Abstract
Background.
Williams-Beuren syndrome (WBS)(OMIM# 194050) is a rare genetic multisystem disorder resulting from a chromosomal microdeletion at 7q11.23. The condition is characterized by distinct facies, intellectual disability, and supravalvar aortic stenosis. Those with WBS have an increased risk of sudden death, but mechanisms underlying this phenotype are incompletely understood.
Objective:
The aim of this study was to quantify and compare autonomic activity as reflected by heart rate variability (HRV) measures in a cohort of individuals with WBS (n=18) subjects and age- and sex-matched controls (n=18).
Methods:
We performed HRV analysis on 24hr ECG recordings using non-linear, time- and frequency-domain analyses on a cohort of subjects with WBS and age- and sex-matched controls enrolled in a prospective cross-sectional study designed to characterize WBS disease natural history.
Results:
WBS subjects demonstrated diminished HRV (reflected by SDNN(P=0.0001), SDANN(P<0.0001), SDNNi(P=0.0002), RMSSD(P=0.0004), SD1(P<0.0001), and SD2(P<0.0001)), and indirect markers of parasympathetic activity (reflected by pNN50(P<0.0007), RMSSD(P=0.0004), lnHF power(P=0.0038), and SD1(P<0.0001)) Additional parameters were also significantly different, including lnVLF power(decreased, P=0.0002), lnLF power(decreased, P=0.0024), and SD1/SD2(decreased, P<0.0001).
Conclusions:
Individuals with WBS demonstrate significant HRV abnormalities consistent with diminished autonomic reserve. Future studies will be needed to determine the relationship between autonomic dysregulation observed and sudden death risk seen in these patients.
Keywords: Williams-Beuren syndrome, Elastin arteriopathy, Heart-rate variability, Autonomic nervous system, Sudden death, Genetic syndrome
Condensed abstract
Williams-Beuren syndrome (WBS) is a rare genetic disorder resulting from a chromosomal microdeletion (7q11.23) that confers increased sudden death risk. The aim of this study was to quantify and compare heart rate variability metrics in a cohort of individuals with WBS with age- and sex-matched controls using non-linear, time- and frequency domain analyses on 24hr ECG recordings as part of a prospective cross-sectional study. People with WBS demonstrate significantly decreased indirect markers of parasympathetic activity and increased sympathetic activity markers consistent with diminished autonomic reserve and increased sudden death risk seen in this population.
Introduction
Williams-Beuren syndrome (WBS) (OMIM# 194050) is a rare multisystem disease, characterized by supravalvar aortic stenosis (SVAS), distinct facies, and intellectual disability(1). The disease arises from a chromosomal microdeletion of 7q11.23, comprising 25–27 genes, among them, elastin(2). Most vascular abnormalities in WBS are caused by elastin haploinsufficiency, although alternate genes within or external to this locus are known to modulate cardiovascular phenotypes in WBS(3). Elastin confers the elastic recoil properties on vessel walls from the arteriole level through the great vessels(4). Following systole, ejected blood expands the vessel lumen resulting in stored potential energy in the vessel walls that that subsequently increases perfusion upon vessel wall recoil. This phenomenon, the “Windkessel effect,” increases circulatory efficiency(5). Diminished elastin results in diminished stored energy and hence diminished perfusion. While great vessel disease is the defining feature of WBS, sudden cardiac death is also prominent, occurring more frequently with anesthesia administration(6–8). Figures vary, but estimates suggest that sudden death risk may be 25–100x higher than risk estimates calculated for general pediatric anesthesia populations(8). WBS sudden death mechanisms remain incompletely understood(8). A portion of cases have been shown to arise from coronary ostial stenosis; however, there are a sizable number of sudden death cases where this abnormality is absent(6). Several disease features have been correlated with sudden death, including bilateral outflow tract obstruction(8). Interestingly, SVAS gradient alone has not been found to correlate with sudden death risk(6,8).
Heart rate variability (HRV) has been a useful tool to investigate cardiovascular disease and sudden death from acute myocardial infarction and heart failure(9–13). Diminished HRV correlates with poor outcome after myocardial infarction, cerebrovascular accident, or with diabetes mellitus(11,13,14). Dysregulated autonomic system function has been further correlated with increased sudden death risk in Rett syndrome(15). HRV represents an attempt to quantify autonomic nervous system activity indirectly through heart rate data. Increased mortality and morbidity have been correlated with diminished HRV(16–18). Based on this, we sought to investigate WBS autonomic activity indirectly through HRV analyses. We hypothesize that WBS patients display diminished HRV. Furthermore, we speculate that this approach will help identify those with Williams syndrome at greatest risk of sudden death.
MATERIALS AND METHODS
Human subjects
Study approval was obtained from the NHLBI IRB. Subjects or legal guardians provided written consent. Assent was obtained when appropriate. Data for all subjects and controls were obtained under the protocol ‘Impact of Elastin Mediated Vascular Stiffness on End Organs’ (ClinicalTrials.gov Identifier: NCT02840448) that recruits people with WBS (n=65), WBS-like conditions (n=8), and controls (n=41). The current study includes only those with WBS (confirmed by clinical and/or research molecular testing to assure the equivalent of ELN fluorescent in situ hybridization (FISH) positive testing) and controls. Standard clinical histories and physical examination were obtained.
Human echocardiographic examination
Complete transthoracic echocardiograms were performed using commercially available systems and measured according to American Society of Echocardiography guidelines.
Ambulatory ECG recordings and HRV analysis
Standard ambulatory ECG monitors were used for 24-hour recordings. All data were reviewed on-site by an ECG telemetry nurse (KS) and by a pediatric cardiologist with advanced clinical electrophysiology training (MDL). The recorded data were analyzed using Spacelabs Impresario (version 3.07.0158) program. Non-normal beats were detected, coded and excluded from subsequent HRV analysis using standard methods. Tracings were reviewed for electrical and mechanical noise artifact and these portions of tracings were similarly excluded from subsequent analysis. Recordings with less than 22 hours of non-artifactual data were excluded.
A text file indicating NN intervals in milliseconds was exported. Rstudio 1.1.463(19) was used to remove non-normal beats and re-order data start-time to 8am. The subsequent text file was imported into Kubios HRV Version 1.0 with an artifact correction threshold of 0.3 seconds. HRV analysis was performed as recommended by the 1996 ESC and NASPE HRV task force(20) and European Heart Rhythm Association(21). No de-trending was performed. Time-domain metrics included NN interval, pNN50, RMSSD, SDNN, SDNNi and SDANN were calculated using 12-hr and 24-hr segmented data. Frequency-domain metrics included VLF(0–0.04 Hz), LF(0.04–0.15 Hz), and HF(0.15–0.4Hz) measured using 5-minute non-overlapping segments of the 24-hour recording. Frequency-domain analysis was conducted using a Lomb-Scargle periodogram with a smoothing window width of 0.02Hz. Frequency-domain parameters were transformed by the natural log and cohorts were compared using unpaired parametric tests. Non-linear metrics included SD1, SD2, SD1/SD2, DFA1(DFA α1 de-trending fluctuation short-ten scaling exponent) and DFA2 (DFA α2, long-term scaling exponent; P=NS, not reported) (22), ApEn (Approximate entropy)(23), and SampEn (Sample Entropy)(24) measured using 1-hr non-overlapping intervals. SampEn and ApEn were calculated using subseries length m=2 and tolerance r = 0.2 multiplied by the standard deviation of the data. For DFA, the limits of short-term (DFA1) and long-term (DFA2) fluctuations were 4–12 beats and 13–64 beats, respectively (DFA2 statistically insignificant, so not included). Hourly segmented Poincare plot morphology was reviewed for each hour of each subject or control and classified as (a) comet-like (b) ball-like (c) asymmetric and/or complex or (d) torpedo-like; classifications were tallied and results were compared by group(25). Sleep abnormalities were investigated by estimating respiratory rate using power spectral density plots and by computing and comparing time in “cyclic variation of heart rate (CVHR)” using low and high amplitude thresholds between cohorts(26–28). High and low amplitude CVHR were based on work by Stein et al: 1.High amplitude CVHR: >3 consecutive cycles of HR changes of at least 20 beats from the minimum HR before the arousal to the maximum HR during the arousal of at least 10 seconds duration and less than 2 minutes between cycles; 2. low amplitude: heart rate changes of 6 or more bpm from prior baseline rate (but retaining all other features of the high amplitude definition)(26). Subjects or controls with “0” minutes of CVHR prior to natural log transformation had values replaced with “0.5” to prevent signal loss.
Statistics
Subsequent analyses between study subjects and controls were performed using GraphPad Prism version 9.0.0 for Mac, (GraphPad Software, San Diego, California USA, www.graphpad.com.) and SAS 9.4 (SAS Institute, Cary, NC). Mann-Whitney tests and Chi-square tests were used to evaluate the difference between WBS subjects and controls. Scatterplot graphs display a bar signifying median of the distribution, except for frequency domain parameters (transformed by the natural log: means graphed). Subjects who underwent cardiothoracic surgery are displayed as open red triangles (no CT surgery who are displayed as closed red squares). Non-linear measures (SD1, SD2, SD1/SD2, DFA1, ApEn, and SampEn) were examined using mixed-effects model to account for 24-hour repeated measurements with adjustments of age, sex, and hour of day; these measures were reported and displayed as least-square mean (lsmean) +/− standard error (stand err.). Stratified daytime and nighttime analyses were conducted using measurements from 8am to 8pm as daytime, and from 8pm to 8am as nighttime. A p-value <0.05 is considered statistically significant, and Supplemental Table 1 displays false discovery rate (FDR) adjusted p-values for all comparisons. P-values less than 0.001 were reported as P<0.001.
Study subjects and control inclusion and exclusion
Patient accrual for the study commenced December 6, 2016. As of March 30, 2021, there have been 65 subjects with WBS (as defined by ELN FISH positivity equivalence) and 41 controls between ages 3–65 years enrolled. Subjects and controls were permitted to opt out of any testing, but inclusion required >22hour ambulatory ECG non-artifactual recording and an age-matched (less than 2 years in children (under 20yo) or within 5 years of adult subject’s age (21 years and over) control with complete ambulatory ECG recording. Further, artifact could comprise no more than 5% of the recording.
Patient exclusion criteria
See Supplemental Figure 1 for full inclusion/exclusion scheme. Four controls were excluded from analysis because HRV results diverged from the remaining controls, including one control with documented anxiety disorder and one control who had spontaneous ventricular tachycardia. Further exclusions occurred because bundle branch morphology precluded accurate rhythm assessment, chronic beta blockade treatment or absence of a Holter meeting quality control metrics. We also excluded children under 12 years of age. Following exclusion, the remaining 23 subjects with WS and 24 controls were matched for age and sex. Five subjects and six controls did not have a suitable match leaving 18 cases with WS and 18 controls for analysis.
Results
Demographics
Subject and control demographic data are summarized in Table 1. There were no differences in age, sex or BMI distribution between WBS subjects and controls (Table 1). By contrast, average 24hr heart rate (HR) was higher in the WBS group(Fig. 1A, P<0.0001)(Supp Fig 2,3). Cardiac systolic function was normal in all study participants(EF=60% or greater). Two WBS subjects had aortic surgery(none with significant residual stenosis); one patient also had pulmonary artery intervention. Three WBS subjects had mild supravalvar aortic stenosis. Controls showed no vessel stenosis.
Table 1.
Patient Demographics
| Control (n=18) |
WBS Subject (n=18) |
P-value | |
|---|---|---|---|
| % female | 72.2 | 72.2 | NS* |
| Age (years) (median; IQR) |
23.1;8.8 | 24.05;10.45 | NS |
| BMI (kg/m2) (median; IQR) |
22.8;6.15 | 22.90;8.47 | NS |
| %EF >60% | 100 | 100 | NS* |
| % CT surgery | 0 | 11.1 | <0.01 |
| % current SVAS | 0 | 16.7 | <0.01 |
Abbreviations: BMI, body-mass index; kg/m2, kilograms/meters2; IQR, inter-quartile range; EF, ejection fraction; CT, cardio-thoracic; SVAS, supra-valvar aortic stenosis. All above values Mean±SD.
P-values were from Chi-Squared(for sex and % EF>60%) and Mann-Whitney U test(remaining variables)
Figure 1. WBS subjects display significant autonomic dysregulation as measured by non-linear, time- and frequency-domain HRV analyses from 24hr ECG recordings.
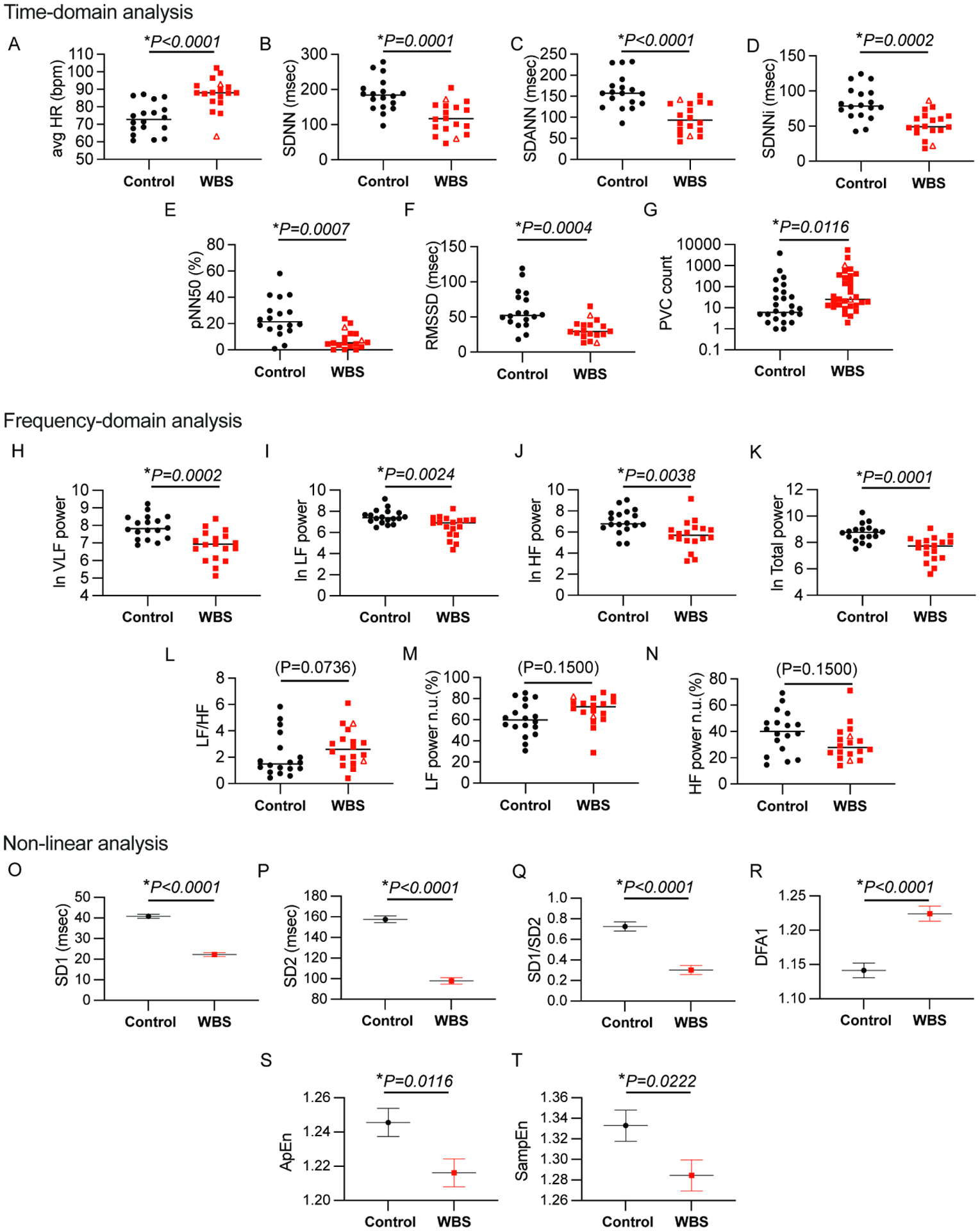
Scatter plots comparing WBS subjects (n=18) and controls (n=18) shown for the following: (1)Time-domain analysis derived from 24 hour data segments: (A)Avg HR (B)SDNN (C)SDANN (D)SDNNi (E)pNN50 (F)RMSSD, (G)PVC count (2)Frequency-domain analysis: (H)lnVLF power (I)lnLF power (J)lnHF power (K)lnTotal Power (L)LF/HF ratio (M) LF power (normalized) (N) HF power (normalized) comparing control and WBS subject cohorts. Mann-Whitney test were performed for comparisons of data not transformed by natural log and unpaired parametric T-tests were performed where data has been transformed by the natural log. (derived from 24hour data segmented into 5 minute intervals) (3)Non-linear analyses using mixed-effect model on 24hour data segmented into 1 hour intervals: (O)SD1, (P)SD2, (Q)SD1/SD2, (R)DFA1, (S)ApEn and (T)SampEn comparing control and WBS subject cohorts. Graphs display median for non-transformed data, mean for transformed data and estimated mean±standard error for mixed model effect data. P-values displayed for all parameters. WBS subjects who underwent CT surgery are displayed as open triangles, while WBS subjects not requiring cardiac surgical palliation are displayed as filled squares.
HRV analysis of complete 24hour recording period
Figure 1 summarizes results for all time- and frequency-domain and non-linear statistics (total 24hour period).
Time-Domain Parameters
All time-domain parameters showed diminished variability in WBS subjects compared to controls. Findings are summarized in Figure 1:(Fig. 1B, SDNN, P=0.0001); (Fig. 1C, SDANN P<0.0001);(Fig. 1D, SDNNi, P=0.0002);(Fig. 1E, pNN50, P=0.0007), and (Fig. 1F, RMSSD, P=0.0004)). Additionally, premature ventricular contraction(PVC) count was significantly higher in the subject group compared to controls(Fig. 1G, PVC, P=0.0116).
Frequency Domain Parameters
Data were segmented into 5-minute non-overlapping intervals for subsequent frequency domain analysis. lnVLF power, lnLF power, lnHF power and lnTotal power all demonstrate significant differences between WBS subjects and controls: WBS lnVLF power(Fig. 1H), lnLF power(Fig. 1I), lnHF power(Fig. 1J) and lnTotal power(Fig. 1K) were lower than control values; LF/HF ratio(Fig. 1L) and normalized LF power(Fig. 1M) values were higher, while normalized HF power(Fig. 1N) was lower than control: (lnVLF, P=0.0002)); (lnLF power P=0.0024)); (lnHF power, P=0.0038)); (LF/HF ratio, P=0.0736)); (normalized LF power, P=0.1500); (normalized HF power, P=0.1500)). Neither respiratory rate determined by hourly power spectral density plot, nor CVHR (low and high amplitude) showed significant differences between cohorts (Supplemental Fig. 4).
Non-linear Parameters
Non-linear measures were quantified using hourly segments and then control and WBS subject cohorts were compared using mixed-effect models to account for 24-hour repeated measurements. These parameters were significantly diminished in subjects vs. controls for the following: SD1, P<0.0001 (Fig. 1O), SD2, P<0.0001 (Fig. 1P), and SD1/SD2, P<0.0001 (Fig. 1Q), ApEn, P=0.0116 (Fig. 1S), and SampEn, P=0.0222 (Fig. 1T). DFA1 was increased in WBS subjects compared to controls, P<0.0001 (Fig.1R). In addition to quantitative SD1, SD2, and SD1/2 non-linear data analysis, hourly Poincaré plot morphology demonstrated two or more “torpedo-like” plots in 11 of 18 subjects compared to 0 of 18 control subjects ((Fisher’s exact, P=0.0001, Fig 3).
Figure 3. Poincaré plot morphologic evaluation from hourly segmented data.
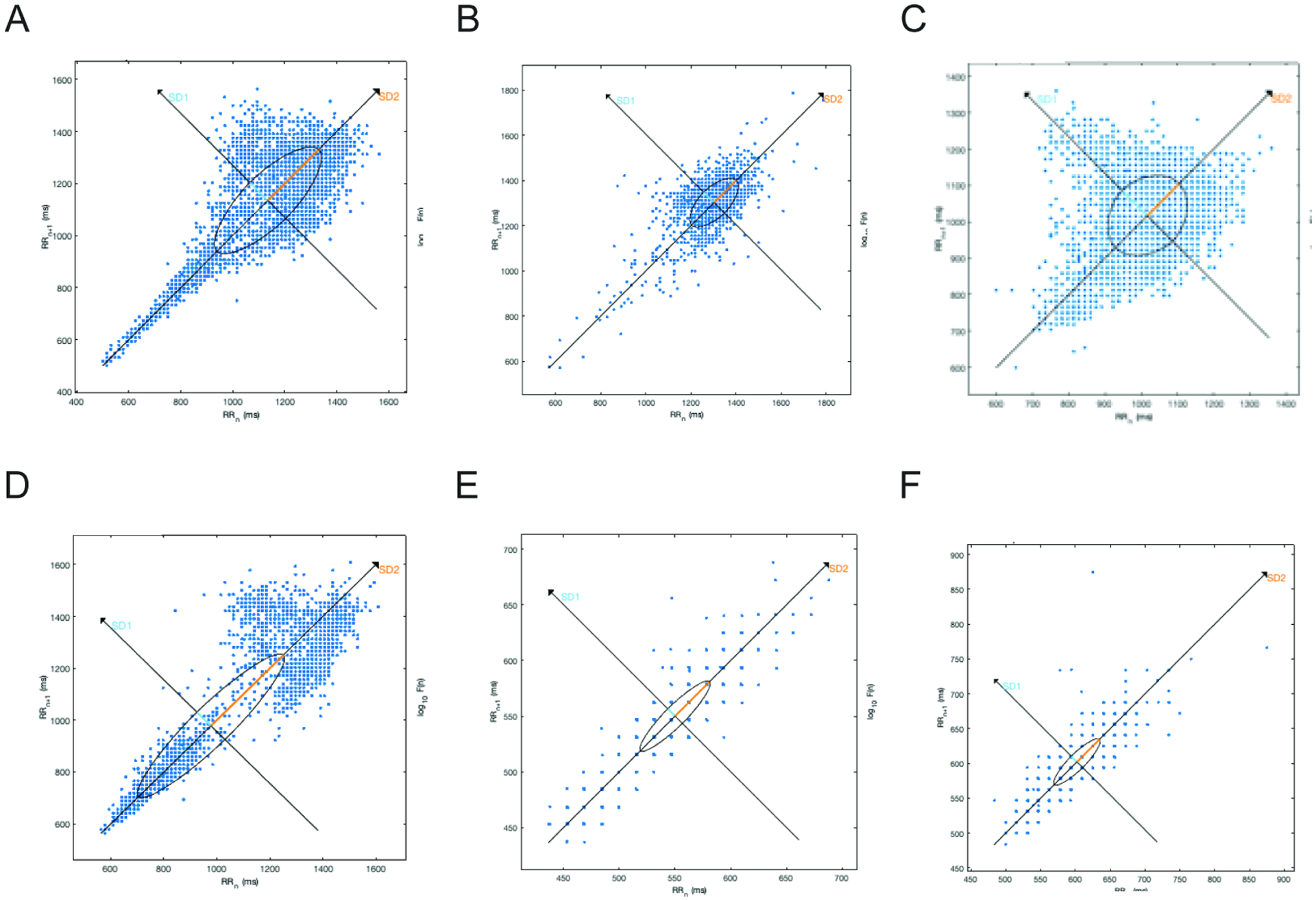
Poincare plots constructed from hourly-segmented data showing: (A) “comet-like” morphology (typical, considered normal) (B) ”ball-like” plot (C) asymmetric “complex” plot and (D) bimodally distributed plot (A-D from controls); Two examples of “torpedo-like” plots from subjects (E) and (F). Eleven of eighteen subjects showed 2 or more torpedo-shaped plots, while no control showed such. Axis scales are not uniform.
Individual subject HR with mean control HR, HF power and LF power 24-hour graphs
Graphical display of variables over 24 hours permits additional insights into subject cohort abnormalities. Figure 2A displays an indicative 24-hour HR trace using data segmented in 5 minute intervals for a 33yo male subject(red) and mean HR from 5 similarly-aged (range 17–31 years) males(mean in black, with S.D. in gray). (Supplemental Figure 3 plots (A)indicative HR trace (red) along with male subjects(n=5, orange) and male controls(n=5 purple) to demonstrate relative similarities of this plot to remainder of cohort (B) mean±SEM HR male subjects vs. mean±SEM HR male controls and (C) mean±SEM HR female subjects vs. mean±SEM HR female controls for comparison). Note the sinusoidal nature of the control trace, with higher HR during daytime and a noticeable drop in HR during sleeping hours(Fig. 2A). By contrast, the individual with WBS displays HRs that are higher than control means throughout the daytime and nighttime. There is a small drop in nighttime HR, but this is modest in comparison. Thus, the sinusoidal nature of the subject waveform is detectable, but dampened.
Figure 2. Indicative trace from WBS subject and sex- and age-matched controls show circadian abnormalities and diminished parasympathetic activity.
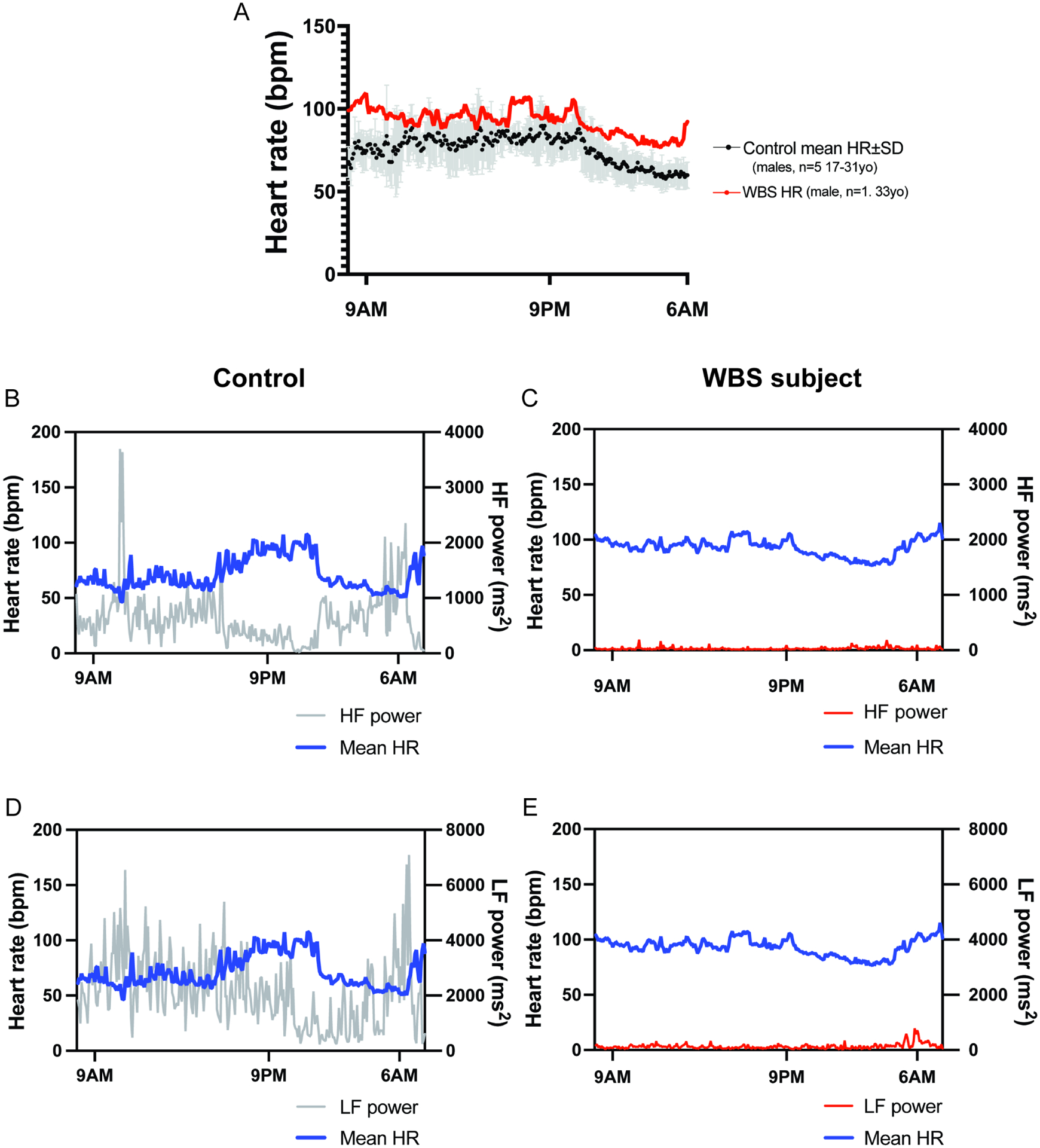
(A) 24hr recording of mean heart rate(bpm) for WBS subject(red, 33yo male) graphed with control HR mean(black)±SD (gray) (males, 26–40yo, (n=5)). Compared to control HR trend(sinusoidal), subject HR trend appears dampened and displaced upward consistent with increased sympathetic activity. Simultaneous graphs of HR(left Y-axis, blue line) and HF power(right Y-axis) (parasympathetic activity correlate), displayed for control (31yo male) (B) and WBS subject (33yo male) (C) simultaneously. Control(B) tracing shows lower HF power during the day and rises through night, peaking just before awakening. By contrast, subject HF tracing(C) shows significantly diminished HF power without significant pre-waking rise. HR(left Y-axis, blue line) and LF power(right Y-axis), graphed simultaneously for control(D) and WBS subject(E).
High frequency power is an indirect indicator of parasympathetic activity(28). Simultaneous graphing of both HR and HF power sheds insight into circadian variation of these parameters (Fig. 2B). Figure 2B displays HR(left y-axis, blue trace) and HF power(right y-axis, gray trace) for one control contained in Figure 2A(31yo male). Two features are characteristic of normal HF power: 1) HF power is low during daylight/waking hours and gradually increases throughout the night during sleep, peaking just before awakening(Fig. 2B), 2) there is an inverse relationship between HR and HF power. The WBS subject’s HF power, by contrast, is significantly diminished, making generalizations difficult(Fig. 2C). Comparable traces showing HR and LF are also provided(control, Fig. 2D; subject, Fig. 2E). LF power was previously considered an indirect sympathetic activity indicator(28), but this is now disputed (29). In the control, one sees a direct relationship between HR and LF power(Fig. 2D). LF power in the WBS subject is significantly diminished(Fig. 2E).
Daytime vs. Nighttime HRV metrics
A breakdown of all daytime and nighttime time- and frequency-domain parameters can be found in Supplemental Figure 2. All time- and frequency-domain parameters at night-time showed statistical differences between control and WBS subjects, and all daytime parameters showed statistically significant differences except LF/HF ratio, normalized LF power and normalized HF power.
Discussion
HRV abnormalities suggest autonomic abnormalities in WBS
This is the first study to detail HRV abnormalities suggesting autonomic dysfunction in WBS patients. We find the following abnormalities: 1. Diminished HRV 2. Diminished indirect measures of parasympathetic activity 3. Indirect measures of increased sympathetic activity(HR) (Central Figure).
Figure 4. Central Illustration.
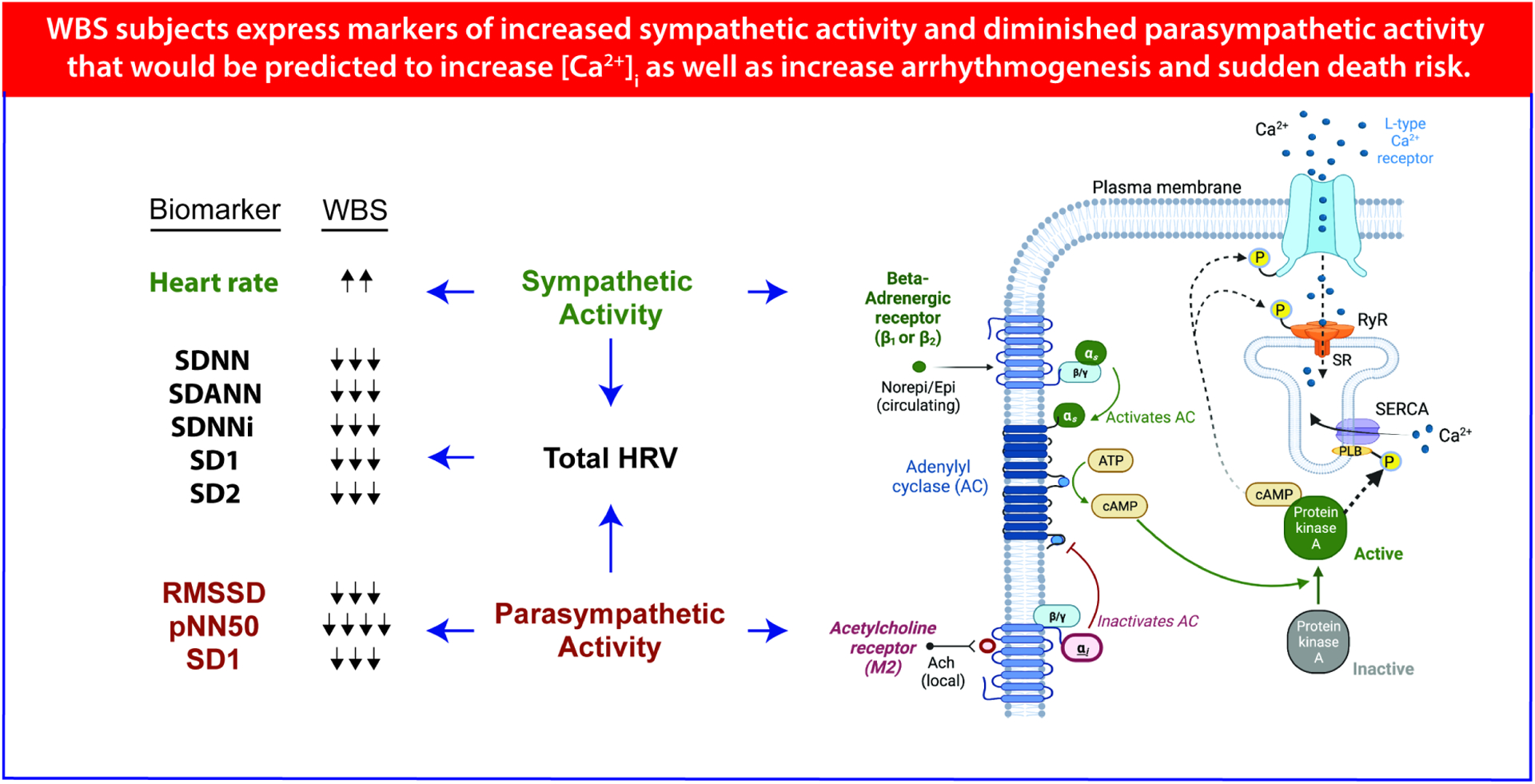
Summary heart rate variability (HRV) findings with graphical explanation of inferred underlying cellular processes. Prospective cross-sectional study of Williams-Beuren Syndrome subjects(n=18) and age/sex matched controls(n=18) reveal autonomic abnormalities that suggest increased sudden death risk: increased sympathetic activity, diminished parasympathetic activity and diminished HRV. Cardiomyocyte firing rate and contractility are modulated by sympathetic and parasympathetic inputs via PKA phosphorylation of calcium handling proteins (L-type Ca2+channel(LTCC), ryanodine receptor (RYR), and phospholamban’s (PLB) effects on smooth ER Ca2+ ATPase (SERCA) that can significantly increase arrhythmia and sudden death risk. Heart rate variability (HRV) analysis enables quantification of sympathetic and parasympathetic activity for subsequent risk stratification.
Diminished HRV
Several approaches were used to probe HRV measures in WBS subjects. Each measure indicated significantly diminished HRV in WBS subjects. Time-domain parameters indicating diminished HRV included: SDNN(Fig. 1B), SDANN (Fig. 1C), and SDNNi(Fig. 1D), and RMSSD(Fig. 1F). As SDANN tracks change throughout the entire 24-hour period, SDANN further reflects circadian rhythm abnormalities(20,30). Frequency-domain analyses similarly demonstrate decreased HRV as evidenced by significantly decreased power of all frequencies: (lnVLF power (Fig. 1H), lnLF power (Fig. 1I), lnHF power (Fig. 1J)), and lnTotal power(Fig. 1K). Non-linear mathematical analyses of a time series measure the unpredictability produced by a system, thereby reflecting variability. Thus, SD1, SD2, SD1/SD2, ApEn, and SampEn are direct measures of HRV(31,32). SD1, SD2, SD1/SD2, ApEn, and SampEn(Fig. 1O–T) all showed significantly diminished values in WBS subjects, suggesting diminished short-term and long-term variability. WBS subjects exhibited significantly lower values of ApEn and SampEn(Fig. 1S,T), suggesting increased “regularity” and reduced complexity of the R-R time series(23,24). Initially ApEn and SampEn were used as markers of neonatal sepsis and drops in these markers corresponded with loss of beat-to-beat variability and preceded or corresponded with incipient sepsis(23,24). These non-linear markers may reflect a less robust system that exhibits diminished capacity to generate fluctuations and, again, possibly represent diminished autonomic reserve. Another non-linear marker, DFA1(Fig. 1R), was also abnormal in WBS subjects compared to controls; DFA1 was significant elevated compared to control values, suggesting a loss of fractal characteristics and loss of short-term variability.
Non-linear qualitative analyses also demonstrated significant differences between control and experimental cohorts. Torpedo-like Poincare plot morphology was seen in at least two hourly plots in 11 of 18 study subjects with WS and no control subjects (Figure 3). This morphology represents decreased RR interval range and the absence of RR interval dispersion commonly seen in healthy controls at longer RR intervals; hence it’s an easily recognizable manifestation of diminished HRV frequently identified in study subjects but not controls(25). Based on these findings, we suggest that Poincare plot morphology analysis may serve as a more easily derived and accessible screen of HRV abnormalities prior to initiating more time-intensive and less intuitive analyses.
Diminished markers of parasympathetic activity
Consensus exists that HRV can reliably assess parasympathetic activity indirectly(28,33–35). We chose the following as indirect measures of parasympathetic activity and found all had significantly diminished values in WBS subjects: RMSSD(Fig. 1F), pNN50(Fig. 1E), HF power (1J), and SD1(Fig. 1O), which likely have mathematical interrelatedness.
Heart rate and circadian abnormalities consistent with possible increased sympathetic activity
HRV sympathetic activity markers remain elusive(34). Initially, LF power was thought to be a reliable indicator of sympathetic activity based on basic experiments using pharmacologic blockers(28); however, subsequent studies refuted this. LF/HF ratio was subsequently proposed as a measure of “sympatho-vagal” balance, but this, too, has proven inconsistent(34). We have included LF power(Fig. 1I, decreased in WBS) and LF/HF ratio (Fig. 1L, increased in WBS) in our analysis as recommended by the HRV task force(20), and because these parameters offer some, albeit imperfect, insight into sympathetic activity. Towards this end, LF power shows significant differences between subjects and controls, and LF/HF ratio shows a trend toward significance(P=0.0736).
The absence of a reliable sympathetic HRV marker is all the more disconcerting given that sympathetic activity underlies well-described arrhythmogenesis and heart failure mechanisms. Heart rate by itself, however, can offer an important window into sympathetic activity(12,30,31). We suggest that increased average HR(24hr)(Figure 1A), as well as increased daytime(Supp Fig. 2A) and increased nighttime(Supp Fig. 2K) HR all suggest increased sympathetic activity in WBS subjects vs. controls. Further evidence of circadian abnormalities can be seen when one graphs WBS HR over 24 hours(Fig. 2A). As demonstrated by the WBS subject trace of HR over time, the subject’s HR waveform is displaced upwards in the higher HR range and appears to have a dampened sinusoidal waveform when compared to the control, characteristic of WBS cohort(Supp Fig. 2,3).
Arrhythmogenesis and sudden death risk: HRV as a tool for risk stratification
An advantage of quantifying autonomic disturbances is that we may collect prognostic data tied to these markers. We caution that restraint should be exercised, however, as significant differences exist between populations where these markers were initially identified (older patients with more severe cardiovascular disease(13,25,31) and our study population. Still, we hypothesize that markers of diminished parasympathetic and increased sympathetic activity will provide prognostic value in WBS cohorts based on the observations that diminished parasympathetic activity and increased sympathetic activity reliably increase arrhythmogenesis in both model systems and human disease(16,17). Sympathetic activity shortens action potential duration, increases dispersion of refractoriness and increases early and late afterdepolarizations. These identical mechanisms also increase sudden death risk(18). Given this mechanistic connection to sudden death, we anticipate these measures will allow caregivers a means to risk stratify WBS patients. While larger WBS cohorts and longer study durations will be needed before HRV tools can be applied with such precision, we remain hopeful that HRV measures will be a valuable tool in uncovering WBS sudden death mechanisms.
Mechanistic insights
In addition to guiding risk stratification, these data also suggest areas for future studies. Increased sympathetic activity directly promotes arrhythmogenesis by shortening refractory periods, increasing dispersion of refractoriness and promoting triggered activity (16,18). Likewise, diminished parasympathetic activity is thought to promote arrhythmogenesis through similar actions (16,18). By contrast, increased parasympathetic activity is thought to counter these pro-arrhythmic activities. Beyond these immediate direct effects, autonomic activity may play a larger role in cardiac health by maintaining a “reserve” for both stress and homeostatic responses, and the lack of “reserve” in WBS patients may predispose these patients to catastrophic consequences when hemodynamic or electrophysiologic stress occurs. Specifically, as has been shown in heart failure models, chronic sympathetic excess ultimately leads to post-translational modification of key ion channel and calcium handling proteins. While a healthy individual naïve to adrenergic sympathetic exposure might be able to muster “super-human” strength in response to an acute insult, the individual with WBS chronically exposed to sympathetic excess might retain only limited capacity to further increase HR or contractility in a similar “stress” situation. Likewise, parasympathetic activity is thought to have healing, restorative and anti-arrhythmic properties. In response to a hemodynamic or electrophysiologic insult, the WBS subject may be balanced on the precipice and easily lapse into VF, while healthy individuals harbor sufficient parasympathetic reserve that their myocardium does not support maintenance of the abnormal rhythm. Those with WBS have diminished autonomic reserve as a result of the described abnormalities and may be predisposed to negative outcomes because they lack the ability to mount an appropriate homeostatic response. Figure 3 summarizes our study findings and provides the inferred cellular framework through which these findings can be understood from a mechanistic standpoint.
Study limitations
Study limitations include the relatively small sample size. Future studies aimed at testing HRV risk stratification usefulness will benefit from a larger enrollment and longer follow-up period. We also caution the application of these methods in clinical care. HRV is a research tool for analyzing cohorts and this analysis is not easily applied to investigating a single patient’s autonomic status at this point.
Conclusions
In conclusion, our results suggest WBS subjects have diminished autonomic reserve, characterized by diminished heart rate variability, circadian abnormalities, diminished parasympathetic activity, and possibly increased sympathetic activity. This diminished reserve may represent diminished capacity for cardiovascular compensation in the face of hemodynamic or electrophysiologic insult, and, thus, may represent a mechanistic link to the increased sudden death risk seen in patients with WBS. Findings here may ultimately aide in patient risk stratification as well as in the quantitative assessment of therapeutic autonomic interventions for WBS patients. Further study will be needed to realize these goals.
Supplementary Material
Clinical competencies.
Competency in medical knowledge and patient care:
People with Williams-Beuren syndrome are at increased risk of sudden death that appears strongly correlated with anesthesia administration. In a cross-sectional prospective study of Williams-Beuren syndrome subjects, heart rate variability and indirect markers of parasympathetic activity were significantly diminished compared to controls, which may help identify those at risk for sudden death.
Translational outlook:
Williams Beuren syndrome patients have increased sudden death risk. This study suggests both a critical biomarker for risk stratifying these patients as well as potential therapeutic intervention that may be enlisted to diminish or prevent this feared outcome. Prospective trials of such interventions in people with WBS should be considered to determine safety and efficacy of this approach.
ACKNOWLEDGEMENTS
Funding for this work was provided by the NHLBI DIR at NIH. The researchers wish to express their sincere gratitude to the subjects, their families and the Williams Syndrome Association for supporting this study. The authors also thank Alex McIntosh for editing the document. No author has any financial relationships to disclose. Central illustration created with BioRender.com.
Funding source:
NHLBI DIR intramural research programs grant ZIA HL 006210 and ZIA HL 006212
ClinicalTrials.gov Identifier: NCT02840448
Abbreviations:
- ApEn
Approximate Entropy
- DFA1
detrended fluctuation analysis, short term scaling exponent, 1α
- HF power
high frequency power, (in ms2)
- HR
heart rate (bpm)
- HRV
heart rate variability
- LF/HF
ratio of low frequency power to high frequency power
- LF n.u.
normalized low frequency power,(%)
- LF power
low frequency power, (in ms2)
- ln
natural log
- NN
normal to normal beat interval
- pNN50
percent of NN intervals different from previous by 50% or more of local average
- RMSSD
root mean square of successive differences of NN intervals, (in msec)
- SampEn
Sample Entropy
- SD1
short axis of Poincaré plot (in msec)
- SD2
long axis of Poincaré plot (in msec)
- SD1/SD2
short axis of Poincaré plot divided by long axis of Poincaré plot (in msec)
- SDANN
standard deviation of average NN for 5-minute intervals over 24 hours, (in msec)
- SDNN
standard deviation of NN intervals (in msec)
- SDNNi
average of 5-minute standard deviations of NN intervals for 24 hours, (in msec)
- VLF power
very low frequency power, (0–0.04Hz), (in ms2)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest declaration: The authors declare no conflict of interest and have no financial relationships to disclose.
References
- 1.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation 1961;24:1311–8. [DOI] [PubMed] [Google Scholar]
- 2.Ewart AK, Morris CA, Atkinson D et al. Hemizygosity at the elastin locus in a developmental disorder, Williams Syndrome. Nat Genet 1993;5:11–6. [DOI] [PubMed] [Google Scholar]
- 3.Li DY, Toland AE, Boak BB et al. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet 1997;6:1021–8. [DOI] [PubMed] [Google Scholar]
- 4.Roach MR. The pattern of elastin in the aorta and large arteries of mammals. Ciba Found Symp 1983;100:37–55. [DOI] [PubMed] [Google Scholar]
- 5.Wille HH, Sauer G, Teebe U, Neuhaus KL, Kreuzer H. Nitroglycerin and afterload: effects of aortic compliance and capacity of the Windkessel. Eur Heart J 1980;1:445–452. [DOI] [PubMed] [Google Scholar]
- 6.Bird LM, Billman GF, Lacro RV et al. Sudden death in Williams syndrome: Report of ten cases. The Journal of Pediatrics 1996;129:926–931. [DOI] [PubMed] [Google Scholar]
- 7.Hornik CP, Collins RT, Jaquiss RDB et al. Adverse cardiac events in children with Williams syndrome undergoing cardiovascular surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. The Journal of Thoracic and Cardiovascular Surgery 2015;149:1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessel A, Gravenhorst V, Buchhorn R, Gosch A, Partsch C-J, Pankau R. Risk of sudden death in the Williams-Beuren syndrome. American Journal of Medical Genetics 1 1994;127A:234–237. [DOI] [PubMed] [Google Scholar]
- 9.Galinier M, Pathak A, Fourcade J et al. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J 2000;21:475–482. [DOI] [PubMed] [Google Scholar]
- 10.Ho KKL, Moody GB, Peng C-K et al. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation 1997;96:842–848. [DOI] [PubMed] [Google Scholar]
- 11.Huikuri HV, Stein PK. Heart rate variability in risk stratification of cardiac patients. Progress in cardiovascular diseases 2013;56:153–159. [DOI] [PubMed] [Google Scholar]
- 12.Bigger JT, Kleiger RE, Fleiss JL, Rolnitzky LM, Steinman RC, Miller JP. Components of heart rate variability measured during healing of acute myocardial infarction. The American Journal of Cardiology 1988;61:208–215. [DOI] [PubMed] [Google Scholar]
- 13.Stein PK, Rich MW, Rottman JN, Kleiger RE. Stability of index of heart rate variability in patients with congestive heart failure. Am Heart J 1995;129:975–81. [DOI] [PubMed] [Google Scholar]
- 14.Stein PK, Domitrovich PP, Kleiger RE, Investigators C. Including patients with diabetes mellitus or coronary artery bypass grafting decreases the association between heart rate variability and mortality after myocardial infarction. American Heart Journal 2004;147:309–16. [DOI] [PubMed] [Google Scholar]
- 15.Guideri F, Acampa M, Hayek G, Zapella M, Pierri TD. Reduced heart rate variability in patients explanation with rett syndrome. A possible explanation for sudden death. Neuropeptides 1999;30:146–148. [DOI] [PubMed] [Google Scholar]
- 16.Shen Mark J, Zipes Douglas P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circulation Research 2014;114:1004–1021. [DOI] [PubMed] [Google Scholar]
- 17.Gardner RT, Ripplinger CM, Myles RC, Habecker BA. Molecular mechanisms of sympathetic remodeling and arrhythmias. Circ Arrhythm Electrophysiol 2017;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. The Journal of Clinical Investigation 2005;215:2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Team R. Integrated Development for R. Boston, MA, 2018. [Google Scholar]
- 20.Malik M, Bigger JT, Camm AJ et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 21.Sassi R, Cerutti S, Lombardi F et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015;17:1341–1353. [DOI] [PubMed] [Google Scholar]
- 22.Mäkikallio TH, Huikuri HV, Mäkikallio A et al. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. Journal of the American College of Cardiology 2001;37:1395–1402. [DOI] [PubMed] [Google Scholar]
- 23.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J Clin Monitor 1991;7:335–345. [DOI] [PubMed] [Google Scholar]
- 24.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiology-regulatory Integr Comp Physiology 2002;283:R789–R797. [DOI] [PubMed] [Google Scholar]
- 25.Woo MA, Stevenson WG, Moser DK, Middlekauff HR. Complex heart rate variability and serum norepinephrine levels in patients with advanced heart failure. Journal of the American College of Cardiology 1994;23:565–569. [DOI] [PubMed] [Google Scholar]
- 26.Stein PK, Duntley SP, Domitrovich PP, Nishith P, Carney RM. A Simple Method to Identify Sleep Apnea Using Holter Recordings. J Cardiovasc Electr 2003;14:467–473. [DOI] [PubMed] [Google Scholar]
- 27.Penttilä J, Helminen A, Jartti T et al. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol 2001;21:365–376. [DOI] [PubMed] [Google Scholar]
- 28.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981;213. [DOI] [PubMed] [Google Scholar]
- 29.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. American Journal of Physiology-Heart and Circulatory Physiology 1990;258:H713–H721. [DOI] [PubMed] [Google Scholar]
- 30.Stein PK, Eric J. Lundequam, Oliveira LPJ et al. Cardiac autonomic modulation: Analyzing circardian and ultradian rhythms. IEEE Egineering in Medicine and Biology Magazine 2007;26:14–18. [DOI] [PubMed] [Google Scholar]
- 31.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. American Heart Journal 1992;123:704–710. [DOI] [PubMed] [Google Scholar]
- 32.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE, Investigators C. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electr 2005;16:13–20. [DOI] [PubMed] [Google Scholar]
- 33.Escorihuela RM, Capdevila L, Castro JR et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2020;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrasco S Gaitán MJ, Gonzalez R, Yanez O. Correlation among Poincare plot indexes and time and frequency domain measures of heart rate variability. Journal of Medical Engineering & Technology 2001;25:240–248. [DOI] [PubMed] [Google Scholar]
- 35.Tulppo MP, Makikallio TH, Takala TE, Seppanen T, Huikuri HV. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol-heart C 1996;271:H244–H252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


