Abstract
Objective:
Cognitive-behavioral therapy (CBT) is considered a first-line treatment for obsessive-compulsive disorder (OCD) in pediatric and adult populations. Nevertheless, some patients show partial or null response. The identification of predictors of CBT response may improve clinical management of patients with OCD. Here, we aimed to identify structural magnetic resonance imaging (MRI) predictors of CBT response in two large series of adults and children with OCD from the worldwide ENIGMA-OCD consortium.
Method:
Data from 16 datasets from 13 international sites were included in the study. We assessed which variations in baseline cortical thickness, cortical surface area and subcortical volume predicted response to CBT (percentage of baseline to post-treatment symptom reduction) in two samples totaling 168 children/adolescent patients (age range 5-17.5) and 318 adult patients (age range 18-63) with OCD. Mixed linear models with random intercept were used to account for potential cross-site differences in imaging values.
Results:
Significant results were exclusively observed in the pediatric sample. Right prefrontal cortex thickness was positively associated with the percentage of CBT response. In a post-hoc analyses, we observed that the specific changes accounting for this relationship were a higher thickness of the frontal pole and the rostral middle frontal gyrus. We observed no significant effects of age, sex, or medication on our findings.
Conclusion:
Higher cortical thickness in specific right prefrontal cortex regions may be important for CBT response in children with OCD. Our findings suggest that the right prefrontal cortex plays a relevant role in the mechanisms of action of CBT in children.
Keywords: obsessive-compulsive disorder, neuroimaging, magnetic resonance imaging, cognitive-behavioral therapy, anxiety disorders
INTRODUCTION
Patients with obsessive-compulsive disorder (OCD) typically present with anxiety-inducing intrusive thoughts, images, or impulses (obsessions), and repetitive behaviors (compulsions). OCD has a lifetime prevalence of 2.3% in the general population1, and its chronic course leads to important socioeconomic costs2. Cognitive-behavioral therapy (CBT) is an effective treatment for anxiety-related disorders, and it is considered a first-line and well-established treatment for OCD in pediatric and adult populations3-5. Although response rates to CBT in patients with OCD (between 62% and 68%) are higher than those for placebo and other treatments6, there is still a significant number of patients showing partial or no response7. The identification of predictors of response to CBT is therefore of paramount importance to select the best candidates for this treatment and ultimately optimize the clinical management of patients. Magnetic resonance imaging (MRI) is currently widely available and provides information about brain function and structure using minimally invasive means; hence, it may help to identify brain circuits underpinning psychological processes relevant for CBT success.
To date, studies using structural and functional MRI to predict treatment response in OCD have been largely inconclusive. In functional studies, a wide variety of approaches have been employed, ranging from different methods for resting-state data analysis to task-based approaches using symptom provocation and other experimental paradigms. The variety of methods and the low statistical power of small-sampled studies have prevented a meaningful integration of findings and drawing clinically relevant conclusions8. Results of brain structural studies have also been heterogeneous, except for evidence that morphometric features in the prefrontal cortex may be useful predictors of treatment outcome. Hoexter et al.9 reported that larger pre-treatment gray matter volumes (derived from a voxel-based morphometric analysis) in the subgenual cingulate cortex predicted greater symptom improvement, while Fullana et al.10 found that lower cortical thickness in another part of the ventromedial prefrontal cortex (vmPFC), i.e., the rostral anterior cingulate cortex, predicted good response to CBT. Despite the apparent opposite direction of these findings, they may point to regulation by the vmPFC of subcortical activity (e.g., amygdala). In addition, smaller volume in other regulatory prefrontal regions, such as the dorsolateral prefrontal cortex (dlPFC), has also been related to poor CBT outcomes11.
Focusing on pediatric populations, a recent whole-brain study reported that thinner cortices in 9 fronto-parietal regions predicted clinical improvement after CBT12. Results in children should be interpreted differently from results in adults. In this population, it is important to consider maturation-related changes13,14. Moreover, in children, CBT has been shown to modulate the development of specific regions of the prefrontal cortex, such as the orbitofrontal cortex, where volume increases were reported after CBT15 and two years after treatment, especially in children between 8 and 12 years of age16.
The aim of this study was to identify structural MRI predictors of CBT response in adults and children with OCD. For this, we pooled data from multiple international sites participating in the worldwide ENIGMA-OCD consortium. This consortium provides the largest sample of patients with OCD with both imaging and clinical information (including response to CBT) available. Further, to aid in the interpretation of findings (i.e., to evaluate whether structural variations associated to CBT response did also differ from normative control values), we also compared regional brain measures of OCD patients with reference values from healthy controls (HC). We did not predict the specific location or the directionality of our findings, because previous research has reported both positive and negative correlations with CBT outcome involving different brain areas.
METHOD
Participants
We included in this analysis 16 datasets from 13 international neuroimaging institutes participating in the ENIGMA-OCD consortium (http://enigma.ini.usc.edu/ongoing/enigma-ocd-working-group/). In total, we analyzed clinical, sociodemographic and structural imaging data from 1057 subjects: 489 patients with OCD (diagnosed with DSM criteria) (168 children and adolescent subjects, <18 years of age; and 318 adult subjects) and 571 healthy control subjects (HCs) free from any psychiatric, neurological or major medical condition (286 children and adolescent subjects, and 285 adult subjects) (Table S1, available online). Patients with OCD underwent CBT after the baseline MRI scan, and were clinically evaluated before and after treatment.
All participants, or their parents or legal guardians, signed an informed consent approved by the ethical committees in clinical research of their corresponding institutions.
CBT characteristics and treatment response
From each site, we collected the following CBT variables: group or individual treatment, total number of sessions (as well as number of introductory/psychoeducational sessions, and of exposure/relapse-prevention sessions), duration of the therapy, frequency of sessions, and use of cognitive tasks or techniques. Treatment response was operationalized as the percentage of symptom reduction between baseline and post-treatment severity assessments:
We also split patient samples into patients with positive response to CBT and patients with non-positive response to CBT using a symptom improvement cut-off of ≥35%17.
Neuroimaging data acquisition and processing
High-resolution structural T1-weighted MRI brain scans were acquired at each site (Table S2, available online), and pre-processed locally in a harmonized way following a common pipeline, described elsewhere18,19. Briefly, images were parcellated using the fully automated and validated segmentation software FreeSurfer v5.320 following standardized ENIGMA protocols to harmonize analysis and quality control processes across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols/). For both hemispheres, thickness and surface areas were estimated from 68 (34 left and 34 right) cortical gray matter regions, which were parcellated based on the Desikan-Killiany atlas21. At this stage, the two whole-hemisphere measures were visually inspected and statistically evaluated for outliers. We also obtained the volume of seven subcortical regions of interest (bilaterally): nucleus accumbens, caudate, putamen, pallidum, amygdala, hippocampus, and thalamus.
Statistical analyses
In line with previous work of the ENIGMA-OCD consortium22, individual level subject data were pooled for one merged adult sample and another children/adolescent dataset.
To better characterize our samples, we first contrasted differences in age, sex, and imaging variables between patient groups and healthy controls. Next, within patient samples, we contrasted sociodemographic, clinical and CBT-related variables between treatment positive response and those who non-achieve a positive-response. In all these analyses, we used independent sample t tests for continuous variables, and chi-squared tests for categorical variables.
Imaging data were analyzed using a mega-analysis strategy with mixed-effects linear models with random intercept to account for cross-site differences. This approach provided lower standard errors and narrower confidence intervals in relation to meta-analytic approaches, while it also displayed better fit indices compared to linear regression methods22. We first assessed which sociodemographic, clinical and CBT-related variables were associated with CBT response, also using mixed linear models. The variables found to be associated with treatment outcome were subsequently used as nuisance covariates in all imaging analyses, along with total intracranial volume (TIV) for surface area and volume analyses. We also performed sensitivity analyses to explicitly evaluate the effects of age, sex, and medication (both previous and during CBT treatment) in our findings.
For cortical thickness and surface area analyses, cortical parcellations were grouped in 6 right/left regions (12 regions in total): sensorimotor strip, prefrontal cortex, temporal cortex, parietal cortex, occipital cortex, and limbic cortex (including the insular cortex). Subcortical volume analyses considered the 7 right/left subcortical regions described above (14 regions in total). These measurements were the focus of separate analyses, with a Bonferroni corrected threshold of P<0.00416 (0.05/12) for cortical thickness and surface area evaluations, and P<0.0036 (0.05/14) in subcortical volume analyses. All analyses were first performed considering treatment response as a quantitative variable (i.e., percentage of symptoms reduction) and then performed considering treatment response as a categorical variable (i.e., positive response vs. non-positive response). Effect sizes were presented as Pearson’s r for quantitative relationships and as Cohen’s d for analyses of treatment response categories, following the equations described in23.
RESULTS
Demographic, clinical and CBT-related characteristics
Demographic and clinical characteristics of the overall adult and children samples are detailed in Table 1, and site-specific information are detailed in Tables S3 and S4, available online.
TABLE 1.
Demographic (Age, Sex) And Clinical (Age of Onset, Illness Duration, Medication Users During Cognitive-Behavioral Therapy (CBT) Trial, Comorbidities, Baseline Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) Severity, Post-CBT CY-BOCS, % of Patients With Positive Response) Data From Pediatric Samples
| Pediatric sample | Adult sample | |||
|---|---|---|---|---|
| HC | OCD | HC | OCD | |
| Male subjects (%) | 49.3 | 55.4 | 42.8 | 49.4 |
| Age, years (mean ± SD) | 13.41 ± 2.74 | 13.10 ± 2.81 | 30.5 ± 9.55 | 31.63 ± 9.65 |
| OCD | OCD | |||
| Age of onset, years (mean ± SD) | 10.43 ± 3.39 | 16.5 ± 8.60 | ||
| Illness duration, years (mean ± SD) | 3.10 ± 2.54 | 15.5 ± 10.85 | ||
| Dimensions, yes (%) | ||||
| Agg. and check | 68.5 | 75.7 | ||
| Cleaning | 64.3 | 67.3 | ||
| Sex. and rel. | 29.2 | 50.3 | ||
| Hoarding | 31.5 | 26.4 | ||
| Ordering | 64.9 | 57.9 | ||
| Medication us at MRI time, yes (%) | 20.8 | 65.7 | ||
| Medication us during CBT, yes (%) | 30.4 | 66.7 | ||
| SSRI | 25.6 | SSRI | 37.7 | |
| Combination | 1.2 | Tricyclics | 2.8 | |
| Other | 3.6 | Combination | 15.1 | |
| Other | 20.4 | |||
| Comorbidities, yes (%) | ||||
| Hx. of anxiety | 30.4 | 31.8 | ||
| Hx. of depression | 6.5 | 36.5 | ||
| Cur. anxiety | 42.3 | 30.2 | ||
| Cur. depression | 7.7 | 23 | ||
| Baseline C/YBOCS severity (mean ± SD) | 24.83 ± 5.21 | 26.29 ± 4.58 | ||
| Post-CBT C/YBOCS severity (mean ± SD) | 14.32 ± 8.67 | 15.45 ± 6.96 | ||
| % C/YBOCS reduction (mean ± SD) / % Patients with positive response | 43.19 ± 32.43/ 56.5 | 40.79 ± 25.29/ 61.6 | ||
Note: Agg and check = Aggressive and checking; Cur = current; Hx = history; OCD = obsessive-compulsive disorder; MRI = magnetic resonance imaging; Sex and rel = sexual and religious; SSRI = selective serotonin reuptake inhibitors.
We did not observe differences between patients with OCD and HCs in mean age or sex distribution, neither in the child/adolescent nor in the adult sample.
CBT-related variables are presented in Table 2. Our sample of adult patients included treatment protocols that were administered individually, in a group, or using an intensive protocol, which can involve both individual and group sessions concentrated in a few days. In children, a large majority of patients were administered individual CBT. It is also important to highlight that cognitive strategies (including techniques such as cognitive restructuring, thought records or behavioral experiments), in addition to exposure and response prevention, were used both in adult and children/adolescent patients, although their use in adult patients was not as common as it was in children/adolescent patients.
TABLE 2.
Cognitive-Behavioral Therapy (CBT) Characteristics in Pediatric and Adult Sample
| Previous CBT trial, yes (%) |
Total n° of CBT sessions (mean ± SD) |
Duration of CBT, weeks (mean ± SD) |
N° psychoeducational/ introductory sessions (mean ± SD) |
N° exposure/relapse prevention sessions (mean ± SD) |
Type CBT: Group/individual/ combination (intensive) (%) |
Use of cognitive techniquesa, yes (%) |
Homework tasks, yes (%) |
|
|---|---|---|---|---|---|---|---|---|
| Pediatric sample | 36.3 | 14.61 ± 3.25 | 16.77 ± 6.43 | 2.35 ± 2.62 | 12.96 ± 3.19 | Group: 14.3 / Individual: 85.1 | 94.0 | 98.8 |
| Adult sample | 5.3 | 15.45 ± 6.33 | 12.15 ± 9.84 | 2.26 ± 1.36 | 12.67 ± 6.23 | Group: 28 / Individual: 15.4 / Intensive: 55.7 | 64.5 | 70.8 |
Note: n° = number
They include cognitive restructuring, thought records, behavioral experiments and others.
Brain structural differences between patients with OCD and Healthy Controls
These results are presented in Supplement 1 and Tables S5-S10, available online.
Clinical and CBT-related variables in patients with positive response and in those with non-positive response.
After CBT completion, 61.6% of adult patients and 56.5% of pediatric patients showed a symptom reduction ≥35%. Tables S11 and S12 (available online) present the main clinical and CBT-related features in these groups for the pediatric and the adult samples, respectively.
Mixed Linear models: Prediction of CBT response
Pediatric sample
The sociodemographic and baseline and CBT clinical variables associated with better CBT response were higher age at disease onset, lower current age, and no previous medication use (Table S13, available online). These variables were therefore controlled for in imaging analyses.
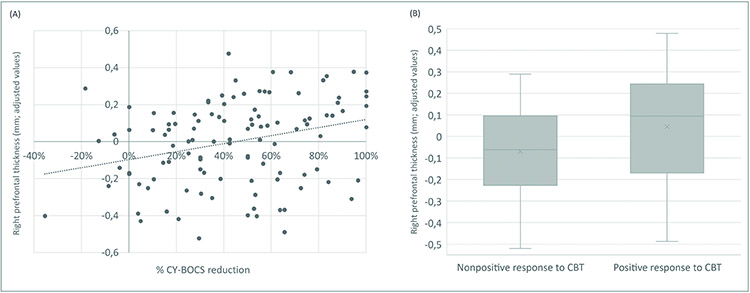
At the cortical level, we observed that right prefrontal cortex thickness was positively associated with the percentage of CBT response (t=3.25 [r=0.25]; P=0.002, see Figure 1 (A) and Table 3). This same measurement was also significantly associated with the dichotomous categorical variable treatment response (positive response vs. non-positive response) (t=3.05 [d=0.48]; P= 0.003, see Figure 1 (B)). No further results were observed at the cortical level. At the subcortical level, we observed two trend level results: left hippocampal volume was positively associated and left accumbens volume negatively associated with CBT response (t=2.84 [r=0.22] and t=−2.85 [r=−0.22], respectively; P=0.006). These results are reported in Tables S14 and S15, available online. These results held when we additionally controlled for sex and medication use during CBT (current age and previous medication use were already included in the model).
FIGURE 1.
Association Between Right Prefrontal Cortex (r-PFC) Thickness and Cognitive-Behavioral Therapy (CBT) Response in Pediatric Patients With Obsessive-Compulsive Disorder (OCD)
Note: (A) Scatter plot depicting the positive correlation between cortical thickness of the right prefrontal cortex (r-PFC) and exposure therapy outcome as measured by the percentage of CY-BOCS reduction. (B) Box plot depicting r-PFC values in patients with positive response to CBT (≥ 35% symptom reduction), and patients with a non-positive response to CBT. Patients with positive response showed a significantly thicker r-PFC in comparison to those with non-positive response.
TABLE 3.
Cortical Thickness Predictors of Clinical Response Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS decrease) in Pediatric Patients (n= 168)
| Standard error |
t | Sig. | 95% confidence interval | ||
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Left prefrontal | 15.507 | 1.530 | 0.134 | −7.637 | 55.089 |
| Right prefrontal | 11.483 | 3.249 | 0.002 a | 14.546 | 60.065 |
| Left temporal | 12.556 | 0.423 | 0.674 | −19.753 | 30.365 |
| Right temporal | 13.194 | −0.278 | 0.782 | −30.059 | 22.281 |
| Left central | 18.579 | 0.631 | 0.531 | −25.570 | 49.002 |
| Right central | 17.703 | 0.857 | 0.394 | −20.074 | 50.415 |
| Left limbic | 16.049 | −0.681 | 0.497 | −42.813 | 20.948 |
| Right limbic | 15.437 | 0.130 | 0.897 | −28.635 | 32.651 |
| Left parietal | 19.291 | 1.742 | 0.086 | −4.832 | 72.056 |
| Right parietal | 17.295 | 0.604 | 0.549 | −24.364 | 45.272 |
| Left occipital | 24.860 | −0.869 | 0.387 | −70.875 | 27.668 |
| Right occipital | 25.179 | 0.756 | 0.451 | −30.895 | 68.966 |
Note:
Significant after Bonferroni correction for multiple testing
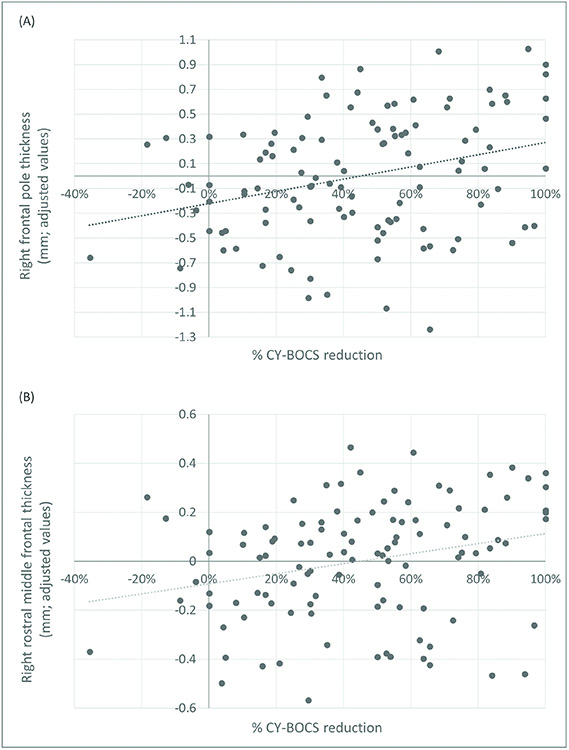
In a post-hoc analysis, we explored which right prefrontal subdivisions were responsible for the significant association between right prefrontal thickness and CBT response. We observed that right frontal pole (t=3.58 [r=0.27]; P=0.001) and right rostral middle gyrus (t=3.04 [r=0.23]; P=0.003) thickness were positively associated with the percentage of treatment response. These results held when we additionally controlled for sex and medication use during CBT, and are presented in Figure 2A/B and Table S16, available online. We also observed that the right superior frontal gyrus (t=2.91 [r=0.22]; P=0.004) was associated with CBT response, but this result was no longer significant when controlling for sex and medication use during CBT.
FIGURE 2.
Association Between Right Frontal Pole And Rostral Middle Frontal Cortex Thickness With Cognitive-Behavioral Therapy (CBT) Response In Pediatric Patients With Obsessive-Compulsive Disorder (OCD)
Note: (A) Scatter plot depicting the positive correlation between right frontal pole thickness and exposure therapy outcome as measured by the percentage of CY-BOCS reduction. (B) Scatter plot depicting the positive correlation between right rostral middle frontal gyrus thickness and exposure therapy outcome as measured by the percentage of CY-BOCS reduction.
These results were largely confirmed when repeating the analyses excluding OCD subjects from centers contributing with less than 10 participants (n=8, 1 center). Right prefrontal cortex findings remained significant (t=3.25 [r=0.25]; P=0.002 and t=3.74 [d=0.61]; P<0.0005 for quantitative and categorical analyses, respectively). Within the right prefrontal cortex, frontal pole findings also remained significant (t=3.38 [r=0.26]; P=0.001), but results from the rostral middle frontal gyrus were only significant at the trend level (t=2.75 [r=0.22]; P=0.007). Previous trend level findings involving left hippocampus and accumbens volumes also diminished their significance. These results are presented in Table S17, S18, S19 and S20, available online.
To better evaluate the potential effects of age in our findings, we split the pediatric sample into two groups: children (≤12 years old) and adolescents (>12 years old), akin to what has been done in previous studies16. Results did not differ between these age groups, and, indeed, we observed no regions significantly predicting CBT response in any of the groups because of the smaller statistical power.
In a final post-hoc analysis, we observed that none of the regional brain measures associated with CBT response significantly differed between patients with OCD and healthy controls.
Adult Sample
The sociodemographic and baseline clinical and CBT variables significantly associated with the percentage of symptom reduction after CBT were age of onset (the higher the age, the better the response) and type of CBT: intensive treatment, in comparison to individual and group treatment, was associated with a better response (see Table S21, available online). These variables were therefore controlled for in imaging analyses. None of the imaging assessments was associated with response to CBT after Bonferroni correction for multiple comparisons (see Tables S22, S23 and S24, available online), and these results did not change after controlling for age, sex, and use of medication prior to or during CBT. To evaluate whether the pattern of results in the adult sample differed between those with early vs. late age of onset, this sample was split in two groups (<≥18 years), and no differences regarding the association between morphometric variables and CBT response were observed.
DISCUSSION
This is the largest study evaluating the anatomical predictors of CBT response in OCD. We explored two large multicenter pooled datasets of adults and children/adolescents with OCD, and we identified significant structural brain characteristics associated with positive CBT outcomes in the pediatric sample. Specifically, and controlling for all the sociodemographic and clinical variables also associated with CBT response, we observed that larger right prefrontal thickness predicted greater symptom improvement after CBT in children/adolescents. Moreover, within this region, we were able to more precisely locate our findings to the frontal pole and the rostral middle frontal gyrus. Our results, therefore, indicate that, in pediatric populations with OCD, responses to psychological interventions may be predicted by anatomical variability in specific brain areas. In adult populations, however, factors other than brain anatomy may more strongly moderate CBT response.
The positive association between right frontopolar cortex thickness and CBT response may be interpreted in relation to previous findings. Electrical stimulation of this region has been recently shown to increase safety learning in OCD, which may critically boost CBT effects24. Moreover, connectivity of the frontopolar cortex with emotion processing regions, such as the bed nucleus of the stria terminalis (BNST), is increased in adults with OCD25, and this can be interpreted as resulting from the control exerted by the frontopolar cortex over abnormally increased BNST activations underpinning sustained anxiety responses26. Partially concurring with these results, graph-based approaches have shown that functional connectivity of the frontopolar cortex with distant structures is disrupted in pediatric OCD samples27. Furthermore, in depression samples, the frontopolar cortex has been linked to specific cognitive processes targeted by CBT, such as negative cognition in ‘future thinking’28. CBT has indeed been shown to modulate frontopolar activity in these clinical samples29, which is germane to the present findings considering the highly prevalent use of cognitive techniques in our sample of children and adolescents with OCD, even though the findings from these two last studies were reported in adult samples.
The other subdivision of the right prefrontal cortex associated with the extent of CBT response was the rostral middle frontal gyrus. This finding resonates with previous studies on the neuroimaging correlates of CBT outcomes in patients with OCD and other psychiatric disorders, which have, however, emphasized different aspects of middle frontal gyrus function. First, this region, together with parietal lobe structures, is part of the dorsal attentional network, whose intrinsic connectivity has been shown to increase after CBT in anxiety disorders in parallel with clinical improvement30. Similarly, in pediatric OCD, increased connectivity of this fronto-parietal network has been shown to predict better CBT response31. These results may be framed within the attentional control theory, which suggests that there exists an inverse relationship between anxiety and attentional control32. Nevertheless, findings from the evaluation of the structural features of this fronto-parietal network have been opposite to our results, and thinner fronto-parietal cortices have been associated with CBT success12. Although this heterogeneity in findings motivates further research, we propose that the methodological differences between the studies, such as the different number of individuals assessed in each study, may partially account for these discrepancies.
Other studies have emphasized other roles of the rostral middle frontal gyrus beyond its participation in attentional networks. This region roughly corresponds to Brodmann areas 9 and 46, and may therefore be labeled as dorsolateral prefrontal cortex, the core area of executive functioning that optimizes information processing and response to external stimuli33. Van der Straten et al.34, for instance, observed excessive middle frontal gyrus activation in a pediatric OCD sample during an executive planning task – a finding that normalized after a CBT trial. In contrast with other areas involved in executive function, such as the anterior cingulate cortex or the superior frontal gyrus, the role of the middle frontal gyrus is probably more related to the processing and regulation of emotional information35. This is important because although regulation of amygdala activity in the context of CBT response in OCD has been related to ventromedial prefrontal cortex function12,36, other studies have shown that the connectivity between the right middle frontal gyrus and the left basolateral amygdala is increased in patients with OCD37. These notions dovetail with the conceptualization of CBT as an intervention aimed at modulating emotion regulation circuits and processes38, in which executive functions play a nodal role39. Our findings of a positive association between right middle frontal gyrus thickness and CBT response could be related to baseline variability in the emotion regulation capacity of children/adolescents. Interestingly, gray matter volume in the right middle frontal gyrus has been observed to increase after group CBT in young students with mild depressive symptoms40.
None of the regions described here to be associated with CBT response significantly differed from controls in our pediatric sample. This suggests that interventions such as CBT do not have to necessarily act upon regions with alleged baseline dysfunction (i.e., restoring their function), as could be expected if they showed abnormal brain volumes. Conversely, CBT may act through compensatory mechanisms by recruiting brain systems (e.g., attentional, top-down regulatory or mnemonic systems) which function may be at least partially independent from disorder’s nuclear symptoms. Improving functioning of these brain systems may result in symptom improvement. These are indeed the principles of cognitive remediation therapy, which, despite the recent negative findings regarding its usefulness as main therapy in treatment protocols for OCD41, might help optimizing the success of CBT treatment strategies through modulation of relevant brain circuits.
Variability in the neural underpinnings of these systems, moreover, should be associated with treatment success and disorder evolution, although not necessarily with disorder severity, or the identification of OCD cases or subjects at increased risk for OCD by means of biologically-informed approaches. An additional implication of this is that regions linked to CBT response may be shared across different disorders, providing a neurobiological explanation to the trans-diagnostic efficacy of CBT42.
In contrast to what was observed in relation to prediction of CBT response, we observed more regions significantly differing between patients with OCD and controls in the adult than in the pediatric sample. Thus, while in children we only observed a thinner left parietal cortex, in adults, thinner cortices were observed in the bilateral parietal and the right prefrontal cortices. Moreover, in adults we observed larger left pallidal and smaller thalamic volumes. These findings are in overall agreement with previous studies18,19,43, and suggest that maturation processes may play a role in the expression of morphometric brain alterations in OCD. Although the direct comparison between pediatric and adult samples is beyond the scope of this study, it is important to discuss why associations with CBT response have been exclusively observed in children. While it is true that initial reports described morphometric changes associated to response to CBT in adults with OCD9,10, more recent research has shown that region-specific prefrontal changes in relation to CBT response are probably more likely to be observed in pediatric samples12, whereas in adult patients, structural changes predicting CBT effects have been reported at the whole-brain network level, also encompassing posterior brain regions44. Despite findings are heterogenous and therefore difficult to integrate with our present results, overall, it seems plausible that region-specific associations with CBT response may be more easily observed in developing brains, where differences in the degree of maturation of particular brain regions may relate to complex behavioral features, than in adult brains, where correlates of individual differences in complex behaviors are better captured by multimodal task-based functional measurements45. In any case, there may be other factors potentially related to the lack of findings in the adult sample, such as the higher variability observed in this sample in the methods of CBT delivery (individual, group and intensive) and other related variables (i.e., use of cognitive techniques and homework tasks).
Effect sizes of our findings fell between the small and medium labels. Although this may not be rare when associating very specific neuroimaging findings with complex behavioral and clinical data, a word of caution should be expressed regarding the immediate use of neuroimaging variables as biomarkers of treatment response. A further limitation of this study is that although multicenter studies of legacy data allow pooling together a large amount of information, this is typically at the cost of significant heterogeneity across the different data sources. In this study we have across-site variability both in technical aspects, such as those related to MRI acquisition, but also in some other aspects, such as patients’ characterization, the criteria for CBT recommendation, and its methodology of application. Although our statistical approach (linear mixed models with random intercept) allowed us to partially control for these sources of variability and, on the pediatric sample, we did not observe significant effects on CBT response from any of the treatment related variables, this issue remains central to the interpretation of our findings. The heterogeneity in the methodology of CBT application may explain, for instance, the lower response rate observed in our study in comparison to the results of clinical trials3,46. The control of these different source of variability could ideally be accomplished in studies exploring large series of individuals with the same MRI scanner and using standardized protocols for patients’ management, although such study is probably difficult to implement in practice.
In summary, in this study, pooling together data from 13 international centers, we identified significant structural predictors of CBT response in children/adolescents with OCD. These results should serve to better define the brain circuits relevant for CBT success, which modulation may potentially boost the effectiveness of this psychological intervention. Moreover, our findings suggest biologically-informed hypotheses about the mechanisms of action of CBT in pediatric samples, which may also apply to different clinical populations in which CBT is the treatment of choice.
Supplementary Material
Acknowledgments
This study was supported by Carlos III Health Institute trough FIS grant (PI18/00856, PI16/00950, PI19/01184 [Alonso, Bertolín, Menchón]); Betolín was supported by Río Hortega grant (CM21/00278); Martínez-Zalacaín was supported by a PFIS grant (FI17/00294); co-funded by European Social Fund (ESF) investing in your future. Jimenez-Murcia was supported by Ministerio de Ciencia, Innovación y Universidades (RTI2018-101837-B-100); and research funded by the Delegación del Gobierno para el Plan Nacional sobre Drogas (2017I067, 2019I47, and 2021I031). Baker was supported by JSPS KAKENHI (26461762 and 16K04344). Fitzgerald was supported by National Institute of Mental Health (NIMH; K23 MH082176) and Charles Dana Foundation (UL1TR000433). Hirano was supported by AMED Brain/MINDS Beyond (JP21dm0307002) and JSPS KAKENHI (19K03309). Jahanshad was supported by NIMH (R01MH117601). Jaspers-Fayer was supported by Micheal Smith Foundation for Health Research. Kuno was supported by JSPS KAKENHI (18K13315). Lazaro was supported by The Marato TV3 Foundation grants (091710). Machado-Sousa was supported by Portuguese Foundation for Science and Technology (2020.07946.BD). Marsh was supported by NIMH (R01MH104648 and R21MH101441). Morgado was supported by National funds, through the Foundation for Science and Technology (FCT) (UIDB/50026/2020 and UIDP/50026/2020). Nakagawa was supported by JSPS KAKENHI (19K03308). O’Neill was supported by NIMH (R01MH081864 and R01MH085900-01A2). Piacentini was supported by NIMH (R01MH081864). Feusner was supported by NIMH (R01MH085900-01A2). Shavitt was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (303754/2018-4 and 573974/2008-0); and FAPESP (2008/57896-8). Shimizu was supported by AMED Brain/MINDS Beyond (JP21dm0307002). Thompson was supported by the National Institutes of Health (NIH; U54 EB020403). VIDI grant awarded to van den Heuvel (91717306). Walitza was supported by the Swiss National Science Foundation (320030_130237) and by the Hartmann Müller Foundation (1460).
The authors thank CERCA Programme/Generalitat de Catalunya for institutional support. This research was supported by CIBER -Consorcio Centro de Investigación Biomédica en Red- (CB07/09/0022), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación. The ENIGMA Working Group acknowledges the NIH Big Data to Knowledge (BD2K) award for foundational support and consortium development (U54 EB020403 to Paul M. Thompson). For a complete list of ENIGMA-related grant support please see here: http://enigma.ini.usc.edu/about-2/funding/.
Appendix
ENIGMA-OCD Working Group members: Eva Real, MD, PhD and Cinto Segalas, MD, PhD are with Bellvitge Biomedical Research Institute-IDIBELL. Eva Real, Cinto Segalas and Astrid Morer, MD, PhD are with CIBERSAM, Barcelona, Spain. Cinto Segalas and Astrid Morer, are also with University of Barcelona. Silvia Brem, PhD is with University Hospital of Psychiatry Zurich, University of Zurich, Switzerland; and Neuroscience Center Zurich, University of Zurich and ETH Zurich, Switzerland. Sonia Ferreira, MSc and Pedro Silva Moreira, PhD are with Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal; and ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal. Sonia Ferreria is also with Clinical Academic Center - Braga, Braga, Portugal. Pedro Silva Moreira are also with Psychological Neuroscience Lab, CIPsi, University of Minho, Braga, Portugal. Kristen Hagen, PhD is with Bergen Center for Brain Plasticity, Haukeland University Hospital, Bergen, Norway; and Hospital of Molde, Molde, Norway. Sayo Hamatani, PhD, Jumpei Takahashi, MD, PhD and Tokiko Yoshida, PhD are with Research Center for Child Mental Development, Chiba University, Chiba, Japan. Maria Alice de Mathis, MD, PhD and Euripedes C. Miguel, MD, PhD are with Hospital das Clinicas HCFMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil. Astrid Morer, MD, PhD is with Hospital Clínic, Barcelona, Spain; and IDIBAPS, Barcelona, Spain. Jose C Pariente, MSc is with Magnetic Resonance Image Core Facility (IDIBAPS). Jinsong Tang, MD, PhD is with Zhejiang University School of Medicine, Hangzhou, China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The ethical committees in clinical research of each participating institution approved the research.
Each center used its own document of consent for collecting the data.
Disclosure: Dr. Feusner has served as a consultant to NOCD, Inc. Dr. Morgado has received CME-related honoraria or consulting fees from Angelini, AstraZeneca, Bial, Biogen, DGS-Portugal, FCT, FLAD, Janssen-Cilag, Gulbenkian Foundation, Lundbeck, Springer Healthcare, Tecnimede, Viatris and 2CA-Braga. Dr. O’Neill has been an unpaid Scientific Advisory Board member for Myriad Genetics and has received funding for clinical trial from Emalex Pharmaceuticals. Dr. Simpson has received royalties from UpToDate, Inc. and Cambridge University Press and a stipend from the American Medical Association for serving as Associate Editor of JAMA-Psychiatry. Dr. Stewart has received grant/research support from the Canadian Institutes for Health Research, the Social Sciences and Humanities Research Council (Canada), the BC Ministry of Health COVID-19 Research Priorities Fund, the BC Centre for Disease Control, the University of British Columbia Faculty of Medicine (Strategic Investment Fund), British Columbia Children’s Hospital, and the BC Mental Health and Substance Use Services Research Institute. She has served on advisory boards/DSMB / speakers bureaus / travel expenses from the International OCD Foundation (IOCDF). She has served on advisory boards/DSMB of Anxiety Canada, the Youth Development Instrument Provincial Practice and Policy Group, and the British Columbia Ministry of Mental Health and Addictions. She has served as consultant / honoraria from the Misophonia Research Foundation and the Milken Institute. She has reported editorship/editorial board of Canadian Journal of Psychiatry and Annals of Clinical Psychiatry. She has reported authorship of assessment tools: Abramovitch A, Abramowitz JS, McKay D, Cham H, Anderson KS, Farrell L, Geller DA, Hanna GL, Mathieu S, McGuire JF, Rosenberg DR, Stewart SE, Storch EA, Wilhelm S. A Revision of the Obsessive-Compulsive Inventory – Child Version: The OCI-CV-R. Journal of Anxiety Disorders (accepted manuscript, January 2022). Dr. Walitza has received royalties from Thieme Hogrefe, Kohlhammer, Springer, Beltz, and Elsevier. Her work was supported by the Swiss National Science Foundation (SNF), diff. EU FP7s, Bfarm Germany, ZInEP, Olga Gertrud Thalmann, Vontobel, Unicentia, Erika Schwarz Fonds. Outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/. Dr. Thompson has received partial research support from Biogen, Inc., for research unrelated to this manuscript. Dr. Van den Heuvel has received consultation honorarium from Lundbeck. Drs. Bertolín, Alonso, Menchón, Jimenez-Murcia, Baker, Bargalló, Batistuzzo, Boedhoe, Brennan, Fitzgerald, Hansen, Hirano, Hoexter, Huyser, Jahanshad, Jaspers-Fayer, Kuno, Kvale, Lazaro, Marsh, Nakagawa, Norman, Nurmi, Ortiz, Piacentini, Picó-Pérez, Shavitt, Shimizu, Thorsen, Wolters, Stein, and Soriano-Mas, Mr. Martínez-Zalacaín, Mss. Fontaine and Machado-Sousa, Mr. Perriello, and Ms. Thomopoulos have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
Clinical Guidance
Contributor Information
Sara Bertolín, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; CIBERSAM, Barcelona, Spain..
Pino Alonso, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; CIBERSAM, Barcelona, Spain; University of Barcelona, Barcelona, Spain..
Ignacio Martínez-Zalacaín, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; University of Barcelona, Barcelona, Spain..
Jose M. Menchón, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; CIBERSAM, Barcelona, Spain; University of Barcelona, Barcelona, Spain..
Susana Jimenez-Murcia, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; CIBERobn, ISCIII, Spain; Psychoneurobiology of Eating and Addictive Behaviors Group, Barcelona, Spain..
Justin T. Baker, McLean Hospital, Belmont, Massachusetts, and Harvard Medical School, Boston, Massachusetts..
Nuria Bargalló, CIBERSAM, Barcelona, Spain; University of Barcelona, Barcelona, Spain; IDIBAPS, Barcelona, Spain; Image Diagnostic Center, Hospital Clinic, Barcelona, Spain; Magnetic Resonance Image Core Facility (IDIBAPS), Barcelona, Spain..
Marcelo Camargo Batistuzzo, Hospital das Clinicas HCFMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil; Pontificial Catholic University of Sao Paulo, Brazil..
Premika S.W. Boedhoe, Amsterdam UMC, Location VUmc, Amsterdam, the Netherlands..
Brian P. Brennan, McLean Hospital, Belmont, Massachusetts, and Harvard Medical School, Boston, Massachusetts..
Jamie D. Feusner, University of California Los Angeles, Los Angeles, California; University of Toronto, Canada; Centre for Addiction and Mental Health, Toronto, Canada; Karolinksa Institutet, Stockholm, Sweden..
Kate D. Fitzgerald, Columbia University, New York, and New York State Psychiatric Institute, New York..
Martine Fontaine, Columbia University Medical College, Columbia University, New York..
Bjarne Hansen, Bergen Center for Brain Plasticity, Haukeland University Hospital, Bergen, Norway; Centre for Crisis Psychology, University of Bergen, Bergen, Norway..
Yoshiyuki Hirano, Research Center for Child Mental Development, Chiba University, Chiba, Japan; United Graduate School of Child Development, Osaka University, Kanazawa University, Hamamatsu University School of Medicine, Chiba University and University of Fukui, Suita, Japan..
Marcelo Q. Hoexter, Hospital das Clinicas HCFMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil; LiNC - Laboratory of Integrative Neuroscience of Universidade Federal de São Paulo (UNIFESP), Brazil..
Chaim Huyser, Levvel, Academic Center for Child and Adolescent Psychiatry, Amsterdam, the Netherlands; Amsterdam UMC, Amsterdam, the Netherlands..
Neda Jahanshad, Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, Marina del Rey, California..
Fern Jaspers-Fayer, University of British Columbia, Vancouver, Canada; British Columbia Children's Hospital Research Institute, Vancouver, Canada..
Masaru Kuno, Research Center for Child Mental Development, Chiba University, Chiba, Japan..
Gerd Kvale, Bergen Center for Brain Plasticity, Haukeland University Hospital, Bergen, Norway; University of Bergen, Bergen, Norway..
Luisa Lazaro, CIBERSAM, Barcelona, Spain; University of Barcelona, Barcelona, Spain; Hospital Clínic, Barcelona, Spain; IDIBAPS, Barcelona, Spain..
Mafalda Machado-Sousa, Life and Health Sciences Research Institute (ICVS), University of Minho, Braga, Portugal; ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal; Clinical Academic Center - Braga, Braga, Portugal..
Rachel Marsh, Columbia University Medical College, Columbia University, New York; The New York State Psychiatric Institute, New York..
Pedro Morgado, Life and Health Sciences Research Institute (ICVS), University of Minho, Braga, Portugal; ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal; Clinical Academic Center - Braga, Braga, Portugal..
Akiko Nakagawa, Research Center for Child Mental Development, Chiba University, Chiba, Japan..
Luke Norman, University of Michigan, Ann Arbor..
Erika L. Nurmi, University of California Los Angeles, Los Angeles, California..
Joseph O’Neill, UCLA Division of Child and Adolescent Psychiatry, Los Angeles, California; UCLA Brain Research Institute, Los Angeles, California..
Ana E. Ortiz, CIBERSAM, Barcelona, Spain; Hospital Clínic, Barcelona, Spain; IDIBAPS, Barcelona, Spain..
Chris Perriello, University of Illinois at Urbana Champaign, Champaign, Illinois..
John Piacentini, UCLA Division of Child and Adolescent Psychiatry, Los Angeles, California; UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, California..
Maria Picó-Pérez, Life and Health Sciences Research Institute (ICVS), University of Minho, Braga, Portugal; ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal; Clinical Academic Center - Braga, Braga, Portugal..
Roseli G. Shavitt, Hospital das Clinicas HCFMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil..
Eiji Shimizu, Research Center for Child Mental Development, Chiba University, Chiba, Japan; United Graduate School of Child Development, Osaka University, Kanazawa University, Hamamatsu University School of Medicine, Chiba University and University of Fukui, Suita, Japan..
Helen Blair Simpson, Columbia University Medical College, Columbia University, New York; The New York State Psychiatric Institute, New York..
S. Evelyn Stewart, University of British Columbia, Vancouver, Canada; British Columbia Children's Hospital Research Institute, Vancouver, Canada; British Columbia Mental Health and Substance Use Services Research Institute, Vancouver, Canada..
Sophia I. Thomopoulos, Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, Marina del Rey, California..
Anders L. Thorsen, Bergen Center for Brain Plasticity, Haukeland University Hospital, Bergen, Norway; Centre for Crisis Psychology, University of Bergen, Bergen, Norway..
L. H. Wolters, Levvel, Academic Center for Child and Adolescent Psychiatry, Amsterdam, the Netherlands..
Susanne Walitza, University Hospital of Psychiatry Zurich, University of Zurich, Switzerland; Neuroscience Center Zurich, University of Zurich and ETH Zurich, Switzerland..
Paul M. Thompson, Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, Marina del Rey, California..
Odile A. van den Heuvel, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, Amsterdam, The Netherlands..
Dan J. Stein, SAMRC Unit on Risk and Resilience in Mental Disorders, Neuroscience Institute, University of Cape Town, Cape Town, South Africa..
Carles Soriano-Mas, Bellvitge Biomedical Research Institute-IDIBELL, Bellvitge University Hospital, Barcelona, Spain; CIBERSAM, Barcelona, Spain; University of Barcelona, Barcelona, Spain..
REFERENCES
- 1.Ruscio A, Stein D, Chiu W, Kessler R. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry [Internet]. 2010;15(1):53–63. 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. WHO/MSD/MER/2017.2. 2017. [Google Scholar]
- 3.POTS Study Team (POTS). Cognitive-Behavior Therapy, Sertraline, and Their Combination for Children and Adolescents With Obsessive-Compulsive Disorder The Pediatric OCD Treatment Study (POTS) Randomized Controlled Trial. JAMA. 2004;292(16):1969–76. 10.1001/jama.292.16.1969 [DOI] [PubMed] [Google Scholar]
- 4.Abramowitz JS, Taylor S, McKay D. Obsessive-Compulsive Disorder. Lancet [Internet]. 2009;374(9688):491–9. 10.1016/S0140-6736(09)60240-3 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence (NICE). Obsessive disorder and body dysmorphic disorder: treatment. 2005;(November 2005):1–12. [PubMed] [Google Scholar]
- 6.Öst LG, Havnen A, Hansen B, Kvale G. Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993-2014. Clin Psychol Rev [Internet]. 2015;40:156–69. Available from: 10.1016/j.cpr.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 7.McKay D, Sookman D, Neziroglu F, Wilhelm S, Stein DJ, Kyrios M, et al. Efficacy of cognitive-behavioral therapy for obsessive-compulsive disorder. Psychiatry Res. 2015;225(3):236–46. 10.1016/j.psychres.2014.11.058 [DOI] [PubMed] [Google Scholar]
- 8.Soriano-Mas C Functional Brain Imaging and OCD. Curr Top Behav Neurosci. 2021;49(February 2021):269–300. 10.1007/7854_2020_203 [DOI] [PubMed] [Google Scholar]
- 9.Hoexter MQ, Dougherty DD, Shavitt RG, D’Alcante CC, Duran FLS, Lopes AC, et al. Differential prefrontal gray matter correlates of treatment response to fluoxetine or cognitive-behavioral therapy in obsessive-compulsive disorder. Eur Neuropsychopharmacol [Internet]. 2013;23(7):569–80. Available from: 10.1016/j.euroneuro.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 10.Fullana MA, Cardoner N, Alonso P, Subirá M, López-sola C, Pujol J, et al. Brain regions related to fear extinction in obsessive-compulsive disorder and its relation to exposure therapy outcome: a morphometric study. Psychol Med. 2014;44:845–56. 10.1017/S0033291713001128 [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiyagaito A, Hirano Y, Asano K, Oshima F, Nagaoka S, Takebayashi Y, et al. Cognitive-behavioral therapy for obsessive–compulsive disorder with and without autism spectrum disorder: Gray matter differences associated with poor outcome. Front Psychiatry. 2017;8(143):1–12. 10.3389/fpsyt.2017.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagliaccio D, Cha J, He X, et al. Structural neural markers of response to cognitive behavioral therapy in pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry. 2020;61(12):1299–1308. 10.1111/jcpp.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual Dimorphism of Brain Developmental Trajectories during Childhood and Adolescence. Neuroimage. 2008;36(4):1065–73. 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka C, Matsui M, Uematsu A, Noguchi K, Miyawaki T. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev Neurosci. 2013;34(6):477–87. 10.1159/000345152 [DOI] [PubMed] [Google Scholar]
- 15.Huyser C, van den Heuvel OA, Wolters LH, De Haan E, Boer F, Veltman DJ. Increased orbital frontal gray matter volume after cognitive behavioural therapy in paediatric obsessive-compulsive disorder. World J Biol Psychiatry. 2012;14(4):319–31. 10.3109/15622975.2012.674215 [DOI] [PubMed] [Google Scholar]
- 16.Huyser C, Van Den Heuvel OA, Wolters L, De Haan E, Lindauer R, Veltman DJ. A longitudinal VBM study in paediatric obsessive-compulsive disorder at 2-year follow-up after cognitive behavioural therapy. World J Biol Psychiatry. 2013;15(6):443–52. 10.3109/15622975.2013.819122 [DOI] [PubMed] [Google Scholar]
- 17.Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J, Marazziti D, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5:181–91. 10.1017/S1461145702002900 [DOI] [PubMed] [Google Scholar]
- 18.Boedhoe PSW, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: A worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174(1):60–70. 10.1176/appi.ajp.2016.16020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: Findings from the enigma obsessive-compulsive disorder working group. Am J Psychiatry. 2018;175(5):453–62. 10.1176/appi.ajp.2017.17050485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;(33):341–55. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- 21.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 22.Boedhoe PSW, Heymans MW, Schmaal L, Abe Y, Alonso P, Ameis SH, et al. An Empirical Comparison of Meta- and Mega-Analysis With Data From the ENIGMA Obsessive-Compulsive Disorder Working Group. Front Neuroinform [Internet]. 2019;12(January):1–8. Available from: 10.3389/fninf.2018.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists [published correction appears in Biol Rev Camb Philos Soc. 2009 Aug;84(3):515]. Biol Rev Camb Philos Soc. 2007;82(4):591–605. 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- 24.Adams TG, Cisler JM, Kelmendi B, et al. Transcranial direct current stimulation targeting the medial prefrontal cortex modulates functional connectivity and enhances safety learning in obsessive-compulsive disorder: Results from two pilot studies. Depress Anxiety. 2022;39(1):37–48. 10.1002/da.23212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano M, Alonso P, Martínez-Zalacaín I, Subirà M, Real E, Segalàs C, et al. Altered functional connectivity of the subthalamus and the bed nucleus of the stria terminalis in obsessive-compulsive disorder. Psychol Med. 2018;48(6):919–28. 10.1017/S0033291717002288 [DOI] [PubMed] [Google Scholar]
- 26.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68(5):416–24. 10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong CC, Moody TD, Feusner JD, McCracken JT, Chang S, Levitt JG, et al. Graph-theoretical analysis of resting-state fMRI in pediatric obsessive-compulsive disorder. J Affect Disord [Internet]. 2016;193:175–84. Available from: 10.1016/j.jad.2015.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama N, Nakagawa A, Umeda S, Terasawa Y, Kurata C, Tabuchi H, et al. Frontopolar cortex activation associated with pessimistic future-thinking in adults with major depressive disorder. NeuroImage Clin [Internet]. 2019;23(January):101877. Available from: 10.1016/j.nicl.2019.101877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama N, Nakagawa A, Umeda S, Terasawa Y, Abe T, Kurata C, et al. Cognitive Behavioral Therapy Effects on Frontopolar Cortex Function During Future Thinking in Major Depressive Disorder: A Randomized Clinical Trial. J Affect Disord. 2021. Nov;S:0165-032(21):1256–8. 10.1016/j.jad.2021.11.034 [DOI] [PubMed] [Google Scholar]
- 30.Neufang S, Geiger MJ, Homola GA, Mahr M, Schiele MA, Gehrmann A, et al. Cognitive-behavioral therapy effects on alerting network activity and effective connectivity in panic disorder. Eur Arch Psychiatry Clin Neurosci [Internet]. 2019;269(5):587–98. Available from: 10.1007/s00406-018-0945-8 [DOI] [PubMed] [Google Scholar]
- 31.Cyr M, Pagliaccio D, Yanes-Lukin P, Fontaine M, Rynn MA, Marsh R. Altered network connectivity predicts response to cognitive-behavioral therapy in pediatric obsessive–compulsive disorder. Neuropsychopharmacology [Internet]. 2020;45(7):1232–40. Available from: 10.1038/s41386-020-0613-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berggren N, Derakshan N. The role of consciousness in attentional control differences in trait anxiety. Cogn Emot. 2013;27(5):923–31. 10.1080/02699931.2012.750235 [DOI] [PubMed] [Google Scholar]
- 33.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141–51. 10.31887/DCNS.2007.9.2/rbonelli [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Straten A, Huyser C, Wolters L, Denys D, van Wingen G. Long-Term Effects of Cognitive Behavioral Therapy on Planning and Prefrontal Cortex Function in Pediatric Obsessive-Compulsive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging [Internet]. 2018;3(4):320–8. Available from: 10.1016/j.bpsc.2017.ll.009 [DOI] [PubMed] [Google Scholar]
- 35.Theiss JD, McHugo M, Zhao M, Zald DH, Olatunji BO. Neural correlates of resolving conflict from emotional and nonemotional distracters in obsessive-compulsive disorder. Psychiatry Res - Neuroimaging [Internet]. 2019;284(May 2018):29–36. Available from: 10.1016/j.pscychresns.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Cyr M, Pagliaccio D, Yanes-Lukin P, Goldberg P, Fontaine M, Rynn MA, et al. Altered fronto-amygdalar functional connectivity predicts response to cognitive behavioral therapy in pediatric obsessive-compulsive disorder. Depress Anxiety. 2021;38(8):836–45. 10.1002/da.23187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Yang X, Chen X, Liu R, Wang P, Meng F, et al. Resting-state functional connectivity of the amygdala subregions in unmedicated patients with obsessive–compulsive disorder before and after cognitive behavioural therapy. J Psychiatry Neurosci. 2021;46(6):E628–38. 10.1503/jpn.210084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin-Falcone H, Weber J, Kishon R, Ochsner K, Delaparte L, Doré B, et al. Neural predictors and effects of cognitive behavioral therapy for depression: The role of emotional reactivity and regulation. Psychol Med. 2021;50(12):146–60. 10.1017/S0033291718004154 [DOI] [PubMed] [Google Scholar]
- 39.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. 10.1111/j.l749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du X, Mao Y, Ran Q, Zhang Q, Luo Q, Qiu J. Short-term group cognitive behavior therapy contributes to recovery from mild depression: Evidence from functional and structural MRI. Psychiatry Res Neuroimaging. 2016. May;251(30):53–9. 10.1016/j.pscychresns.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 41.van Passel B, Danner UN, Dingemans AE, et al. Cognitive Remediation Therapy Does Not Enhance Treatment Effect in Obsessive-Compulsive Disorder and Anorexia Nervosa: A Randomized Controlled Trial. Psychother Psychosom. 2020;89(4):228–241. 10.1159/000505733 [DOI] [PubMed] [Google Scholar]
- 42.Nakao M, Shirotsuki K, Sugaya N. Cognitive–behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc Med. 2021;15(1):16. 10.1186/s13030-021-00219-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fouche JP, du Plessis S, Hattingh C, et al. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry. 2017;210(1):67–74. 10.1192/bjp.bp.115.164020 [DOI] [PubMed] [Google Scholar]
- 44.Cao R, Yang X, Luo J, et al. The effects of cognitive behavioral therapy on the whole brain structural connectome in unmedicated patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110037. 10.1016/j.pnpbp.2020.110037 [DOI] [PubMed] [Google Scholar]
- 45.Tetereva A, Li J, Deng JD, Stringaris A, Pat N Capturing Brain-Cognition Relationship: Integrating Task-Based fMRI Across Tasks Markedly Boosts Prediction and Reliability and Reveals the Role of Frontoparietal Areas. bioRxiv 2021.10.31.466638; 10.1101/2021.10.31.466638 [DOI] [Google Scholar]
- 46.Torp NC, Dahl K, Skarphedinsson G, et al. Effectiveness of cognitive behavior treatment for pediatric obsessive-compulsive disorder: acute outcomes from the Nordic Long-term OCD Treatment Study (NordLOTS). Behav Res Ther. 2015;64:15–23. 10.1016/j.brat.2014.11.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.