Abstract
Background:
Maternal obesity complicates a high number of pregnancies. The degree to which neonatal outcomes are adversely affected is unclear.
Objective:
To evaluate neonatal outcomes of pregnancies complicated by maternal obesity.
Study Design:
Secondary analysis of a cohort of deliveries occurring on randomly selected days at 25 hospitals from 2008-2011. Data were collected by certified abstractors. This analysis included singleton deliveries between 24 and 42 weeks. BMI was calculated based on maternal height and most recent weight prior to delivery. Normal/overweight (reference group; BMI 18.5-29.9 kg/m2), obese (OB; BMI 30-39.9 kg/m2), morbidly obese (MO; BMI 40-49.9 kg/m2) and super morbidly obese (SMO; BMI ≥ 50 kg/m2) patients were compared. Patients in the reference group were matched 1:1 with those in all other obesity groups using the baseline characteristics of age, race-ethnicity, previous cesarean, pre-existing diabetes, chronic hypertension, parity, cigarette use, and insurance status. The primary outcome was composite neonatal morbidity, including fetal or neonatal death, hypoxic ischemic encephalopathy, respiratory distress syndrome, Grade III-IV intraventricular hemorrhage, necrotizing enterocolitis, sepsis, birth injury, seizures, or ventilator use. We used modified Poisson regression to examine the associations between BMI and composite neonatal outcome. Preterm delivery < 37 weeks and the presence of maternal preeclampsia/eclampsia were included in the final model because of their known associations with neonatal outcomes.
Results:
52,162 patients and their neonates were included after propensity score matching. Of these, 21,704 (41.6%) were OB, 3787 (7.3%) were MO and 590 (1.1%) were SMO. A total of 2103 (4.0%) neonates had the composite outcome. Neonates born to pregnant people with morbidy obesity had a 33% increased risk of composite neonatal morbidity compared with those in the reference group (aRR 1.33; 95%CI 1.17-1.52), but no significant association was observed for persons with obesity (aRR 1.05; 95%CI 0.97-1.14) or with super morbid obesity (aRR 1.18; 95%CI (0.86-1.64).
Conclusion:
Compared with the reference group, gravidas with morbid obesity are at higher risk for composite neonatal morbidity.
Keywords: Obesity, obesity in pregnancy, neonatal morbidity, neonatal mortality, BMI
Introduction
Maternal obesity complicates almost one-third of pregnancies in the United States and is associated with increased risks of miscarriage, stillbirth, congenital anomalies, shoulder dystocia, macrosomia and neonatal death.1–12 Others have also reported an increased risk for meconium aspiration.10 However, obesity is also associated with maternal comorbidities such as diabetes, hypertension, and preeclampsia, which are also associated with neonatal morbidity. It is unclear if people with obesity but without these comorbidities are at increased risk for neonatal morbidity.
Obesity is a chronic disease state characterized by chronic inflammation.13 Maternal obesity impacts neonatal immune system development and long-term outcomes of children born to obese women include increased risks for cancer as well as for chronic inflammatory diseases such as cardiovascular disease, diabetes, and asthma4. There is also evidence that abnormal neurodevelopment (e.g. lower mental development scores, higher rates of ADHD and possibly autism) is more common in the offspring of women with obesity.14–16
We sought to determine whether maternal obesity is a risk factor independent of pregestational diabetes and chronic hypertension for poor short term neonatal outcomes, and if that risk is increased with increasing maternal BMI, in a progressive fashion.
Materials and Methods
This is a secondary analysis of an observational study of a cohort of deliveries on randomly selected days at 25 hospitals from 2008-2011.17,18 People who arrived on the selected days and who delivered at 23 weeks’ gestation or above with a live fetus at presentation were included in the original reports. Patients with fetal deaths on presentation were not included, but if the fetal death occurred following presentation, the pregnant person and their data were included in the study. Maternal and neonatal charts were reviewed by trained and certified abstractors. Demographic data as well as detailed medical and obstetric histories, intrapartum and postpartum care, and obstetric and neonatal outcomes were collected. Maternal data were collected until hospital discharge and neonatal data were collected until discharge or 120 days of life, whichever came first. Details of the study design have been previously published.17,18
The current analysis includes gravidas with singleton deliveries between 24 and 42 weeks’ gestation. Maternal BMI was calculated using maternal height and most recent weight prior to delivery, as prepregnancy weight was not available for most subjects. The reference group (REF) was defined as persons with a BMI of 18.5-29.9 kg/m2, obese (OB) as persons with a BMI of 30-39.9 kg/m2, morbidly obese (MO) as persons with a BMI of 40-49.9 kg/m2, and super morbidly obese (SMO) as persons with a BMI ≥ 50 kg/m2. Normal and overweight people were combined in the reference group due to the high frequency of overweight people in the United States. Preterm delivery was analyzed as delivery < 37 weeks, < 32 weeks and < 28 weeks’ gestation. We defined a composite outcome of neonatal morbidity that included fetal or neonatal death, hypoxic ischemic encephalopathy, respiratory distress syndrome, grade III-IV intraventricular hemorrhage, necrotizing enterocolitis, sepsis, birth injury, seizures, or ventilator use. Definitions and criteria for these outcomes have been fully described in previous publications.17,18
We used propensity scores to address significant differences in baseline characteristics. People in the three obesity groups were matched with those in the REF group based on propensity score using the baseline characteristics of age (≤ 19, 20-34, ≥ 35 years), race-ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, not documented), previous cesarean (yes/no), pre-existing diabetes (yes/no), chronic hypertension (yes/no), parity (nulliparous, multiparous), cigarette use (yes/no), and government insurance (yes/no). Individuals were assigned a propensity score based on the above baseline characteristics. For purposes of matching, people in the REF group were considered unexposed and all other groups were considered exposed. Those in the OB, MO and SMO groups were matched to the REF group in a 1:1 ratio where scores differed by no more than 0.01. Individuals were excluded if no match was found. Individuals in REF were also excluded if they were a duplicate match for an exposed individual.
We evaluated the relationship between maternal BMI and baseline demographics and neonatal complications using the Cochran-Armitage trend test, Monte-Carlo estimate for exact trend test, and the Jonckheere-Terpstra test. We used modified Poisson regression to compute unadjusted and adjusted relative risks (aRR) and 95% confidence intervals (CI) for the associations between BMI and the composite neonatal outcome. Because significant group differences remained after propensity score matching, multivariable models were initially adjusted for maternal age, pre-existing diabetes, chronic hypertension, and smoking. However, we retained only those covariates that were statistically significant in the final model. Preterm delivery < 37 weeks and the presence of maternal preeclampsia/eclampsia were included in the final model because of their known associations with neonatal outcomes. We considered a p-value of < 0.05 to be significant. We did not adjust for multiple comparisons. No imputation for missing data was performed. This study was approved by the institutional review board at each participating facility under a waiver of informed consent.
Results
Between March 2008 and February 2011, data were collected on 115,502 patients in 25 hospitals. After propensity score assignment, 52,162 patients with singleton deliveries between 24 and 42 weeks’ gestation (inclusive), a BMI > 18.5 kg/m2 based on most recent weight prior to delivery, and data on maternal complications were included in this analysis. (Figure 1) Of these, 26,081 (50%) were normal/overweight (REF), 21,704 (42%) were OB, 3787 (7%) were MO and 590 (1%) were SMO. Demographics are shown in Table 1, and reveal progressively increased frequencies of pregestational diabetes, chronic hypertension and cigarette use with increasing BMI, although the frequency in patients with OB was lower than that in the REF group. While alcohol and drug use among pregnancies affected by OB, MO, and SMO progressively increased, the frequencies in all three categories were lower than that in REF. In addition, OB were more likely to be Hispanic people while MO and SMO were more likely to be non-Hispanic Black people and to have had a prior cesarean delivery. Pregnancies affected by OB, MO and SMO were progressively less likely to have been conceived via ART. Although the trend in maternal age was statistically significant, the difference was not clinically significant. The frequency of preeclampsia/eclampsia and cesarean delivery also progressively increased with increasing BMI.
Figure 1.
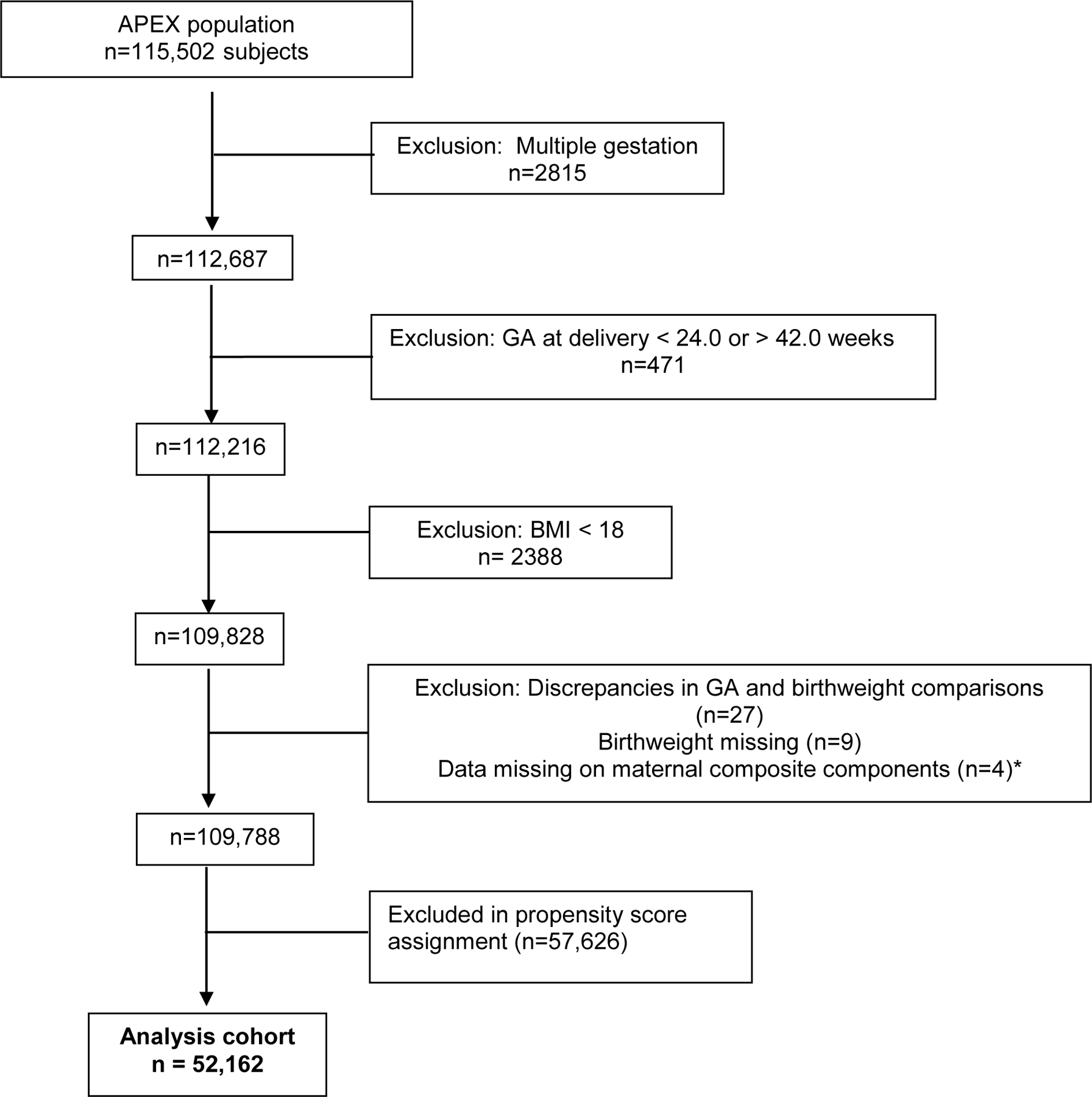
Study population
*Cohort includes maternal patients matched using propensity scores, and their neonates.
Table 1.
Patient demographics and obstetrical outcomes by BMI category at delivery
| Reference (REF) | Obese (OB) | Morbidly Obese (MO) | Super Morbidly Obese (SMO) | P-value for trend* | |
|---|---|---|---|---|---|
| N=26,081 | N=21,704 | N=3,787 | N=590 | ||
| Maternal Age (yrs) | 27.9 ± 6.3 | 28.1 ± 6.0 | 27.9 ± 5.9 | 27.9 ± 5.8 | <.001 |
| Age ≥ 35 yrs | 4356 (16.7) | 3258 (15.0) | 523 (13.8) | 83 (14.1) | <.001 |
| Nulliparous | 9944 (38.1) | 8419 (38.8) | 1456 (38.5) | 230 (39.0) | 0.24 |
| Race/ethnicity | <.001** | ||||
| NH White | 10657 (40.9) | 9383 (43.2) | 1556 (41.1) | 194(32.9) | |
| NH Black | 6449 (24.7) | 4701 (21.7) | 1252 (33.1) | 262 (44.4) | |
| NH Asian | 739 (2.8) | 668 (3.1) | 32 (0.8) | 6 (1.0) | |
| NH Native Hawaiian | 49 (0.2) | 48 (0.2) | 11 (0.3) | 2 (0.3) | |
| NH American Indian | 59 (0.2) | 38 (0.2) | 10 (0.3) | 1 (0.2) | |
| Hispanic | 6730 (25.8) | 5854 (27.0) | 799 (21.1) | 101 (17.1) | |
| ND | 1506 (5.8) | 1098 (5.1) | 148 (3.9) | 27 (4.6) | |
| Pregestational diabetes | 326 (1.3) | 182 (0.8) | 80 (2.1) | 26 (4.4) | .001 |
| Chronic hypertension | 602 (2.3) | 342 (1.6) | 176 (4.7) | 61 (10.3) | <.001 |
| Cigarette use | 3110 (11.9) | 1906 (8.8) | 470 (12.4) | 76 (12.9) | <.001 |
| Alcohol Use | 901 (3.5) | 605 (2.8) | 109 (2.9) | 18 (3.1) | <.001 |
| Drug Use | 1250 (4.8) | 637 (2.9) | 139 (3.7) | 25 (4.2) | <.001 |
| Government Insurance | 11397 (43.7) | 8542 (39.4) | 1880 (49.6) | 347 (58.8) | 0.05 |
| Preeclampsia/eclampsia | 1055 (4.1) | 1362 (6.3) | 445 (11.8) | 95 (16.1) | < .001 |
| Cesarean delivery | 7197 (27.6) | 7191 (33.1) | 1711 (45.2) | 311 (52.7) | < .001 |
Data presented are mean ± SD or n (%)
Cochran-Armitage or Jonckheere-Terpstra tests for trend.
NH Black versus all other race/ethnicities combined.
NH=non-Hispanic; ND= not documented; ART=assisted reproductive technology
Missing: alcohol use (51), drug use (74), ART (25)
The frequency of preterm delivery < 37 weeks was lowest in the OB group compared with the other groups. (Table 2) However, frequencies of preterm birth at less than 37 weeks and less than 28 weeks progressively increased as BMI weight group increased from OB to SMO. There was a significant trend towards higher birth weights across BMI groups, and babies born to pregnancies affected by OB, MO and SMO were more likely to be > 4000gm at delivery (LGA) and to have an anomaly, compared with babies born to REF pregnant people.
Table 2.
Neonatal Characteristics by Maternal BMI
| Reference (REF) | Obese (OB) | Morbidly Obese (MO) | Super Morbidly Obese (SMO) | P value for trend* | |
|---|---|---|---|---|---|
| N=26,081 | N=21,704 | N=3,787 | N=590 | ||
| GA at delivery (wks) | 39.1 (38.3-40.0) | 39.3 (38.6-40.1) | 39.1 (38.1-40.1) | 39.1 (38.0-40.0) | <.001 |
| GA < 37 wks | 3062 (11.7) | 1889 (8.7) | 458 (12.1) | 73 (12.4) | <.001 |
| GA < 32 wks | 649 (2.5) | 291 (1.3) | 101 (2.7) | 14 (2.4) | <.001 |
| GA < 28 wks | 242 (0.9) | 99 (0.5) | 42 (1.1) | 7 (1.2) | 0.03 |
| BW (gm) | 3212 (2887-3522) | 3390 (3064-3721) | 3430 (3058-3785) | 3445 (3055-3850) | <.001 |
| BW > 4000 gm | 1190 (4.6) | 2290 (10.6) | 521 (13.8) | 96 (16.3) | <.001 |
| NICU admit | 3331 (12.8) | 2624 (12.1) | 592 (15.6) | 94 (15.9) | 0.008 |
| LOS > 5 days | 2146 (8.2) | 1420 (6.6) | 363 (9.6) | 55 (9.4) | 0.14 |
| Anomaly | 731 (2.8) | 628 (2.9) | 140 (3.7) | 18 (3.1) | 0.02 |
Data presented are n (%) or Median (IQR)
Jonckheere-Terpstra test for continuous variables and Cochran-Armitage test for categorical variables
GA = gestational age, wks = weeks, BW = birth weight, gm = grams, NICU = neonatal intensive care unit, LOS = length of stay
Missing: Birthweight (7); NICU admission (11); length of stay (61); anomaly (3)
A total of 2,103 (4.0%) neonates developed the primary outcome, including 4.1% in the REF group, 3.6% in the OB group, 5.9% in the MO group and 5.6% in the SMO group. Neonates born to pregnant people in the MO group had a 33% increased risk of composite neonatal morbidity compared with those born to pregnant people in the REF group (aRR 1.33; 95% CI [1.17-1.52]). (Table 3) The risk of composite neonatal morbidity was not significantly increased in the other BMI groups.
Table 3.
Neonatal Composite Outcome by Maternal BMI
| Reference (REF) | Obese (OB) | Morbidly Obese (MO) | Super Morbidly Obese (SMO) | P value for trend* | |
|---|---|---|---|---|---|
| N=26,081 | N=21,704 | N=3,787 | N=590 | ||
| Composite morbidity | 1057 (4.1) | 790 (3.6) | 223 (5.9) | 33 (5.6) | 0.006 |
| Unadj RR (95%CI) | - | 0.90 (0.82-0.98) | 1.45 (1.26-1.68) | 1.38 (0.98-1.95) | |
| aRR (95%CI) ‡ | - | 1.05 (0.97-1.14) | 1.33 (1.17-1.52) | 1.18 (0.86-1.64) | |
| Birth injury | 67 (0.3) | 94 (0.4) | 35 (0.9) | 4 (0.7) | <0.001** |
| Death | 87 (0.3) | 45 (0.2) | 21 (0.6) | 1 (0.2) | 0.86** |
| HIE | 128 (0.5) | 86 (0.4) | 28 (0.7) | 7 (1.2) | 0.18** |
| RDS | 571 (2.2) | 416 (1.9) | 98 (2.6) | 14 (2.4) | 1.00 |
| IVH Grade 3 or 4 | 38 (0.2) | 21 (0.1) | 8 (0.2) | 2 (0.3) | 0.79** |
| NEC | 73 (0.3) | 45 (0.2) | 13 (0.3) | 3 (0.5) | 1.00** |
| Seizures | 30 (0.1) | 31 (0.1) | 6 (0.2) | 1 (0.2) | 0.31** |
| Sepsis | 10 (0.04) | 10 (0.1) | 3 (0.1) | 1 (0.2) | 0.17** |
| Ventilator use | 637 (2.4) | 410 (1.9) | 116 (3.1) | 15 (2.5) | 0.54 |
Composite morbidity includes birth injury, fetal or neonatal death, hypoxic ischemic encephalopathy, respiratory distress syndrome, intraventricular hemorrhage grades 3 or 4, necrotizing enterocolitis, seizures, sepsis or ventilator use.
Adjusted for pre-existing diabetes, preterm delivery and pre-eclampsia or eclampsia.
Data presented for composite morbidity and its components are n (%)
Cochran-Armitage test
Monte-Carlo estimate for exact trend.
Death=fetal or neonatal, HIE = hypoxic ischemic encephalopathy, RDS = respiratory distress syndrome, IVH = intraventricular hemorrhage, NEC = necrotizing enterocolitis
Missing: death (22); HIE (142); RDS (15); IVH (22); NEC (14), seizure (14), sepsis (1), and ventilator use (15); missing data were evenly distributed across BMI categories
Discussion
Principal Findings
Serious composite neonatal morbidity was increased in patients with MO compared with normal/overweight patients, adjusting for maternal diabetes, preeclampsia/eclampsia, and preterm delivery. There was no significant progressive increase in composite neonatal morbidity with increasing maternal BMI.
Results
Kim and colleagues reported that a composite of neonatal morbidity was increased with increasing BMI at delivery, reaching 32% at a BMI of ≥ 50kg/m2.19 The final adjusted model included BMI category, chronic hypertension, and preeclampsia. However, the adjusted odds ratio was not significantly higher except in the group with a BMI of ≥ 60kg/m2. Their definition of serious morbidity included low Apgar scores, hypoglycemia, NICU admission and length of stay > 5 days, in addition to RDS, sepsis and death. A significant trend towards composite neonatal morbidity with class of obesity was also reported by Marshall and colleagues, reaching 12.9% in those with a BMI of ≥ 50kg/m2. 20 Their analysis was limited to term infants, without congenital anomalies, and morbidity was defined as low Apgar scores, birth trauma, neonatal infection, neonatal hypoglycemia, respiratory distress syndrome, neonatal seizures, neonatal length of stay > 5 days, and/or meconium aspiration. Maternal BMI was based on self-reported prepregnancy weight, and pregnant people with diabetes (pre-gestational and gestational) and chronic hypertension were excluded.
The finding of an increase in large for gestational age infants and in congenital anomalies confirms work by other authors.5–7,9–12 The association between obesity and preterm birth is less clear. Prior authors have reported an increased risk for both medically indicated and spontaneous preterm delivery in women with obesity,5,21while others have reported decreases in the rates of preterm birth in women with obesity.21–23 Others have found no significant difference in preterm birth rates, particularly in those with milder degrees of obesity.5,12 We also found a decreased frequency of preterm birth at < 37, < 32 weeks and < 28 weeks in the OB group, with a significant trend towards an increasing occurrence of preterm birth at < 37 and < 28 weeks, as BMI increased.
Clinical Implications
After controlling for chronic hypertension and pre-gestational diabetes, both comorbidties of high BMI and precursors for neonatal morbidity, a progressive increase in composite neonatal morbidity with increasing maternal BMI at delivery was not seen in this large and diverse patient population.
Research Implications
The pattern of short-term neonatal morbidity in our cohort suggests that factors other than obesity and the co-morbidities of diabetes, preeclampsia/eclampsia and preterm birth play significant roles. These additional factors remain to be elucidated.
Strengths and Limitations
The strengths of our study include the large and diverse population studied, comprising over 110,000 pregnant people across the United States, delivering at both academic and community hospitals. This study also includes a large number of pregnant people affected by super morbid obesity (SMO; BMI ≥ 50 kg/m2). This allowed us to evaluate the progression of risk with increasing BMI, but may not have the power to detect small differences among the BMI groups. We also controlled for the presence of chronic hypertension and diabetes, which have both been independently associated with neonatal morbidity.
Due to the retrospective nature of this secondary analysis, we were limited by the data that had already been collected. Embryonic losses and fetal deaths prior to presentation were not recorded in the database, so pregnancy loss rates are underestimated and cannot be addressed by this analysis. Neonatal follow up was limited to date of discharge or 120 days, and thus our results may also underestimate the rates of congenital anomalies and neonatal death. Because the prenatal diagnosis of anomalies in persons with obesity may be limited, this may also lead to underestimates of the rates of congenital anomalies.24
Conclusions
Our study examined the relationship between BMI at delivery and short-term neonatal outcomes. Although obesity at the time of delivery was significantly associated with composite neonatal morbidity only in the MO group, obesity prior to pregnancy and early in pregnancy appears to be a predictor of poor neonatal outcomes.7,9–12,24–26 Although initial reports are not uniformly encouraging, attaining a normal weight prior to pregnancy may improve short term neonatal outcomes, while also reducing comorbidities such as hypertension and diabetes.27–29
Supplementary Material
AJOG at a Glance:
Why was the study conducted? To evaluate whether a composite of neonatal mortality and short-term morbidity increases as maternal BMI at delivery increases.
- What are the key findings?
- Maternal comorbidities, including chronic hypertension, pre-gestational diabetes, and cesarean delivery are more frequent in higher BMI categories.
- Compared with the reference group (BMI 18.5-29.9 kg/m2), only neonates born to pregnant people with a BMI of 40-49.9 kg/m2 were at increased risk for composite morbidity.
What does this study add to what is already known? After adjusting for other comorbidities, such as chronic hypertension, diabetes, preeclampsia/eclampsia and preterm birth, increasing maternal BMI is not associated with an increase in composite neonatal morbidity.
Acknowledgments:
The authors thank William A. Grobman, M.D., M.B.A., Elizabeth Thom, Ph.D., Madeline M. Rice, Ph.D., Brian M. Mercer, M.D. and Catherine Y. Spong, M.D. for protocol development and oversight.
Funding:
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD21410, HD27869, HD27915, HD27917, HD34116, HD34208, U10 HD36801, HD40500, HD40512, HD40544, HD40545, HD40560, HD40485, HD53097, HD53118] and the National Center for Research Resources [UL1 RR024989; 5UL1 RR025764]. Comments and views of the authors do not necessarily represent views of the NIH.
Appendix
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
Northwestern University, Chicago, IL – W. Grobman, G. Mallett, M. Ramos-Brinson, A. Roy, L. Stein, P. Campbell, C. Collins, N. Jackson, J. Senka (NorthShore University HealthSystem), K. Paychek (NorthShore University HealthSystem), A. Peaceman Columbia University, New York, NY – M. Talucci, M. Zylfijaj, Z. Reid (Drexel U.), R. Leed (Drexel U.), J. Benson (Christiana H.), S. Forester (Christiana H.), C. Kitto (Christiana H.), S. Davis (St. Peter’s UH.), M. Falk (St. Peter’s UH.), C. Perez (St. Peter’s UH)
University of Utah Health Sciences Center, Salt Lake City, UT – K. Hill, A. Sowles, J. Postma (LDS Hospital), S. Alexander (LDS Hospital), G. Andersen (LDS Hospital), V. Scott (McKay-Dee), V. Morby (McKay-Dee), K. Jolley (UVRMC), J. Miller (UVRMC), B. Berg (UVRMC)
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Dorman, J. Mitchell, E. Kaluta, K. Clark (WakeMed), K. Spicer (WakeMed), S. Timlin (Rex), K. Wilson (Rex)
University of Texas Southwestern Medical Center, Dallas, TX – L. Moseley, K. Leveno (deceased), M. Santillan, J. Price, K. Buentipo, V. Bludau, T. Thomas, L. Fay, C. Melton, J. Kingsbery, R. Benezue
University of Pittsburgh, Pittsburgh, PA – H. Simhan, M. Bickus, D. Fischer, T. Kamon (deceased), D. DeAngelis
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH – B. Mercer, C. Milluzzi, W. Dalton, T. Dotson, P. McDonald, C. Brezine, A. McGrail
The Ohio State University, Columbus, OH – C. Latimer, L. Guzzo (St. Ann’s), F. Johnson, L. Gerwig (St. Ann’s), S. Fyffe, D. Loux (St. Ann’s), S. Frantz, D. Cline, S. Wylie, J. Iams
University of Alabama at Birmingham, Birmingham, AL – M. Wallace, A. Northen, J. Grant, C. Colquitt, D. Rouse, W. Andrews
University of Texas Medical Branch, Galveston, TX – J. Moss, A. Salazar, A. Acosta, G. Hankins
Wayne State University, Detroit, MI – N. Hauff, L. Palmer, P. Lockhart, D. Driscoll, L. Wynn, C. Sudz, D. Dengate, C. Girard, S. Field
Brown University, Providence, RI – P. Breault, F. Smith, N. Annunziata, D. Allard, J. Silva, M. Gamage, J. Hunt, J. Tillinghast, N. Corcoran, M. Jimenez
The University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX– F. Ortiz, P. Givens, B. Rech, C. Moran, M. Hutchinson, Z. Spears, C. Carreno, B. Heaps, G. Zamora
Oregon Health & Science University, Portland, OR – J. Seguin, M. Rincon, J. Snyder, C. Farrar, E. Lairson, C. Bonino, W. Smith (Kaiser Permanente), K. Beach (Kaiser Permanente), S. Van Dyke (Kaiser Permanente), S. Butcher (Kaiser Permanente)
The George Washington University Biostatistics Center, Washington, D.C. – E. Thom, M. Rice, Y. Zhao, V. Momirova, R. Palugod, B. Reamer, M. Larsen
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
MFMU Network Steering Committee Chair (Medical University of South Carolina, Charleston, SC) – J. P. VanDorsten, M.D.
Footnotes
See Appendix for a list of other members of the NICHD MFMU Network
Presented in part at the 38th annual meeting of the Society for Maternal-Fetal Medicine, January 29-February 3, 2018, Dallas, TX.
The authors have no conflicts of interest or financial disclosures.
Condensation: Increasing maternal BMI is not associated with a progressive increase in composite neonatal morbidity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Statement:
Mara J. DINSMOOR, M.D., M.P.H. - conceptualization, methodology, investigation, writing (original, edit and review), visualization,
Lynda G. UGWU, Ph.D. - methodology, validation, formal analysis, investigation, writing (original, edit and review), visualization
Jennifer L. BAILIT, M.D., M.P.H. - methodology, investigation, writing (edit and review), supervision, project administration
Uma M. REDDY, M.D., M.P.H., Mona PRASAD, D.O., M.P.H. - investigation, writing (edit and review)
Ronald J. WAPNER, M.D., Michael W. VARNER, M.D., John M. THORP, Jr., M.D., Steve N. CARITIS, M.D., Alan T. N. TITA, M.D., Ph.D., George R. SAADE, M.D., Yoram SOROKIN, M.D., Dwight J. ROUSE, M.D., Sean C. BLACKWELL, M.D., Jorge E. TOLOSA, M.D., M.S.C.E. - methodology, investigation, resources, writing (edit and review), supervision, project administration, funding acquisition
Contributor Information
Mara J. DINSMOOR, Departments of Obstetrics and Gynecology of Northwestern University, Chicago, IL
Lynda G. UGWU, George Washington University Biostatistics Center, Washington, DC
Jennifer L. BAILIT, MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH
Uma M. REDDY, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
Ronald J. WAPNER, Columbia University, New York, NY
Michael W. VARNER, University of Utah Health Sciences Center, Salt Lake City, UT
John M. THORP, Jr., University of North Carolina at Chapel Hill, Chapel Hill, NC
Steve N. CARITIS, University of Pittsburgh, Pittsburgh, PA
Mona PRASAD, The Ohio State University, Columbus, OH
Alan T. N. TITA, University of Alabama at Birmingham, Birmingham, AL
George R. SAADE, University of Texas Medical Branch, Galveston, TX
Yoram SOROKIN, Wayne State University, Detroit, MI
Dwight J. ROUSE, Brown University, Providence, RI
Sean C. BLACKWELL, University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX
Jorge E. TOLOSA, Oregon Health & Science University, Portland, OR
References
- 1.Lim CC, Mahmood T. Obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol 2015;29:309–319. doi: 10.1016/j.bpobgyn.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 2.Mission JF, Marshall NE, Caughey AB Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv. 2013;68(5):389–399. doi: 10.1097/ogx.0b013e31828738ce [DOI] [PubMed] [Google Scholar]
- 3.Lashen H, Fear K, Sturdee DW Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod 2004;19(7):1644–1646. doi: 10.1093/humrep/deh277 [DOI] [PubMed] [Google Scholar]
- 4.Wilson RM, Messaoudi I. The impact of maternal obesity during pregnancy on offspring immunity. Mol Cell Endocrinol. 2015;418 Pt 2:134–142. doi: 10.1016/j.mce.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews: Obesity in pregnancy - a review of reviews. Obes Rev 2015;16:621–638. doi: 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- 6.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate–a population-based screening study. Am J Obstet Gynecol 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058 [DOI] [PubMed] [Google Scholar]
- 7.Gaillard R, Durmuş B, Hofman A, Mackenbach JP, Steegers EAP, Jaddoe VWV. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013;21:1046–1055. doi: 10.1002/oby.20088 [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536–1546. doi: 10.1001/jama.2014.2269 [DOI] [PubMed] [Google Scholar]
- 9.Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011;118(2, Part 1):305–312. doi: 10.1097/AOG.0b013e3182245d49 [DOI] [PubMed] [Google Scholar]
- 10.Cedergren M Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00 [DOI] [PubMed] [Google Scholar]
- 11.Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009;301(6):636. doi: 10.1001/jama.2009.113 [DOI] [PubMed] [Google Scholar]
- 12.Mantakas A, Farrell T. The influence of increasing BMI in nulliparous women on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2010;153(1):43–46. doi: 10.1016/j.ejogrb.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 14.van der Burg JW, Sen S, Chomitz VR, Seidell JC, Leviton A, Dammann O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr Res 2016;79:3–12. doi: 10.1038/pr.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mina TH, Lahti M, Drake AJ, et al. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr Res 2017;82:47–54. doi: 10.1038/pr.2017.43 [DOI] [PubMed] [Google Scholar]
- 16.Mina TH, Lahti M, Drake AJ, et al. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol Med 2017;47:353–362. doi: 10.1017/S0033291716002452 [DOI] [PubMed] [Google Scholar]
- 17.Bailit JL, Grobman WA, Rice MM, et al. Risk-adjusted models for adverse obstetric outcomes and variation in risk-adjusted outcomes across hospitals. Am J Obstet Gynecol 2013;209(5):446.e1-446.e30. doi: 10.1016/j.ajog.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobman WA, Bailit JL, Rice MM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX study. Am J Obstet Gynecol 2014;211(2):147.e1-147.e16. doi: 10.1016/j.ajog.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim T, Burn SC, Bangdiwala A, Pace S, Rauk P. Neonatal morbidity and maternal complication rates in women with a delivery body mass index of 60 or higher. Obstet Gynecol 2017;130(5):988–993. doi: 10.1097/AOG.0000000000002316 [DOI] [PubMed] [Google Scholar]
- 20.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol 2012;206:417.e1-417.e6. doi: 10.1016/j.ajog.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torloni MR, Betrán AP, Daher S, et al. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. J Matern Fetal Neonatal Med 2009;22(11):957–970. doi: 10.3109/14767050903042561 [DOI] [PubMed] [Google Scholar]
- 22.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord J Int Assoc Study Obes 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670 [DOI] [PubMed] [Google Scholar]
- 23.Korkmaz L, Baştuğ O, Kurtoğlu S. Maternal obesity and its short- and long-term maternal and infantile effects. J Clin Res Pediatr Endocrinol 2016;8(2):114–124. doi: 10.4274/jcrpe.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dashe JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses: Obstet Gynecol 2009;113(5):1001–1007. doi: 10.1097/AOG.0b013e3181a1d2f5 [DOI] [PubMed] [Google Scholar]
- 25.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of Adverse Pregnancy Outcomes by Prepregnancy Body Mass Index: A Population-Based Study to Inform Prepregnancy Weight Loss Counseling. Obstet Gynecol 2015;125(1):133–143. doi: 10.1097/AOG.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg TJ, Garbers S, Chavkin W, Chiasson MA. Prepregnancy weight and adverse perinatal outcomes in an ethnically diverse population. Obstet Gynecol 2003;102:1022–1027. [DOI] [PubMed] [Google Scholar]
- 27.Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014;348:g1285. doi: 10.1136/bmj.g1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodd JM, McPhee AJ, Turnbull D, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on neonatal health outcomes: the LIMIT randomised trial. BMC Med 2014;12:163. doi: 10.1186/s12916-014-0163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson K, Cnattingius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med 2015;372(9):814–824. doi: 10.1056/NEJMoa1405789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


