Abstract
Objective-
Prevotella copri (Pc), a gut commensal, has been reported to be an immune relevant organism in individuals with rheumatoid arthritis (RA). Our goal was to evaluate anti-Pc antibody responses in our participant cohorts and determine when in the natural history of RA such responses develop.
Methods-
Serum levels of IgA and IgG anti-Pc-p27, an immunogenic Pc protein, were analyzed in study participants at-risk for the development of RA, those who transitioned to RA, in those with early RA (< one year of disease), and in those with established RA, compared to matched controls. Additionally, levels of anti-Pc-p27 antibodies were evaluated in individuals stratified by RA-related autoantibody status.
Results-
Overall, participants with RA had significantly higher levels of IgA anti-Pc-p27 antibodies and trends towards higher levels of IgG anti-Pc-p27 antibodies when compared to their matched controls. When stratified by early versus established RA, early RA participants had median values of IgG anti-Pc-p27 antibodies that were overall higher, whereas median values of IgA anti-Pc-p27 were statistically significantly higher in participants with established RA, compared with their matched controls. In the autoantibody specific analyses, the at-risk population with anti-CCP antibodies, but not RF, demonstrated trends towards increased levels of IgG anti-Pc-p27. Additionally, RA participants who were CCP+/RF+ had significantly increased levels of IgA anti-Pc-p27 antibodies and a trend toward levels of IgG anti-Pc-p27 antibodies when compared to their matched controls.
Conclusion-
These findings support a potential etiologic role for this microorganism in both RA preclinical evolution and the subsequent pathogenesis of synovitis.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is associated with immune responses to self-antigens and characterized by inflammation and substantial joint destruction1–3. RA pathogenesis is understood to involve biologically complex interactions between environmental and genetic factors that transcend beyond the generation of neoantigens by citrullination; among them are the Human Leukocyte Antigen (HLA) susceptibility genes known as the shared epitope (SE), smoking, diet, and intestinal dysbiosis4–7.
Amongst individuals with RA, there are two distinct autoantibody subgroups, seropositive and seronegative8. Seropositive RA individuals exhibit circulating autoantibodies, including rheumatoid factor (RF) and antibodies to citrullinated protein/peptide antigens (ACPAs) most often measured as anti-cyclic citrullinated peptide (CCP) antibodies. In the great majority of seropositive cases, there is a period of autoantibody positivity, typically an average of 3–5 and up to 17–18 years, prior to the onset of clinically apparent and classified RA9–12. During the phase prior to the development of arthritis, RA-related autoantibody positive individuals can be defined as being at-risk for the future development of classified RA13.
Manifestation of systemic autoantibodies in serum prior to inflammation in joints, the well-established primary tissue target of the disease, suggests initiation of the autoimmune process elsewhere. Mucosal sites such as the periodontium, lungs, gut, and vaginal tract have garnered attention as initiating sites for the autoimmune process in RA14–18. Recent developments in the identification and categorization of bacterial commensals and pathobionts at mucosal sites have helped to advance our understanding of the immune consequences when dysbiosis exists. Barra et al. found that the proportion of ACPAs of the IgA isotype (a mucosal-associated immunoglobulin) was higher in new onset RA than in those with established RA in whom IgG ACPAs were more abundant, providing further evidence that the autoimmune process may originate at mucosal sites19.
Using a discovery-based approach, Scher et al., identified Prevotella species, particularly P. copri (Pc), as expanded in stool samples in patients with new onset RA (mean duration of disease 5.4 months) when compared to patients with established RA, psoriatic arthritis and healthy controls20. They also found that the presence of the human leukocyte-antigen (HLA) Class II locus, primarily in the DRB1 region, also known as the shared epitope (SE), is inversely correlated with the relative abundance of Pc in new onset RA patients. The SE is a highly specific set of alleles which is thought to contribute to roughly 40% of the genetic risk of developing RA21–24. In their New York study population, 33 of 44 patients (75%) with new-onset RA had an overabundance of Prevotella spp., especially Pc, in stool samples compared with 37.5% (6/16) patients with chronic RA or psoriatic arthritis and 11.5% (3/26) of healthy controls20.
To further explore these findings, Seward et al. and Wang et al. developed a novel technique to identify HLA-DR-presented microbial or self-peptides from synovial tissue, synovial fluid mononuclear cells, or peripheral blood mononuclear cells (PBMC) of arthritis patients, followed by determination of antigenicity of the peptides using patients’ samples25,26. From the PBMC of 1 of 2 RA patients tested, one HLA-DR-presented Pc peptide was identified, which was derived from the signal domain of a 27-kD protein (Pc-p27) of the organism25. In their Boston cohort, Pianta et al reported that 42% of 40 RA patients had Th1 responses to this single T cell epitope of Pc-p27, and among 127 RA patients, 24% had IgG or IgA antibodies, but usually not both, to the Pc-p27 protein27. In the subgroup with IgA Pc-p27 antibody responses, the antibodies correlated with Th17 responses and a high frequency of ACPAs, suggestive of a mucosal immune response. In contrast, in the subgroup with IgG Pc-p27 antibody reactivity, the antibodies correlated with Prevotella DNA in synovial fluid, Pc-specific Th1 responses, and less frequent ACPAs, suggestive of possible invasion of Pc or its components in joints accompanied by a systemic immune response.
In a follow-up study, Pianta et al. reported the identification of 4 additional immunogenic HLA-DR-presented Pc peptides or proteins in RA patients, but the greatest reactivity was still with the previously identified Pc-p27 protein28. Additionally, 74% of RA patients who had IgA antibodies to ≥ 1 of the 5 Pc proteins demonstrated ACPA reactivity, compared with 49% who lacked IgA Pc antibodies (P=0.05), and IgA Pc antibody levels correlated with ACPA values. Although IgA and IgG responses to Pc-p27 were generally higher in RA patients than in comparison groups, IgA anti-Pc-p27 responses were more specific for RA than IgG anti-Pc-p27 responses, which could sometimes be found in patients with PsA or AS, or in healthy controls (HC)27,28. In contrast, IgA and IgG antibody responses to Bacteroides fragilis or Escherichia coli were similar in RA patients and in those with other rheumatic diseases or in healthy controls27.
In two European cohorts, Wells et al. demonstrated an association between Prevotella_7 and Prevotella 9, two amplicon sequence variants derived from 16S sequencing, and genetic risk for RA, via evaluation of polygenetic risk scores (PRSs) and SE positivity29. Specifically, they found Prevotella_7 (annotated to multiple Prevotella spp) had the strongest taxon association with the PRS for RA and Prevotella_9 (predicted to be P. copri) was associated with preclinical RA.
In the current study, we hypothesized immune reactivity to Pc may be found in additional RA populations and potentially in individuals at-risk for developing RA. We found that at-risk individuals and those with early RA had overall higher levels of IgG Pc-p27 antibodies and those with established RA had significantly increased levels of IgA anti-Pc-p27 antibodies.
METHODS
Studies of the Etiology of Rheumatoid Arthritis
Participants were selected from the Studies of the Etiology of Rheumatoid Arthritis (SERA) cohort, described previously30. Briefly, SERA is a multicenter, longitudinal study that follows individuals at-risk for future development of RA (at-risk defined as individuals with positive levels of circulating RA-specific autoantibodies and/or first-degree relatives (FDRs) of a proband with RA, which confers a higher genetic risk for the development of RA)31–33, patients with classifiable RA (by 1987 ACR criteria), and healthy controls.
Selection of Participants
Participants were selected as a subset from the larger SERA population as described below. RA participants were recruited from rheumatology clinics, academic centers, Veteran’s hospitals, and local advertisement. Given the associations with ACPA and Pc found in previous studies27–28, both seropositive and seronegative RA participants were selected for inclusion. Seropositive was defined by positivity for any of the following autoantibodies: RF isotypes: IgA and IgM, and the ACPA serologic test anti-CCP3 (IgG). Anti-CCP3 was used to define ACPA positive participants in both the RA and at-risk cohorts given the predictive value of anti-CCP3 for future RA development34. In one analysis, RA participants were stratified as either early RA (≤ 1 year from RA diagnosis) or established RA (> 1 year from RA diagnosis).
At-risk participants were selected based on positivity for anti-CCP3. Of the 67 anti-CCP3+ at-risk participants, 22 were FDRs. At-risk participants were recruited from community-based health fairs, general population screenings, and rheumatology clinics.
Control participants were negative for RA-related autoantibodies and without current or past inflammatory arthritis (IA) at the selected visit. Control participants were recruited from community-based health fairs or local advertisement. RA, and at-risk cases were matched to the pool of control participants (1:1 ratio) by sex and age within ± 10 years. SERA subjects that did not have blood samples available for complete autoantibody profiling, or that could not be sex and age matched as described, were not included in this study.
Study Visits
All participants completed questionnaires that assessed demographics, self and family history of disease, and past and current environmental exposures. Blood was drawn into serum separation tubes (Fisher Scientific BD VacutainerTM)), allowed to clot for 15 minutes at room temperature and then centrifuged at 3000 rpm for 10 minutes. Serum was aliquoted into 2ml graduated tubes and stored at −80°C until the sample was thawed and sent for analysis. A 68-joint examination was performed by a rheumatologist or trained study nurse in the at-risk and control participants to confirm the absence, or in the case of the at-risk participants who transitioned to disease, the presence of IA.
Autoantibody Testing
Serum samples were tested for the following autoantibodies: anti-CCP3 (IgG ELISA; Inova Diagnostics), RF isotypes IgA and IgM by ELISA (Quanta Lite kits; Inova Diagnostics). Positivity for the anti-CCP3 test was determined using the manufacturer’s recommended cutoff level of ≥20 units. Serum positivity for each RF isotype was established based on levels >95th percentile of 491 blood donor controls.
Shared Epitope Testing
DNA from buffy coat samples was genotyped at the Benaroya Research Institute for the presence of HLA alleles containing the SE, using methods described elsewhere30. Participants were considered SE positive if one or more allele included the following subtypes: DRB1*0401, *0404, *0405, *0408, *0409, *0410, and *0413; DRB1*0101, *0102, and *1001. SE was assessed as a dichotomous positive/negative variable.
Anti-Pc-p27 Antibody Assessments
Participants were tested for IgA and IgG antibody responses to Pc-p27, as determined by ELISA in the RA research laboratory at Massachusetts General Hospital (ACS). Two reports, which present methods and results of the Pc-p27 assays in RA patients and control subjects, have been published previously27, 28. Recombinant Pc-p27 was made by GenScript using an Escherichia coli expression vector (pET30a). Target protein purity was estimated to be ~90%. For standardization, the same high-positive, low-positive, and control serum samples were included on each plate and were required to have <10% variation in values to account for day-to-day variation in test performance. Samples were tested without knowledge of case/control status; however, the sample organization was constructed so that each case (RA or at-risk) sample and its matched control were included on the same plate. On the day before each assay, the plates were coated overnight at 4°C with recombinant Pc-p27 protein (1 μg/ml). The next morning, the plates were incubated with blocking buffer (PBST, 5% milk) at room temperature for 1 hour. After washing between each step, serum samples (diluted 1:50 for IgA and 1:100 for IgG) were added in duplicate for 2 hours, followed by horseradish-peroxidase (HRP)-conjugated goat anti-human IgG (1:3,000) or IgA (1:2,000) for 1.5 hours and then by TMB substrate for 10–15 minutes. The plates were then read at OD450. Assays were performed in Heteroblock to limit interference with RF35. The results of the high-positive value on each plate were adjusted to the same OD, and the correction fraction for each plate was used to normalize all values on that plate.
Establishment of the 95th Percentile Anti-Pc-p27+ Cut-off
In addition to analyzing levels of anti-Pc-p27, positivity for IgG or IgA antibody responses to anti-Pc-p27 was established using a cut-off level higher than that observed in 95% of all selected controls. The 95% cut-off for IgA anti-Pc-p27 was ≥0.29 and for IgG anti-Pc-p27 was ≥0.85.
Cross-sectional Study
From the populations described above, we selected, for a cross-sectional analysis of levels of IgA and IgG anti-Pc-p27, 98 RA participants and 98 matched controls and 67 at-risk participants and 67 matched controls. The first anti-CCP3+ study visit for the at-risk participants and the first study visit for the RA and control participants were selected for inclusion in the current analyses.
Longitudinal Study
During the course of study follow-up, 23 at-risk participants transitioned to IA as defined by the presence of ≥1 swollen joint on physical examination consistent with RA-like synovitis, of which 22 met ACR/EULAR criteria for RA. Twenty-one of the 23 at-risk participants had IgA anti-Pc-p27 testing at both selected visits and all at-risk participants had IgG anti-Pc-p27 testing at both selected visits. We compared levels of IgA and IgG anti-Pc-p27 from the first selected visit and the first visit after which the participant had transitioned to IA/RA for those with complete longitudinal data.
Statistical Analyses
Categorical variables were compared between RA and at-risk participants and their matched controls using chi-square, or Fisher’s exact test when cell size was less than 5. Continuous variables were analyzed using either a Students t-test (age) or Mann-Whitney U test (anti-Pc-p27 antibody biomarkers); results are presented in Table 1. Unadjusted comparisons of anti-Pc-p27 biomarker levels were made between RA or at-risk participants and their matched controls and are presented in Figure 1. Conditional logistic regression models were also used to assess whether levels of either IgA or IgG anti-Pc-p27 antibodies differ between RA participants and matched controls or at-risk participants and matched controls (Figure 1), accounting only for matching strategy since no covariate listed in Table 1 was associated with anti-Pc-p27 (data not presented). Given the previous findings in new onset RA populations24, and the association with the development of ACPA positivity27, we also evaluated SE as a potential effect modifier. Only matched pairs with complete data are included in the models (IgA anti-Pc-p27 at-risk n=66 matched pairs, RA n=95 matched pairs; IgG anti-Pc-p27 at-risk n=65 matched, RA=92 matched pairs). IgA and IgG anti-Pc-p27 levels were log2-transformed and results are presented for a 1-unit increase in log2 anti-Pc-p27 levels in logistic regression models. Stratified analyses are also presented among the RA participants and their matched controls by duration of disease. Finally, comparisons in levels of anti-Pc-p27 were assessed among RA and at-risk participants compared to controls by autoantibody status to see if certain RA-related autoantibodies were driving differences in IgA or IgG anti-Pc-p27 levels. P-values were also adjusted for multiple comparisons of the IgA and IgG anti-Pc-p27 levels for the Mann-Whitney test using the false discovery rate method of Benjamin-Hochberg36. Both unadjusted and adjusted p-values are presented. An adjusted p-value ≤0.05 was considered significant for all analyses. All statistical analyses were performed in SAS version 9.4 (Cary, NC).
Table 1.
Characteristics of the study populations
| RA (n = 98) |
Controls (n = 98) 2) |
P | At-Risk (n = 67) |
Controls (n = 67) |
P | |
|---|---|---|---|---|---|---|
| Sex: % female | 80 | 80 | 1.00 | 73 | 73 | 1.00 |
| Age: mean ± SD | 47 ± 15 | 44± 16 | 0.21 | 48 ± 14 | 47 ± 16 | 0.61 |
| Race/ethnicity: % non-Hispanic white | 75 | 74 | 0.90 | 82 | 77 | 0.49 |
| Education: % >High School | 77 | 96 | <0.01 | 80 | 99 | <0.01 |
| Income: % > $40,000 | 64 | 70 | 0.37 | 67 | 65 | 0.80 |
| Ever smoker: % yes | 37 | 23 | 0.04 | 45 | 20 | <0.01 |
| Current Smoker: % yes | 10 | 2 | 0.02 | 11 | 0 | 0.01 |
| Shared Epitope: % positive | 60 | 49 | 0.12 | 51 | 42 | 0.29 |
Missing Data Summary:
| RA | At-Risk |
| 3 subjects missing race/ethnicity | 3 subjects missing race/ethnicity |
| 5 subjects declined to answer education | 3 subjects declined to answer education |
| 16 subjects declined to answer income | 10 subjects delinked to answer income |
| 1 subject missing ever smoker | 2 subjects missing ever smoker |
| 1 subject missing current smoker | 2 subjects missing current smoker |
| 9 subjects missing shared epitope | 2 subjects missing shared epitope |
Figure 1.
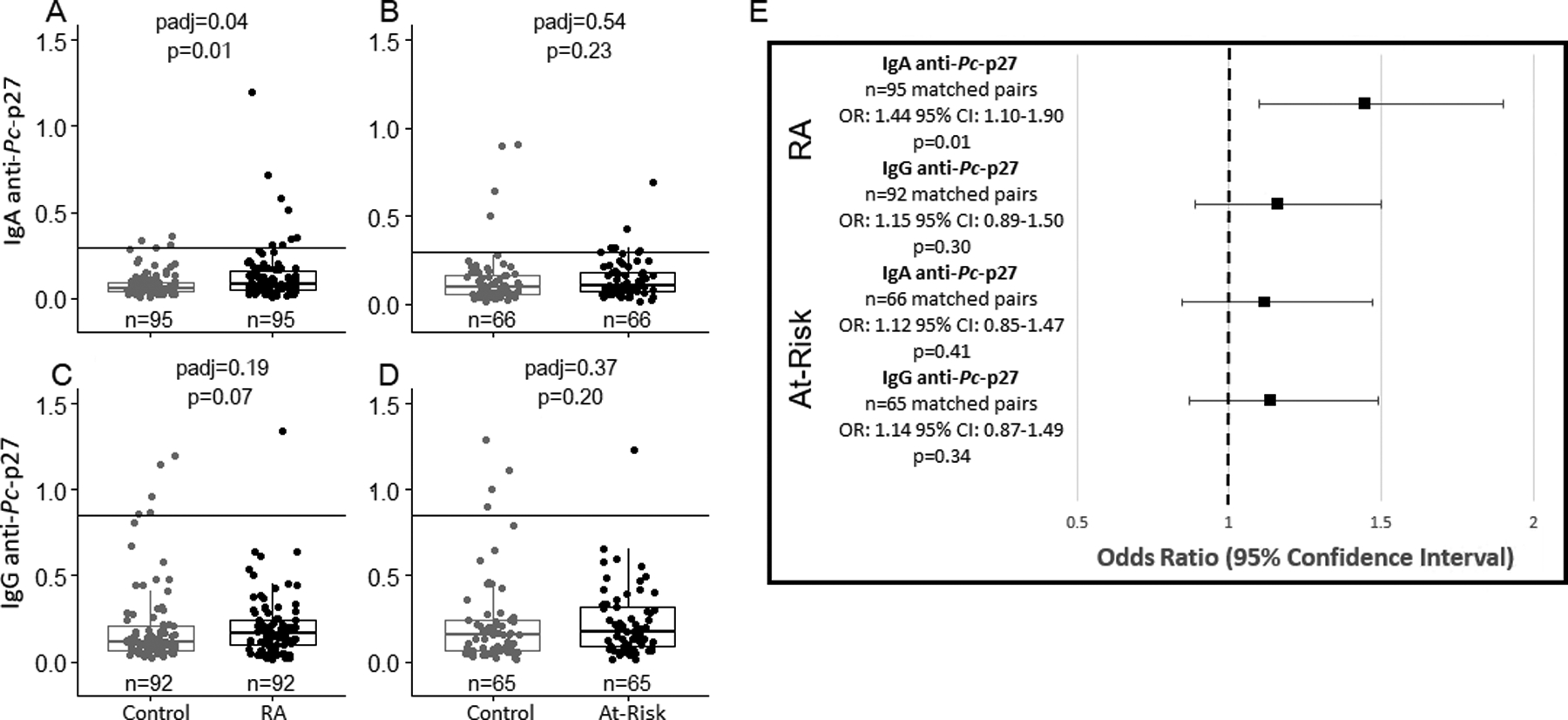
Unadjusted and adjusted comparisons of IgA and IgG anti-Pc-p27 levels among RA and at-risk participants compared to matched controls. The solid line on 1a-1d represents the positive cutoff. 1a, levels of IgA anti-Pc-p27 are significantly higher in RA cases compared to matched controls (median [IQR] Control= 0.06 [0.04–0.10], median [IQR] RA= 0.08 [0.05–0.17]). 1b, levels of IgA anti-Pc-p27 are not different in at-risk participants compared to matched controls (median [IQR] Control= 0.10 [0.05–0.17], median [IQR] At-Risk= 0.11 [0.07–0.18]). 1c, levels of IgG anti-Pc-p27 are not different in RA participants compared to matched controls (median [IQR] Control= 0.11 [0.07–0.21], median [IQR] RA= 0.17 [0.10–0.24]). 1d, levels of IgG anti-Pc-p27 are not different in at-risk participants compared to matched controls (median [IQR] Control= 0.16 [0.06–0.24], median [IQR] At-Risk= 0.18 [0.09–0.32]). 1e, association between IgA and IgG anti-Pc-p27 among RA and at-risk participants compared to matched controls. Results are shown as odds ratios with error bars showing the 95% confidence intervals. Estimates are conditioned on matched pair. RA participants are more likely to have higher levels of IgA anti-Pc-p27 compared to matched controls (OR: 1.44; 95% CI: 1.10–1.90; p=0.01).
Ethical Considerations
All study procedures were approved by institutional review boards at participating institutions.
RESULTS
Study participant characteristics are presented in Table 1. Both the RA and at-risk controls are more likely to have greater than a high school level education when compared to RA and at-risk participants, respectively. The RA and at-risk participants had a higher proportion of ever and current smokers compared to RA controls and at-risk controls, respectively.
Cross-Sectional Analysis of anti-Pc-p27 Levels
RA participants had significantly higher levels of IgA anti-Pc-p27 when compared to their matched controls (Figure 1a). RA participants also exhibited higher levels of IgG anti-Pc-p27 over RA controls (Figure 1c). While numerically higher, both IgA and IgG anti-Pc-p27 levels were not significantly different in at-risk individuals compared with at-risk controls (Figure 1b and 1d). Using conditional logistic regression models, we found, again, that RA participants exhibited higher levels of IgA anti-Pc-p27 compared to RA controls, (Figure 1e). Therein, for every one-unit (log2) increase in IgA anti-Pc-p27, an individual is 1.44 times more likely to be an RA participant compared to RA control (95% CI:1.10–1.90, p=0.01).
Early vs Established RA Analysis
Disease-stage heterogeneity following development of classified RA and levels of anti-Pc-p27 has been observed in a previous study27. To evaluate this in our study, we stratified by early and established RA and tested for associations with antibody levels. Study participant characteristics are represented in Table 2 and findings from the analyses are presented in Figure 2. In early RA participants, we observed a trend toward increased levels of IgG anti-Pc-p27 when compared to their matched controls (Figure 2c, p= 0.01, padj=0.11). In addition, median values of IgA anti-Pc-p27 were significantly higher in established RA participants when compared to their matched controls (Figure 2b, p = 0.01, padj=0.04). Similarly, in the conditional regression model, IgA anti-Pc-p27 was significantly higher in the established RA group when compared to their matched controls (Figure 2e) (OR: 1.43; 95%, CI: 1.04–1.96; p=0.03). Thus, the results from the early RA analysis demonstrated a trend in increasing IgG anti-Pc-p27 antibody levels, and established RA participants had a significant elevation in IgA anti-Pc-p27 antibodies compared to controls. When conditioning on matched pairs in the logistic regression model, the established RA association remained significant.
Table 2.
Characteristics of early RA and established RA compared to matched controls
| Early RA (n = 27) |
Controls (n = 27) |
P | Established RA (n = 71) |
Controls (n = 71) |
P | |
|---|---|---|---|---|---|---|
| Sex: % female | 7 | 78 | 1.00 | 80 | 80 | 1.00 |
| Age: mean ± SD | 39 ± 14 | 36 ± 14 | 0.50 | 50 ± 15 | 47 ± 16 | 0.27 |
| Race/ethnicity: % non-Hispanic white | 81 | 78 | 0.79 | 73 | 73 | 1.00 |
| Education: % >High School | 88 | 93 | 0.66 | 72 | 97 | <0.01 |
| Income: % > $40,000 | 65 | 77 | 0.37 | 64 | 68 | 0.63 |
| Ever smoker: % yes | 44 | 11 | 0.01 | 34 | 28 | 0.43 |
| Current Smoker: % yes | 15 | 0 | 0.11 | 9 | 3 | 0.17 |
| Shared Epitope: % positive | 48 | 50 | 0.89 | 65 | 49 | 0.06 |
Missing data summary:
| Early RA | Established RA |
| 1 subject missing race/ethnicity | 2 subjects missing race/ethnicity |
| 2 subjects declined to answer education | 3 subjects declined to answer education |
| 5 subjects declined to answer income | 11 subjects declined to answer income |
| 5 subjects missing shared epitope | 1 subject missing ever smoker |
| 1 subject missing current smoker | |
| 4 subjects missing shared epitope |
Figure 2.
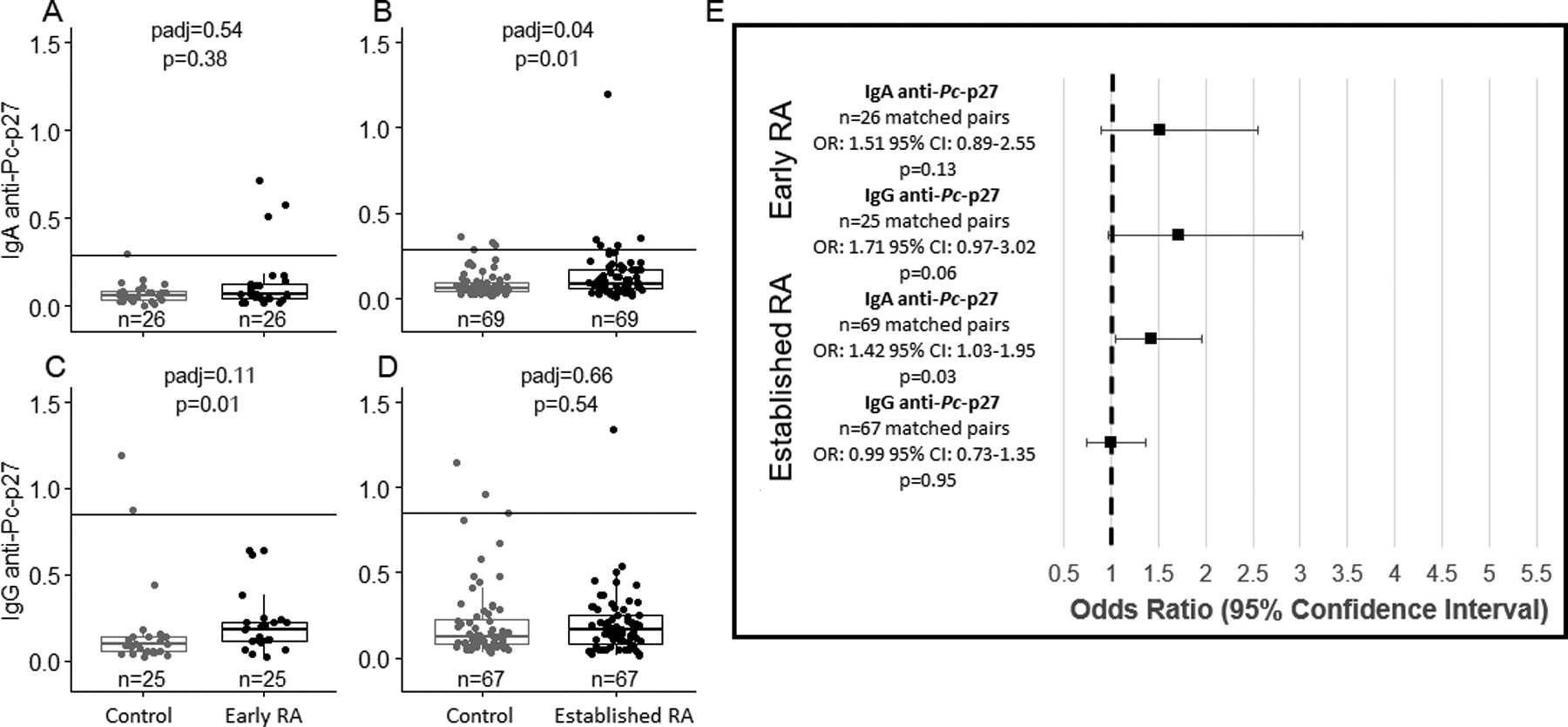
Unadjusted and adjusted comparisons of IgA and IgG anti-Pc-p27 levels among early RA and established RA participants compared to matched controls. The solid line on 2A-2D represents the positive cutoff. 2A, levels of IgA anti-Pc-p27 do not significantly differ between early RA participants and matched controls (median [IQR] Control= 0.06 [0.03–0.08], median [IQR] Early RA= 0.07 [0.04–0.13]). 2B, levels of IgA anti-Pc-p27 are significantly higher in established RA participants compared to matched controls (median [IQR] Control= 0.06 [0.04–0.10], median [IQR] Established RA= 0.09 [0.06–0.17]). 2C, levels of IgG anti-Pc-p27 are higher in early RA participants compared to matched controls (median [IQR] Control= 0.09 [0.05–0.14], median [IQR] Early RA= 0.18 [0.12–0.22]), however, non-significant after multiple comparison adjustment (p=0.01, padj=0.11). 2D, levels of IgG anti-Pc-p27 do not significantly differ between established RA participants compared to matched controls (median [IQR] Control= 0.12 [0.08–0.24], median [IQR] Established RA= 0.17 [0.08–0.25]). 2E, association between IgA and IgG anti-Pc-p27 levels among early RA and established RA compared to matched controls. Results shown as odds ratios with error bars showing 95% confidence intervals. Established RA participants are more likely to have higher levels of IgA anti-Pc-p27 compared to matched controls (OR: 1.42; 95% CI:1.03–1.95; p=0.03).
Longitudinal Analysis of anti-Pc-p27 Levels in At-Risk Individuals Who Have Transitioned to Develop IA
There were 23 at-risk participants who developed IA during the study over an average of 2.06 years between their first selected visit and the IA visit (range=0.05 years to 7.78 years). The first selected visit was an average of 2.06 years (SD±2.33) prior to date of IA diagnosis, and the IA visit was an average of 0.75 years (SD±1.52) after the date of IA diagnosis. Among at-risk participants who develop IA and had samples such that both IgA (n =20) and IgG (n = 23) anti-Pc-p27 were measured at both visits, there was not a significant elevation in either IgA or IgG anti-Pc-p27 antibodies between the pre-IA and post-IA visit (Figures 3a and 3b).
Figure 3.
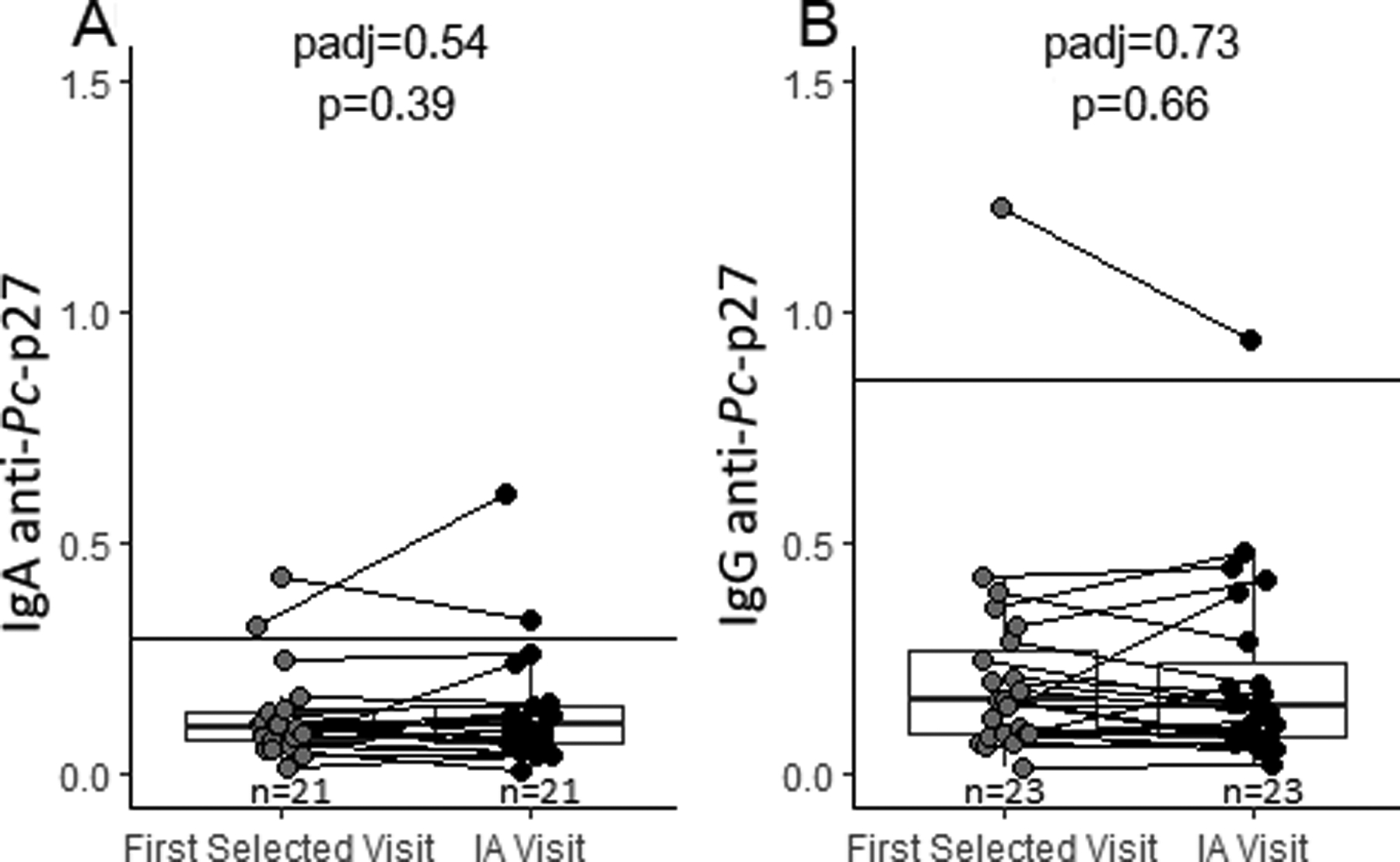
Longitudinal analysis of IgA and IgG anti-Pc-p27 among participants who transitioned to IA during follow-up. The solid line on each plot represents the positive cutoff. 3A, levels of IgA anti-Pc-p27 did not significantly change from the first selected study visit to the study visit where IA was determined. 3B, levels of IgG anti-Pc-p27 did not significantly change from the first selected study visit to the study visit where IA was determined.
RA-related Autoantibody Subset Analysis
To explore if RA-related autoantibodies were driving the observed associations, the analysis of IgA or IgG anti-Pc-p27 antibodies was stratified by the aforementioned RA-related autoantibodies known to have a direct correlation with RA development and disease onset (Figure 4). ACPA and RF isotype positivity across both the RA and at-risk populations are described in Supplemental Table 1. Since all at-risk participants were ACPA(CCP3)+, we assessed the difference in levels of IgA and IgG anti-Pc-p27 in this subgroup by whether the at-risk participant was RF+. There was not a significant difference in the levels of IgA anti-Pc-p27 among the at-risk group by the presence of RF (Figure 4b). However, IgG anti-Pc-p27 levels were trending higher among CCP+/RF− at-risk participants compared to their matched controls (Figure 4d; p=0.05, padj=0.19). In the RA population, we observed a significant elevation in IgA anti-Pc-p27 antibodies among participants who were positive for both CCP and RF (CCP+/RF+) compared to matched controls (Figure 4a; p=<0.01, padj=0.01). With regard to IgG anti-Pc-p27 (Figure 4c), both CCP+/RF+ and CCP+/RF− levels were also trending higher compared with matched controls (p=0.09, padj=0.20 and p=0.06, padj=0.19, respectively). In summary, CCP+/RF− at-risk participants and RA CCP+/RF+ participants had higher IgG anti-Pc-p27 antibody levels (although not significant after p-value adjustment), and CCP+/RF+ RA participants had significantly higher IgA anti-Pc-p27 antibody values compared to matched controls.
Figure 4.
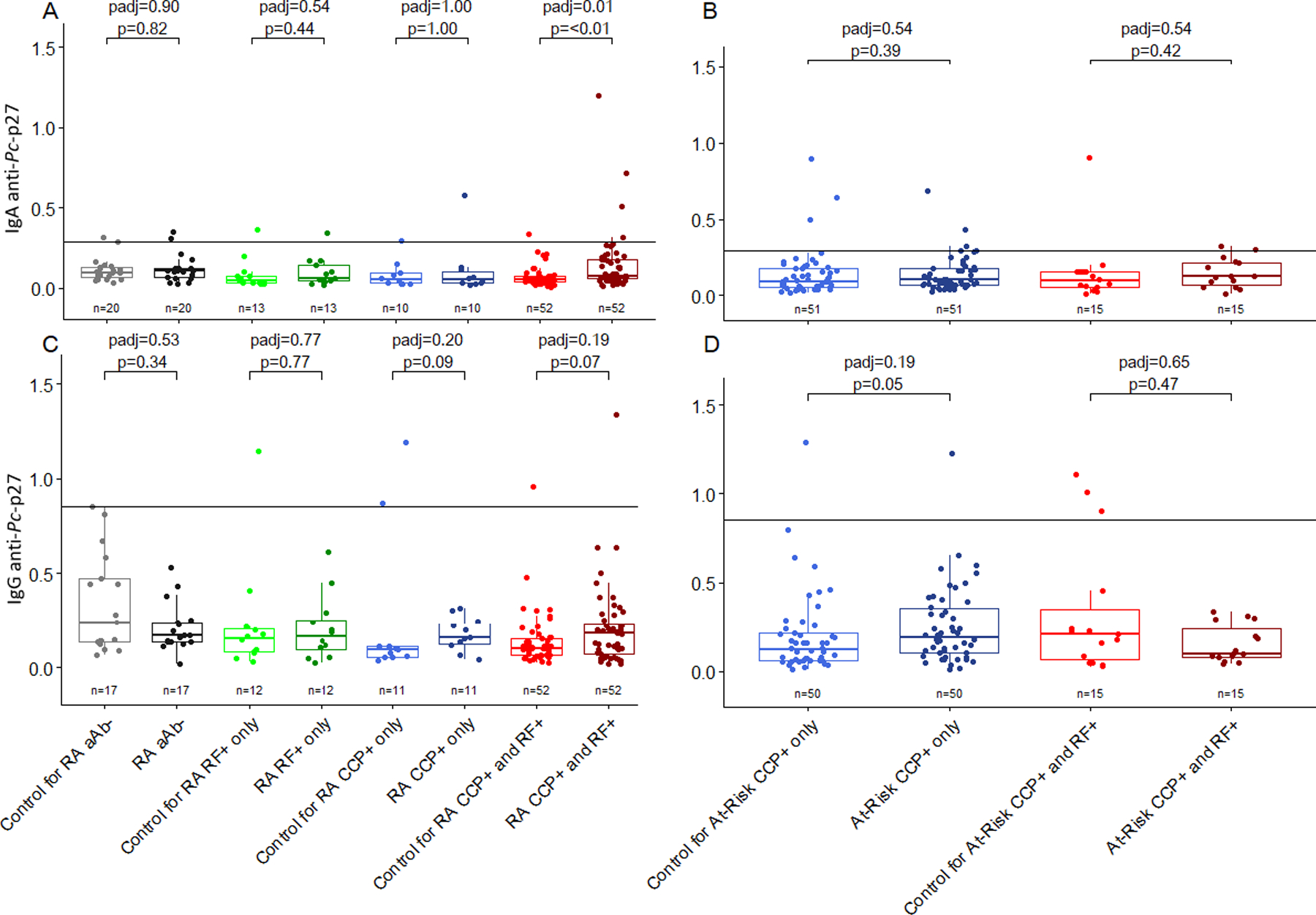
Unadjusted and adjusted comparisons of IgA and IgG anti-Pc-p27 levels among RA and at-risk participants compared to matched controls by autoantibody status. The solid line on 4a-4d represents the positive cutoff. 4A, levels of IgA anti-Pc-p27 are significantly higher in RA participants who are anti-CCP+ and RF+ (IgA and/or IgM) compared to matched controls (p<0.01, padj=0.01). 4B, levels of IgA anti-Pc-p27 do not significantly differ between at-risk participants compared to matched controls. 4C, levels of IgG anti-Pc-p27 do not significantly differ between RA participants compared to matched controls. 4D, levels of IgG anti-Pc-p27 are overall higher in at-risk participants who are anti-CCP+/RF-compared to matched controls, however, non-significant after multiple comparison adjustment(p=0.05, padj=0.19).
DISCUSSION
Although not yet well understood mechanistically, an increasing number of observations suggest an important role for immune dysregulation and dysbiosis at mucosal sites in the initial break in tolerance that ultimately leads to the development of RA, as well as in the transition to synovitis and classified RA. Functionally, the gut microbiome is pivotal to this process via its interaction with both systemic circulation and Peyer’s patches (the gut-associated lymphoid tissues). The gut microbiota is largely responsible for the modulation of immune functions that are integral to regulation of inflammation and when in disequilibrium, may lead to autoimmune disease37. Thus, dysbiosis of the gut microbiota may tip the scales in favor of bacteria that elicit inflammatory and/or autoantibody responses which can be detrimental to host homeostasis.
Wells et al. observed an association with fecal Prevotella spp. in a genetically at-risk FDR population, where a PRS was calculated as a continuous variable based on 233 genome-wide association study-identified single nucleotide polymorphisms known to be associated with RA29. In addition, Wells reported only the presence of modestly elevated 16S RNA levels of Prevotella spp. in stool samples, and did not determine immune responses to this group of organisms.
The studies by Pianta et al. provided evidence of immune relevance of Pc in RA patients, as determined primarily by T cell reactivity with a single Pc protein, Pc-p27, antibody responses to Pc-p27, and to a sonicate preparation of whole P. copri. In our study, we demonstrated associations with increased levels of anti-Pc-p27 in at-risk CCP+/RF− participants and in new-onset or established RA participants compared to their matched controls. Thus, the current study supports the immune relevance of this gut commensal in another population and extends these observations by identifying subgroups who are likely to develop these responses and when reactivity may develop. Importantly, both CCP+ and early RA participants had overall higher levels of IgG anti-Pc-p27 antibodies.
Zhao et al. found a correlation between Pc DNA in both synovial tissue and fluids and synovial cell proliferation, further suggesting that these mucosal bacteria known to originate in the gut, circulate systemically and then settle in joint-specific tissues38. Similarly, in the study by Pianta et al., 3 of 5 patients with IgG Pc antibodies had positive PCR results for Prevotella DNA in synovial fluid as compared with none of 13 patients with IgA Pc antibodies or no Pc antibodies27. Based on these observations, we hypothesize that certain Prevotella spp., especially Pc, may bind to the gut mucosa and periodically escape into the systemic circulation where they stimulate systemic immune responses. Moreover, these organisms or their components may sometimes reach joints, perhaps within cells, where they may shape or amplify immune responses. There is an emerging literature about the distant spread of commensal organisms resulting not only in autoimmunity, but also in malignancies or adverse treatment outcomes39, 40.
The lack of association of IgG Pc-p27 antibodies in at-risk individuals with both CCP and RF autoantibodies might be explained by the fact that RF may be beneficial in controlling the organism in the mucosa. For example, in a rat model, IgG responses to Trypanosoma lewisi followed by treatment with IgM RF terminated the infection41. The beneficial effect of RF was thought to result from cross-linking IgG antibodies, thereby allowing more effective phagocytosis of the parasite. Perhaps RF can play a similar role in controlling certain gut commensals. In contrast, RF in joints, in which autoimmunity is the problem rather than infection, has been shown to be detrimental by increasing immune complex-associated inflammation42, 43. The lack of other significant differences by autoantibody status could also be due to the small sample size of some of the ACPA/RF group combinations (e.g. at-risk CCP+/RF+ participants n=15 (IgA), n=15 (IgG), and CCP−/RF− RA participants n=20 (IgA), n=17 (IgG)).
In the current study, increased IgA anti-Pc-p27 antibody responses were found primarily in individuals with established RA; however, previous studies have shown that IgA anti-Pc-p27 antibody levels correlate with ACPA values27, 28, findings suggestive of a mucosal immune response. Thus, similar to the situation with RF, we postulate that an intermittent antibody response to microbial citrullinated proteins at mucosal sites may be beneficial in controlling the spread of this organism, whereas chronic responses to host citrullinated proteins in joints are associated with more severe disease44. In a previous study, HLA-DR-presented peptides in synovial tissue derived from 2 highly expressed self-proteins, N-acetylglucoasimne-6-phosphatase (GNS) and filamin A (FLNA), had sequence homology with T cell epitopes of Prevotella spp, and those of several other related microbes45. GNS appeared to be citrullinated in vivo, and GNS antibody values correlated with ACPA levels. Thus, sequence homology between microbial and host T or B cell epitopes and their association with ACPA may provide another mechanism by which immune responses to commensal microbes may be advantageous in the mucosa, but disadvantageous in the amplification of autoimmune joint inflammation.
The studies on SE and abundance of Pc have yielded mixed results16. Similar to the findings in the studies by Pianta et al., the SE in our studies herein was not an effect modifier when evaluating the association of levels Pc-p27 antibodies either in those with new-onset or established RA (data not shown). However, in the Pianta et al. studies, immune responses to Pc-p27-related autoantigens, GNS and FLNA, were associated with SE alleles45. This suggests that SE alleles may have their effect in propagating autoimmune responses but not in recognition of the microbe.
Limitations of our study include that recognition of Pc immune responses was based on reactivity with only one Pc protein, Pc-p27, which may have limited the power to detect reactivity with this microbe and may have contributed to the loss of significance in some adjusted analyses. Additionally, the pathogenesis of this protein may have immunologic relevance in only a subset of individuals with RA, as some of our analyses, when stratified by group, demonstrated trends towards differences when comparing cases versus controls but were not statistically significant. In addition, it is not clear why in our population the most pronounced IgA response to Pc-p27 occurs in Established rather than Early RA, a finding which may point to an increased role of this organism at later time points. Moreover, most of our established RA participants were receiving DMARD therapy, which likely affected the constitution of the microbiome, as has been shown in a spondyloarthritis population46. Lastly, insufficient numbers of fecal samples were available from SERA participants to perform 16S sequencing to assess prevalence of Prevotella abundance.
Despite these limitations, we were able to show statistically significant correlations with IgA anti-Pc-p27 antibody responses in the RA groups and higher levels of IgG anti-Pc-p27 in the CCP+ at-risk participants and in those with early RA. However, P. copri, as identified by Pc-p27 antibody responses, is likely only one of a number of organisms that may have immune relevance in RA.
In conclusion, we found that at-risk participants and those with early RA had overall higher levels of IgG anti-Pc-p27 antibodies and those with established RA had significantly increased levels of IgA anti-Pc-p27 antibodies. These findings are consistent with and extend previous studies. As such, further investigation into the roles of this and other microorganisms in RA evolution and pathogenesis of disease is warranted. Such knowledge may lead to the development of therapies targeting immunogenic bacterial commensals and/or pathobionts, which may prevent the development of RA in at-risk individuals and may have a beneficial effect in those with new-onset or established RA.
Supplementary Material
Acknowledgments
This study was supported in part by National Institutes of Health grants U01 AI101981, R01 AR051394, P30 AR079369, and American College of Rheumatology Innovative Grant Program “Within our Reach, Finding a Cure for RA” (VMH); the Ounsworth-Fitzgerald Foundation, Mathers Foundation, English, Bonter, Mitchell Foundation, Littauer Foundation, Lillian B. Davey Foundation, and the Eshe Fund (ACS)
Footnotes
Competing interests: The authors declare no competing financial interests.
REFERENCES
- 1.Deane KD, Holers VM. The natural history of rheumatoid arthritis. Clin Therapeutics. 2019;41(7):1256–1269. [DOI] [PubMed] [Google Scholar]
- 2.McInnes I, Schett G The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8. [DOI] [PubMed] [Google Scholar]
- 4.Deane KD, Holers VM. Rheumatoid arthritis pathogenesis, prediction, and prevention: An Emerging Paradigm Shift. Arthritis Rheum. 2021;73(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tracy A, Buckley CD, Raza K. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Semin Immunopathol. 2017;39(4):423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlag DM, Norris JM, Tak PP. Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford). 2016;55(4):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catrina AI, Deane KD, Scher JU. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford). 2016;55(3):391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(1055):2023–38. [DOI] [PubMed] [Google Scholar]
- 9.Raza K, Holers VM, Gerlag D. Nomenclature for the phases of the development of rheumatoid arthritis. Clin Ther. 2019;41(7):1279–1285. [DOI] [PubMed] [Google Scholar]
- 10.Kelmenson LB, Wagner BD, McNair BK, Frazer-Abel A, Demoruelle MK, Bergstedt DT, et al. Timing of elevations of autoantibody isotypes prior to diagnosis of rheumatoid arthritis. Arthritis Rheum. 2020;72(2):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated Peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum. 2013;65, 2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majka DS, Holers VM. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 2003;48(10):2701–5. [DOI] [PubMed] [Google Scholar]
- 13.Gerlag DM, Raza K, van Baarsen LG, Brouwer E, Buckley CD, Burmester GR, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the study group for risk factors for rheumatoid arthritis. Ann Rheum Dis. 2012;71(5):638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31(1), 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheum. 2017;69(6):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HH, Huang N, Chen YM, Chen TJ, Chou P, Lee YL, et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: a nationwide, population-based, case-control study. Ann Rheum Dis 2013;72:1206–11. [DOI] [PubMed] [Google Scholar]
- 18.Kishikawa T, Maeda Y, Nii T, Motooka D, Matsumoto Y, Matsushita M, et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann Rheum Dis. 2020;79(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barra L, Scinocca M, Saunders S, Bhayana R, Rohekar S, Racapé M, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013;65:1439–47. [DOI] [PubMed] [Google Scholar]
- 20.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30(11):1205–13. [DOI] [PubMed] [Google Scholar]
- 23.de Vries N, Tijssen H, van Riel PL, van de Putte LB. Reshaping the shared epitope hypothesis: HLA-associated risk for rheumatoid arthritis is encoded by amino acid substitutions at positions 67–74 of the HLA-DRB1 molecule. Arthritis Rheum 2002;46, 921–928. [DOI] [PubMed] [Google Scholar]
- 24.de Vries RR, Huizinga TW, Toes RE. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J Autoimmun. 2005;25 Suppl:21–5. [DOI] [PubMed] [Google Scholar]
- 25.Seward RJ, Drouin EE, Steere AC, Costello CE. Peptides presented by HLA-DR molecules in synovia of patients with rheumatoid arthritis or antibiotic-refractory Lyme arthritis. Mol Cell Proteomics. 2011;10(3):M110.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Drouin EE, Yao C, Zhang J, Huang Y, Leon DR, et al. Immunogenic HLA-DR-presented self-peptides identified directly from clinical samples of synovial tissue, synovial fluid, or peripheral blood in patients with rheumatoid arthritis or lyme arthritis. J Proteome Res. 2017;16(1):122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pianta A, Arvikar S, Strle K, Drouin E, Wang Q, Costello C, et al. Evidence for immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheum. 2017;69(5):964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pianta A, Chiumento G, Ramsden K, Wang Q, Strle K, Arvikar S, et al. Identification of novel, immunogenic HLA-DR-presented Prevotella copri peptides in patients with rheumatoid arthritis. Arthritis Rheum. 2021;27(73):2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells PM, Adebayo AS, Bowyer RCE, Freidin MB, Finckh A, Strowig T, et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 2020;2(7):e418–e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolfenbach JR, Deane KD, Derber LA, O LA OR, Deane KD, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silman AJ, Hennessy E, Ollier B. Incidence of rheumatoid arthritis in a genetically predisposed population. Br J Rheumatol. 1992;31(6):36565. [DOI] [PubMed] [Google Scholar]
- 32.McDonagh JE, Walker DJ. Incidence of rheumatoid arthritis in a 10-year follow-up study of extended pedigree multicase families. Br J Rheumatol. 1994; 33(9):826–31. [DOI] [PubMed] [Google Scholar]
- 33.Silman AJ, Ollier B, Mageed RA. Rheumatoid factor detection in the unaffected first degree relatives in families with multicase rheumatoid arthritis. J Rheumatol. 1991; 18(4):512–5. [PubMed] [Google Scholar]
- 34.Martinez-Prat L, Nissen MJ, Lamacchia C, Bentow C, Cesana L, Roux-Lombard P, Gabay C, Mahler M. Comparison of serological biomarkers in rheumatoid arthritis and their combination to improve diagnostic performance. Front Immunol. 2018;9:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson P, Theander E, Bergström U, Jovinge S, Jacobsson L, Turesson C. Multiplex cytokine analyses in patients with rheumatoid arthritis require use of agents blocking heterophilic antibody activity. Scand J Rheumatol. 2017;46(1):1–10. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini Y and Hochberg Y, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 1995;57:289–300. [Google Scholar]
- 37.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept Diabetologia. 2011;54:3055–3061. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Chen B, Li S, Yang L, Zhu D, Wang Y, et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep. 2018;8(1)14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarkson AB Jr, Mellow GH. Rheumatoid factor-like immunoglobulin M protects previously uninfected rat pups and dams from Trypanosoma lewisi. Science. 1981;214(4517):186–8 [DOI] [PubMed] [Google Scholar]
- 42.Veale DJ, Orr C, Fearon U. Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):343–354. [DOI] [PubMed] [Google Scholar]
- 43.Birchmore DA, Taylor RP, Waller SJ, Davis JS IV, Morley KW. Interaction between rheumatoid factor and antibody/DNA complexes. Arthritis Rheum 1981;24:527–33. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7(3):R458–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pianta A, Arvikar SL, Strle K, Drouin EE, Wang Q, Costello CE, Steere AC. Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J Clin Invest. 2017;127(8):2946–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazin T, Hooks KB, Barnetche T, Truchetet ME, Enaud R, Richez C, et al. Microbiota composition may predict anti-TNF alpha response in spondyloarthritis patients: an exploratory study. Sci Rep. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


