Abstract
Environmental enteric dysfunction (EED) is a subclinical syndrome of intestinal inflammation, malabsorption and barrier disruption that is highly prevalent in low- and middle-income countries in which poverty, food insecurity and frequent exposure to enteric pathogens impair growth, immunity and neurodevelopment in children. In this Review, we discuss advances in our understanding of EED, intestinal adaptation and the gut microbiome over the ‘first 1,000 days’ of life, spanning pregnancy and early childhood. Data on maternal EED are emerging, and they mirror earlier findings of increased risks for preterm birth and fetal growth restriction in mothers with either active inflammatory bowel disease or coeliac disease. The intense metabolic demands of pregnancy and lactation drive gut adaptation, including dramatic changes in the composition, function and mother-to-child transmission of the gut microbiota. We urgently need to elucidate the mechanisms by which EED undermines these critical processes so that we can improve global strategies to prevent and reverse intergenerational cycles of undernutrition.
Introduction
In low- and middle-income countries (LMICs), maternal stunting is a risk factor for low birthweight and subsequent childhood stunting, thereby perpetuating a vicious intergenerational cycle of undernutrition. This cycle has adverse consequences for children’s survival, growth and neurodevelopment1. Maternal gut function during pregnancy is critical to healthy fetal and child development2, asdemonstrated by studies of other enteropathies such as coeliac disease and inflammatory bowel disease. Environmental enteric dysfunction (EED), or the ‘impoverished gut’3, is a subclinical condition of small intestinal crypt–villus architectural derangements, mucosal and systemic inflammation, malabsorption and gut barrier dysfunction. It is ascribed to the interplay of food insecurity, poor diet quality, inadequate sanitation and hygiene, and health inequity in resource-limited settings4-6. EED is challenging to diagnose, because the only definitive gold standard diagnostic test is small intestinal biopsy. Despite this limitation, biomarkers of EED-associated barrier dysfunction and intestinal inflammation have been proposed, including dual sugar absorption tests, and faecal myeloperoxidase, neopterin and α1-antitrypsin, among others7-11. These biomarkers are associated with linear growth faltering in early childhood as well as the underperformance of oral vaccines in LMICs across age groups8,12,13. Tragically and unacceptably, one in five children worldwide has stunted growth14. Childhood stunting increases the risk of death from infectious diseases 3-to-6-fold15, and oral vaccines against rotavirus, poliovirus and other gut pathogens underperform in the settings with the highest burden of these devastating infections16,17.
It is not yet known to what extent EED affects mothers in LMICs, whether pregnancy modulates the gut inflammation resulting from this syndrome, and whether EED contributes to the intergenerational transmission of growth stunting. In this Review, we summarize current insights into maternal and child enteric function and the gut microbiome during pregnancy and early life, drawing lessons from studies in LMICs, enteropathies in high-income countries/regions, and animal models. Preventing and reversing the intergenerational cycle of undernutrition will be crucial to achieving two of the United Nations Sustainable Development Goals: Goal 2: “End hunger, achieve food security and improve nutrition” and Goal 3: “To ensure healthy lives and promote well-being for all at all ages” by 2030 (ref. 18). Africa, Asia and Oceania (excluding Australia and New Zealand) are the global regions most affected, with a prevalence of childhood stunting of 30.7%, 21.8% and 41.4%, respectively14. Ongoing global crises are expected to exacerbate food insecurity and extreme poverty in LMICs19, highlighting the importance of closing critical knowledge gaps surrounding gut adaptation and microbiota function during pregnancy, lactation and infancy.
EED and the first 1,000 days
Undernutrition-associated linear growth stunting in children is largely irreversible beyond 2 years of age20, so early-life interventions to prevent or reverse the factors contributing to stunting – including EED – offer the best opportunity for improving outcomes. Measures taken against stunting during the first 1,000 days of life can lead to improvements in neurodevelopment and biomarkers of central nervous system development1,21. However, some evidence suggests that later interventions, between the ages of 1 and 8 years, also yield measurable growth, nutritional, cognitive and educational benefits for children22,23. Disappointingly, even the best-designed and most rigorous postnatal nutritional, water, sanitation and hygiene interventions typically produce only modest benefits for children’s linear growth in high-risk settings in which the mean height-for-age Z score at 2 years of age is two or more standard deviations below the World Health Organization (WHO) median24-30. EED-associated linear growth faltering is analogous to chronic inflammatory causes of intestinal failure in children, in which, despite seemingly adequate oral nutrition, ponderal and linear growth are limited by decreased small intestinal surface area, inflammation and compromised absorptive function31.
Multi-country/region birth cohort studies of EED and growth faltering consistently reveal that the strongest individual predictors of childhood stunting at 2 years of age are maternal height and neonatal anthropometry25-29,32,33. Food insecurity, pathogens, disruption of the gut microbiome, birth practices, genetics and environmental toxins are all key components of postnatal growth trajectories; however, the individual contributions of these factors are modest relative to maternal and neonatal anthropometry. This observation suggests an underappreciated role for maternal nutrition and gut health in shaping the intrauterine environment, the gut microbiota, and epigenetic determinants of early childhood growth (Fig. 1). The 2006 WHO international growth charts remain the current standard against which all other infants are compared34. Three recently completed longitudinal cohort studies have developed intrauterine fetal growth charts, one in the USA and two international: the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies35, The International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st)36 and the WHO Multicentre Growth Reference Study (WHO Fetal)37. These results will provide additional context in which to assess the extent of stunting in utero in undernourished populations. Intrauterine growth restriction in the context of maternal stunting has been proposed as an adaptation that reduces the risk of cephalo-pelvic disproportion and obstructed labour in high-risk mothers; however, maternal metabolic constraints probably also contribute to fetal growth38,39. Ultimately, multipronged approaches that optimize diet and environment in children and women across the lifespan are needed to promote healthy birth, children’s growth and neurodevelopment23.
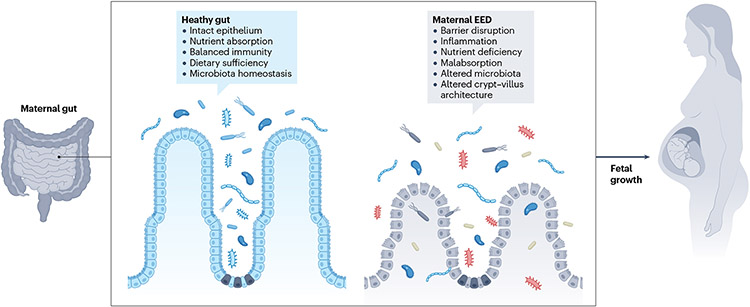
Fig. 1 ∣. Maternal gut health and fetal growth in the context of environmental enteric dysfunction.
The energy demands of pregnancy dictate adaptations in the maternal gut that enable increased nutrient absorption. In women with environmental enteric dysfunction (EED), accommodating the nutritional demands of pregnancy could be challenged by increased intestinal epithelial barrier disruption, altered microbial communities and malabsorption. These features of EED might also contribute to impaired fetal growth. Maternal intestinal inflammation, which is itself energetically costly, might exacerbate this condition.
The first 1,000 days of life encompass the approximately 270 days of a term pregnancy and the 730 days spanning birth to a child’s second birthday and are thought to be a critical window for promoting healthy growth and cognitive development40. In contrast to the explosion in knowledge gained by applying next-generation sequencing techniques such as RNA sequencing, 16S ribosomal RNA sequencing, and metagenomics to childhood stunting and EED in LMICs25-29, remarkably little is known about EED in the context of pregnancy and lactation41. The extent to which this syndrome affects mothers in LMICs is not yet clear, nor is it known whether maternal EED contributes to the intergenerational transmission of growth stunting. Intriguingly, in a study of 258 pregnant Ugandan women, Lauer et al. reported that maternal anti-flagellin and anti-lipopolysaccharide (LPS) immunoglobulin G (IgG) serum concentrations (which are serum markers of gut-to-blood bacterial translocation in EED) were associated with shorter gestation and reduced infant length at birth41. They also observed higher urinary lactulose-to-mannitol ratios (dual sugar biomarkers of gut permeability and surface area, respectively) in mothers who delivered preterm, and higher lactulose excretion in mothers whose babies were born wasted (defined as a weight-for-height Z score two or more standard deviations below the US Centers for Disease Control and Prevention (CDC) and WHO median), suggesting a link between maternal EED and birth outcomes41. Additionally, in a cohort of 706 pregnant women infected with HIV in Dar es Salaam, Tanzania, the same group of investigators found no correlations of EED biomarkers at 32 weeks gestation related to microbial translocation or gut epithelial damage with the primary outcomes of birthweight, gestation durations or birthweight for gestational age42. However, among secondary outcomes, higher levels of the serum EED biomarkers faecal intestinal fatty acid-binding protein (I-FABP) and anti-LPS immunoglobulin A (IgA) were associated with stillbirth42. Notably, the Ugandan study analysed samples at 18 weeks gestation and excluded pregnant women living with HIV, which might account for the discrepancies between the two cohorts.
Lessons learned from enteropathies in high-income countries/regions demonstrate that coeliac disease (also known as non-tropical sprue) and inflammatory bowel disease are maternal risk factors for miscarriage and stillbirth, fetal growth restriction and intrauterine growth restriction, low birthweight and prematurity43,44. Moreover, in inflammatory bowel disease, pregnancy can either trigger flares or induce remission45. The strongest epidemiological data linking coeliac disease and inflammatory bowel disease with adverse pregnancy and perinatal outcomes is from contemporary case–control studies in high-income countries/regions43,44. These studies suggest that enteropathy, independent of either undernutrition or health inequity, is a risk factor for adverse outcomes during the first 1,000 days43,44. Gluten-free diets improve clinical symptoms and substantially reduce the risk of adverse perinatal outcomes in coeliac disease; however, coeliac enteropathy can persist on a gluten-free diet46. Moreover, gluten-free diets do not eliminate the increased risk of intrauterine growth restriction, low birthweight, and premature birth seen in coeliac disease43. The histopathological, transcriptomic and microbiome signatures of EED and coeliac disease have overlapping features26,47. Therefore, EED, like coeliac disease in high-income countries/regions, might be an under-recognized risk factor for pregnancy loss, intrauterine growth restriction and prematurity in LMICs.
Maternal gut adaptation
Framing the potential adverse effects of maternal EED on small intestinal adaptation requires an appreciation of the remarkable transformation in structure and function of the small bowel that occurs during the first 1,000 days to support healthy pregnancy and lactation – which have the energetic equivalent requirements of an ultramarathon48 (Fig. 2a). Several lines of evidence suggest that the upper limit of metabolic adaptation to the caloric demands of pregnancy and lactation is set by the small intestine, which undergoes a rapid and sustained increase in mass to absorb the higher quantity of food necessary to fuel maternal, fetal and suckling infant growth39,48-51 (Fig. 2b,c). Surprisingly, it is not yet known how the intestinal epithelium expands so quickly to provide the increased surface area that is necessary to absorb approximately 80,000 additional kilocalories during pregnancy and 500 kcal per day during lactation52-54. The sexually dimorphic proliferation of haematopoietic stem cells in response to pregnancy hormones – a key mechanism by which blood volume expands during pregnancy – might hold clues55. Gut trophic effects of nutrition, direct effects of sex hormones on intestinal epithelial cells or other mucosal compartments, shifts in gut microbial communities, or combinations thereof, might also be drivers50.
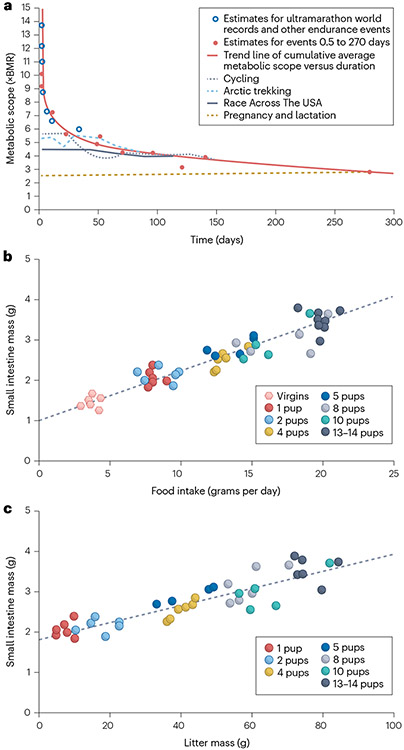
Fig. 2 ∣. Expansion of small intestinal absorptive capacity during pregnancy and lactation.
The extreme metabolic demands of pregnancy and lactation require the expansion of small intestinal absorptive capacity. a, Maximum sustained human metabolic scope (fold-increase in basal metabolic rate, BMR) versus duration of extreme endurance events flattens out at 2.5× BMR. Cumulative average metabolic scope is shown for elite cyclists over a touring season, Arctic trekking, Race Across The USA runners, and pregnancy and lactation. The sustained metabolic demands over the duration of pregnancy and lactation are equivalent to model estimates for ultramarathons and other extreme endurance events lasting 9 months or more. b, Direct changes in small intestinal mass due to feeding in lactating mice. c, Indirect changes in small intestinal mass due to increasing litter mass. Part a adapted with permission of AAAS from ref. 48, © The Authors, some rights reserved; exclusive licensee AAAS, distributed under a CC BY-NC 4.0 License (http://creativecommons.org/licenses/by-nc/4.0/). Parts b and c adapted from ref. 51, Springer Nature Limited. Part b also adapted with permission from ref. 49, The University of Chicago Press.
Preclinical studies
Given the understandable paucity of tissue-based studies of the structural and functional changes of the small intestine during human pregnancy and lactation, some insights must be gleaned from preclinical models. Pregnant rats have an average gestation of 21–23 days and undergo a progressive increase in duodenal villous width and jejunal villous width and height during pregnancy56,57. During lactation, this villous hypertrophy extends to the entire small intestine57,58. Similar patterns are observed in pregnant and lactating mice, with some adaptations persisting after cessation of lactation, thereby contributing to postpartum weight retention56,59,60. This small bowel adaptation is closely coupled to caloric requirements, showing striking linear correlations between daily food intake, litter size and small intestine mass in mice49.
Progesterone is a key pregnancy hormone that prevents both inflammation and preterm birth. In pregnant women, Zhou et al. found decreased plasma bacterial LPS (a biomarker of gut microbial translocation) at 24-to-28 weeks gestation compared with 8-to-12 weeks gestation as well as an inverse correlation between rising progesterone levels and levels of plasma LPS and tumour necrosis factor61. Complementing these observations, progesterone increased barrier function in primary human colon explants and Caco-2 cells by upregulating occludin and inhibiting nuclear factor-κB activation following LPS stimulation61.
Intriguingly, the structural (increased length and width of villi) and functional (decreased gastrointestinal motility and increased absorption) changes of the small intestine seen during pregnancy and lactation in rats resemble those observed after administering the glucagon-like peptide 2 (GLP2) analogue teduglutide to patients with short bowel syndrome62. Endogenous GLP2 is produced by the L cells of the intestinal epithelium, and the GLP2 receptor resides primarily on enteric neurons63. Elevated serum GLP2 levels are observed in both pregnancy and obesity, which suggests that there are common mechanisms by which the gut expands to accommodate increased consumption of calories64. Relaxin levels also increase during pregnancy, slowing GI motility and enabling additional absorption65. In pregnant mice, increases in circulating neuregulin drive remodelling of the cardiac ventricles66. Neuregulin has also been shown to accelerate maturation of inducible-pluripotent-stem-cell-derived human intestinal organoids67. It remains to be seen whether neuregulin similarly promotes gut adaptation during pregnancy. The extent to which these hormonal regulators are altered by maternal EED is not yet clear, but these observations warrant further investigation. Moreover, changes in gut structure and function have profound implications for members of the gut microbiota, which in turn might regulate EED and growth outcomes.
The gut microbiome during pregnancy
In healthy human cohorts in high-income countries/regions, gut microbial communities and overall bacterial load shift over the course of gestation68. Microbial diversity increases during the third trimester, with marked elevations in Proteobacteria and Actinobacteria noted relative to the first trimester in a study of 91 women in Finland69. Faecal microbiota samples taken from women in the third trimester of pregnancy conferred increased faecal inflammatory cytokines, adiposity and insulin-insensitivity when transferred into germ-free mice compared with microbiota from the first trimester69.
Hormonal shifts during pregnancy could influence these changes. For example, oestrogen and progesterone are known to alter microbial communities70. In a murine model, pregnancy induced shifts in gut microbial communities and could be further modified by diet71. Bifidobacteria have been reported to be elevated in the gut microbiota of pregnant women and in pregnant mice, and this increase is thought to be mediated by elevated progesterone levels72. By contrast, other studies have reported stable microbial communities over the course of gestation73. The diet of participants and study design might explain these discrepancies, highlighting the need for additional investigation of the interplay between maternal perinatal immunity, diet and the gut microbiome, particularly in states of undernutrition and enteropathy74.
EED is associated with alterations in gut microbial communities25. In pregnant women in Zimbabwe, gut microbiota composition and metabolic function predicted birthweight and weight-for-age Z score more accurately than gestational age75. In addition, animal models demonstrate that alterations in the maternal gut microbiota during pregnancy profoundly shape maternal health and offspring behavioural, developmental and immune characteristics75-79. These findings suggest that the maternal microbiome could be altered in women with EED during gestation, but to what extent this occurs remains to be determined.
Maternal nutrition and the gut microbiota
Gut microorganisms play an important part in nutrient absorption, facilitating energy harvest from dietary components80. Although the prevalence and consequences of EED during pregnancy are unclear, dietary intake is particularly critical during this period of increased metabolic expenditure (Fig. 2). Pregnancy is known to exacerbate nutritional deficiencies, and the contribution of gut microorganisms to maternal nutrition warrants further study.
Micronutrient deficiencies are common in populations experiencing undernutrition81. Vitamin A deficiency during pregnancy is a major public health concern that causes visual impairment and increased risk of illness and death from infection in children82. Vitamin A also regulates proliferation and differentiation of intestinal epithelial cells to help maintain the gut barrier. The gut microbiota can influence levels of retinoic acid, a metabolite of vitamin A, and animals that lack retinoic acid receptor-α (RARα), which binds to retinoic acid, have underdeveloped lymphoid follicles and are more susceptible to infection by the intestinal pathogen Citrobacter rodentium83,84. Vitamin A deficiency can directly affect microbial communities, and combined vitamin A and zinc deficiency in mice results in lower levels of serum and intestinal mucosal IgA, a known regulator of microbiota composition85,86. Similarly, rodent diets that are deficient in the methyl donor nutrients choline and folate reduce gut microbial diversity, with associated reductions in growth and dysmorphic intestinal development87. Other essential micronutrients, such as iron, zinc, vitamin B12 and vitamin D, also influence the composition of the gut microbiota in mice88. Thus, nutritional deficiencies can directly affect immune function as well as shift gut microbial communities, with profound consequences for host development.
Dietary macronutrients are also important in shaping fetal development and the gut microbiota. Protein deficiency is a common feature of undernutrition and markedly affects gut microbial communities in murine models89,90. Maternal dietary protein deficiency has also been shown to reduce expression of brain-derived neurotrophic factor (BDNF) in the neonatal rat brain. BDNF contributes to learning and memory, suggesting that the physiological effects of this type of deficiency might be wide-ranging91. Importantly, alterations in the uptake of the amino acid tryptophan in Ace2-mutant mice led to reduced antimicrobial peptide production in intestinal epithelial cells and an altered gut microbial community that could confer increased susceptibility to colitis in recipient germ-free mice92. The microbiota also has a critical role in dietary fat absorption, and insufficient dietary fat intake is another common feature of undernutrition93. Gut microorganisms stimulate fatty acid uptake in the intestinal epithelium and can also deconjugate bile acids, which in turn influence lipid absorption94,95. Although the role of maternal lipid intake is best understood in the context of high-fat diet and obesity, it is likely that lipid absorption could affect fetal development in states of undernutrition as well.
Other critical dietary components include non-digestible dietary carbohydrates that are fermented by the microbiota to produce short-chain fatty acids (SCFAs)96. In humans, maternal serum acetate was linked with maternal weight gain, whereas serum propionate was negatively correlated with newborn weight and length97. SCFAs can serve as energy sources for intestinal epithelial cells and influence a wide range of host physiological processes. These processes include formation of the intestinal mucus layer and protection of the intestinal barrier, maturation of the immune system (including stimulating the development of regulatory T (Treg) cells), and acidification of the gut to improve mineral solubility and absorption96. In a murine model, a high-fibre diet during pregnancy led to elevated levels of plasma SCFAs and a concomitant increase in the number of both thymic and peripheral Treg cells98. A separate murine study reported that a high-fibre diet during pregnancy resulted in resistance to the development of allergic airway disease, implicating systemic effects of maternal gut production of SCFAs99. These microbial fermentation products might also have beneficial effects on nutrient absorption by regulating the function of intestinal epithelial cells, including enteroendocrine L-cells, and stimulating production of glucagon-like peptide 1 (GLP1)100.
Although the role of these mediators is yet to be investigated in maternal undernutrition, increasing their production could potentially benefit both mother and infant by reducing intestinal permeability and increasing intestinal absorption during gestation based on preclinical evidence101,102. It is unclear to what extent current prenatal nutritional interventions such as dietary supplements repair alterations to the undernourished maternal microbiome. Owing to the major contribution of the gut microbiota to nutrient processing and absorption, consideration of this feature of EED has the potential to improve the efficacy of nutritional therapies.
Maternal microbiome and birth outcomes
Changes in the maternal microbiome have been associated with pregnancy outcome and birth anthropometry in LMICs, where rates of small-for-gestational-age birth, fetal growth restriction and intrauterine growth restriction are higher than in high-income countries/regions103. An analysis of 19 longitudinal birth cohorts estimated that small-for-gestational-age births account for 20% of childhood stunting and 30% of childhood wasting globally104, and infants with fetal growth restriction are at increased risk of preterm delivery, which is a major cause of perinatal morbidity and mortality105. In mothers in rural Zimbabwe, the composition of the gut microbiota and its metabolic functions predicted infant birthweight more accurately than gestational age or length-for-age75. For example, increased abundance of Roseburia intestinalis and Butyrivibrio sp. CAG:318 were predictive of higher birthweight75. Intriguingly, these taxa are capable of degrading plant fibres to produce the SCFA butyrate. However, a causal role for these changes has yet to be demonstrated, and alterations in the microbiota might result from changes in dietary patterns that in turn influence birthweight. These findings further emphasize the importance of gnotobiotic animal models in testing causal roles for the microbiota in host phenotypes.
Insight into the role of the maternal microbiome can also be gleaned from studies in high-income countries/regions. In a prospective cohort study of Japanese mother–child dyads, newborn head circumference of male infants, but not female infants, was positively associated with maternal faecal microbial alpha-diversity106. The same study reported negative correlations between head circumference at birth and abundance of the genera Parabacteroides and Eggerthella in maternal stool samples. Japanese mothers of preterm infants were also found to have alterations in intestinal microbial communities at 28 weeks gestation compared with mothers who delivered term babies, with a noted increase in the abundance of members of the Lactobacillales order and reduced abundance of the Bacteroides and Clostridium genera107. In a separate study from Norway, increased gut microbiota alpha-diversity four days postpartum was associated with reduced odds of spontaneous preterm birth, and mothers of premature infants showed lower levels of Bifidobacterium, Streptococcus and Clostridiales compared with mothers of term infants108.
Although our understanding of the role of the maternal microbiome in undernutrition and EED is currently limited, evidence from other disease states and animal models suggests an important role in regulating immunity, metabolism and energy acquisition. Intriguingly, in mice, the maternal microbiome during pregnancy imparts resistance to later-life obesity caused by a high-fat diet109. Mice born to germ-free mothers and reared by conventionally colonized mothers were more susceptible to metabolic syndrome associated with obesity than animals both born to and reared by conventionally colonized mothers109.
The maternal gut microbiota has also been implicated in the neurocognitive development of offspring. A study in mice reported that depletion of the maternal microbiota with antibiotics during gestation led to persistent neurodevelopmental changes in offspring, including reduced expression of genes related to axonogenesis, impaired outgrowth of thalamic axons and altered tactile sensitivity79. In a murine model of maternal immune activation, changes to offspring behaviour were dependent on IL-17 production driven by the maternal gut microbiota76,77. Maternal immune activation during gestation also altered social behaviour in non-human primate models110,111.
Further evidence for the importance of the maternal microbiome includes reports of altered offspring immunity following maternal exposure to antibiotics during gestation and lactation. In a murine model, administration of vancomycin during gestation altered offspring microbial communities and immunity, leading to increased numbers of splenic T and B cells compared with untreated controls112. Immunity was also altered in a mouse model of maternal gestational antibiotic exposure using Il10−/− mice113. These animals were treated with cefoperazone during pregnancy and lactation, leading to decreased numbers of Treg cells in the mesenteric lymph nodes of their offspring, which was associated with an increased susceptibility to spontaneous and chemically induced colitis113. Complementing these findings, an elegant study by Agüero et al. used transient colonization of pregnant female mice with Escherichia coli to show increased levels of group 3 innate lymphoid cells in their offspring, which protected against bacterial translocation114. Collectively, these results suggest that the maternal microbiome is capable of shaping offspring growth, cognition and immunity in animal models. It is currently unclear whether similar findings will emerge from human studies, and whether and how the EED-associated microbiota plays a role in shaping fetal development during pregnancy (Fig. 3). Elucidating these connections will require detailed insights from human cohorts as well as gnotobiotic animal models to investigate causal roles for specific microbial communities and functions. Nevertheless, current results present early and promising clues into the role of maternal microorganisms and immune signals, which might be even more critical when mother and child are faced with the adverse environmental conditions observed in EED.
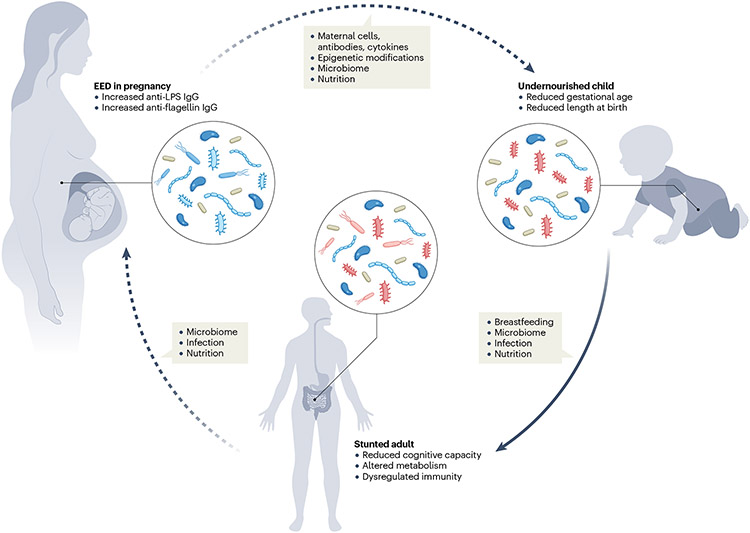
Fig. 3 ∣. The effect of maternal environmental enteric dysfunction on child development: potential mechanisms and consequences.
Mothers with environmental enteric dysfunction (EED) might harbour altered immune cells and signals, epigenetic modifications, nutrition and microbiota during pregnancy36. These signals could influence development in utero and thereafter be passed on to infants. In early life, infant growth is shaped by breast milk, the gut microbiota, infection and nutrition. These factors are likely to be interrelated and act in concert to shape attained height, metabolism, immunity and cognition at adulthood. Dotted arrows indicate areas requiring additional investigation, whereas solid arrows indicate more established connections. Anti-LPS IgG, anti-lipopolysaccharide immunoglobulin G.
Intergenerational microbial transfer
At birth, infants encounter a vast new microbial world. Although evidence exists for some level of exposure to microbial products in utero, colonization with microorganisms increases dramatically during the first days of life115-118. Many of these microorganisms are derived from maternal skin, oral and vaginal microbial communities119,120. A substantial proportion also originate from the maternal gut microbiome, and evidence suggests that maternal-gut-derived strains might be more-persistent colonizers of the infant gut microbiota than environmentally derived strains121. Maternal breast milk can also contain microorganisms that seed the infant gut122-124. These patterns of microbial inheritance are modified by birth mode. Initial observations using fluorescence in situ hybridization, quantitative reverse transcription PCR (RT-qPCR) and culture-based methods demonstrated alterations in Bifidobacterium and Bacteroides abundance in the gut microbiota of infants delivered by caesarean section125-127. These findings were largely supported by later studies employing 16s rRNA or metagenomic sequencing approaches128-130. These taxonomic changes could be partially restored by exposure of caesarean-delivered infants to maternal vaginal fluid at birth or more fully by maternal to infant faecal microbiota transplant in breast milk131,132. Caesarean delivery has also been associated with an increased risk of asthma, coeliac disease and obesity in childhood, further highlighting the importance of immune and microbiome development during infancy133,134. These findings present the intriguing possibility that the inheritance of maternal microorganisms at birth could alter the trajectory of microbial community assembly in infants born to mothers who experience growth stunting or EED.
Intergenerational growth stunting
Other features of undernutrition and EED might also be transmitted through generations. A study in Norway of 3,497 women and 5,010 children demonstrated that shorter women are at a higher risk of preterm birth compared with taller women135. This association does not hold true for fathers, which is consistent with the relative importance of intrauterine conditions compared with genetics135. A birth cohort of 3,485 mother–infant pairs from three Nordic countries (Finland, Denmark and Norway) suggested that both genetic factors and non-genetic factors influenced infant height, and non-genetic factors had a more important role in determining gestational age136. In LMICs, these factors could include shared mother–infant environments with substantial sanitation and dietary challenges, pathogen encounters and epigenetic changes determined by the mother’s prior life history that are transmitted to her offspring1 (Fig. 3).
Across LMICs, maternal stature is inversely related to child stunting and overall child mortality137. This observation is probably due at least in part to physical constraints upon fetal growth in smaller mothers, but additional factors, including maternal inflammation, gut function, microbiota and epigenetics, might also have a role138. Substantial changes in DNA methylation were noted in duodenal biopsy samples from children with EED in rural Pakistan (n = 33) relative to healthy controls from the USA (n = 21), with DNA hypomethylation identified in genes linked to immune activation and cell division26. In turn, genes linked to enterocytes and metabolic processes were hyper-methylated26. In rodents, the suckling period is critical for epigenetic development of intestinal stem cells139. The gut microbiome facilitates these postnatal epigenetic processes, and disruption of intestinal epithelial cell methylation by cell-type specific deletion of the DNA methyltransferase Dnmt1 induced enteropathy at postnatal day 7 in a murine model139. It remains to be determined whether similar patterns of DNA methylation are present in mothers with EED, and whether or how these changes are passed from mother to child.
Breast milk might also serve as a source of intergenerational signals influencing infant development. Maternal milk contains a multitude of bioactive components, including immune cells, antibodies, growth factors and cytokines. Breast milk contains human milk oligosaccharides (HMOs) that are minimally absorbed in the infant gut, but transit to the large intestine where they serve as prebiotic compounds that shape the infant microbiota140. Maternal diet can shape HMO composition and the functional capacity of milk-resident bacteria141. Indeed, mothers of healthy infants in Malawi were shown to have elevated levels of sialylated oligosaccharides in breast milk compared with mothers of infants with stunted growth. In a murine model, sialylated bovine milk oligosaccharides led to greater weight gain and bone volume when given to gnotobiotic mice colonized with microbiota from a Malawian infant with severely stunted growth compared with mice fed an unsupplemented diet142. These sialylated structures were also able to shape immune function both locally and systemically in this model, boosting the number of small intestinal tuft cells and reducing the number of bone-resorbing osteoclasts in femoral bone143. A biomarker of bone resorption was elevated in children with stunting prior to nutritional therapy, after which it was decreased143. Because bone-resorbing osteoclasts develop from immune cells, these results suggest a potential mechanism by which sialylated HMOs might influence immunity and, in turn, linear growth. HMOs are primarily consumed by members of the genus Bifidobacterium, which can themselves regulate immune development, presenting an intriguing potential avenue by which to shape the early-life microbiome and its functional properties144.
Maternal immune cells can also be found in breast milk, and these cells can have persistent effects on infant immunity. Specifically, breast milk contains neutrophils, macrophages, epithelial cells, stem cells and lymphocytes (including innate lymphoid cells and natural killer cells)145,146. Maternal immune cells can adhere to the infant gut and traffic to other organs147. In murine models, this process can impart an antigen-specific immune response to Mycobacterium tuberculosis in fostered, unimmunized pups reared by immunized dams148. Transferred cells also included maternal FoxP3+ T cells that could be identified in the thymus and spleen of fostered pups149. Maternally derived cells have been identified in the bone marrow of offspring, and these cells showed long-term persistence and tolerance to non-inherited maternal antigens in a murine model150,151. Intriguingly, similar results have also been reported in lambs and piglets, in whom labelled lymphocytes were absorbed in the gastrointestinal tract and entered the circulation152,153. Maternal antibodies, in addition to providing passive immunity, might also have a role in determining offspring immune reactivity154.
In addition to HMOs and immune cells, breast milk also contains maternal-gut-derived microorganisms155. The composition of breast milk microorganisms is shaped by delivery mode, gestational age and lactation stage156. The mechanisms by which maternal gut microorganisms are delivered to this site are still unclear, although it has been postulated that maternal gut dendritic cells and macrophages might sample microorganisms from the intestinal lumen and transport these microbial cells to breast milk157. Supporting this idea, intestinal immune cells, including IgA-producing B cells, are known to traffic to the mammary gland during pregnancy and lactation157. Further research is needed to determine the source and mechanisms behind the presence of gut-derived microorganisms in breast milk, and to identify how these microorganisms are altered by maternal undernutrition and EED.
Early-life microbiome
A child’s microbiome matures gradually over the first 3 years of life, eventually reaching an adult-like configuration158,159. These changes correlate broadly with changes in diet. Milk-consuming microorganisms of the genus Bifidobacterium dominate the gut during periods of exclusive breastfeeding. By contrast, taxa more adapted to the consumption of complex sugars and starch expand upon the introduction of complementary foods160,161. The microbial metagenome mirrors these trends, with changes in the presence of functional genes for degradation of milk sugars and plant polysaccharides, which are enriched during exclusive breastfeeding and after the introduction of complementary foods, respectively160.
In a study of 64 Bangladeshi children with severe acute malnutrition, patterns of microbial community assembly during the first 20 months of life were altered compared with healthy individuals as controls162. The gut microbiota of children with severe acute malnutrition seemed to be more similar to microbial communities present in healthy children of a younger age, leading to the conclusion that these children harboured ‘immature’ microbial communities. Diarrhoeal episodes were also associated with alterations in the microbiota in this study. In a study of seven Bangladeshi adults, the composition of the microbiota during recovery from infection with Vibrio cholerae closely resembled the pattern of community assembly observed in early life163. Heavy burdens of early childhood diarrhoea are linked to adverse developmental and cognitive outcomes, and diarrhoeal episodes in early life could also affect the timing and process of microbiota maturation164. Indeed, pathogenic infections are common in areas with high burdens of undernutrition and EED, and detection of Shigella spp., enteroaggregative E. coli, Campylobacter spp. and Giardia in stool samples negatively correlates with linear growth in the first 2 years of life165. In animal models, colonization with pathogenic E.coli and Campylobacter jejuni can recapitulate some of the effects of EED observed in children166-169 (Table 1). By contrast, no correlation was found between length-for-age Z score and pathogen burden in duodenal aspirates of children with EED, although specific members of the duodenal microbiota did correlate with reduced length-for-age Z score, including Veillonella spp., Streptococcus spp. and Rothia mucilaginosa25. In a non-human primate model, growth faltering during infancy was associated with intestinal inflammation and ‘decompartmentalization’ of specific microbial taxa between the small and large intestine170. For example, Streptococcus was identified in the small intestine in healthy animals, but found in increased abundance in the colon of animals with growth faltering. By contrast, Prevotella, Catenibacterium and Lachnospiraceae were primarily found in the large intestine of healthy animals, but were identified in increased abundance of the small intestine of animals with growth faltering170. These data suggest that both the composition and the physical geography of the gut microbiota might be altered in undernutrition and EED.
Table 1 ∣.
Mammalian models of EED and the microbiome
| Animal | Sex | Age | Diet | Colonization | Outcome | Refs |
|---|---|---|---|---|---|---|
| C57BL/6J mice | Male | 5.5 weeks | 10.5% protein, 21.1% fat | Gnotobiotic: cultured strains from duodenal aspirates of children with EED | ↑ Bacterial translocation ↑ Inflammatory infiltrate ↑ Crypt elongation ↑ Duodenal REG3β, REG3γ ↑ Serum and tissue MMP8 |
25 |
| C57BL/6J mice | Male | Adult | 10.4% protein, 0.9% fat | Gnotobiotic: IgA+ bacteria from 21-month-old twins ± kwashiorkor | ↑ Diet-dependent weight loss ↑ Barrier disruption ↑ Serum inflammatory cytokines ↓ Proliferation of IEC progenitors |
180 |
| C57BL/6 mice | Female | 3 weeks | 7% protein, 5% fat | SPF plus Bacteroides vulgatus, Bacteroides dorei, Bacteroides fragilis, Parabacteroides distasonis, Bacteroides ovatus and Escherichia coli | ↑ Epithelial disruption, permeability ↑ IL-6, calprotectin ↑ Small intestine IELs ↓ Salmonella resistance |
166 |
| C57BL/6Tac mice | Male and female | 3 weeks | 7% protein, 7% fat | SPF plus adherent-invasive E. coli | ↓ Weight gain; tail length ↑ Permeability, villous blunting ↑ Tissue IFNγ, LCN2 ↑ Small intestine lamina propria Treg cells ↓ Oral vaccine response |
181 |
| C57BL/6 | Male | 3 weeks | 7% protein, 5% fat | SPF plus enteroaggerative E. coli | ↑ Enteroaggerative E. coli shedding ↑ Enteroaggerative E. coli small intestine burden |
167 |
| C57BL/6 | Not identified | 6 days | 2% protein | SPF plus enteroaggerative E. coli | ↓ Weight gain ↑ Enteroaggerative E. coli burden |
168 |
| Piglets | Not identified | 4 days | Low (7.5%) protein | Gnotobiotic: healthy infant faecal microbiota | ↓ Growth ↓ Rotavirus challenge response |
182,183 |
| Piglets | Not identified | 5–7 days | Sufficient | Gnotobiotic: infant microbiota from rotavirus vaccine responder and non-responder | ↑ Duration of rotavirus shedding ↑ Viral titre ↑ Duration of diarrhoea |
184 |
| Macaques | Male and female | 6–12 months | Not identified | Endogenous microbiota in animals with and without growth faltering and/or diarrhoea | ↑ Inflammatory cytokines ↑ Microbiota disruption ↑ Campylobacter abundance |
170 |
Although critical work has been published in other model systems, in Table 1 we focus on mammalian studies incorporating features of altered gut microbial communities that result in characteristic features of environmental enteric dysfunction (EED). These include studies in conventional and gnotobiotic mice, piglets and macaques. The microbial communities studied include human infant microbiota, as well as endogenous mouse or macaque microbial species. These models replicate some of the most critical features of EED, and permit the mechanistic study of biological pathways underlying this disorder. Developing animal models of maternal EED presents a major opportunity to discover critical biology and improve the growth of children around the world. IEC, intestinal epithelial cell; IEL, intraepithelial lymphocyte; IFNγ, interferon-γ; IgA, immunoglobulin A; LCN2, lipocalin 2; MMP8, matrix metalloproteinase 8; SPF, specific-pathogen-free; Treg cells, regulatory T cells.
Microbial communities in early life have long-term implications for health, influencing phenotypes as diverse as linear growth, cognitive function, susceptibility to infection and metabolic disease. Many of these findings have emerged from comparison of germ-free mice with those colonized with human microbiota, or gnotobiotic mice colonized with specific microbial taxa whose functions have been implicated in host phenotypes. For example, germ-free mouse pups experienced reduced growth and bone volume compared with pups raised with conventional microbiota, and this was linked to a reduction in systemic insulin-like growth factor 1 (IGF1)171. Administration of a strain of Lactobacillus plantarum improved growth on a malnourished diet and increased IGF1 levels. A separate study reported that microbiota-derived SCFAs drove IGF1 production in conventionally raised mice, providing a link between gut microbial metabolism and host development172.
Inflammation is another potential pathway by which the gut microbiota might influence development. Interestingly, the negative effects of inflammation on cognitive development have been demonstrated both during pregnancy as well as during the postnatal period. A study of mother-infant pairs in Bangladesh identified a negative association between the inflammatory biomarkers C-reactive protein, soluble CD14, IL-1β and IL-6 in serum and neurodevelopmental outcomes173. Early-life infection with E. coli in rats was also shown to influence brain development in adulthood, altering microglia and neurogenesis in these animals174. In addition to shaping immunity and protecting against infection via colonization resistance, the microbiota can also have a role in regulating neurotransmitter production in the host gastrointestinal tract in a murine model, presenting another potential avenue for the modulation of host behaviour175. Thus, the potential effects of the gut microbiota extend beyond pregnancy and into early life.
Immunity and oral vaccination
Mouse studies have demonstrated a critical window during early development around the time of weaning in which transient opening of goblet-cell-associated antigen passages enables immune recognition of luminal bacteria176. This ‘weaning reaction’ led to a temporary increase in inflammatory cells and cytokines in the ileum, which helped to constrain later inflammatory responses through production of Treg cells177. Another study demonstrated that the number of Treg cells in the colon was determined by a non-genetic, heritable mechanism in which maternal IgA responses to the microbiota modulate Treg cell number178. This information was transmitted to offspring through breast milk during a tight window of development in early life. Most strikingly, this Treg cell set point could then be further transmitted to multiple generations of female offspring. Mice with elevated Treg cells were more susceptible to intestinal pathogens but less susceptible to cancer, allergy and colitis178. Intriguingly, IgA responses to the microbiota were increased in children with undernutrition and EED in a study of 138 individuals in Madagascar and the Central African Republic179. In a murine model, IgA targeted members of the microbiota that could transmit diet-dependent enteropathy180.
These findings on intestinal Treg cells also have major implications for the efficacy of oral vaccines. Work by Bhattacharjee et al. demonstrated that Treg cells were elevated in the small intestine in a murine model consisting of a low-protein diet and adherent-invasive E. coli to induce EED-like weight loss, inflammation and intestinal barrier disruption181 (Table 1). Treg cells reduced oral vaccine efficacy against an attenuated E. coli heat labile toxin in a microbiota-dependent manner, but their ablation reduced weight gain. Immunity was also altered in a gnotobiotic piglet model in which animals were colonized with healthy infant microbiota and fed a protein-deficient diet, leading to reduced vaccine-mediated protection from challenge with rotavirus182,183. The gut microbiota might have a role in regulating these responses, given that transplantation of gut microbiota from a healthy human infant into gnotobiotic piglets led to increased rotavirus-specific T cell responses compared with transplanting microbiota from an infant with enteropathy184. These findings provide intriguing preliminary evidence for a link between the gut microbiota and regulatory immune responses that could act as a double-edged sword in EED. Specifically, Treg cells might be necessary to constrain inflammation and improve growth, but at the same time they could enhance susceptibility to infection and reduce oral vaccine responses. In turn, inflammatory responses might be protective against infectious agents in the short term but harmful to host growth and development in the long term.
The extent to which these immunological phenomena apply to humans remains to be determined. The human gut is functionally immature at birth, with greater permeability to bacteria as well as maternal-derived immune mediators185,186. A study of full-term healthy infants demonstrated that neonatal intestinal permeability, assessed via the lactulose–mannitol dual sugar absorption test, declined substantially over the first 30 days of life. This decline was accelerated in breastfed compared with formula-fed infants187. Investigating how neonatal gut permeability, the microbiota and its links to immunity are affected by maternal undernutrition and EED might present additional therapeutic targets to improve child growth and protect against infection.
Emerging technologies and therapies
Advances in modelling EED using human and mouse enteroids have further enabled mechanistic investigation into epithelial cell function in this disorder. Glutamine deprivation and methyl donor deficiency recapitulate some isolated features of undernutrition and EED in murine enteroids87,188. Human enteroids have been generated from Pakistani children with refractory EED and incorporated into organ-on-a chip platforms189. When exposed to microorganisms or nutritionally depleted culture media, these EED-on-a-chip platforms display barrier defects and produce higher levels of cytokines than chips derived from children without EED. Despite lacking the full complexity of components that comprise the small intestine, human enteroids are an attractive preclinical platform for understanding gut adaptation to pregnancy and lactation and the effect of EED on these processes.
As microbiome-directed, bulk and single-cell RNA sequencing, and metabolomic studies of EED increase, the deluge of data is being analysed using cutting-edge data science approaches190. Of these, deep-learning image analysis of EED versus healthy features in duodenal tissue is particularly exciting. For example, Syed and colleagues applied a deep-learning convolutional neural network to images from duodenal biopsy samples from patients with EED, achieving 93.4% case-detection accuracy, with a false-negative rate of 2.4%, and automated learning of cellular features of EED such as loss of secretory lineages191. Computer vision techniques will prove particularly useful if technologies that can safely visualize and sample the small intestinal mucosa and microbiota as well as assess barrier function repeatedly over the first 1,000 days, without the need for sedation, can be scaled up. For example, tethered capsule endomicroscopy is currently being explored in infants and pregnant women in the USA and Pakistan192,193. Tissue-based techniques will ultimately enable the development and validation of robust EED biomarkers that can be detected non-invasively, shedding light on the ‘black boxes’ of maternal and infant EED.
Identifying durably effective therapies for EED and undernutrition has been both a major global health priority and a seemingly insurmountable challenge. While great gains have been made in the treatment of acute undernutrition (wasting and underweight) and diarrhoeal diseases, attention has necessarily shifted towards improving both quality and quantity of life in areas affected by EED. Prevention or reversal of linear growth faltering has been more challenging to achieve, with only modest gains seen following intensive preconception194 and prenatal nutritional supplementation1; water, sanitation and hygiene (WASH) interventions195; anti-inflammatory medications196; and antibiotics30.
With the critical role of the gut microbiota in mind, new avenues for therapeutic intervention have emerged. A clinical trial demonstrated the promise of employing ‘microbiota-directed complementary foods’ (MDCFs) to boost levels of microbial taxa associated with healthy development in early life21. This work used preclinical mouse models to identify dietary ingredients that enriched growth-associated taxa, and identified a combination of four food ingredients (chickpea, peanut, banana and soyflour) that seemed to be effective in both gnotobiotic mice and piglets at enriching growth-associated taxa in young animals197. In children aged 12–18 months with moderate acute malnutrition, this MDCF formulation led to statistically significant increases in mean rate of increase in weight-for-length and weight-for-age Z scores relative to controls given a ready-to-use supplementary food (difference in change 0.011 (95% CI 0.001–0.021) and 0.008 (95% CI 0.001–0.015), respectively). These increases were associated with elevations in plasma proteins linked to bone growth, neurodevelopment and inflammation21; however, length-for-age scores were unchanged between children receiving the MDCF treatment versus traditional ready-to-use therapeutic foods. Whether longer therapeutic treatment with MDCFs is more effective at improving length-for-age scores, and whether these changes are beneficial for cognitive function, remains to be seen. Complex probiotic consortia and fermented foods that might assist in the recovery of the gut microbiota during EED and pregnancy also deserve further exploration198-200.
HMOs might also be an appealing addition to current therapies. The ability of HMOs to modulate host immunity, boost beneficial microbial taxa and protect against gastrointestinal infection might prove to be another critical tool against growth stunting201. Current commercially available HMOs include 2′-fucosyllactose and lacto-N-tetraose. These compounds are currently being added to some infant formulas in the USA after achieving GRAS (generally recognized as safe) status from the USA Food and Drug Administration (FDA)202. However, major questions remain regarding the potential utility of HMOs in relation to EED. Different HMO structures have diverse effects on host physiology, and it is unclear which HMOs could be useful in this disorder. Additionally, the question of when HMOs might be effective will need to be addressed. In infancy, an abundance of Actinobacteria (including Bifidobacterium longum subsp. infantis, a major consumer of HMOs) correlated with immune responses to vaccination203. However, beyond the first year of life, data indicate that the transition away from a microbial community dominated by Bifidobacterium might be advantageous for growth21.
Many questions also remain regarding maternal EED (Fig. 4). With an estimated 20% of stunting originating in utero, intervening in the first years of life might not be early enough to prevent some of the worst effects of growth impairment1,204. More work is needed to develop and adequately characterize model systems that incorporate components of maternal nutrition and microbiota, and to relate their findings to existing data from human studies. These models might uncover novel target pathways, and will serve as critical tools in the investigation of therapies for maternal and child health during EED.
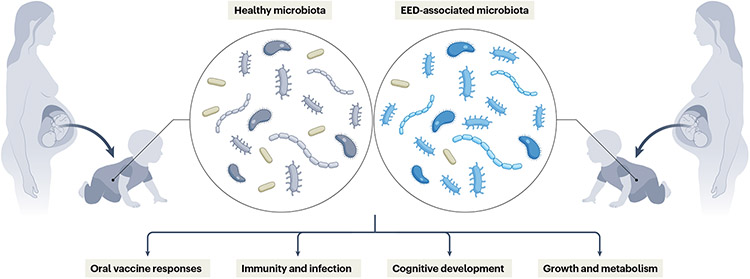
Fig. 4 ∣. Long-term implications of maternal environmental enteric dysfunction.
Maternal gut inflammation is a risk factor for poor fetal growth and adverse birth outcomes in multiple enteropathies. Infants inherit a substantial proportion of their gut microbiota from their mothers; thus, the maternal microbiome can play a part in shaping immunity in both mother and child. Altered maternal and infant gut function can have lifelong health consequences, including susceptibility to infection, oral vaccine responses, cognitive development and metabolic tone. EED, environmental enteric dysfunction.
Conclusions
The exquisite remodelling of the human gut in symbiosis with its microbial inhabitants during the 1,000-day period spanning the metabolic ‘ultramarathon’ of pregnancy, lactation and the first 2 years of a child’s life is truly remarkable. The extent to which EED undermines this adaptation to contribute to an intergenerational burden of chronic undernutrition in LMICs is far from clear (Box 1). However, enteropathies that are prevalent in high-income countries/regions and preclinical animal models provide important clues. More directly, the heroic work by LMIC investigators and the application of new scientific tools to address one of the great challenges of our time are collectively closing the knowledge gap. Political will, implementation science and discoveries hold the promise of accelerating progress towards the United Nations Sustainable Development Goals 2030 targets. Ultimately, improving the gut health of women, and of children in their first 1,000 days, is critical to unlocking individual potential and enabling communities to thrive.
Box 1. Future directions and open research questions.
How prevalent is maternal environmental enteric dysfunction (EED), and to what extent does it contribute to poor fetal growth, birth outcomes and child growth? Does pregnancy, in turn, modulate EED?
How do alterations in the maternal microbiome and diet regulate gut function during pregnancy, and what effect does this have on fetal development?
How do maternal undernutrition and EED influence milk composition and immune and microbial signals received by infants during pregnancy and lactation?
What is the critical window of opportunity in which to intervene to promote child growth and immune development through nutritional or microbiome-based therapies?
How do alterations in microbiota composition and function shape developing immunity in the first 1,000 days of life?
Can the microbiome promote appropriate regulatory and inflammatory responses to oral vaccines and pathogens while maintaining healthy growth?
To what degree are microbiome-directed therapies scalable across diverse geographies?
Key points.
Maternal and neonatal anthropometry are key predictors of childhood stunting, highlighting the intergenerational nature of undernutrition and pinpointing the first 1,000 days of life as a critical window for development.
Pregnancy and lactation are metabolically demanding, requiring an expansion of small intestinal absorptive capacity; enteropathies adversely affect perinatal outcomes.
Environmental enteric dysfunction (EED) is characterized by inflammation, increased barrier permeability, and reduced absorptive capacity. Its prevalence and consequences in mothers in low and middle-income countries warrant urgent investigation.
Gut microbial communities are disrupted during EED and undernutrition in humans, and confer aspects of these phenotypes to gnotobiotic mice; nutrient processing, absorption and regulation of immunity are potential mechanisms.
Infants inherit a substantial portion of their microbiome from their mothers. Maternal microorganisms, breast milk and epigenetics are implicated in intergenerational undernutrition.
Gut microbial communities in early life shape host immunity, with potential consequences for survival, growth and cognitive development.
Acknowledgements
The authors were supported by the US National Institutes of Health (awards R01HD105729 (to C.A.C.), D43TW007585 (to S.R.M., S.S., Z.J. and S.A.A.), K23DK117061 (to S.S.), K43TW010697 (to N.I.) and U19AI116491 (to S.R.M.)); and by the Bill and Melinda Gates Foundation (grants OPP1144149 (to S.R.M.) and OPP1138727 (to S.A.A.)). The authors gratefully acknowledge insights derived from conversations with Bill and Melinda Gates Foundation programme officers R. Elliott, C. Damman, J. Yan, H. Gammill and V. Ridaura.
Footnotes
Competing interests
S.R.M. was a paid consultant for Takeda on paediatric short bowel syndrome in 2020. S.R.M. receives royalties from UpToDate for a chapter entitled “Persistent diarrhoea in children in resource-limited countries”. The other authors declare no competing interests.
References
- 1.Prendergast AJ & Humphrey JH The stunting syndrome in developing countries. Paediatr. Int. Child. Health 34, 250–265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes CF, Sousa M, Lourenço I, Martins D & Torres J Gastrointestinal diseases during pregnancy: what does the gastroenterologist need to know? Ann. Gastroenterol 31, 385–394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ & Lima AAM The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol 10, 220–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syed S, Ali A & Duggan C Environmental enteric dysfunction in children. J. Pediatr. Gastroenterol. Nutr 63, 6–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe K & Petri WA Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine 10, 25–32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagundes-Neto U, Viaro T, Wehba J, Patrício FR & Machado NL Tropical enteropathy (environmental enteropathy) in early childhood: a syndrome caused by contaminated environment. J. Trop. Pediatr 30, 204–209 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Campbell RK et al. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. J. Pediatr. Gastroenterol. Nutr 65, 40–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosek M et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg 88, 390–396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick BJJ et al. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. Am. J. Trop. Med. Hyg 96, 465–472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tickell KD, Atlas HE & Walson JL Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 17, 181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosek MN & MAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 18, 109–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marie C, Ali A, Chandwe K, Petri WA & Kelly P Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol. 11, 1290–1298 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Levine MM Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 129 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2021 edition. WHO; https://www.who.int/publications-detail-redirect/9789240025257 (2021). [Google Scholar]
- 15.Olofin I et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS ONE 8, e64636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black RE et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Moore SR, Lima AAM & Guerrant RL Preventing 5 million child deaths from diarrhea in the next 5 years. Nat. Rev. Gastroenterol. Hepatol 8, 363–364 (2011). [DOI] [PubMed] [Google Scholar]
- 18.United Nations. Transforming our world: the 2030 agenda for sustainable development. United Nations Department of Economic and Social Affairs; https://sdgs.un.org/2030agenda (2015). [Google Scholar]
- 19.Matonhodze CR Leaving no one behind: impact of COVID-19 on the Sustainable Development Goals (SDGs). United Nations Development Programme; https://www.undp.org/publications/leaving-no-one-behind-impact-covid-19-sustainable-development-goals-sdgs (2021). [Google Scholar]
- 20.de Onis M & Branca F Childhood stunting: a global perspective. Matern. Child. Nutr 12, 12–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RY et al. A microbiota-directed food intervention for undernourished children. N. Engl. J. Med 384, 1517–1528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crookston BT et al. Postinfancy growth, schooling, and cognitive achievement: young lives. Am. J. Clin. Nutr 98, 1555–1563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice AM et al. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr 97, 911–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima AAM et al. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr 40, 28–35 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Chen RY et al. Duodenal microbiota in stunted undernourished children with enteropathy. N. Engl. J. Med 383, 321–333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberman Y et al. Mucosal genomics implicate lymphocyte activation and lipid metabolism in refractory environmental enteric dysfunction. Gastroenterology 160, 2055–2071.e0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodges P, Tembo M & Kelly P Intestinal biopsies for the evaluation of environmental enteropathy and environmental enteric dysfunction. J. Infect. Dis 224, S856–S863 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonaesch P et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl Acad. Sci 115, E8489–E8498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordiz MI et al. Environmental enteric dysfunction is associated with poor linear growth and can be identified by host fecal mRNAs. J. Pediatr. Gastroenterol. Nutr 63, 453–459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBoer MD et al. Effect of scheduled antimicrobial and nicotinamide treatment on linear growth in children in rural Tanzania: a factorial randomized, double-blind, placebo-controlled trial. PLoS Med. 18, e1003617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tickell KD & Walson JL Nutritional enteric failure: neglected tropical diseases and childhood stunting. PLoS Negl. Trop. Dis 10, e0004523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard SA et al. Enteric dysfunction and other factors associated with attained size at 5 years: MAL-ED birth cohort study findings. Am. J. Clin. Nutr 10.1093/ajcn/nqz004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arndt MB et al. Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am. J. Trop. Med. Hyg 95, 694–701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr. Suppl 450, 76–85 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Grantz KL et al. Unified standard for fetal growth: the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies. Am. J. Obstet. Gynecol 226, 576–587.e2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papageorghiou AT et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am. J. Obstet. Gynecol 218, S630–S640 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Kiserud T et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 14, e1002220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells JCK The new ‘obstetrical dilemma’: stunting, obesity and the risk of obstructed labour. Anat. Rec 300, 716–731 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Dunsworth HM, Warrener AG, Deacon T, Ellison PT & Pontzer H Metabolic hypothesis for human altriciality. Proc. Natl Acad. Sci. USA 109, 15212–15216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarzenberg SJ, Georgieff MK & Committee on Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 141, e20173716 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Lauer JM et al. Biomarkers of maternal environmental enteric dysfunction are associated with shorter gestation and reduced length in newborn infants in Uganda. Am. J. Clin. Nutr 108, 889–896 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirby MA et al. Biomarkers of environmental enteric dysfunction and adverse birth outcomes: an observational study among pregnant women living with HIV in Tanzania. eBioMedicine 84, 104257 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tersigni C et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 20, 582–593 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Cornish J et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 56, 830–837 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alstead EM & Nelson-Piercy C Inflammatory bowel disease in pregnancy. Gut 52, 159–161 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leonard MM et al. Value of IgA tTG in predicting mucosal recovery in children with celiac disease on a gluten-free diet. J. Pediatr. Gastroenterol. Nutr 64, 286–291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T-C et al. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl. Trop. Dis 14, e0007975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurber C et al. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv 5, eaaw0341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond KA, Konarzewski M, Torres RM & Diamond J Metabolic ceilings under a combination of peak energy demands. Physiological Zool. 67, 1479–1506 (1994). [Google Scholar]
- 50.Hammond KA & Diamond J Maximal sustained energy budgets in humans and animals. Nature 386, 457–462 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Hammond KA Adaptation of the maternal intestine during lactation. J. Mammary Gland. Biol. Neoplasia 2, 243–252 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Butte NF, Wong WW, Treuth MS, Ellis KJ & O’Brian Smith E Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr 79, 1078–1087 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Kominiarek MA & Rajan P Nutrition recommendations in pregnancy and lactation. Med. Clin. North Am 100, 1199–1215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butte NF & King JC Energy requirements during pregnancy and lactation. Public Health Nutr. 8, 1010–1027 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Nakada D et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabet Sarvestani F, Rahmanifar F & Tamadon A Histomorphometric changes of small intestine in pregnant rat. Vet. Res. Forum 6, 69–73 (2015). [PMC free article] [PubMed] [Google Scholar]
- 57.Prieto RM, Ferrer M, Fe JM, Rayó JM & Tur JA Morphological adaptive changes of small intestinal tract regions due to pregnancy and lactation in rats. Ann. Nutr. Metab 38, 295–300 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Boyne R, Fell BF & Robb I The surface area of the intestinal mucosa in the lactating rat. J. Physiol 183, 570–575 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casirola DM & Ferraris RP Role of the small intestine in postpartum weight retention in mice. Am. J. Clin. Nutr 78, 1178–1187 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Şensoy E & Öznurlu Y Determination of the changes on the small intestine of pregnant mice by histological, enzyme histochemical, and immunohistochemical methods. Turk. J. Gastroenterol 30, 917–924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Z et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep 9, 8367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drucker DJ The discovery of GLP-2 and development of teduglutide for short bowel syndrome. ACS Pharmacol. Transl. Sci 2, 134–142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan X et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130, 150–164 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Kahr MK et al. SERUM GLP-2 is increased in association with excess gestational weight gain. Am. J. Perinatol 10.1055/s-0041-1728828 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garella R, Squecco R & Baccari MC Site-related effects of relaxin in the gastrointestinal tract through nitric oxide signalling: an updated report. Curr. Protein Pept. Sci 18, 1254–1262 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Lemmens K, Doggen K & De Keulenaer GW Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am. J. Physiol. Heart Circ. Physiol 300, H931–H942 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Kilik U et al. Maturation of human intestinal epithelium from pluripotency in vitro. Preprint at bioRxiv 10.1101/2021.09.24.460132 (2021). [DOI] [Google Scholar]
- 68.Collado MC, Isolauri E, Laitinen K & Salminen S Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr 88, 894–899 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Koren O et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.García-Gómez E, González-Pedrajo B & Camacho-Arroyo I Role of sex steroid hormones in bacterial–host interactions. BioMed. Res. Int 2013, e928290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gohir W et al. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 6, 310–320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nuriel-Ohayon M et al. Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 73.DiGiulio DB et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl Acad. Sci. USA 112, 11060–11065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bisanz JE et al. Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of moringa-supplemented probiotic yogurt. Appl. Environ. Microbiol 81, 4965–4975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gough EK et al. Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. EBioMedicine 68, 103421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lammert CR et al. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J. Immunol 10.4049/jimmunol.1701755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi GB et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vuong HE et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 10.1038/s41586-020-2745-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Ahmed T, Hossain M & Sanin KI Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann. Nutr. Metab 61, 8–17 (2012). [DOI] [PubMed] [Google Scholar]
- 82.McGuire S WHO guideline: vitamin A supplementation in pregnant women. Geneva: WHO, 2011; WHO guideline: vitamin A supplementation in postpartum women. Geneva: WHO, 2011. Adv. Nutr 3, 215–216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grizotte-Lake M et al. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49, 1103–1115.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jijon HB et al. Intestinal epithelial cell-specific RARα depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 11, 703–715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kheirouri S & Alizadeh M Decreased serum and mucosa immunoglobulin A levels in vitamin A and zinc-deficient mice. Cent. Eur. J. Immunol 39, 165–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hibberd MC et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci. Transl. Med 9, eaal4069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alves da Silva AV et al. Murine methyl donor deficiency impairs early growth in association with dysmorphic small intestinal crypts and reduced gut microbial community diversity. Curr. Dev. Nutr 3, nzy070 (2019). [Google Scholar]
- 88.Forgie AJ et al. The impact of maternal and early life malnutrition on health: a diet-microbe perspective. BMC Med. 18, 135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayneris-Perxachs J et al. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr 104, 1253–1262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Preidis GA et al. Composition and function of the undernourished neonatal mouse intestinal microbiome. J. Nutr. Biochem 26, 1050–1057 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Marwarha G, Claycombe-Larson K, Schommer J & Ghribi O Maternal low protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J. Nutr. Biochem 45, 54–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hashimoto T et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Guryn K et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23, 458–469.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sayin SI et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Semova I et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12, 277–288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makki K, Deehan EC, Walter J & Bäckhed F The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Priyadarshini M et al. Maternal short-chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl. Res 164, 153–157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakajima A et al. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J. Immunol 199, 3516–3524 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Thorburn AN et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun 6, 7320 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Tolhurst G et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cani PD et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y, Raka F & Adeli K The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med 8, 2227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Onis M, Blössner M & Villar J Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr 52, S5–S15 (1998). [PubMed] [Google Scholar]
- 104.Christian P et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol 42, 1340–1355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arifeen SE et al. Infant growth patterns in the slums of Dhaka in relation to birth weight, intrauterine growth retardation, and prematurity. Am. J. Clin. Nutr 72, 1010–1017 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Sato Y et al. Maternal gut microbiota is associated with newborn anthropometrics in a sex-specific manner. J. Dev. Orig. Health Dis 10.1017/S2040174419000138 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Shiozaki A et al. Intestinal microbiota is different in women with preterm birth: results from terminal restriction fragment length polymorphism analysis. PLoS ONE 9, e111374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dahl C et al. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PLoS ONE 12, e0184336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kimura I et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367, eaaw8429 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Machado CJ, Whitaker AM, Smith SEP, Patterson PH & Bauman MD Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiat 77, 823–832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bauman MD et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiat 75, 332–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nyangahu DD et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 6, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyoshi J et al. Peripartum exposure to antibiotics promotes persistent gut dysbiosis, immune imbalance, and inflammatory bowel disease in genetically prone offspring. Cell Rep. 20, 491–504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agüero M Gde et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Leiby JS et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lauder AP et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mishra A et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409.e20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aagaard K et al. The placenta harbors a unique microbiome. Sci. Transl. Med 6, 237ra65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asnicar F et al. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems 2, e00164–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yassour M et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe 24, 146–154.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferretti P et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martín R et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Env. Microbiol 75, 965–969 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Collado MC, Delgado S, Maldonado A & Rodríguez JM Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett. Appl. Microbiol 48, 523–528 (2009). [DOI] [PubMed] [Google Scholar]
- 124.Perez PF et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119, e724–e732 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Grölund M-M, Lehtonen O-P, Eerola E & Kero P Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr 28, 19–25 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Penders J et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Biasucci G, Benenati B, Morelli L, Bessi E & Boehm G Cesarean delivery may affect the early biodiversity of intestinal bacteria. J. Nutr 138, 1796S–1800S (2008). [DOI] [PubMed] [Google Scholar]
- 128.Dominguez-Bello MG et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shao Y et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chu DM et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med 23, 314–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dominguez-Bello MG et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med 22, 250–253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Korpela K et al. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell 183, 324–334.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 133.Mårild K, Stephansson O, Montgomery S, Murray JA & Ludvigsson JF Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 142, 39–45.e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keag OE, Norman JE & Stock SJ Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 15, e1002494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Myklestad K, Vatten LJ, Magnussen EB, Salvesen KÅ & Romundstad PR Do parental heights influence pregnancy length?: a population-based prospective study, HUNT 2. BMC Pregnancy Childbirth 13, 33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang G et al. Assessing the causal relationship of maternal height on birth size and gestational age at birth: a Mendelian randomization analysis. PLoS Med. 12, e1001865 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Özaltin E, Hill K & Subramanian SV Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA 303, 1507–1516 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Addo OY et al. Maternal height and child growth patterns. J. Pediatr 163, 549–554.e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu D-H et al. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol. 16, 211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zivkovic AM, German JB, Lebrilla CB & Mills DA Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl Acad. Sci 108, 4653–4658 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seferovic MD et al. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep 10, 22092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Charbonneau MR et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164, 859–871 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cowardin CA et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc. Natl Acad. Sci 116, 11988–11996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Henrick BM et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Goldman AS & Goldblum RM Transfer of maternal leukocytes to the infant by human milk. In Reproductive Immunology (ed. Olding LB) 205–213 (Springer, 1997). [DOI] [PubMed] [Google Scholar]
- 146.Baban B, Malik A, Bhatia J & Yu JC Presence and profile of innate lymphoid cells in human breast milk. JAMA Pediatr. 172, 594–596 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cabinian A et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS ONE 11, e0156762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghosh MK, Nguyen V, Muller HK & Walker AM Maternal milk T cells drive development of transgenerational Th1 immunity in offspring thymus. J. Immunol 197, 2290–2296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghosh MK, Muller HK & Walker AM Lactation-based maternal educational immunity crosses MHC class I barriers and can impart Th1 immunity to Th2-biased recipients. J. Immunol 199, 1729–1736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dutta P & Burlingham WJ Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism 1, 2–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kinder JM, Stelzer IA, Arck PC & Way SS Immunological implications of pregnancy-induced microchimerism. Nat. Rev. Immunol 17, 483–494 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tuboly S, Bernáth S, Glávits R, Kovács A & Megyeri Z Intestinal absorption of colostral lymphocytes in newborn lambs and their role in the development of immune status. Acta Vet. Hung 43, 105–115 (1995). [PubMed] [Google Scholar]
- 153.Tuboly S, Bernáth S, Glávits R & Medveczky I Intestinal absorption of colostral lymphoid cells in newborn piglets. Vet. Immunol. Immunopathol 20, 75–85 (1988). [DOI] [PubMed] [Google Scholar]
- 154.Lemke H, Coutinho A & Lange H Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 25, 180–186 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Martín R et al. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediat 143, 754–758 (2003). [DOI] [PubMed] [Google Scholar]
- 156.Khodayar-Pardo P, Mira-Pascual L, Collado MC & Martínez-Costa C Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol 34, 599–605 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Wilson E & Butcher EC CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J. Exp. Med 200, 805–809 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yatsunenko T et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bokulich NA et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med 8, 343ra82–343ra82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vatanen T et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bäckhed F et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 162.Subramanian S et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hsiao A et al. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 515, 423–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guerrant DI et al. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg 61, 707–713 (1999). [DOI] [PubMed] [Google Scholar]
- 165.Rogawski ET et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health 6, e1319–e1328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown EM et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun 6, 7806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bolick DT et al. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J. Med. Microbiol 62, 896–905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roche JK, Cabel A, Sevilleja J, Nataro J & Guerrant RL Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J. Infect. Dis 202, 506–514 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Giallourou N et al. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog. 14, e1007083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rhoades NS et al. Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen. Mucosal Immunol. 10.1038/s41385-021-00418-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schwarzer M et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351, 854–857 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Yan J et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl Acad. Sci 113, E7554–E7563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang NM et al. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am. J. Trop. Med. Hyg 97, 974–979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bland ST et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav. Immun 24, 329–338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Knoop KA et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol 2, eaao1314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Al Nabhani Z et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 10.1016/j.immuni.2019.02.014 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Ramanan D et al. An immunologic mode of multigenerational transmission governs a gut treg setpoint. Cell 181, 1276–1290.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huus KE et al. Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: findings from the Afribiota study. Microbiome 8, 113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kau AL et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med 7, 276ra24–276ra24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bhattacharjee A et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy. Immunity 54, 1745–1757.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miyazaki A et al. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine 36, 6270–6281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vlasova AN et al. Protein malnutrition modifies innate immunity and gene expression by intestinal epithelial cells and human rotavirus infection in neonatal gnotobiotic pigs. mSphere 2, e00046–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Twitchell EL et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 8, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Molès J-P et al. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol 29, 133–143 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Weström B, Arévalo Sureda E, Pierzynowska K, Pierzynowski SG & Pérez-Cano F-J The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol 11, 1153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Catassi C, Bonucci A, Coppa GV, Carlucci A & Giorgi PL Intestinal permeability. Changes during the first month: effect of natural versus artificial feeding. J. Pediatr. Gastroenterol. Nutr 21, 383–386 (1995). [DOI] [PubMed] [Google Scholar]
- 188.Moore SR et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am. J. Physiol. Gastrointest. Liver Physiol 308, G831–G839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bein A et al. Nutritional deficiency in an intestine-on-a-chip recapitulates injury hallmarks associated with environmental enteric dysfunction. Nat. Biomed. Eng 10.1038/s41551-022-00899-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kummerlowe C et al. Single-cell profiling of environmental enteropathy reveals signatures of epithelial remodeling and immune activation. Sci. Transl. Med 14, eabi8633 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Syed S et al. Assessment of machine learning detection of environmental enteropathy and celiac disease in children. JAMA Netw. Open 2, e195822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gora MJ et al. Tethered capsule endomicroscopy: from bench to bedside at a primary care practice. J. Biomed. Opt 21, 104001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thompson AJ et al. The potential role of optical biopsy in the study and diagnosis of environmental enteric dysfunction. Nat. Rev. Gastroenterol. Hepatol 14, 727–738 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Hambidge KM et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the women first trial. Am. J. Clin. Nutr 109, 457–469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pickering AJ et al. The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob. Health 7, e1139–e1146 (2019). [DOI] [PubMed] [Google Scholar]
- 196.Jones KD et al. Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Med. 12, 133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gehrig JL et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 365, eaau4732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ito M et al. Fermented foods and preterm birth risk from a prospective large cohort study: the Japan environment and children’s study. Env. Health Prev. Med 24, 25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wastyk HC et al. Gut-microbiota-targeted diets modulate human immune status. Cell 10.1016/j.cell.2021.06.019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bobilev D et al. 1953. VE303, a rationally designed bacterial consortium for prevention of recurrent Clostridioides difficile (C. Difficile) infection (rCDI), stably restores the gut microbiota after vancomycin (vanco)-induced dysbiosis in adult healthy volunteers (HV). Open Forum Infect. Dis 6, S60 (2019). [Google Scholar]
- 201.Fukuda S et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011). [DOI] [PubMed] [Google Scholar]
- 202.Walsh C, Lane JA, van Sinderen D & Hickey RM From lab bench to formulated ingredient: characterization, production, and commercialization of human milk oligosaccharides. J. Funct. Foods 72, 104052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huda MN et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–e372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de Rooij SR, Wouters H, Yonker JE, Painter RC & Roseboom TJ Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl Acad. Sci. USA 107, 16881–16886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]