Abstract
Purpose:
to report the first case to date of delayed suprachoroidal hemorrhage not associated with hypotony two weeks after placement of the Xen45 gel stent.
Case summary:
An 84 year old Caucasian male with significant cardiovascular comorbidities underwent uneventful ab externo implantation of a Xen45 gel stent for asymmetric progression of severe primary open angle glaucoma. The patient had a reduction in intraocular pressure by 11 mm Hg on postoperative day 1 and maintained preoperative visual acuity. The IOP remained stable at 8 mm Hg on multiple postoperative visits until the patient developed a suprachoroidal hemorrhage (SCH) at postoperative week 2 immediately following a light session of physical therapy. The patient was treated medically with topical cycloplegic, steroid and aqueous suppressants. He maintained preoperative visual acuity throughout the postoperative course and had resolving SCH without the need for surgical intervention.
Conclusions:
This is the first report of a delayed presentation of suprachoroidal hemorrhage in the absence of hypotony after ab externo implantation of the Xen45 device. The possibility of this vision threatening complication should be considered as part of the risk assessment and included in the consent process for the gel stent. In patients with significant preoperative comorbidities, prolonged activity restrictions beyond two weeks following Xen45 surgery may mitigate the risks of delayed SCH.
Keywords: MIGS complication, delayed suprachoroidal hemorrhage, Xen45 gel stent, glaucoma
Background
The Xen45 gel stent (Allergan©, Dublin, Ireland) is a microshunt approved for the treatment of refractory and moderate to severe open angle glaucoma.1, 2 The gel stent is a biocompatible hydrophilic device, derived from porcine gelatin cross-linked with glutaraldehyde, that is flexible when hydrated to minimize movement and conjunctival erosion.3 Engineered based on the Hagen-Poiseuille principle of fluid dynamics through a cylindrical tube, the length of the stent and the inner and outer lumen diameter are designed to avoid hypotony, defined as an IOP less than or equal to 5 mm Hg. 3 The Xen gel stent is a transscleral implant that creates a subconjunctival bleb similar to trabeculectomy and is reported to have superior safety profile.1–3 However, trabeculectomy is the gold standard procedure due to its proven ability to achieve excellent reduction in IOP, reduce medication burden and glaucoma progression.3
The formation of a subconjunctival bleb makes the gel stent prone to many complications encountered with traditional glaucoma filtering procedure, including hypotony, fibrosis, choroidal effusion, wound leak, bleb failure, and suprachoroidal hemorrhage (SCH), in addition to implant related complications such as exposure.4–6 A SCH occurs from rupture of the short or long posterior ciliary arteries between the uvea and the sclera. It is the most devastating complication after conventional glaucoma surgery because of its grave consequences on visual function. SCH is attributed to hypotony secondary to globe decompression or wound dehiscence reducing scleral rigidity and precipitating sheer stress on the vasculature.7 Delayed SCH occurring after incisional ocular surgery is thought to be triggered by persistent hypotony and inflammation.7, 8 The incidence of delayed SCH is reported to be 2–8% after glaucoma drainage device implants and trabeculectomy with and without antimetabolite.8 Postoperative SCH associated with the Xen gel stent has not been reported in the pivotal trials for the device.2, 4, 9–11 Several case reports have since described the occurrence of SCH after Xen45 implantation intraoperatively or associated with postoperative hypotony.6, 12, 13
To the best of our knowledge, we report the first case of delayed non-expulsive suprachoroidal hemorrhage, at postoperative week 2 after an uneventful implantation of the Xen45 gel stent, unrelated to postoperative hypotony. Our case demonstrates the importance of preoperative risk assessment and postoperative risk management and precautions to reduce vision threatening complications associated with the Xen45 gel stent.
Case report
We present the case of an 84-year-old Caucasian man with significant cardiovascular risk factors and severe primary open angle glaucoma. His medical history is significant for congestive heart failure, diabetic neuropathy with bilateral foot ulcers, orthostatic hypotension, deep vein thrombosis, frailty, and chronic back pain treated with Aspirin 325 mg. His ocular history included pseudophakia, dry age-related macular degeneration, and vitreomacular traction in both eyes as well as amblyopia in the right eye. The visual field on presentation showed double arcuate depression with depleted retinal nerve fiber layer in both eyes. He developed asymmetric progression in the left eye, involving the central five degrees of fixation, over the course of three years with IOP in the range of 11–16 mm Hg on topical medical therapy. A brain and orbit MRI revealed no evidence an intracranial lesion, and thereby the vision loss was consistent with progressive glaucomatous damage.
The patient and family were resistant to consider surgical intervention because of his frailty and comorbidities. The Xen gel stent was selected for the potential IOP reduction comparable to trabeculectomy, minimal manipulation of tissues and reduced intraoperative time. At the preoperative exam for the left eye, the best corrected visual acuity was 20/150 with an IOP of 19 mm Hg on maximally tolerated medications (Latanoprostene bunod, Dorzolamide-timolol, Brimonidine, and Apraclonidine).
As the patient was pseudophakic, he underwent an uneventful ab externo Xen implantation without conjunctival dissection. Briefly, a corneal traction suture was placed for mobilization of the globe. The preloaded injector with a beveled 27-gauge needle entered the subconjunctival space approximately 8–9 mm posterior to the limbus and carefully tunneled towards the limbus. The needle penetrated the sclera at 2.5 mm posterior to the gray-white line of the corneoscleral junction and entered the anterior chamber. The needle tip was verified to be within the trabecular meshwork on gonioscopy before the gel stent was deployed. A diffuse bleb was noted surrounding the gel stent and 0.1 cc of Mitomycin C 0.1 mg/ml was injected into the subconjunctival space without complications.
On postoperative day 1, the visual acuity (VA) was 20/200 and IOP improved to 8 mm Hg with a diffuse bleb and a formed anterior chamber. All aqueous suppressants were held in the immediate postoperative period. The patient’s VA returned to preoperative baseline and the IOP remained stable off aqueous suppressants on postoperative week 1. On postoperative week 2, the patient participated in physical therapy with light endurance exercises, gait training and wheelchair transfers. He reported worsening headaches the same evening and was noted to have a hemorrhagic choroidal detachment on head CT in the emergency room. Slit lamp exam showed a flat bleb with a deep chamber and 360° of non-appositional hemorrhagic choroidal detachment (Figure 1). His VA was unchanged at 20/150, and the IOP was elevated to 30 mm Hg. The patient was managed conservatively with the addition of aqueous suppressants (Methazolamide, Dorzolamide-Timolol, Apraclonidine, Brimonidine), steroids and cycloplegics. His IOP improved and VA remained stable at preoperative levels. At postoperative month 3, the SCH improved without surgical intervention as noted on ultrasound (Figure 2).
Figure 1.
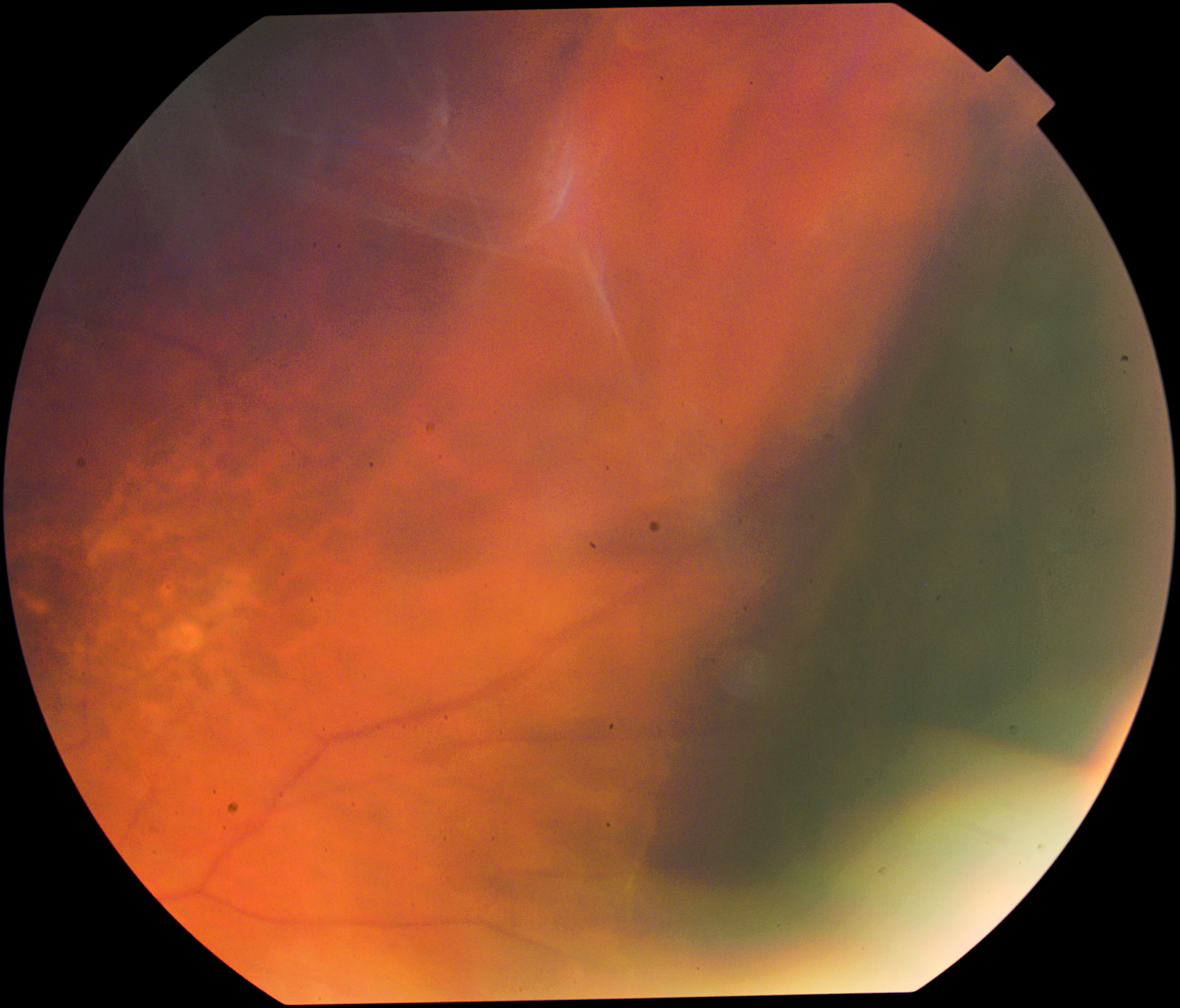
Fundus photo of the suprachoroidal hemorrhage (delineated by the dark shadow) in the retinal periphery.
Figure 2.
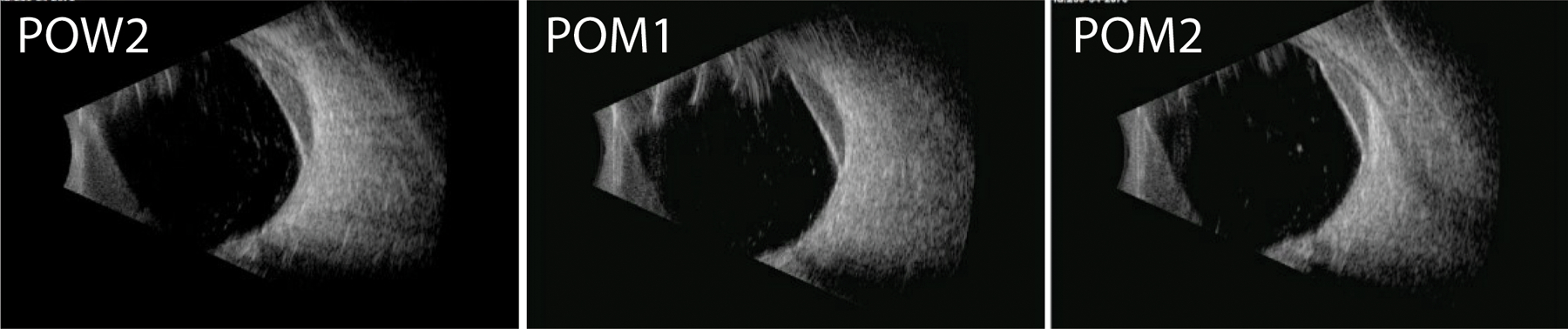
Serial B scan ultrasonography performed in the longitudinal axis of the macula over the postoperative period. There is a reduction in the size of the suprachoroidal hemorrhage by postoperative month 2.
Discussion
The Xen45 gel stent is a subconjunctival MIGS device designed to improve the reproducibility and safety profile of bleb-forming procedure, but real-world experience show it is also susceptible to the same complications of filtering surgery.3 Since the approval of the device by the FDA in 2016, there have been more than 15 prospective, retrospective and comparative studies, including more than 1700 patients who received the device in a stand-alone procedure or in combination with cataract surgery.1, 2, 9 The novel implant can achieve significant IOP reduction (22–51% from baseline) and decrease medication burden (by 1–3 medications), comparable to traditional filtering surgery such as trabeculectomy and glaucoma drainage device. 4, 11, 14 The most common reported early postoperative complications included needling (22–49%) and early hypotony (4–30%) within postoperative week 1.4, 10, 14 The incidences of vision threatening persistent hypotony and/or choroidal effusion are 0–3% compared to the incidence of 13–31% associated with trabeculectomy.2, 15, 16 Notably, the success and adverse events for most of the Xen trials have concluded at 12 months after surgery, thus the long-term risk of failure and serious complications for the Xen45 device remain to be determined.
A suprachoroidal hemorrhage is the most dreaded complication after incisional ocular surgery. Glaucoma surgery poses the highest risks for SCH and is estimated to occur 1.4–2% for trabeculectomy and 2.7–8.3% for glaucoma drainage devices.17 Reports of SCH associated with Xen implantation have been reported several times following intraoperative or postoperative hypotony. Of the many clinical trials for the device, only one case of focal SCH was observed by Kalina and colleagues in a prospective study of Xen for medically refractory open angle glaucoma.1 The case was attributed to postoperative hypotony at day 1 and fully resolved with conservative measures by 2 weeks. A case report by Liu et al. described the occurrence of intraoperative, non-expulsive SCH during deployment of the gel stent that was associated with anterior chamber instability.13 Prokosch-Willing et al. presented another case of SCH at postoperative day 2 that was associated with an IOP of 4 mm Hg.12 A short case series by Rooney and colleagues described a patient with combined SCH and rhegmatogenous retinal detachment following Xen implantation at postoperative day 2 with IOP of 4 mm Hg.6
To the best of our knowledge, this case report is unique in its delayed presentation and absence of hypotony after an uneventful ab externo Xen implantation. In contrast to the previously described cases, our patient maintained a stable IOP of 8 mm Hg on multiple postoperative exams within the first two weeks. The SCH occurred incidentally after a session of light physical therapy for gait and stability training at postoperative week 2. We propose two distinct mechanisms for perioperative SCH with and without associated postoperative hypotony. In the presence of hypotony, scleral deformation and choroidal fluid can exert sheer stress on the vasculature and cause rupture of the choroidal vessels. This mechanism may explain the early perioperative SCH described in the previous case reports. In contrast, under normal range of IOP, the delayed rupture of the posterior ciliary arteries may be attributed to the intrinsic strength of the vessels. Our patient had significant risk factors for SCH including age, diabetes mellitus, atherosclerosis, hypertension, frailty, and fall risks, that predisposed him to weak vascular integrity. Even at postoperative week two, possible Valsalva and light physical therapy under low normal transscleral tension may have induced spontaneous rupture of susceptible vessels. This is the first report of delayed SCH occurring after ab externo placement of the device. We opted to implant the device via an ab externo approach to minimize operative time and avoid intraoperative IOP fluctuations from a large corneal incision and intracameral injection of cohesive viscoelastic. Given the infrequent incidence of SCH associated with the Xen gel stent, it is unclear whether the implantation approach can affect the presentation and mechanism of SCH.
This case report demonstrates that patients who are poor candidates for traditional glaucoma surgery are also likely poor candidates for Xen45 gel stent. Recognition that the Xen device is prone to similar complications as conventional filtering surgery will aid in early detection and management of vision threatening sequelae. It is important to mitigate the preoperative and postsurgical risk factors in patients undergoing placement of Xen45 gel stent and possibly other subconjunctival MIGS devices. Despite the safety features in the design, the Xen device is prone to early postoperative hypotony (25–30% 4, 18). Avoiding drastic reduction in IOP is critical for reducing fluid shifts in the suprachoroidal space postoperatively. Our patient exhibited a delayed presentation associated with his systemic comorbidities and physical activity rather than hypotony. The patient had resumed physical therapy and full dose Aspirin for pain control by postoperative week 2. Limiting anticoagulation, avoidance of Valsalva activity and aggressive treatment of hypertension postoperatively may mitigate the cardiovascular risks factors. Although there is no established postoperative timeline for restrictions of anticoagulation and exercises after the XEN45 implant, our report suggests that two weeks may be insufficient in high-risk patients.
The management of SCH is controversial and can vary from conservative medical therapy to surgical drainage. Several large case series on SCH after filtering surgeries have described devastating vision loss (20/200 to hand motion) and support early surgical drainage to improve visual recovery. 7, 8, 17 Notably, in 4 of the 5 reported cases of SCH associated with the Xen gel stent, patients retained preoperative vision on topical therapy. The difference in visual prognosis suggests that SCH associated with MIGS device may have better visual outcome and require less invasive interventions compared to traditional filtering surgery. The singular case described by Reitsamer and colleagues had poor visual outcome due to several comorbidities, including rhegmatogenous retinal detachment, repeat surgery for Xen device truncation and persistent hypotony.14 Our case report and others highlight the need for prospective longitudinal trials to examine the long-term safety profile and prognosis of vision threatening complications of the Xen microshunt.
References
- 1.Kalina AG, Kalina PH & Brown MM XEN(®) Gel Stent in Medically Refractory Open-Angle Glaucoma: Results and Observations After One Year of Use in the United States. Ophthalmol. Ther 8, 435–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fea AM et al. XEN(®) Gel Stent: A Comprehensive Review on Its Use as a Treatment Option for Refractory Glaucoma. Clin. Ophthalmol 14, 1805–1832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheybani A, Reitsamer H & Ahmed II Fluid Dynamics of a Novel Micro-Fistula Implant for the Surgical Treatment of Glaucoma. Invest. Ophthalmol. Vis. Sci 56, 4789–4795 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Grover DS et al. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am. J. Ophthalmol 183, 25–36 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Karri B, Gupta C & Mathews D Endophthalmitis Following XEN Stent Exposure. J. Glaucoma 27, 931–933 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rooney DM et al. Postoperative Complications of Ab Interno Gelatin Microstent. J. Glaucoma 28, e77–e81 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Gressel MG & Parrish RK,2nd. Fluorouracil and suprachoroidal hemorrhage. Arch. Ophthalmol 105, 169 (1987). [DOI] [PubMed] [Google Scholar]
- 8.Chu TG & Green RL Suprachoroidal hemorrhage. Surv. Ophthalmol 43, 471–486 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Schlenker MB et al. Efficacy, Safety, and Risk Factors for Failure of Standalone Ab Interno Gelatin Microstent Implantation versus Standalone Trabeculectomy. Ophthalmology 124, 1579–1588 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Tan SZ, Walkden A & Au L One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye (Lond) 32, 324–332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widder RA et al. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch. Clin. Exp. Ophthalmol 256, 765–771 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Prokosch-Willing V, Vossmerbaeumer U, Hoffmann E & Pfeiffer N Suprachoroidal Bleeding After XEN Gel Implantation. J. Glaucoma 26, e261–e263 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Liu JC, Green W, Sheybani A & Lind JT Intraoperative suprachoroidal hemorrhage during Xen gel stent implantation. Am. J. Ophthalmol. Case Rep 17, 100600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitsamer H et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 257, 983–996 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Gedde SJ et al. Treatment Outcomes in the Primary Tube Versus Trabeculectomy (PTVT) Study After 5 Years of Follow-up. Ophthalmology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gedde SJ et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol 153, 789–803.e2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Learned D & Eliott D Management of Delayed Suprachoroidal Hemorrhage after Glaucoma Surgery. Semin. Ophthalmol 33, 59–63 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Karimi A et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond) 33, 469–477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


