Abstract
Objectives:
Delirium is a common postoperative complication associated with death and long-term cognitive impairment. We studied the association between opioid-sparing anesthetics incorporating Enhanced Recovery After Cardiac Surgery (ERACS) guided analgesics and postoperative delirium.
Design:
We performed a retrospective review of non-emergent coronary, valve, or ascending aorta surgery patients.
Setting:
A tertiary academic medical institution.
Participants:
We analyzed a dataset of elective adult cardiac surgical patients. All patients ≥18 years undergoing elective cardiac surgery from 11/2/2017 until 2/2/21 were eligible for inclusion.
Interventions:
The ERACS-guided multimodal pain regimen included preoperative oral acetaminophen and gabapentin, and intraoperative intravenous lidocaine, ketamine, and dexmedetomidine.
Measurements and Main Results:
Delirium was measured by bedside nurses using Confusion Assessment Method for ICU (CAM-ICU). Delirium occurred in 220 of the 1,675 patients (13.7%). Use of any component of the multimodal pain regimen was not associated with delirium (Odds Ratio: 0.85 [95% confidence interval: 0.63, 1.16]). Individually, acetaminophen was associated with reduced odds of delirium (OR: 0.60 [95% CI: 0.37, 0.95]). Gabapentin (OR: 1.36 [95% CI: 0.97, 2.21]), lidocaine (OR: 0.86 [95% CI: 0.53, 1.37]), ketamine (OR: 1.15 [95% CI: 0.72, 1.83]), and dexmedetomidine (OR: 0.79 [95% CI: 0.46, 1.31]) were not individually associated with postoperative delirium. Individual ERACS elements were associated with secondary outcomes hospital length of stay, ICU duration, postoperative opioid administration, and postoperative intubation duration.
Conclusions:
Use of an opioid sparing perioperative ERACS pain regimen was not associated with reduced postoperative delirium, opioid consumption, or additional poor outcomes. Individually, acetaminophen was associated with reduced delirium.
Keywords: Delirium, Enhanced Recovery After Cardiac Surgery (ERACS), pain, cardiac, multimodal analgesia
Introduction
Postoperative delirium is a known predictor of other surgical outcomes, including increased hospital length of stay, cost, risk of cognitive impairment, functional decline, and mortality.1–10 Moreover, it is common—with the rate in cardiac surgical patients estimated to vary from 26-52%, depending on the assessment method used, and as high as 80% in critically ill noncardiac patients.1–3 Data from our institution suggest 1 in 4 cardiology and cardiac surgery patients develop postoperative delirium, with the majority of cases presenting as hypoactive delirium.1 Strategies aimed at preventing delirium, such as early mobilization, frequent reorientation, ensuring sleep hygiene, and avoiding medications that may promote delirium, have emerged as the most promising treatment for delirium—by attempting to prevent rather than treat delirium11. Benzodiazepine and opiate use have been associated with increased risk of postoperative delirium,1, 6 and in non-cardiac surgery, use of Enhanced Recovery After Surgery (ERAS) pathways—which in part aim to minimize opioid exposure—have been associated with reduced incidence of postoperative delirium.1 In cardiac surgery patients more evidence is needed to guide pharmacologic interventions to impact delirium and neurologic recovery.2
Within cardiac surgery patient management, strategies to reduce delirium remain limited. It is unclear if ERAS-derived multimodal analgesia and sedation medications impact this patient population. Some elements of ERAS pathways include limiting perioperative opioid use by administering non-opioid adjuvant analgesics.1,12 Dexmedetomidine use as a sedative agent has reduced delirium in several large trials of non-cardiac surgery patients.12–14 Acetaminophen in combination with dexmedetomidine has also been shown to reduce postoperative delirium.15 These studies provide a rationale that a multi-modal approach aimed to reduce opioid use and provide alternative analgesics may provide pain control, reduce delirium, and improve functional recovery following cardiac surgery.16 The evidence to support a multi-modal anesthesia, sedation, and pain medicine approach to impact delirium following cardiac surgery remains uncertain. We tested the hypothesis that administration of any ERACS pathway guided analgesic medications was associated with reduced delirium following cardiac surgery and additional poor outcomes.
Methods
Study design
We performed a historical cohort study of patient data from a tertiary academic medical institution to examine the associations between ERACS treatments and the development of postoperative delirium and additional organ injuries and indicators of protracted recovery. This study was approved by our university’s Institutional Review Board, with a waiver of the requirement for written, informed consent, and adheres to the STROBE guidelines.17
Study sample
We analyzed a dataset of elective adult cardiac surgical patient data gathered from the Perioperative Data Warehouse (PDW) at our institution. All patients ≥18 years undergoing elective cardiac surgery from 11/2/2017 until 2/2/21 were eligible for inclusion. Patients undergoing a transplant, ventricular assist device implantation, emergency surgery, pulmonary endarterectomy, or placement of or removal from mechanical circulatory support devices were excluded. Patients who died during surgery, were not admitted to the ICU postoperatively, or were missing data required for analysis were excluded from the study.
ERACS medications
Opioid-sparing ERACS perioperative medications prescribed as included preoperative acetaminophen (650-1000mg) per os (PO), preoperative gabapentin (100-600mg) PO, intraoperative dexmedetomidine infusion (0.3-1 mcg/kg/hr), intraoperative lidocaine infusion (1-2 mg/min), and intraoperative ketamine infusion (2.5-5 mcg/kg/min). Dichotomous logical variables were created to indicate whether a patient received an individual, any of the 5 ERACS drugs.
Outcomes
The primary outcome was the postoperative incidence of delirium, defined as a positive Confusion Assessment Method for ICU (CAM-ICU) test at any point during their hospital encounter following surgery. The CAM-ICU is currently the gold-standard of delirium assessment tools and can be performed quickly by healthcare providers in the ICU.1–6, 18, 19 It consists of four main features: (1) alteration or fluctuation in patient mental status, (2) inattention, (3) altered level of consciousness, and (4) disorganized thinking. If both features (1) and (2) were present with either features (3) or (4), then the CAM-ICU was positive for delirium. At our hospital, the CAM-ICU is typically performed at least twice a day during an ICU stay.
Secondary outcomes included opioids received following surgery measured in morphine milligram equivalents, the incidence of atrial fibrillation, incidence of acute kidney injury (AKI), postoperative ventilation free days, ICU length of stay, total hospital length of stay, and in-hospital mortality. Atrial fibrillation was defined as new onset atrial fibrillation or atrial flutter after anesthesia stop requiring antiarrhythmic treatment. Atrial fibrillation and flutter were detected using continuous telemetry and electrocardiograms. AKI was defined using KDIGO criteria.20 Stage 1 AKI was defined as an increase in serum creatinine (SCr) of >0.3mg/dL from baseline or >1.5 times baseline within 7 days, stage 2 as an increase in SCr >2.0 times baseline, and stage 3 as an increase in SCr >3.0 times baseline or new onset dialysis. By definition, patients with end stage renal disease (ESRD) were considered not eligible for onset of AKI. Patients with ESRD were defined as patients who had renal replacement therapy within the same hospital encounter, had estimated glomerular filtration rate (eGFR) < 15 ml/min, or had ever undergone peritoneal dialysis. Postoperative ventilation free days were defined as the number of days that a patient was alive and free of invasive ventilation, calculated from the end of surgery. For example, a patient who was not intubated postoperatively would have 28 ventilation free days, whereas a patient who died while intubated or spent the entire postoperative period intubated would have zero ventilation free days.
Missing data
Data missingness among study variables was reported using missing frequencies and rates. Based on prior work with these data, we anticipated low rates of missingness, and that data were considered as missing completely at random (i.e., not associated with the outcome). Thus, patients with missing covariate data were removed from the analysis.
Statistical analysis
Demographic and procedural variables were summarized as median and interquartile ranges for continuous variables, and with counts and percentages for categorical variables. We measured the association between ERACS medication use and delirium using two distinct logistic regressions. The first regression, which we call the “collective” model assessed the association between postoperative delirium and the administration of any ERACS medication vs. the administration of no ERACS medications. The second, or the “individual” model, assessed the association between delirium and individual ERACS components. Confounding bias was managed by including as many possible confounders as could be permitted to the model. To minimize the risk of overfitting, the number of predictors included in the model was limited to provide an events per degree of freedom ratio ≥15. Included covariates were sex, race, ASA status, surgical complexity (defined as coronary artery bypass grafting (CABG)/valve, 2 or more valves, aorta valve, or aorta CABG), renal impairment, liver injury, age, BMI, preoperative opioid use, and preoperative midazolam exposure. Due to the low count of patients receiving them, exposure to opioids was dichotomized into a binomial logical variable. Renal impairment was defined as an estimated baseline glomerular filtration rate (eGFR) of 60 mL/min/1.73m2 or lower using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and liver injury by using the Elixhauser comorbidity index.21
We then repeated this analysis for secondary outcomes. For categorical secondary outcomes, logistic regression was used. As few patients had high-stage acute kidney injury, we elected to dichotomize the incidence of AKI as a logical Yes/No variable. For continuous secondary outcomes, Poisson regression was used due to the distribution of the variables.
Odds ratios (OR) or Poisson coefficients (β), depending on the distribution of the outcome variable, with corresponding 95% confidence intervals (CI) were reported to quantify the association between ERACS medications and both primary and secondary postoperative outcomes. The receiver operating characteristic (ROC) curve and corresponding concordance statistic (i.e., area under the ROC curve) assessed discriminative ability of the model, while a calibration plot measured the predictive accuracy.22, 23 In addition to calibration plot, a Brier Score was calculated as a quantitative measure of predictive accuracy. Variance inflation factors (VIF) were calculated to measure multicollinearity. All analyses were performed with R (Version 3.6.3; R Core Team; Vienna, Austria).
Power calculation
A power analysis was conducted based on a type-I error rate of 5%, a power of 80%, a projected sample size of 1500 patients, and number of included covariates in the model.24 Considering these factors, we were able to detect a small treatment effect.
Sensitivity analysis
As a post hoc sensitivity analysis, we studied the association between postoperative delirium and the administration of all 5 ERACS medications.
Results
Patient characteristics
Of the 1,695 total patients, 20 (1.3%) were excluded from the analysis—2 for a lack of recorded height and BMI, 3 for not being admitted to the ICU postoperatively, 3 for dying within the OR, and 12 for a lack of baseline creatinine measurement. One thousand patients (59.7%) received at least one of the 5 ERACS medications, while 675 patients (40.3%) did not receive any. Acetaminophen and gabapentin were administered to 465 (27.8%) and 405 (24.2%) patients, respectively. Ketamine, lidocaine, and dexmedetomidine infusions were administered to 700 (41.8%), 681 (40.7%), and 189 (11.3%) patients, respectively. All 5 ERACS medications were administered to 44 (2.6%) patients (Table 1).
Table 1.
Patient Characteristics Categorical data are reported as number (%) and continuous data as median (25th percentile, 75th percentile).
| Overall (N=1675) | Any ERACS drug (N=1000) | No ERACS drug (N=675) | |
|---|---|---|---|
| Age, years | 64 [55, 71] | 63 [54, 70] | 65 [57, 72] |
| Men | 1176 (70.2) | 707 (70.7) | 469 (69.5) |
| Race | |||
| White | 1482 (88.5) | 881 (88.1) | 601 (89.0) |
| American Indian or Alaska Native | 3 (0.2) | 2 (0.2) | 1 (0.1) |
| Asian | 20 (1.2) | 8 (0.8) | 12 (1.8) |
| Black or African American | 130 (7.8) | 83 (8.3) | 47 (7.0) |
| Unknown or Other | 40 (2.4) | 26 (2.6) | 14 (2.1) |
| Ethnicity | |||
| Hispanic or Latino/a | 14 (0.8) | 6 (0.6) | 8 (1.2) |
| Not Hispanic or Latino/a | 1617 (96.5) | 968 (96.8) | 649 (96.1) |
| Unknown | 44 (2.6) | 26 (2.6) | 18 (2.7) |
| Height, cm | 175 [166, 180] | 175 [168, 180] | 175 [165, 180] |
| Weight, kg | 89.0 [75.8, 102.1] | 88.5 [74.8, 102.5] | 89.8 [77.1, 102.1] |
| BMI, kg/m2 | 29.0 [25.3, 33.5] | 28.9 [25.1, 33.5] | 29.3 [25.8, 33.4] |
| ASA Status (%) | |||
| 1 | 1 (0.1) | 1 (0.1) | 0 (0.0) |
| 2 | 9 (0.5) | 4 (0.4) | 5 (0.7) |
| 3 | 291 (17.4) | 189 (18.9) | 102 (15.1) |
| 4 | 1368 (81.7) | 803 (80.3) | 565 (83.7) |
| 5 | 6 (0.4) | 3 (0.3) | 3 (0.4) |
| COPD | 61 (3.6) | 30 (3.0) | 31 (4.6) |
| History of Atrial Fibrillation | 752 (44.9) | 439 (43.9) | 313 (46.4) |
| HbA1c, mmol/mol | 5.8 [5.4, 6.6] | 5.8 [5.4, 6.6] | 5.8 [5.4, 6.7] |
| Creatinine, mg/dL | 1.01 [0.85, 1.24] | 1.00 [0.84, 1.22] | 1.03 [0.86, 1.27] |
| eGFR, mL/min | 74.04 [56.88, 88.48] | 71.96 [54.43, 86.55] | 75.30 [59.84, 90.11] |
| Chronic kidney disease (eGFR<60) | 480 (28.7) | 227 (33.6) | 253 (25.3) |
| Surgery type | |||
| Coronary artery bypass graft | 836 (49.9) | 496 (49.6) | 340 (50.4) |
| Heart valve procedures | 467 (27.9) | 279 (27.9) | 188 (27.9) |
| Combined valve/CABG | 172 (10.3) | 100 (10.0) | 72 (10.7) |
| Aorta | 152 (9.1) | 92 (9.2) | 60 (8.9) |
| Myectomy | 14 (0.8) | 9 (0.9) | 5 (0.7) |
| Other | 34 (2.0) | 24 (2.4) | 10 (1.5) |
| ICU before Anesthesia Start | 113 (6.7) | 68 (6.8) | 45 (6.7) |
| Complex Surgery | 1160 (69.3) | 688 (68.8) | 472 (69.9) |
| Reoperations in 24 Hours | 59 (3.5) | 37 (3.7) | 22 (3.3) |
| Reoperations in 48 Hours | 73 (4.4) | 45 (4.5) | 28 (4.1) |
| Operation time, hours | 6 [5, 6] | 6 [5, 6] | 6 [5, 6] |
| ERACS medications | |||
| Acetaminophen | 465 (27.8) | 46.5% | 0 (00.0) |
| Gabapentin | 405 (24.2) | 40.5% | 0 (00.0) |
| Lidocaine | 681 (40.7) | 68.1% | 0 (00.0) |
| Ketamine | 700 (41.8) | 70.0% | 0 (00.0) |
| Dexmedetomidine | 189 (11.3) | 18.9% | 0 (00.0) |
| Any ERACS drug | 1000 (59.7) | 1000 (100.0) | 0 (00.0) |
| All ERACS drugs | 44 (2.7) | 44 (4.4%) | 0 (00.0) |
| Delirium | 220 (13.7) | 122 (12.2) | 98 (14.5) |
| Death in Hospital | 107 (6.4) | 62 (6.2) | 45 (6.7) |
| Postop Atrial Fibrillation | 644 (38.4) | 370 (37.0) | 274 (40.6) |
| Hospital Length of Stay Days | 7.9 [5.5, 11.8] | 7.7 [5.4, 11.4] | 8.1 [6.0, 12.5] |
| Postop Opioid, MME | 36.3 [15.0, 58.8] | 38.8 [14.9, 60.0] | 33.8 [16.3, 57.5] |
| Acute Kidney Injury (%) | |||
| None | 1501 (89.6) | 908 (90.8) | 593 (87.9) |
| Stage 1 | 110 (6.6) | 57 (5.7) | 53 (7.9) |
| Stage 2 | 11 (0.7) | 7 (0.7) | 4 (0.6) |
| Stage 3 | 53 (3.2) | 28 (2.8) | 25 (3.7) |
| Renal Replacement Therapy (%) | 68 (4.1) | 40 (4.0) | 28 (4.1) |
| ICU Length of Stay, hours | 45 [25, 75] | 45 [26, 73] | 44 [25, 81.5] |
BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ERACS, Enhanced Recovery After Surgery; HbA1c, hemoglobin A1c; ICU, intensive care unit; IQR, interquartile range; M, male; MME, morphine milligram equivalent; postop, postoperative.
Postoperative delirium occurred in 122 patients (12.2%) patients who received any of the ERACS medications and in 98 patients (14.5%) who did not. There was a median of 4 postoperative CAM-ICU assessments performed on each patient. There were 108 patients who did not have a documented CAM-ICU assessment (Table 1).
Statistical analysis
Administration of any one of the five ERACS medications vs. administration of no ERACS medications was not associated with postoperative delirium (OR: 0.85 [95% CI: 0.63, 1.16]). Individually, acetaminophen was associated with reduced delirium (OR: 0.60 [95% CI: 0.37, 0.95]). Gabapentin (OR: 1.36 [95% CI: 0.97, 2.21]), lidocaine (OR: 0.86 [95% CI: 0.53, 1.37]), ketamine (OR: 1.15 [95% CI: 0.72, 1.83]), and dexmedetomidine (OR: 0.79 [95% CI: 0.46, 1.31]) were not associated with postoperative delirium (Table 2).
Table 2.
Associations between ERACS drug administrations and postoperative delirium
| ERACS Treatment | Odds Ratio (OR) | 95% Confidence Interval | P-value |
|---|---|---|---|
| Any ERACS guided analgesic treatment vs. No ERACS treatment (i.e., “collective” model | 0.85 | 0.63, 1.16 | 0.31 |
| Other significant covariates in “collective” model | |||
| Age (Years) | 1.01 | 1.00, 1.03 | 0.03 |
| BMI | 0.98 | 0.95, 0.99 | 0.04 |
| Intraoperative Opioid MME | 0.99 | 0.99, 0.99 | 0.01 |
| Liver Elixhauser index | 2.62 | 1.55, 4.34 | <0.001 |
| eGFR (<60 = Yes) | 2.15 | 1.58, 2.93 | <0.001 |
| Individual ERACS treatments (i.e., “individual” model) | |||
| Acetaminophen | 0.60 | 0.37, 0.95 | 0.03 |
| Gabapentin | 1.36 | 0.97, 2.21 | 0.21 |
| Ketamine | 1.15 | 0.72, 1.83 | 0.55 |
| Lidocaine | 0.86 | 0.53, 1.37 | 0.52 |
| Dexmedetomidine | 0.79 | 0.46, 1.31 | 0.39 |
Model diagnostics revealed that the model discriminated between patients who did and did not experience postoperative delirium 70% of the time—area under the receiver operating characteristic curve (AUROC) = 0.696. The model was well calibrated and capable of making accurate predictions in outside data sample (Figure 1). Complementing the calibration plot, a calculated Brier score of 0.11 further indicated strong predictive capabilities. Variance inflation factors (VIF) were low (≤ 2), indicating no multicollinearity within the model.
Figure 1.
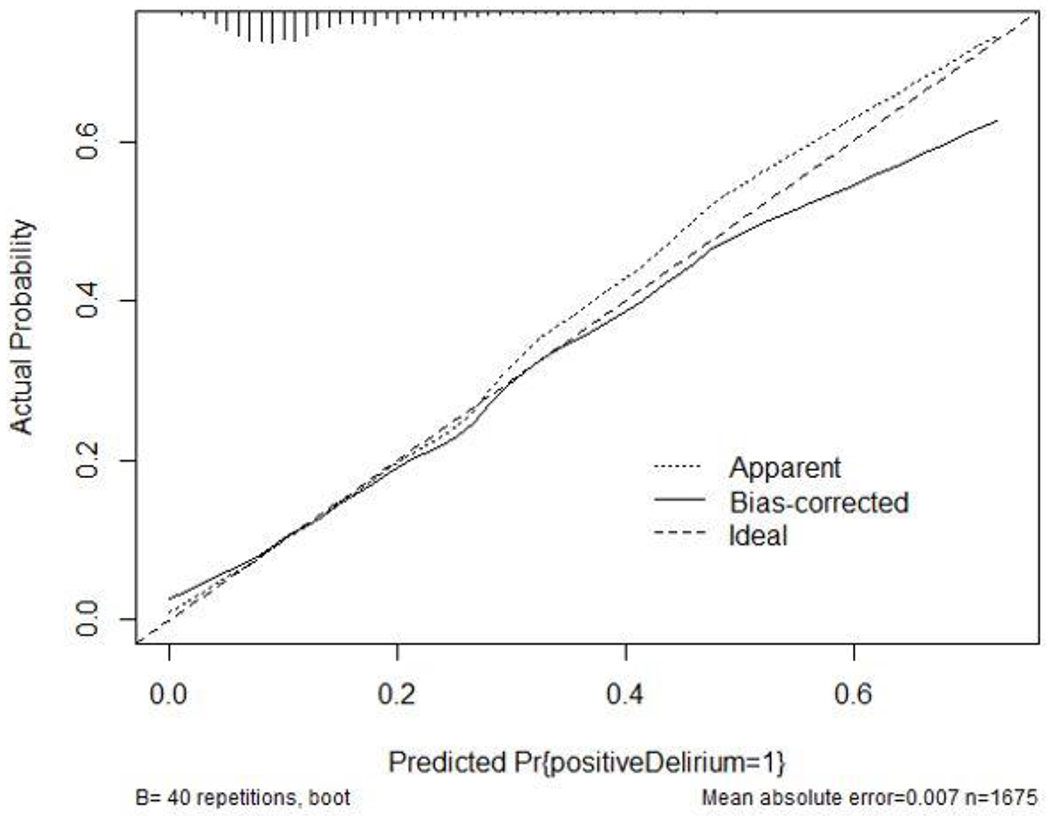
Model Calibration plot. Shown here are bias-corrected (overfitting-corrected) estimates of predicted vs. observed values of postoperative delirium. Pr{positiveDelirium=1}; predicted probability of a patients having a positive CAM-ICU assessment. n; number.
ERACS medication treatments were statistically significantly associated with hospital length of stay (days), ICU length of stay (hours), postoperative opioid administration, and postoperative intubation duration, but for the most part this association was not practically significant (Table 3 and Table 4). Preoperative acetaminophen was associated with 7% reduced ICU length of stay (β: 0.93 [95% CI: 0.91, 0.94]) and 15% reduced postoperative intubation duration (β: 0.85 [95% CI: 0.82, 0.88]). Preoperative gabapentin was associated with 3% increased ICU length of stay (β: 1.03 [95% CI: 1.01, 1.05]), 5% increased postoperative opioid administration (β: 1.05 [95% CI: 1.02, 1.07]), and 8% increased postoperative intubation duration (β: 1.08 [95% CI: 1.04, 1.12]). Intraoperative ketamine was associated with 5% increased ICU length of stay (β: 1.05 [95% CI: 1.03, 1.07]) and 27% increased postoperative intubation duration (β: 1.27 [95% CI: 1.22, 1.31]) but 3% reduced postoperative opioid administration (β: 0.97 [95% CI: 0.95, 0.99]). Intraoperative lidocaine was associated with 7% reduced ICU length of stay (β: 0.93 [95% CI: 0.91, 0.94]) and 6% reduced postoperative intubation duration (β: 0.94 [95% CI: 0.91, 0.98]). Intraoperative dexmedetomidine was associated with 7% reduced hospital length of stay (β: 0.93 [95% CI: 0.88, 0.98]), 2% reduced ICU length of stay (β: 0.98 [95% CI: 0.96, 0.99]), 6% reduced postoperative opioid administration (β: 0.94 [95% CI: 0.91, 0.96]), and 20% reduced postoperative intubation duration (β: 0.80 [95% CI: 0.77, 0.84]).
Table 3.
Associations between use of any ERACS drugs and development of secondary outcomes
| Outcome | Odds Ratio (OR) or Poisson Coefficient | 95% Confidence Interval | P-value |
|---|---|---|---|
| In-Hospital mortality | 1.00 | 0.99, 1.03 | 0.98 |
| Postoperative atrial fibrillation | 0.95 | 0.76, 1.17 | 0.61 |
| Acute Kidney Injury | 0.83 | 0.56, 1.04 | 0.34 |
| Length of stay days | 0.95 | 0.92, 0.98 | 0.003 |
| ICU length of stay, hours | 0.95 | 0.94, 0.97 | <0.001 |
| Postoperative opioid MME | 0.97 | 0.96, 0.99 | <0.001 |
| Postoperative Intubation, hours | 1.11 | 1.08, 1.14 | <0.001 |
Table 4.
Associations between individual use of ERACS drugs and development of secondary outcomes
| Outcomes | ERACS drug | Odds Ratio or Linear Coefficient | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| In-hospital mortality | Acetaminophen | 1.75 | 0.98, 3.07 | 0.05 |
| Gabapentin | 0.54 | 0.27, 1.04 | 0.07 | |
| Ketamine | 1.86 | 0.98, 3.45 | 0.05 | |
| Lidocaine | 0.53 | 0.28, 1.03 | 0.06 | |
| Dexmedetomidine | 1.09 | 0.52, 2.09 | 0.81 | |
|
| ||||
| Atrial fibrillation | Acetaminophen | 0.92 | 0.66, 1.27 | 0.60 |
| Gabapentin | 0.95 | 0.67, 1.36 | 0.79 | |
| Ketamine | 1.15 | 0.83, 1.59 | 0.39 | |
| Lidocaine | 0.86 | 0.62, 1.19 | 0.36 | |
| Dexmedetomidine | 1.02 | 0.72, 1.43 | 0.93 | |
|
| ||||
| Acute kidney injury | Acetaminophen | 1.62 | 0.92, 2.87 | 0.09 |
| Gabapentin | 0.52 | 0.26, 1.01 | 0.06 | |
| Ketamine | 0.94 | 0.46, 1.91 | 0.87 | |
| Lidocaine | 1.13 | 0.62, 2.06 | 0.68 | |
| Dexmedetomidine | 0.89 | 0.48, 1.64 | 0.71 | |
|
| ||||
| Length of stay, days | Acetaminophen | 1.02 | 0.97, 1.06 | 0.47 |
| Gabapentin | 0.96 | 0.91, 1.01 | 0.11 | |
| Ketamine | 1.01 | 0.96, 1.06 | 0.79 | |
| Lidocaine | 0.98 | 0.94, 1.03 | 0.51 | |
| Dexmedetomidine | 0.93 | 0.88, 0.98 | 0.004 | |
|
| ||||
| ICU length of stay, hours | Acetaminophen | 0.93 | 0.91, 0.94 | <0.001 |
| Gabapentin | 1.03 | 1.01, 1.05 | 0.003 | |
| Ketamine | 1.05 | 1.03, 1.07 | <0.001 | |
| Lidocaine | 0.93 | 0.91, 0.94 | <0.001 | |
| Dexmedetomidine | 0.98 | 0.96, 0.99 | 0.02 | |
|
| ||||
| Postoperative opioids, MME | Acetaminophen | 0.98 | 0.96, 1.01 | 0.15 |
| Gabapentin | 1.05 | 1.02, 1.07 | <0.001 | |
| Ketamine | 0.97 | 0.95, 0.99 | 0.01 | |
| Lidocaine | 0.98 | 0.96, 1.00 | 0.09 | |
| Dexmedetomidine | 0.94 | 0.91, 0.96 | <0.001 | |
|
| ||||
| Postoperative Intubation, hours | Acetaminophen | 0.85 | 0.82, 0.88 | <0.001 |
| Gabapentin | 1.08 | 1.04, 1.12 | <0.001 | |
| Ketamine | 1.27 | 1.22, 1.31 | <0.001 | |
| Lidocaine | 0.94 | 0.91, 0.98 | 0.003 | |
| Dexmedetomidine | 0.80 | 0.77, 0.84 | <0.001 | |
Sensitivity analysis
We failed to detect an association between postoperative delirium and the administration of all five ERACS medications (OR: 0.85; 95% CI: 0.62, 1.15).
Discussion
In this single-center, retrospective cohort study of cardiac surgery patients, there was little association between use of opioid-sparing ERACS medication and development of delirium. Acetaminophen, however, was associated with nearly half the odds of postoperative delirium. We failed to detect any other association between delirium and ERACS treatment.
Postoperative systematic delirium screening is recommended in the guidelines for perioperative care in cardiac surgery provided by the ERAS Society.16 These recommendations also suggest that a balanced multimodal analgesia and sedation protocol that aims to reduce pain and anxiety may mitigate postoperative delirium and improve recovery. Inadequate treatment of pain has been known to increase the risk for delirium.11 However, treatment with excessive use of opioids may be associated with a higher risk of delirium, although no clear association between opioid use and delirium has been found in the cardiac surgical population.1, 25–27 A multimodal analgesia strategy has emerged as an essential component of all ERAS pathways. Concurrent use of multiple, primarily non-opioid analgesics may have an additive or synergistic effect to decrease overall opioid consumption while addressing postoperative pain. A small study of patients undergoing CABG procedures demonstrated that ERACS pathways were associated with decreased opioid use and increased incidence of successful extubation in the OR.28 A randomized clinical trial of 226 patients comparing the addition of ERAS pathways in patients undergoing valvular procedures found that patients treated with ERAS pathways had reduced lengths of ICU and hospital stay, postoperative complications, and cost. The current study did not find evidence that use opioid-sparing ERACS medications impacted postoperative delirium, opioid use, of duration of hospitalization.
The current study does provide evidence that one element of the ERACS treatment bundle, preoperative oral acetaminophen may impact postoperative delirium. Acetaminophen use is known to reduce opioid consumption and pain but may also reduce pro-inflammatory cytokines and have potential central nervous system modifying effects which may reduce delirium. The DEXACET study, which randomized 121 cardiac surgery patients to IV acetaminophen versus placebo as an analgesic adjunct in addition to dexmedetomidine versus propofol as a sedative, found that patients who received acetaminophen had a significantly lower incidence of in-hospital delirium compared to placebo.15 Patients assigned acetaminophen also had a shorter duration of delirium, as well as a shorter ICU length of stay, and received less opioids compared to placebo. In other cardiac surgery clinical trials, perioperative acetaminophen reduced plasma concentrations of isofurans, sensitive markers of oxidative damage, and perioperative oxidative damage contributes to postoperative delirium.30,31 The results of our study comport with the findings of prior studies in cardiac patients on the beneficial effect of acetaminophen on the incidence of delirium.
The robust, easily replicable statistical methodology used in this study to isolate the effect of ERACS medications on the odds of postoperative delirium is a strength. Similarly, the data collection resulted in a highly reproducible process that minimized missingness and accurately captured all required data elements. We avoided overfitting, as evidenced by the model calibration, or introducing bias from multicollinearity, which both add to the study’s generalizability. Consequently, the model demonstrated high, bias-corrected predictive ability and discriminated between cardiac patients who did and did not have delirium. For these reasons, the study results may be generalizable, although further work with a larger dataset of cardiac surgical patients could increase the statistical power.
The study has several limitations. The study only included cases from a single center. The administration of ERACS medications was not random. It should be noted, however, that the decision to administer ERACS medications was largely driven by the anesthesiologist provider rather than influenced by patient factors that could confound the relationship between ERACS medications and organ injury. This observation is supported by the similar patient characteristics between those who did and did not receive ERACS medication. Yet, the different analgesic elements of the ERAS protocol were utilized to varying degrees within the cardiac surgery population. The present study did not include other programmatic changes included in ERAS programs but looked exclusively at use of opioid sparing sedative and analgesic medications.
Another limitation is that patients without a CAM-ICU evaluation were assumed to be “negative” for delirium—which could lead to underestimation of delirium rates if hypoactive symptoms simply went unnoticed. Manual review of patient charts revealed that, for many of these patients, delirium was unable to be measured (i.e., patient still sedated, RASS score of −4 or −5). Considering this, we elected to use the CAM-ICU assessment method because it was developed to diagnose delirium in ICU patients, who are often nonverbal due to mechanical ventilation.3 Thus, we believe any bias of delirium rates to be minimal. Also, there were no familywise error adjustments made for the model with multiple treatment variables because such adjustments inappropriately and unjustifiably localize Type I error.32
In summary, the use of opioid sparing ERACS medications was not associated with postoperative delirium, postoperative opioid use, nor addition secondary outcomes. Acetaminophen was associated with reduced risk of delirium. Additional evidence is required to determine if strict adherence to the ERACS medications bundle affect delirium, opioid consumption, and postoperative outcomes in cardiac surgery patients.
Funding:
Dr. Billings’ research is supported by R01GM11287 from the NIH, and Dr. Robert Freundlich’s research is supported by K23HL148640 from the NIH-NHLBI and UL1TR002243 from the NCATS.
Glossary of Terms:
- ERAS
Enhanced Recovery After Surgery
- ERACS
Enhanced Recovery After Cardiac Surgery
- CAM-ICU
Confusion Assessment Method for ICU
- PDW
Perioperative Data Warehouse
- SCr
serum creatinine
- AKI
acute kidney injury
- ESRD
end stage renal disease
- eGFR
estimated glomerular filtration rate
- CABG
coronary artery bypass grafting
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- OR
odds ratios
- CI
confidence interval
- ROC
receiver operating characteristic
- VIF
variance inflation factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: No relevant conflict of interest.
References
- 1.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med 2013; 41: 405–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011; 112: 1202–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CH. Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol 2014; 27: 117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004; 32: 955–62 [DOI] [PubMed] [Google Scholar]
- 5.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009; 37: 1881–5 [DOI] [PubMed] [Google Scholar]
- 6.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008; 65: 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crocker E, Beggs T, Hassan A, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg 2016; 102 4: 1391–9 [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Minhas JS, Chung EML. Risk factors associated with cognitive decline after cardiac surgery: a systematic review. Cardiovascular Psychiatry and Neurology 2015; 2015: 370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman MF, Grocott HP, Mathew JP, et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke 2001; 32: 2874–81 [DOI] [PubMed] [Google Scholar]
- 11.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018; 46(9):e825–873. [DOI] [PubMed] [Google Scholar]
- 12.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301: 489–99 [DOI] [PubMed] [Google Scholar]
- 13.Pandhardipande P, Pun BT, Herr DL, et al. Effect of sedation with Dexmedetomidine vs Lorazepam on Acute Brain Dysfunction in Mechanically Ventilated Patients: The MENDS Randomized Controlled Trial. JAMA 2007; 298(22):2644–2653. [DOI] [PubMed] [Google Scholar]
- 14.Shehabi Y, Grant P, Wolfenden H, et al. Prevalance of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetdomine Compared to Morphine- DEXCOM study). Anesthesiology 2009; 111(5): 1075–84. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA 2019; 321: 686–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: Enhanced Recovery After Surgery Society recommendations. JAMA Surg 2019; 154: 755–66 [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–7 [DOI] [PubMed] [Google Scholar]
- 18.Freundlich RE, Boncyk CS. Clearly-defined outcomes improve the quality of health outcomes research. Brit J Anaesth 2019; 122: 14–6 [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 20.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012;120(4):c179–84. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21: 128–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook N. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008; 54: 17–23 [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis. Current Directions in Psychological Science 1992; 1: 98–101 [Google Scholar]
- 25.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306 [DOI] [PubMed] [Google Scholar]
- 26.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg 2006; 102: 1267–73 [DOI] [PubMed] [Google Scholar]
- 27.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med 2009; 37: 177–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markham T, Wegner R, Hernandez N, et al. Assessment of a multimodal analgesia protocol to allow the implementation of enhanced recovery after cardiac surgery: retrospective analysis of patient outcomes. J Clin Anesth 2019; 54: 76–80 [DOI] [PubMed] [Google Scholar]
- 29.Li M, Zhang J, Gan TJ, et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: a randomized clinical trial. Eur J Cardiothorac Surg 2018; 54: 491–7 [DOI] [PubMed] [Google Scholar]
- 30.Billings FT, Petracek MR, Jackson Roberts L II, et al. Perioperative Intravenous Acetaminophen Attenuates Lipid Peroxidation in Adults Undergoing Cardiopulmonary Bypass: A Randomized Clinical Trial. PLoS One 2015. 10(2): e0117625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez MG, Hughes CG, DeMatteo A, et al. Intraoperative Oxidative Damage and Delirium after Cardiac Surgery Lopez. Anesthesiology 2020; 132:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Keefe DJ. Colloquy: should familywise alpha be adjusted?: against familywise alpha adjustment. Human Communication Research 2006; 29: 431–47 [Google Scholar]


