Abstract
Objectives
There is paucity of population-based studies investigating the epidemiology of lupus nephritis (LN) in the US and long-term secular trends of the disease and its outcomes. We aimed to examine the epidemiology of LN in a well-defined eight-county region in the US.
Methods
Patients with incident LN between 1976 and 2018 (1976-2009 Olmsted County, 2010-2018 eight-county region) in Minnesota were identified. Age- and sex-specific incidence rates and point prevalence for four decades, adjusted to the projected 2000 US population, were reported. Standardized mortality ratios (SMR), survival rates, and time to end-stage renal disease (ESRD) were estimated.
Results
There were 72 patients with incident LN between 1976–2018. Mean age at diagnosis was 38.4 years (SD 16.24), 76% were female, and 69% non-Hispanic White. Average annual LN incidence between 1976 and 2018 was 1 per 100,000 population (95%CI 0.8-1.3) and highest in the 30-39 age group. Between 1976–1989 and 2000–2018 periods, overall incidence of LN increased from 0.7 to 1.3 per 100,000, but this was not statistically significant. Estimated LN prevalence increased from 16.8 in 1985 to 21.2 per 100,000 in 2015. LN had an SMR of 6.33 (95% CI 3.81-9.89) with no improvement in mortality gap in the last four decades. At 10 years, survival was 70%, and 13% had ESRD.
Conclusion
The incidence and prevalence of LN in this area increased in the last four decades. LN patients have poor outcomes with high rates of ESRD and mortality rates six times that of the general population.
Keywords: Epidemiology, lupus nephritis, incidence, prevalence, standardized mortality ratio, LUMEN
Introduction:
Lupus nephritis (LN) is one of the most severe manifestations of systemic lupus erythematosus (SLE) and can result in end-stage renal disease (ESRD). It is estimated that LN affects 20% to 65% of patients with SLE based on prior cohort studies (1-3). LN patients often require significant immunosuppression, increasing the risk of comorbid conditions and infections.
Given the fragmentation of the US healthcare system, it is difficult to estimate the incidence and prevalence of LN in the general population. While LN has been a clearly identified phenotype that has different long-term outcomes and has received substantial attention as an independent subset of SLE requiring its own therapeutic strategies, clinical trials, and clinical practice guidelines, the characterization of the epidemiology of LN has been rarely done as an individual subgroup (4,5). In addition, in earlier SLE classification criteria, patients with lupus nephritis (even if biopsy-proven and positive to anti dsDNA) without other non-renal manifestations did not classify for SLE, thus potentially resulting in the under-representation of patients with LN in SLE studies that relied on the American College of Rheumatology 1997 (or earlier versions) classification criteria for case identification. (6). Finally, patients with LN are seen by providers in multiple specialties, including nephrology, rheumatology, and internal medicine. Therefore, cohorts based on specialty clinics may not capture the whole spectrum of the disease. While the Centers for Disease Control and Prevention (CDC) lupus registries have provided high-quality population-based data regarding the epidemiology of SLE, LN epidemiology was not reported as an independent subgroup (7-11). Recent data showed that the incidence and prevalence of SLE are rising in the US, partly due to increasing racial diversity in the US population (12). It is unknown whether these changes also led to an increase in the incidence of LN.
A prior study using US Medicaid claims for LN demonstrated an incidence of 6.92/100,000 person-years and a prevalence of 30.9/100,000 among Medicaid beneficiaries (13). However, this study used a claims-based algorithm relying on ICD-9 codes for case identification (13). This algorithm misclassifies around >20% of the cases (14). In addition, the data was from almost two decades ago with short ascertainment periods; it may under-ascertain individuals with renal disease since patients with end-stage renal disease are insured mainly by Medicare. Thus, they could not examine the long-term secular trends of LN epidemiology and its outcomes. The literature on mortality of LN from cohort studies in Asia and Europe report standardized mortality rates (SMR) relative to the general population ranging from 5.9 to 9.0 (15,16); however, there are no population-based SMR estimates from the US.
In this population-based study of LN in the US, we aimed to characterize the incidence, prevalence, renal outcomes, and mortality of LN in an eight-county region of Minnesota.
Patients and Methods:
Study design
The Lupus Midwest Network (LUMEN) is the newest population-based lupus registry funded by the CDC. LUMEN is a population-based registry nested in the Rochester Epidemiology Project (REP), a record-linkage system. The REP has included patients from Olmsted County, Minnesota, since 1966 and, in 2010, expanded to additional counties. Eight contiguous counties with more than 95% population capture in the REP census compared to the US Census (Olmsted, Mower, Freeborn, Waseca, Steele, Dodge, Wabasha, Goodhue) were used for this epidemiologic study. Olmsted County comprised 48% of the total eight-county region population at the time of expansion in 2010. According to US Census data, Olmsted County had a population of 92,006 in 1980, 29.5% being <18 years of age and 98% White. In 2010, the population was 144,248, with 25.3% being <18 years of age. The ethnic distribution in 2010 was 85.7% White, 5.5% Asian/Native Hawaiian/Pacific Islander, 4.8% African American, 4.2% Hispanic and 0.2% American Indian/Alaska Native. The population for the eight-county region in 2010 was 311,266. (17) The REP allows ready access to the medical records from healthcare providers for this population, including the Mayo Clinic and the Olmsted Medical Center and their affiliated hospitals, local nursing homes, etc. This system ensures virtually complete ascertainment of all clinically recognized cases of LN in patients living in the eight-county region, independently of insurance coverage (18). The demographics, distribution of morbidity, and death rates in the REP region are like those in the upper Midwest. The characteristics and strengths of the REP, as well as its generalizability, have been described elsewhere (19,20).
Case finding and ascertainment
Potential LN cases in Olmsted County from 1976-2009, and the eight-county region from 2010-2018 were identified through two different strategies by examining all medical records: 1) through hospital International Classification of Disease Adaptation (HICDA), International Classification of Diseases (ICD)-9, and ICD-10 codes for SLE, cutaneous lupus erythematosus, and other associated diseases and 2) through laboratory measures associated with SLE: anti-nuclear antibodies (ANA; with abnormal titers defined as ≥1:80), low complement, anti-double-stranded DNA, anti-Sm, lupus anticoagulant anticardiolipin (IgG, IgM, and IgA), and anti-beta 2 glycoprotein 1 (IgG, IgM, and IgA) antibodies (Supplemental Table S1). The medical records of all potential LN patients were reviewed in detail, including clinical notes, pathology reports, and medical photography, for the following inclusion criteria: either 1)biopsy-proven LN in the presence of a positive ANA or ds-DNA antibody or 2) meeting 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria (21) and having documented proteinuria (500 mg in 24 hrs urine collection or protein/creatinine ratio ≥0.5) that did not have a better explanation than SLE (e.g., patients with diabetes mellitus or other causes of proteinuria who did not have a kidney biopsy to confirm LN were excluded).
Data collection
Complete medical records from all healthcare providers were reviewed for each patient using a standardized, pre-tested Research Electronic Data Capture (REDCap) application. Information regarding demographics, clinical characteristics, laboratory data were obtained through the review of the clinical records. Vital status and causes of death were collected through the REP mortality data which is obtained regularly from the Minnesota State Death Certificates and National Death Index. The discharge summaries describing the clinical episode that led to death and autopsies (if available) were reviewed to cross-validate the cause of death data. Cases were assigned to one of the five mutually exclusive race/ethnicity groups based on the self-reported information in the medical records: Hispanic (any race), Asian, White, Black, Other. There were no reported patients of other races/ethnicity. LN class information was defined according to the 2018 revised International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification criteria for LN (22). Date of end-stage renal disease (ESRD) (time of first dialysis session or renal transplant, whichever occurred first) was collected. Dialysis for acute kidney injury or temporary dialysis was not considered ESRD. Patients were followed until death, migration out of the 8-county region, or December 31, 2019. Data abstractors (MH, MVA, JD) were extensively trained until each abstractor achieved 95% agreement with the senior author for all elements in the data dictionary. Audits of 10% random samples of the abstracted patients were performed, and retraining was done as needed to maintain the 95% agreement. AD performed an independent review of all LN cases to confirm that they met the case definition. An incident case had to be a resident in one of the eight counties before meeting the case definition for LN; the earlier date that the patient fulfilled either case definition was considered the incidence date. A prevalent case needed to reside in the eight-county region and meet either case definition before or at the time of our four dates of point prevalence: January 1st of 1985, 1995, 2005, and 2015.
Statistical analysis
Descriptive statistics (percentages, means, etc.) were used to summarize the demographics. Average annual incidence rates were calculated using the number of incident cases as the numerator, and yearly population counts for residents from the REP census as the denominator (17). Overall incidence rates were age-adjusted and/or age/sex-adjusted to the 2000 projected US population (23) using eight age groups (<18 years, 18–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, 80 years and older). To compute 95% confidence intervals (95% CI) for incidence rates, we assumed that the number of incident cases followed a Poisson distribution. The point prevalences of LN were calculated on January 1, 1985, January 1, 1995, and January 1, 2005, based on residents of the Olmsted County population, and on January 1, 2015, based on residents of the eight counties, as REP included data only for Olmsted County residents before 2010. Given the expansion of the REP in 2010 to multiple counties, we performed a sensitivity analysis comparing the incidence rates of Olmsted County vs. the other 7 Counties included in the study. We estimated the survival rates following the diagnosis of LN using Kaplan-Meier methods and compared them to expected survival obtained from lifetables of the Minnesota population. Trends in incidence, prevalence, and standardized mortality ratios (direct standardization) were examined using Poisson regression models, including age, sex, and calendar year of incidence date. Two-way interactions between combinations of age, sex and calendar year were also examined. Cumulative incidence of ESRD adjusted for competing risk of death was estimated. Statistical analyses were performed using SAS version V.9.4 (SAS Institute, Cary, North Carolina, USA) and R 3.6.2 (R Foundation for Statistical Computing). This study was approved by the institutional review boards of Mayo Clinic (IRB #20-006485) and Olmsted Medical Center (IRB #036-OMC-20).
Results
Demographics and clinical characteristics of the incident and prevalent LN cohorts
Our study identified 72 incident LN cases between January 1st, 1976, and December 31st, 2018. The mean age at diagnosis was 38.4 years (range 6.4–76.8). Eight patients were younger than 18 years of age at diagnosis. The incident cohort was predominantly female, with a female to male ratio of 3.2:1. The overall racial distribution was 69% non-Hispanic White, 15% non-Hispanic Asian, 6% non-Hispanic Black, and 10% Hispanic (of any race). The proportion of non-Hispanic White decreased in the last decades while the proportion of non-Hispanic Asian, Hispanic, and non-Hispanic Black patients increased (Table 1).
Table 1.
Demographics and LN class of 72 patients with incident lupus nephritis, Olmsted County, MN (1976–2009) and eight-county region (2010–2018) in southeast Minnesota
| 1976 to 1989 N=10 |
1990 to 1999 N=8 |
2000 to 2009 N=16 |
2010 to 2018 N=38 |
1976-2018 N=72 |
|
|---|---|---|---|---|---|
| Age, years, mean (SD) | 36.6 (16.6) | 29.4 (15) | 37.7 (13.23) | 41.1 (17.31) | 38.4 (16.24) |
| Female sex, n (%) | 9 (90) | 8 (100) | 12 (75) | 26 (68) | 55 (76) |
| Race/Ethnicity*, n (%) | |||||
| Non-Hispanic Asian | 0 (0) | 2 (25) | 5 (31) | 4 (11) | 11 (15) |
| Non-Hispanic Black | 0 (0) | 0 (0) | 1 (6) | 3 (8) | 4 (6) |
| Non-Hispanic White | 10 (100) | 6 (75) | 9 (57) | 25 (66) | 50 (69) |
| Hispanic | 0 (0) | 0 (0) | 1 (6) | 6 (16) | 7 (10) |
| LN Class**, n (%) | |||||
| Class II | 1 (17) | 0 (0) | 1 (7) | 5 (19) | 7 (14) |
| Class III | 1 (17) | 0 (0) | 4 (29) | 4 (15) | 9 (18) |
| Class IV | 2 (33) | 3 (75) | 5 (36) | 10 (37) | 20 (39) |
| Class V | 1 (17) | 1 (25) | 4 (29) | 3 (11) | 9 (18) |
| Class III +V | 0 (0) | 0 (0.0) | 0 (0) | 1 (4) | 1 (2) |
| Class IV + V | 1 (17) | 0 (0.0) | 0 (0) | 4 (15) | 5 (10) |
| No biopsy | 4 (40) | 4 (50) | 2 (13) | 11 (29) | 21 (29) |
Cases were assigned to one of the five mutually exclusive race/ethnicity groups based on the self-reported information in the medical records: Hispanic (any race), Asian, White, Black, Other. There were no patients of Other races/ethnicity reported.
LN class information was reported according to the 2018 revised International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification criteria for LN.
Abbreviations: ESRD End-stage renal disease.
Fifty-one (71%) patients had biopsy-proven LN; class IV was the most common class (39%), followed by class III (18%), class V (18%), class II (14%), mixed class IV/V (10%), and mixed class III/V (2%).
Incidence and prevalence of LN in the study population
The overall LN incidence was 1.0 (95% CI 0.8, 1.3) per 100,000 population between 1976 and 2018; it nearly doubled from 0.7 in 1976–1989 to 1.3 per 100,000 in 2010–2018 (Table 2), however this increase did not reach statistical significance (rate ratio: 1.00 per calendar year; 95% CI: 0.98-1.01). The overall LN incidence rate for females was 1.5 per 100,000 (95% CI 1.1, 1.9) and the trend showed a non-statistically significant increase in females from 1.2 in 1976–1989 to 1.7 per 100,000 in 2010–2018 (rate ratio: 0.99 per calendar year; 95% CI: 0.99-1.01). Similarly, the overall LN incidence rate for males was 0.5 per 100,000 (95% CI 0.3, 0.8) and the trend showed a four-fold increase from 0.2 in 1976–1989 to 0.8 per 100,000 in 2010–2018 in males (Table 2), but this did not reach statistical significance (rate ratio: 0.98 per calendar year; 95% CI: 0.96-1.00). Our analysis did not find evidence that the increase over calendar time differed significantly between men and women (interaction p=0.48). Age- and sex-specific incidence rates peaked for females at 2.6 per 100,000 at 30-39 years of age and for males at 1.0 per 100,000 for 40-59 years of age (Supplementary Table S2). The overall LN prevalence showed a statistically significant increase from 16.8 (95% Cl 8.3, 25.3) per 100,000 population in 1985 to 21.2 (95% CI 16.3, 26.2) per 100,000 in 2015 (rate ratio: 1.02 per calendar year; 95% CI:1.00-1.04; p=0.046). LN was more prevalent in females than males for all four decades in our study period (Table 2). In our sensitivity analyses, there were no differences between the incidence rates of LN in Olmsted County vs. the other counties included in the study. (Supplementary Table S3)
Table 2.
Age-adjusted average annual incidence (1976–2018) and point prevalence (1985, 1995, 2005, 2015) rates of lupus nephritis (LN), Olmsted County, MN (1976–2009) and eight-county region (2010-2018) in southeast Minnesota
| Age-adjusted average annual LN incidence rates/ 100,000 population (95% CI)* |
Age-adjusted LN Point Prevalence/ 100,000 population (95% CI)* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1976-1989 | 1990-1999 | 2000-2009 | 2010-2018 | 1976-2018 | 01/01/1985 | 01/01/1995 | 01/01/2005 | 01/01/2015 | |
| Females | 1.2 (0.4, 2.0) |
1.3 (0.4, 2.2) |
1.7 (0.7, 2.7) |
1.7 (1.0, 2.4) |
1.5 (1.1, 1.9) |
25.9 (11.4, 40.5) |
30.7 (16.7, 44.7) |
36.5 (22.4, 50.7) |
33.4 (24.7, 42.1) |
| Males | 0.2 (0.0, 0.6) |
0.0 (0.0, 0.0) |
0.6 (0.0, 1.2) |
0.8 (0.4, 1.3) |
0.5 (0.3, 0.8) |
7.1 (0.0, 15.1) |
6.0 (0.0, 13.0) |
1.4 (0.0, 4.3) |
8.6 (4.1, 13.0) |
| Overall | 0.7 (0.2, 1.2) |
0.6 (0.2, 1.1) |
1.2 (0.6, 1.8) |
1.3 (0.9, 1.7) |
1.0 (0.8, 1.3) |
16.8 (8.3, 25.3) |
18.5 (10.7, 26.4) |
19.4 (12.1, 26.8) |
21.2 (16.3, 26.2) |
Rates for females and males were age-adjusted to the 2000 projected US population using eight age categories. Overall rates were age- and sex-adjusted to the 2000 projected US population. Rates are per 100,000 population. Denominator data are based on the Rochester Epidemiology Project census (see reference in the text).
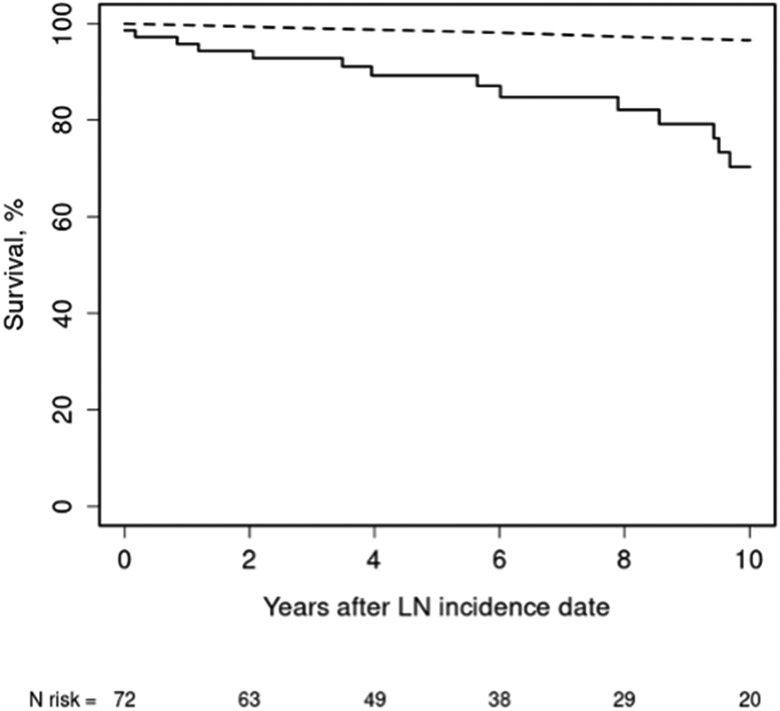
Mortality and renal outcomes
In our incidence cohort, there were 19 deaths during a median follow-up of 6.2 years (Table 3). Based on the Minnesota lifetables, only three deaths were expected. The survival in our cohort was 89% at five years and 70% at ten years after diagnosis of LN (Figure 1). Compared with the general population, the standardized mortality ratio (SMR) of LN patients was 6.33 (95% CI 3.81, 9.89). When stratified by age, LN patients younger than 40 years at LN diagnosis had a 12-fold increase in mortality, while those older than 40 years had a four-fold increase. Analysis of trends in SMR over time showed no improvement in the mortality gap over time (p=0.54). Infection was the most common cause of death, followed by SLE disease activity and cardiovascular disease.
Table 3.
Standardized mortality ratios (SMR) for 72 patients with incident lupus nephritis (LN), by decade and by age group, Olmsted County, MN (1976–2009) and eight-county region in southeast Minnesota (2010–2018)
| Decade | Number of deaths | Expected number of deaths | SMR (95% CI) |
|---|---|---|---|
| 1976-1989 | 6 | 1.0 | 5.74 (2.11, 12.51) |
| 1990-1999 | 3 | 0.3 | 9.34 (1.93, 27.3) |
| 2000-2009 | 6 | 0.9 | 6.72 (2.47, 14.63) |
| 2010-2018 | 4 | 0.7 | 5.38 (1.47,13.79) |
| Total (1976-2018) | 19 | 3.0 | 6.33 (3.81, 9.89) |
| Age | |||
| <40 | 9 | 0.7 | 12.34 (5.64, 23.42) |
| ≥40 | 10 | 2.3 | 4.40 (2.11, 8.10) |
Figure 1.
Survival in 72 patients with incident lupus nephritis (LN) compared with expected survival obtained from the Minnesota lifetables. Olmsted County (1976–2009) or an eight-county region in southeast Minnesota (2010–2018) (Standardized mortality ratio 6.33; 95% CI: 3.81-9.89).
Observed (solid line) and expected (dashed line).
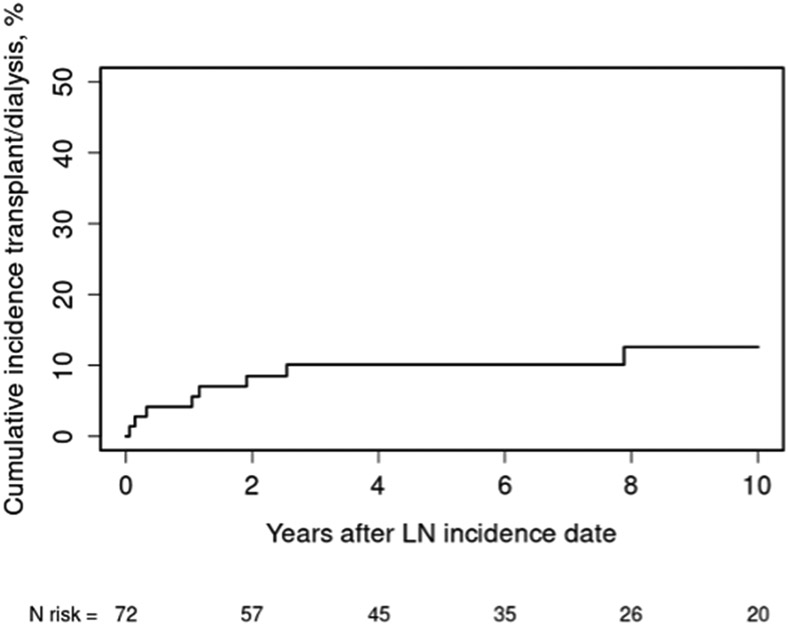
The cumulative incidence of ESRD in the entire cohort was 10% at five years and 13% at ten years. (Figure 2) At ten years of follow-up, 61% of the patients were alive and did not have ESRD or renal transplants.
Figure 2.
Cumulative incidence of end-stage renal disease (i.e., dialysis or transplant) in 72 patients with incident lupus nephritis (LN) adjusted for the competing risk of death living in Olmsted County (1976–2009) or an eight-county region in southeast Minnesota (2010–2018).
Discussion
In this population-based study of the epidemiology of LN in the United States, our findings showed that the average annual incidence of LN was 1.0 per 100,000 population, with a male: female ratio of 1:3. The incidence remained largely the same over the study period. However, the prevalence trends showed a modest increase. Our results regarding mortality demonstrated that LN patients had six times the mortality of the general population, and the mortality gap has not improved in the last forty years. In our LN cohort, 13% of patients developed ESRD; only 61% were alive and did not have ESRD or renal transplant at ten years of follow-up. Among the CDC-funded lupus registries (7-11), this study provides incidence and standardized mortality rate estimates and its secular trends data specifically for LN.
Our estimates of the incidence and prevalence of LN are lower than a previous report from the adult Medicaid population in the United States (1.0 vs. 6.9 per 100,000 person-years for incidence and 16.8 to 21.2 vs. 30.9 per 100,000 for prevalence) (13). This likely reflects the differences in socioeconomic and racial backgrounds of the studied populations, as well as differences in the methodology, given that the cases identified in the prior study were based on ICD-9 codes. Previous research showed that the incidence and prevalence of LN were higher in racial minorities and populations with low socioeconomic status (24). As our study population in southeast Minnesota was predominantly non-Hispanic White—more than 80% of the eight-county region was non-Hispanic White in 2019 (25)—the lower incidence of LN that we observed compared with the estimates from a Medicaid population could underscore the racial disparities in LN epidemiology. Furthermore, the difference in case identification strategy between the two studies played a role in the discrepant results since we eliminated the risk of misclassification in our approach with manual verification of every case, compared to using ICD-9 codes for disease definition. On the other hand, incidence estimates from Europe were closer to our estimate at 0.4 per 100,000 person-years for the United Kingdom (UK) and 0.45 per 100,000 person-years for Denmark (24,26). The differences in racial background and research methodology likely account for this difference as well. For example, the Denmark study relied on coding information for case identification and had a near-exclusive White cohort (94% White). In contrast, the UK study included only biopsy-proven cases but did have a significant number of minorities, including Indo-Asian, Chinese, and Afro-Caribbean.
The literature on the secular trends of LN incidence is limited due to the short ascertainment period of prior epidemiological studies. In our study period, the incidence of LN nearly doubled, with an increase observed in both males and females, but not reaching statistical significance. The driver of this potential trend could be the study population's increased racial/ethnic diversity, with a rising proportion of Hispanic and Asian individuals. We have recently demonstrated that the incidence of SLE rose in the same US population, with increased incidence observed in racial and ethnic minority populations than non-Hispanic whites. (12) (We did not have the statistical power to provide specific incidence estimates for patients with different racial backgrounds. It is possible that the potential rise in LN incidence could also be due to the increasing diversity of this US population. On the other hand, the possible role of environmental risk factors on this trend is unknown and warrants further study.
There are a few studies that have investigated the mortality of LN, but no prior SMR data is available in the US. Several population-based studies from Norway reported SMRs of 3.8 and 5.6 during 14 to 20 years of follow-up (27,28), while single-center studies from Asia reported SMRs of 3.59 and 9.0 during 10 to 15 years of follow-up (16, 29). Previous data on survival in LN stemmed mainly from cohorts from academic centers with mortality estimates ranging between 4.3% and 17% after ten years (27, 29, 30, 31) and 5% to 42% after 20 years (15, 28, 32). Our estimates for SMR and mortality were generally higher than these prior estimates. The differences in racial background and research methodology limit the comparability of these studies. For example, most studies mentioned above were based on data from single or referral centers. Our population-based cohort collected data from patients seen across different practices and specialties. Furthermore, our study period is over four decades long, thus encompassing different clinical treatment eras for LN (15, 29, 31, 32). Despite changes in clinical practice, we found no improvement in the mortality gap over four decades. On the other hand, the ESRD outcomes were similar to estimates from previous cohort studies. Around 10%-22% of patients with LN develop ESRD and require either transplant or dialysis (33).
This study has multiple strengths. First, a population-based study provides estimates on the full spectrum of LN without referral bias, independently of which clinicians saw the patients or restrictions based on insurance coverage, thus addressing several limitations from prior studies. Second, access to comprehensive medical records for all patients in our population-based cohort allowed us to ascertain cases, and outcomes, including causes of death, with high accuracy by reviewing pathology reports and applying validated EULAR/ACR 2019 criteria for SLE, thus minimizing misclassification. Third, the forty-year ascertainment period enabled us to track secular trends of LN incidence, prevalence, and outcomes. Fourth, our population-based approach enabled us to provide SMR estimates and mortality gap trends for LN not previously available in the US.
This study has several limitations. Our population-based cohort was ascertained in a geographic area with a predominantly White population which might limit its generalizability to regions of the country with more racially and ethnically diverse populations. However, more than 30% of the cases were in minorities. Only cases that reached medical attention could be identified; therefore, undiagnosed cases are not included, but it is improbable that patients would remain undiagnosed with a severe disease like LN without ever having contact with the healthcare system. Not all the patients included in the study had a pathological diagnosis which may introduce misclassification. However, this is an insurmountable issue given that in clinical practice is impossible to have biopsies in all patients with LN due to individual risks and preferences (e.g., bleeding, hypertension, patient not consenting to kidney biopsies). Thus, we minimized this potential bias by including patients with proteinuria without other explanation than LN.
In conclusion, this population-based study showed a rising disease burden in the United States due to LN. Long-term outcomes in patients with LN remain poor. There was no improvement in the mortality gap between patients with LN and the general population. Our findings highlight the urgent need to develop new therapies and optimize the treatment of patients with LN.
Supplementary Material
Acknowledgments:
The study team would like to thank Barbara Abbott for her support accessing the Rochester Epidemiology Project data. The Rochester Epidemiology Project was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
The Lupus Midwest Network (LUMEN) project is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) under Grant number U01 DP006491 as part of a financial assistance award totaling $1,750,000 with 100 percent funded by CDC/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS or the U.S. Government.
Footnotes
Disclosure Statement: Alí Duarte-García is supported by the Rheumatology Research Foundation Scientist Development Award and the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. The rest of the authors have nothing to disclose.
Data availability statement:
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1.Alarcon GS. Multiethnic lupus cohorts: what have they taught us? Reumatol Clin 2011; 7(1): 3–6. [DOI] [PubMed] [Google Scholar]
- 2.Bastian HM, Roseman JM, McGwin G Jr., et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002; 11(3): 152–60. [DOI] [PubMed] [Google Scholar]
- 3.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1999; 78(3): 167–75. [DOI] [PubMed] [Google Scholar]
- 4.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64(6): 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020; 79(6): 713–23. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40(9): 1725. [DOI] [PubMed] [Google Scholar]
- 7.Ferucci ED, Johnston JM, Gaddy JR, et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007-2009. Arthritis Rheumatol 2014; 66(9): 2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol 2014; 66(2): 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dall'Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol 2017; 69(10): 1996–2005. [DOI] [PubMed] [Google Scholar]
- 10.Izmirly PM, Wan I, Sahl S, et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017; 69(10): 2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers EC, Marder W, Cagnoli P, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014; 66(2): 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte-García A, Hocaoglu M, Valenzuela-Almada M, et al. Rising incidence and prevalence of systemic lupus erythematosus: a population-based study over four decades. Ann Rheum Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum 2013; 65(3): 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010; 19(6): 741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 2012; 27(8): 3248–54. [DOI] [PubMed] [Google Scholar]
- 16.Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013; 65(8): 2154–60. [DOI] [PubMed] [Google Scholar]
- 17. https://rochesterproject.org/for-researchers/population-overview/.
- 18.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011; 173(9): 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Grossardt BR, Brue SM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018; 47(2): 368–j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012; 87(2): 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019; 71(9): 1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018; 93(4): 789–96. [DOI] [PubMed] [Google Scholar]
- 23.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001; (20): 1–10. [PubMed] [Google Scholar]
- 24.Patel M, Clarke AM, Bruce IN, Symmons DP. The prevalence and incidence of biopsy-proven lupus nephritis in the UK: Evidence of an ethnic gradient. Arthritis Rheum 2006; 54(9): 2963–9. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Census. QuickFacts. Accessed October 9, 2021. https://www.census.gov/quickfacts/.
- 26.Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. Incidence of Systemic Lupus Erythematosus and Lupus Nephritis in Denmark: A Nationwide Cohort Study. J Rheumatol 2016; 43(7): 1335–9. [DOI] [PubMed] [Google Scholar]
- 27.Reppe Moe SE, Molberg O, Strom EH, Lerang K. Assessing the relative impact of lupus nephritis on mortality in a population-based systemic lupus erythematosus cohort. Lupus 2019; 28(7): 818–25. [DOI] [PubMed] [Google Scholar]
- 28.Norby GE, Mjoen G, Bjorneklett R, et al. Outcome in biopsy-proven Lupus nephritis: Evaluation of biopsies from the Norwegian Kidney Biopsy Registry. Lupus 2017; 26(8): 881–5. [DOI] [PubMed] [Google Scholar]
- 29.Kono M, Yasuda S, Kato M, et al. Long-term outcome in Japanese patients with lupus nephritis. Lupus 2014; 23(11): 1124–32. [DOI] [PubMed] [Google Scholar]
- 30.Hanly JG, O'Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55(2): 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bono L, Cameron JS, Hicks JA. The very long-term prognosis and complications of lupus nephritis and its treatment. QJM 1999; 92(4): 211–8. [DOI] [PubMed] [Google Scholar]
- 32.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. Progressive improvement of patient and renal survival and reduction of morbidity over time in patients with lupus nephritis (LN) followed for 20 years. Lupus 2013; 22(8): 810–8. [DOI] [PubMed] [Google Scholar]
- 33.Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971-2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol 2016; 68(6): 1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.