMR data across participating centers for research purposes. In addition, there are standardized schema such as the PCORnet Common Data Model which allow for abstraction and comparison of data from multiple sites despite differences in data naming and storage between sites and EMR vendors.
However, EMR data has significant limitations. Like administrative data, it is collected for purposes other than research (clinical care/documentation and billing) and thus not always stored in ways that facilitate analyses or captured in formats optimal for quality improvement or research applications.22 Key data elements may only exist as free text, adding significant complexity to projects. While there is hope for translating these free text variables using natural language processing, those tools are in development and not yet broadly available, expensive, and have unproven efficacy for obstetric variables. Similarly, while some data elements in common EMR platforms are harmonized to facilitate analyses, data models for obstetrics can vary significantly even between sites using the same EMR vendor.
Prospective Multicenter Research Registries
Prospective data collection performed in multi-center settings allows customization of the data collection process to obtain detailed, purpose-driven data. An example of a successful, large multicenter obstetric study is the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b), one of many studies facilitated through the Maternal Fetal Medicine Units network.23 Advantages of multicenter prospective studies include extraordinarily detailed and standardized data collection with abstraction by trained study personnel, and correlation with biospecimen collection. However, their value for quality improvement is limited as they are not designed for that purpose. Other disadvantages include cost, as they are resource-intensive, and scope, as they are limited to a defined study period. Additionally, participating hospitals tend to be large research hospitals raising concern for generalizability.
Alliance for Innovation on Maternal Health (AIM)
The AIM data center coordinates the submission of standardized QI data from participating hospitals, produces hospital reports and comparisons of specific quality measures, and facilitates tracking the implementation of AIM bundles. A major advantage is that, unlike the above data sources, AIM was designed specifically for quality assessment and improvement, and data collection is timely. Disadvantages are that only a subset of hospitals participate, and data are primarily available to hospital administrators rather than for more general research purposes.
REGISTRIES IN OTHER SPECIALTIES
Several other specialties, including cardiovascular medicine, surgery, and anesthesiology, have created registries similar to what we advocate for obstetrics (Table 1). While each registry is different, they share several common features:
Combine quality improvement and research functions. Most registries aim to provide direct benefit to participating sites, through quality improvement reporting to the institution and in some cases, through public recognition of care quality. Concurrently, they have analytic centers where involved sites can propose research projects using the data.
They use a hybrid EMR-assisted data capture model. To reduce the burden of data entry on hospitals, registries often use models in which some data are abstracted via an automated extract from the EMR, while data elements that are more clinically nuanced or poorly captured are submitted by trained abstractors.
Primary funding by hospital user fees. While some registries are funded by government agencies or corporate sponsors, most are funded by fees paid by hospitals - typically $5,000-$20,000 annually.24
Data only leaves the source institution as a Limited Data Set. Inclusion of all deliveries at a given facility is essential to reliable quality indexing and ensuring the representativeness of registry results. The only approach that would be practicable and ethical would be to ensure that the data leaving the source institution for the centralized data center is in the form of a limited data set to protect the privacy of participants.
Table 1:
Examples of registries in other specialties
| Registry | Population | Outcomes | Research | Quality Improvement | Funding/Oversight | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| United States Renal Data System | All new dialysis initiates | Death Transplant | Standard analysis files (free) Linked Medicare claims ($) |
Publication of annual data report | NIH/NIDDK | Cleaned and focused research data sets “special studies” | Detailed follow-up data only available for those with Medicare as primary insurance (non-generalizeable, especially for patients < 65) |
| Scientific Registry of Transplant Recipients | Patients who join the waitlist +/− get a solid organ transplant | Transplant Graft Loss Death | Standard analysis files ($) | - Program specific reports generated with standardized risk adjustment - OPTN and CMS use these data to determine which programs need further scrutiny |
HRSA contract; Mandated public reporting of program specific performance | Easily accessible Program reports risk adjusted, publicly available Detailed research datasets |
Concern that program specific reports lead to risk aversion, “cherry picking” patients |
| NCDR CathPCI Registry* | Patients undergoing left heart cardiac catheterization, with or without intervention | Procedural outcomes and complications | By application to publications committee (free) or via grant funding ($) | Quarterly risk-adjusted benchmarks, provider-specific dashboards, and public reporting | American College of Cardiology / Society for Cardiovascular Angiography and Interventions, funded by hospital participation fees | Detailed procedural and outcomes information | Participation is voluntary (but 90% of United States hospitals performing PCI do) |
| Get-with the Guidelines STroke | Patients being treated for stroke | Delivery of evidence-based care and clinical outcomes (up to 30 days) | By application to publications committee (free) or via grant funding ($) | Risk-adjusted outcomes, public reporting as “awards” | American Heart Association, funded by hospital participation fees | Detailed procedural and outcomes information | Participation is voluntary (but 50% of United States hospitals with stroke programs do) |
| MPOG | Patients undergoing surgery with anesthesiology records at MPOG site hospitals | Multiple perioperative endpoints | Measure variation in perioperative clinical practice | Identification and implement of best practice | “The Executive Board serves without pay and is comprised of anesthesiology department chairs or head of practice of active/contributing member institutions, as well as the Executive Director, Research Director, and Quality Improvement Director of MPOG.” | Detailed data from perioperative encounter (vital signs, medications administered) | Awaiting incorporation of longitudinal data outside of the perioperative arena. Participation/data is limited to MPOG hospitals. |
NCDR and Get with the Guidelines both encompass numerous databases; examples provided for illustration
Most registries not arising from a government mandate are managed and overseen by a multidisciplinary team under the auspices of a non-profit professional society (such as the American College of Cardiology) and we would favor a similar model in obstetrics. From a research standpoint, national registries provide rich data and infrastructure on which further research studies can be built, and provide important information on questions not practical to answer with clinical trials. For example, the Get with the Guidelines Stroke Registry, demonstrated the safety of Tissue Plasminogen Activator for stroke treatment in patients receiving warfarin therapy.25 Registries can also lower the cost of observational research by providing a common data set already being collected by trained abstractors; this model was used for post-approval surveillance of safety and efficacy of prasugrel for acute coronary syndromes treated with percutaneous coronary intervention.26 Finally, registries can serve as a common base data set (with augmentation) for randomized controlled trials as was done in the Study of Access Site for Enhancement of PCI for Women trial.27
A NATIONAL OBSTETRICS REGISTRY
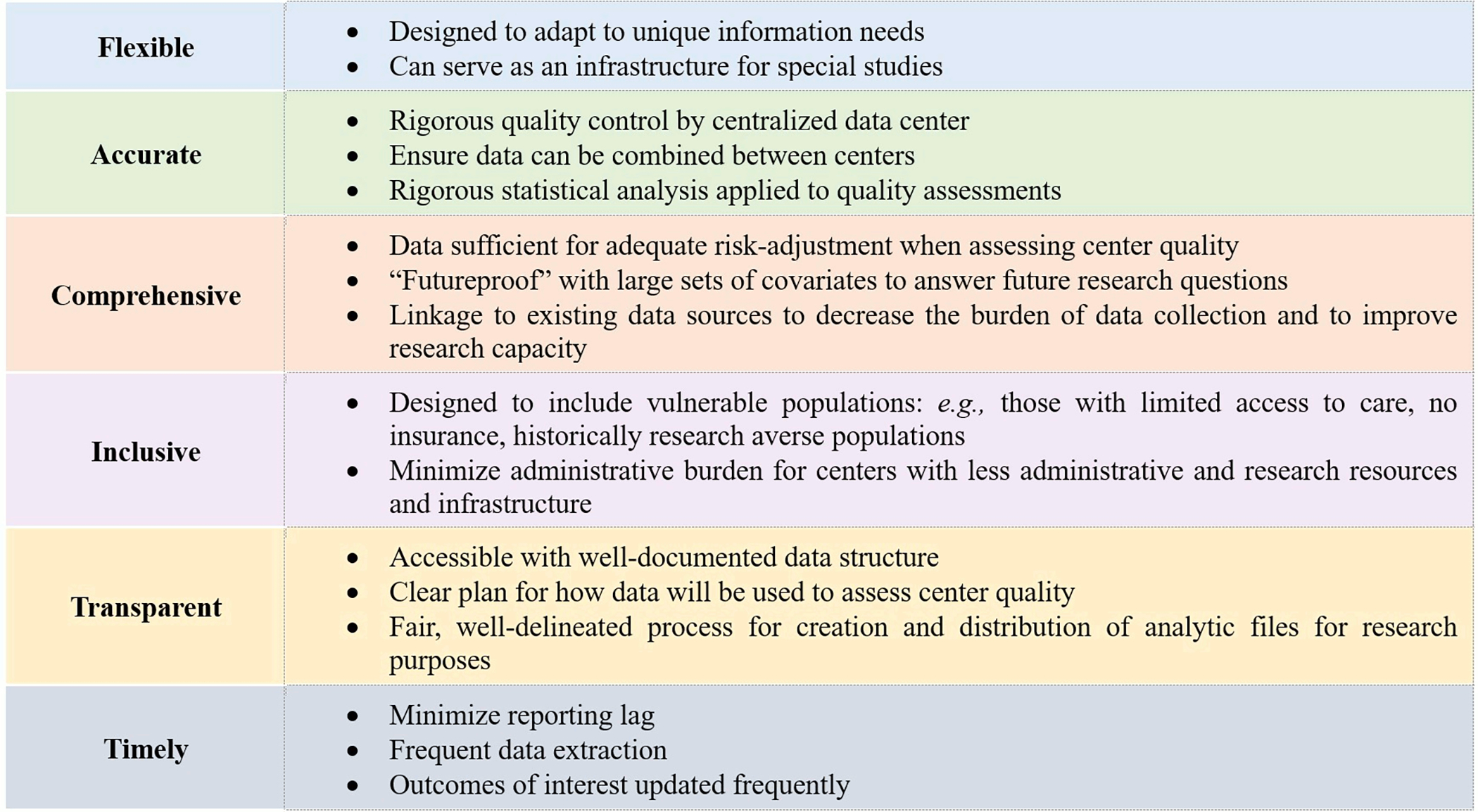
We propose the creation of a national EHR-assisted obstetrics registry to address the unique challenges to improving quality of care and evidence-based practice in obstetrics. While the logistics of such an endeavor are complex, this has been achieved successfully and in a financially sustainable manner in other specialties. As a first step we outline a set of guiding principles we consider important for development of a registry (summarized in Figure 1.)
Inclusive: Many data sources used for medical research rely on administrative or billing data alone. These often exclude some of the most vulnerable populations: persons without insurance and those with limited access to care. This lack of inclusivity introduces bias, limits generalizability of research findings, and hinders quality improvement efforts. The goal should be the equitable collection of accurate, comprehensive data that represents all obstetric patients, with special efforts to capture self-reported race, ethnicity, and gender; those who are unrepresented or underrepresented, uninsured or underinsured, non-English speaking, and/or have limited healthcare access. Quality metrics should include social determinants of health to inform quality improvement efforts to address health inequities.
Comprehensive: Comprehensive data are critical to answer research questions that cannot be addressed with single center or administrative data. An ideal registry is “future-proof”, such that capture of comprehensive covariates allows for future questions to be answered, or unexpected health challenges addressed. When possible, linkage to existing data sources such as the Social Security Master Death File, and birth certificate data can allow the study of a wider variety of outcomes without increasing the data collection burden for individual centers. In addition, we must collect sufficient data for adequate risk adjustment given a major goal of this effort is quality assessment and improvement. If case mix is not properly accounted for, centers who routinely care for medically complex patients may be unfairly labeled as “low quality.” Experience in other fields suggests limitations in case mix adjustment can lead to “cherry picking” patients and ultimately worse outcomes.28 It is critical to capture social determinants of health to facilitate efforts to identify and reduce health disparities. A comprehensive registry will require careful design, consciously balancing the need for comprehensiveness with the administrative burden of data collection.
Transparent: Strengths and limitations of the source data should be transparent. A registry must be accessible to clinicians, researchers, and stakeholders with a clear, well-documented data structure. Plans should be developed and updated for how the data will be used to assess center quality and what will be done with the resultant information. The registry’s focus should be on improving care rather than penalizing underperforming centers. There should be a plan in place to routinely provide accessible data reports to the public. A fair, clearly delineated process for the creation and distribution of analytic files for research purposes should be in place. This process should be equitable so that data accessibility is not limited to those with connections and resources, but available to all with solid analytic plans. The creation of standard analytic files available for free or for a modest fee to researchers with well-considered analytic proposals as determined by an independent review committee would improve equity in research conduct. Each center should have unrestricted access to their own data for quality improvement and research purposes.
Flexible: Once the infrastructure for an obstetrics registry is in place, it should be designed to adapt to special circumstances and unique information needs. For example, if a national registry with a centralized data center was in place at the beginning of the COVID-19 pandemic, COVID-19 studies could more rapidly be implemented across multiple centers to allow for prompt insights in care and outcomes to be realized. As an example, it was April 2021 before the first data on COVID-19 vaccine safety in pregnant women was published and included only 827 completed pregnancies29- despite the fact that over 100,000 pregnant women had received the vaccine by that time. Additionally, it had no comparison group, leading to criticism that inferences about the presence or absence of adverse outcomes could not be made. Though there were extraordinary efforts by many researchers to mobilize and gather data,30 the process would have been quicker and more efficient if a national research infrastructure was in place.
Accurate: Rigorous quality control mechanisms would ensure the data are as accurate as possible. Center-level differences in reporting or measuring outcomes should be avoided and when necessary, accounted for transparently. Adoption of uniform definitions, such as those outlined in ACOG’s ReVITALize project, will be critical.31 We propose a model, commonly used in other registries, in which most data elements are extracted in an automated fashion from the EMR, with manual review and cleaning for the most essential data elements with consistent application of clinical criteria. All efforts should be made to understand weaknesses in the data and develop plans to account for them, either at the level of data collation or by using appropriate statistical methods in analyses.
Timely: Efforts should be made to minimize the lag time between when data are reported and when they are processed and made available. Priority should be given to providing individual centers with processed data for quality improvement and research, as well as providing thoughtful reports on center performance.
Figure 1:
Guiding principles for development of an obstetrics registry
NEXT STEPS AND FUTURE DIRECTIONS
We propose that next steps in this effort should center on identifying proposed data elements to be captured in the registry across a few institutions which share a common EMR vendor. Once completed, we propose a “pilot period” in which 5–10 sites participate in prospective data capture, with an iterative process used to refine how data are extracted from the EMR and reviewed prior to submission. This pilot period will provide an understanding of the time and staffing investments needed for this effort and an estimate of the cost needed to provide the core data center and quality reporting services.
Creation of a national obstetrics registry would be a monumental undertaking requiring significant resources, funding, and commitment from centers. Buy-in from stakeholders is essential. A critical next step is identifying professional organizations, policy makers, and national agencies willing to invest their time and resources. We anticipate concerns that this proposal is overly ambitious and resource intensive. We counter that 1) experience in other specialties shows national registries are attainable, and 2) there is a unique and critical need in obstetrics owing to the previously outlined challenges to the conduct of research and quality assessment, as well as a historic lack of funding and support for women’s health. Creation of a national registry would signify a national willingness to invest in the health of women and children in the United States and would be a crucial step towards expanding our evidence base, improving patient outcomes, and reducing the stark, unacceptable disparities that plague obstetric care.
Funding:
The project described was supported by the National Center for Advancing Translational Sciences (NCATS) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, through grant award numbers UL1TR002489, UL1TR001117, 1KL2TR002554, TL1TR002555 and K12HD103083. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Meng was additionally supported by the Foundation for Anesthesia Education and Research.
REFERENCES
- 1.Tikkanen R, Gunja MZ, Fitzgerald M, Zephyrin L. Maternal mortality and maternity care in the United States compared to 10 other developed countries, 2020. [Google Scholar]
- 2.Howell EA, Zeitlin J. Quality of Care and Disparities in Obstetrics. Obstet Gynecol Clin North Am 2017;44:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gingrey JP. Maternal Mortality: A US Public Health Crisis. Am J Public Health 2020;110:462–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marill MC. Getting To The Heart Of America’s Maternal Mortality Crisis. Health Aff (Millwood) 2021;40:1824–29. [DOI] [PubMed] [Google Scholar]
- 5.Pettker CM, Grobman WA. Obstetric Safety and Quality. Obstet Gynecol 2015;126:196–206. [DOI] [PubMed] [Google Scholar]
- 6.Group TL. Healthy Moms, Healthy Babies: Hospital Performance on Leapfrog’s Maternity Care Standards Based on Results of the 2020 Leapfrog Hospital Survey.
- 7.Thomas Craig KJ, Mckillop MM, Huang HT, George J, Punwani ES, Rhee KB. U.S. hospital performance methodologies: a scoping review to identify opportunities for crossing the quality chasm. BMC Health Serv Res 2020;20:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Main EK, Chang SC, Dhurjati R, Cape V, Profit J, Gould JB. Reduction in racial disparities in severe maternal morbidity from hemorrhage in a large-scale quality improvement collaborative. Am J Obstet Gynecol 2020;223:123 e1–23 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenstein MG, Chang SC, Sakowski C, et al. Hospital Quality Improvement Interventions, Statewide Policy Initiatives, and Rates of Cesarean Delivery for Nulliparous, Term, Singleton, Vertex Births in California. JAMA 2021;325:1631–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington R, Washington D, Paliani S, Thompson K, Rouse L, Anderson A. A New Effort To Address Racial And Ethnic Disparities In Care Through Quality Measurement. Health Affairs: Forefront 2021. [Google Scholar]
- 11.Gold R, Bunce A, Cowburn S, et al. Adoption of Social Determinants of Health EHR Tools by Community Health Centers. Ann Fam Med 2018;16:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gyneologists. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy . Obstet Gynecol 2018;132:e1–e17. [DOI] [PubMed] [Google Scholar]
- 13.Federspiel JJ, Wein LE, Addae-Konadu KL, et al. Venous thromboembolism incidence among patients recommended for pharmacologic thromboembolism prophylaxis after cesarean delivery in selected guidelines. J Thromb Haemost 2021;19:830–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyert DL. Maternal Mortality Rates in the United States, 2019. National Center for Health Statistics: Health E-Stats 2021;April. [Google Scholar]
- 15.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 1995;108:978–81. [DOI] [PubMed] [Google Scholar]
- 16.Kaye DK. The moral imperative to approve pregnant women’s participation in randomized clinical trials for pregnancy and newborn complications. Philos Ethics Humanit Med 2019;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Databases HCUP. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/databases.jsp. [PubMed] [Google Scholar]
- 18.Glance LG, Hasley S, Glantz JC, et al. Measuring Childbirth Outcomes Using Administrative and Birth Certificate Data. Anesthesiology 2019;131:238–53. [DOI] [PubMed] [Google Scholar]
- 19.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA 2012;307:1433–5. [DOI] [PubMed] [Google Scholar]
- 20.Snowden JM, Lyndon A, Kan P, El Ayadi A, Main E, Carmichael SL. Severe Maternal Morbidity: A Comparison of Definitions and Data Sources. Am J Epidemiol 2021;190:1890–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh KK, Strauss RA, Grotegut CA, et al. Machine Learning and Statistical Models to Predict Postpartum Hemorrhage. Obstet Gynecol 2020;135:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfrancesco MA, Goldstein ND. A narrative review on the validity of electronic health record-based research in epidemiology. BMC Med Res Methodol 2021;21:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theilen LH, Greenland P, Varagic J, et al. Association between aspirin use during pregnancy and cardiovascular risk factors 2–7 years after delivery: The nuMoM2b Heart Health Study. Pregnancy Hypertens 2022;28:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Heart Association. Get With the Guidelines Pricing Sheet. https://www.stroke.org/-/media/files/affiliates/swa/qi-files/2020-get-with-the-guidelines-pricing-sheet.pdf?la=en
- 25.Xian Y, Liang L, Smith EE, et al. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA 2012;307:2600–8. [DOI] [PubMed] [Google Scholar]
- 26.Federspiel JJ, Anstrom KJ, Xian Y, et al. Comparing Inverse Probability of Treatment Weighting and Instrumental Variable Methods for the Evaluation of Adenosine Diphosphate Receptor Inhibitors After Percutaneous Coronary Intervention. JAMA Cardiol 2016;1:655–65. [DOI] [PubMed] [Google Scholar]
- 27.Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv 2014;7:857–67. [DOI] [PubMed] [Google Scholar]
- 28.Jay C, Schold JD. Measuring transplant center performance: The goals are not controversial but the methods and consequences can be. Curr Transplant Rep 2017;4:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley LE. mRNA Covid-19 Vaccines in Pregnant Women. N Engl J Med 2021;384:2342–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical Presentation of Coronavirus Disease 2019 (COVID-19) in Pregnant and Recently Pregnant People. Obstet Gynecol 2020;136:1117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol 2014;124:150–53. [DOI] [PubMed] [Google Scholar]


