Abstract
Purpose
Cancers deficient in homologous recombination DNA repair, such as those with BRCA1 or BRCA2 (BRCA1/2) mutations rely on a pathway mediated by the enzyme poly(adenosine diphosphate-ribose) polymerase (PARP). PARP inhibitors (PARPi’s) have demonstrated efficacy in treating patients with germline (g)BRCA1/2, somatic (s)BRCA1/2, and gPALB2 mutations in clinical trials. However, patients with a poor performance status (PS) and those with severe organ impairment are often excluded from clinical trials and cancer-directed treatment.
Methods
We report the cases of two patients with metastatic breast cancer who had poor PS, significant visceral disease, and gPALB2 and sBRCA mutations, who derived significant clinical benefit from treatment with PARP inhibition.
Results
Patient A had germline testing demonstrating a heterozygous PALB2 pathogenic mutation (c.3323delA) and a BRCA2 variant of unknown significance (c.9353T>C), and tumor sequencing revealed PALB2 (c.228_229del and c.3323del) and ESR1 (c.1610A>C) mutations. Patient B was negative for pathologic BRCA mutations upon germline testing, but tumor sequencing demonstrated somatic BRCA2 copy number loss and a PIK3CA mutation (c.1633G>A). Treatment with PARPi’s in these two patients with an initial PS of 3–4 and significant visceral disease resulted in prolonged clinical benefit.
Conclusion
Patients with a poor PS, such as those described here, may still have meaningful clinical responses to cancer treatments targeting oncogenic drivers. More studies evaluating PARPi’s beyond gBRCA1/2 mutations and in sub-optimal PS would help identify patients who may benefit from these therapies.
Keywords: Breast cancer, BRCA, PALB2, PARP, Performance status
Introduction
DNA repair aberrations are a hallmark of cancer, as they play a central role in giving rise to mutations that contribute to cancer development [1, 2]. Cancers deficient in homologous recombination (HR) DNA repair, such as those with BRCA1 or BRCA2 (BRCA1/2) mutations, rely on a repair pathway for single-strand breaks mediated by the enzyme poly(adenosine diphosphate-ribose) polymerase (PARP) [1–3]. PARP inhibitors (PARPi’s) cause irreparable DNA damage in HR-deficient cancer cells, leading to death via synthetic lethality [1–4].
The OlympiAD and EMBRACA trials were phase III randomized studies in which patients with metastatic human epidermal growth factor receptor 2 (HER2/neu) negative breast cancer with germline (g)BRCA1/2 mutations demonstrated significantly longer median progression-free survival (PFS) and better quality of life in groups treated with PARPi’s compared to those treated with standard chemotherapy [3, 5, 6]. The phase III OlympiA trial, which compared the PARPi olaparib to placebo in patients with early stage HER2/neu-negative breast cancer with BRCA1/2 mutations, showed significant improvements in invasive disease-free survival (85.9% for olaparib vs. 77.1% for placebo) [7]. The TBCRC048 trial was a nonrandomized, multicenter, phase II trial that assessed the response to olaparib in: (1) those with germline mutations in non-BRCA1/2 HR-related genes such as PALB2, ATM, or CHEK2 (cohort 1), or (2) those with somatic (s) mutations in these genes or BRCA1/2 (cohort 2) [8]. Median PFS was 13.3 months for patients with gPALB2 mutations and 6.3 months for those with sBRCA1/2 mutations, suggesting benefit from PARPi’s for patients with these mutations [8].
These trials demonstrated the efficacy of PARP inhibition for patients with g/sBRCA1/2 and gPALB2 mutations. However, the OlympiA, OlympiAD, and TBCRC048 trials only enrolled patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, as did the EMBRACA trial, with the exception of a few patients with an ECOG PS of 2 [3, 5, 7, 8]. Patients with poor PS or with severe organ impairment are often excluded from clinical trials and few studies have explored the effects of chemotherapy or other novel drugs versus supportive care in these patients [9, 10]. We present two cases which demonstrate significant clinical benefit derived from PARPi’s in patients with germline PALB2 and somatic BRCA alterations with poor PS and in oncologic crisis.
Case report
Patient A was a 53-year-old woman with a history of coronary artery disease, hyperlipidemia, hypertension, morbid obesity, neuropathy, osteoarthritis, osteoporosis, and pulmonary embolism who noted a breast mass, for which imaging confirmed breast and axillary lesions. Biopsy revealed her tumor to be estrogen receptor (ER) positive at 75%, progesterone receptor (PR) 0, and HER2/neu 1+ (DISH HER2/neu to CEN17 ratio: 1; average HER2 copy number: 1.9), with a Ki-67 of 30%. Her family history was notable for breast cancer in her mother and aunt. A 17-gene germline genetic testing panel performed in 2015 by Ambry Genetics revealed a heterozygous PALB2 pathogenic mutation (c.3323delA) and a BRCA2 variant of unknown significance (c.9353T>C). The patient underwent six cycles of neoadjuvant docetaxel and cyclophosphamide. She subsequently underwent a left partial mastectomy with axillary lymph node dissection demonstrating a pathologic complete response. She received radiation therapy (XRT) to the left breast and left supraclavicular nodal region, then started on tamoxifen but took it for less than 3 months before self-discontinuing therapy (Table 1).
Table 1.
Germline and somatic genetic sequencing
| Patient | Tumor mutational burden | Sequencing type | Gene | Mutation | VAF (%) | Biological relevancea |
|---|---|---|---|---|---|---|
| Patient A |
15.8 m/MB 93rd percentile |
Germlineb | BRCA2 |
c.9353T>C p.M3118T |
VUS | |
| PALB2 | c.3323delA | Pathogenic | ||||
| Tumor NGS | PALB2 |
c.228_229del p.I76fs |
62.8 | Potentially actionable | ||
| ESR1 |
c.1610A>C p.Y537S |
33.5 | Potentially actionable | |||
| PALB2 |
c.3323del p.Y1108fs |
15.7 | Potentially actionable | |||
| MAP3K1 |
c.3236dup p.N1079fs |
31.8 | Biologically relevant | |||
| LRP1B |
c.8787_8811del p.E2929fs |
11.9 | Biologically relevant | |||
| KMT2C (MLL3) |
c.3471_3496del p.Q1158fs |
11.9 | Biologically relevant | |||
| CKS1B | Copy number gain | Biologically relevant | ||||
| ELF3 | Copy number gain | Biologically relevant | ||||
| ERCC4 |
c.2585A>G p.N862S |
87.1 | VUS | |||
| BRCA1 |
c.1561G>A p.A521T |
82.2 | VUS | |||
| BRCA2 |
c.9353T>C p.M3118T |
61.2 | VUS | |||
| ACVR1 |
c.656A>T p.Y219F |
59.0 | VUS | |||
| MAP3K7 |
c.54G>A p.M18I |
51.0 | VUS | |||
| ZNF471 |
c.1315G>T p.D439Y |
39.2 | VUS | |||
| WNK1 |
c.2268_2270delinsCCT p.PP756PL |
37.6 | VUS | |||
| CXCR4 |
c.544G>A p.D182N |
33.6 | VUS | |||
| SMARCA4 |
c.3539C>G p.P1180R |
33.6 | VUS | |||
| MYCN |
c.250C>T p.Q84 |
33.1 | VUS | |||
| ATR |
c.6286G>C p.D2096H |
30.9 | VUS | |||
| SRP14 |
c.275A>C p.D92A |
30.3 | VUS | |||
| MEF2B |
c.925G>A p.A309T |
30.1 | VUS | |||
| DEFB119 |
c.83_84delinsGT p.H28R |
30.1 | VUS | |||
| ALK |
c.1432T>A p.F478I |
29.8 | VUS | |||
| PDGFRB |
c.2109C>G p.H703Q |
29.0 | VUS | |||
| CTNNA1 |
c.1872_1894del p.I625fs |
27.5 | VUS | |||
| ATIC |
c.1211T>C p.V404A |
25.5 | VUS | |||
| CBL |
c.1566T>C p.A522A |
24.4 | VUS | |||
| LRP1B | c.8663-6_8663-5del | 20.1 | VUS | |||
| ACVR1B |
c.1569_1583del p.A524_A528del |
17.0 | VUS | |||
| SYNE1 |
c.13909G>A p.D4637N |
16.6 | VUS | |||
| TANC1 |
c.388A>C p.S130R |
10.4 | VUS | |||
| ERCC1 |
c.925C>A p.L309I |
9.8 | VUS | |||
| KMT2C (MLL3) |
c.1127C>T p.T376I |
5.0 | VUS | |||
| Patient B |
7.4 m/MB 84th percentile |
Germlinec | None | |||
| Tumor NGS | PIK3CA |
c.1633G>A p.E545K |
10.1 | Potentially actionable | ||
| BRCA2 | Copy number loss | Potentially actionable | ||||
| LRP1B |
c.6412C>T p.R2138 |
27.5 | Biologically relevant | |||
| RUNX1 | c.508+1G>T | 25.5 | Biologically relevant | |||
| ARID1B |
c.5309C>T p.A1770V |
82.6 | VUS | |||
| RAD21 |
c.100G>C p.E34Q |
44.9 | VUS | |||
| MIB1 |
c.2716C>T p.R906 |
35.5 | VUS | |||
| ERBB4 |
c.1806T>A p.S602R |
21.8 | VUS | |||
| MKI67 (Ki-67) |
c.2195G>A p.R732Q |
20.8 | VUS | |||
| CALR | c.194-3C>A | 20.7 | VUS | |||
| HIST1H1E |
c.62A>G p.K21R |
20.1 | VUS | |||
| LAG3 |
c.1533_1538dup p.E513_P514insPE |
19.9 | VUS | |||
| MYH11 |
c.2005C>T p.R669C |
19.0 | VUS | |||
| CALR | c.397+5G>T | 14.2 | VUS | |||
| QKI |
c.164A>G p.D55G |
14.0 | VUS | |||
| TBX3 |
c.734_739del p.M245_H246del |
9.9 | VUS | |||
| SYNE1 |
c.16667G>A p.W5556 |
8.7 | VUS | |||
| ARID1B |
c.2510G>T p.S837I |
8.5 | VUS | |||
| APOB |
c.13146G>A p.M4382I |
8.3 | VUS | |||
| PTPN13 |
c.4570G>T p.D1524Y |
5.5 | VUS |
NGS next-generation sequencing; LOF loss of function; GOF gain of function; VAF variant allele fraction; VUS variant of unknown significance
aTumor NGS was conducted by TEMPUS. Biological relevance is as determined and reported by TEMPUS in 2022 for Patient A and in 2020 for Patient B
bGermline sequencing for Patient A was reported by Ambry Genetics in 2015. Seventeen genes were analyzed as part of this panel: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, MRE11A, MUTYH, NBN, NF1, PALB2, PTEN, RAD50, RAD51C, RAD51D, TP53
cGermline sequencing for Patient B was reported by Color Health in 2018. Thirty-one genes were analyzed as part of this panel: APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A (p14ARF), CDKN2A (p16INK4a), CHEK2, EPCAM, GREM1, MITF, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLD1, POLE, PTEN, RAD51C, RAD51D, SMAD4, STK11, TP53
The patient developed left buttock pain and back pain approximately 3 years later and was found to have a compression fracture at L4. A biopsy of L4 revealed breast cancer that was ER > 90%, PR 5–10%, and HER-2/neu 1+. Tumor next-generation sequencing (NGS) by TEMPUS was notable for PALB2 (c.228_229del and c.3323del) and ESR1 (c.1610A>C) mutations, and a tumor mutational burden (TMB) of 15.8 m/MB (Table 2). The c.3323del PALB2 mutation detected by tumor NGS was consistent with that found in the patient’s initial germline testing, with the second somatic mutation indicating possible bi-allelic inactivation. The patient received radiation to L2–L5 and was subsequently restarted on tamoxifen. Two months later, she underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy to allow for transition to endocrine therapy, consisting of letrozole and palbociclib with intravenous zoledronic acid. While she initially experienced disease control, restaging scans 8 months later revealed progression of the patient’s cancer to her liver. She was started on nab-paclitaxel in addition to her letrozole; however, she experienced progression in her liver and bones 6 months later, prompting transition to gemcitabine and carboplatin.
Table 2.
Treatment summary
| Patient | Receptor status | Treatment historya |
|---|---|---|
| Patient A |
Early stage: ER 75%, PR 0, HER2/neu 1+ Metastatic (bone): ER > 90%, PR 5–10%, HER-2/neu 1+ |
Early stage – Neoadjuvant docetaxel and cyclophosphamide – Left partial mastectomy with axillary lymph node dissection – Radiation therapy to the left breast and left supraclavicular nodal region Metastatic – Radiation therapy to L2–L5 – Tamoxifen – Total abdominal hysterectomy and bilateral salpingo-oophorectomy – Letrozole and palbociclib with intravenous zoledronic acid – Nab-paclitaxel – Gemcitabine and carboplatin – Olaparib – Trastuzumab-deruxtecan |
| Patient B |
Breast: ER > 90%, PR > 90%, HER2/neu 1+ Metastatic (bone): ER 80%, PR 80%, HER2/neu 0 |
Metastatic – Tamoxifen and palbociclib with denosumab and zoledronic acid – Radiation therapy to spine – Vertebroplasty – Exemestane, goserelin, and everolimus – Olaparib |
ER estrogen receptor; PR progesterone receptor; HER2/neu human epidermal growth factor receptor 2
aTreatments are listed in chronological order by date of initiation
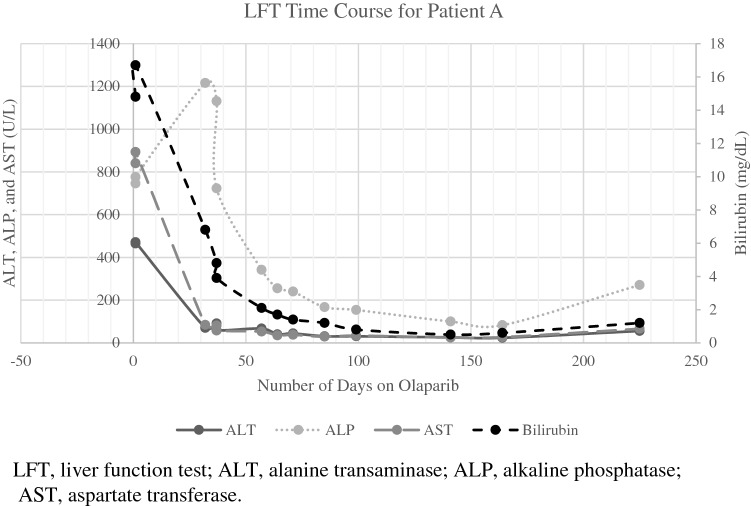
Patient A was referred to the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center at that time. Laboratory evaluation showed a total bilirubin of 4.5 mg/dL. However, less than 2 weeks later, she presented to the emergency department for jaundice, nausea, and fatigue. Liver function tests (LFTs) demonstrated markedly elevated total bilirubin (16.7 mg/dL), alkaline phosphatase (ALP, 776 U/L), aspartate transferase (AST, 893 U/L), and alanine transaminase (ALT, 471 U/L), with evidence of hepatic insufficiency [low albumin (3.3 g/dL) and platelets (67 K/cu mm); elevated INR (1.24) and prothrombin time (12.9 s)]. Her ECOG PS was 4 at the time of her emergency department visit. After discussions regarding her prognosis and goals of care, given her PALB2 mutations, the patient and her treating oncologist decided to discontinue chemotherapy and start olaparib at a dose of 300 mg twice daily, and she was co-enrolled in hospice. Within 2 months, her condition had significantly improved; her LFTs began to normalize (Fig. 1A), she reported increased strength and mobility, and her ECOG PS decreased to 2. Her main treatment-related toxicity was anemia, which required red blood cell transfusions. After a few months, she decided to return to work. She met Response Evaluation Criteria in Solid Tumors (RECIST) for radiographically stable disease in her liver on olaparib for 7 months (Fig. 2). However, restaging scans at month 9 demonstrated new hepatic metastases, indicative of progressive disease (Fig. 2). At this time, she was transitioned from olaparib to trastuzumab deruxtecan given her HER2-low status; unfortunately, she acutely decompensated and passed away shortly after her first dose.
Fig. 1.
LFT time course for Patient A
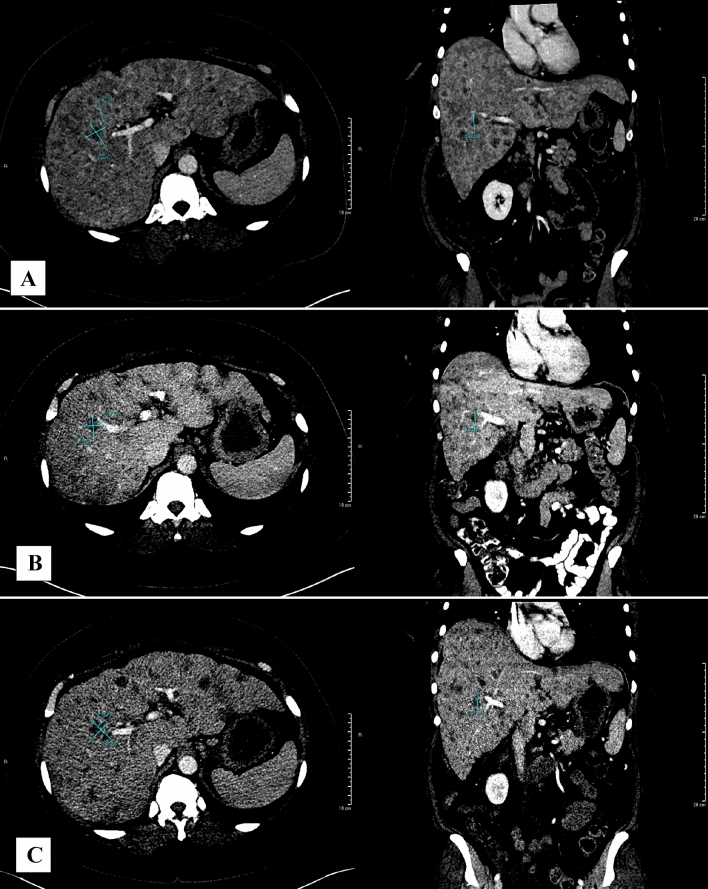
Fig. 2.
CT imaging for Patient A. CT abdomen/pelvis demonstrating a segment 5/8 lesion measuring 2.5 × 2.4 × 2.7 cm at baseline (initiation of olaparib) (A), 2.2 × 2.1 × 2.3 cm post-treatment at 7 months (B), and 3.3 × 2.3 × 2.5 cm post-treatment at 9 months (C). RECIST stable disease was observed between A and B. RECIST progressive disease was observed between B and C
Patient B is a 66-year-old man with a history of type 2 diabetes mellitus, hypertension, obesity, hepatitis C with hepatic fibrosis, prescription drug use disorder, and 73.5 pack-year tobacco use disorder, who noted a breast mass, with imaging confirming a breast abnormality. Biopsy of the left breast mass revealed poorly differentiated invasive carcinoma with signet ring cell features, ER > 90%, PR > 90%, HER2/neu 1+, and Ki-67 of 40–50%. Staging scans demonstrated metastases in his lumbar spine, which biopsy confirmed to be ER 80%, PR 80%, and HER2/neu 0 breast cancer. His family history was significant for breast cancer in his mother, colon cancer in his maternal aunt, and colon cancer in his maternal uncle. A 31-gene germline genetic testing panel performed in 2018 by Color Health was negative for pathologic BRCA mutations; however, tumor NGS by TEMPUS indicated somatic BRCA2 copy number loss, a PIK3CA mutation (c.1633G>A), and TMB of 7.4 m/MB (84th percentile) (Table 2). The patient was started on tamoxifen, denosumab (later transitioned to zoledronic acid), and received XRT for his spinal metastases, followed by vertebroplasty for pathologic fractures of his L1 and L2 vertebrae. Palbociclib (125 mg) was then added. However, after three cycles of therapy, restaging scans revealed progressive disease in his thoracic and lumbar spine. Tamoxifen and palbociclib were discontinued and he was started on exemestane, goserelin, and everolimus. While he experienced approximately 2 years of disease control with this regimen, his course was complicated by osteonecrosis of the jaw and back pain secondary to his pathologic compression fracture status post-radiation therapy and vertebroplasty. He also developed a brain abscess requiring IV antibiotics, and had bilateral lower extremity edema and pain. Restaging scans approximately 2 years later demonstrated mildly progressed diffuse osseous metastatic lesions, new multiple FDG-avid hepatic lesions, and an increase in size of non-FDG-avid mediastinal lymph nodes, consistent with progressive disease. Unfortunately, his course was further complicated by SARS-CoV2 infection at this time, and he continued to clinically deteriorate, demonstrating forgetfulness, difficulty ambulating, and increased pain. The cumulative burden of these conditions along with progressive cancer caused his performance status to deteriorate.
His ECOG PS was 4; thus, after discussions of prognosis and goals of care, the patient and his oncology team decided to discontinue exemestane, goserelin, and everolimus, and to start olaparib at a reduced dose of 200 mg twice daily, based on his elevated serum creatinine of 1.4 mg/dL (creatinine clearance of 46.8 mL/min). The patient responded well to olaparib, as his functional mobility improved, and his PS improved to 3. Restaging scans after 4 months exhibited markedly decreased radiotracer avidity of hepatic and osseous lesions and decrease in activity at the left breast mass and axilla, compatible with treatment response. After 1 year of stable disease on olaparib, scans revealed diffuse extensive mixed osteolytic and sclerotic lesions in the calvarium, facial bones, cervical spine, and upper thorax, suggesting possible progression, as well as worsening mandibular osteonecrosis. The patient was hospitalized again for altered mental status, lethargy, and hypercalcemia. He ultimately enrolled in hospice and passed away 3 months later.
Discussion
The American Society of Clinical Oncology (ASCO) recommends against cancer-directed treatment for patients with a poor performance status (ECOG PS 3–4), as low PS has been associated with poor survival, reduced response, and worse chemotherapy-related toxicity [9]. Poor PS and inadequate organ function also typically preclude inclusion in clinical trials, further limiting treatment options for these patients [3, 5, 8, 10]. However, targeting oncogenic drivers of disease may possibly result in rapid and profound treatment responses that can potentially reverse oncologic burden and improve patient outcomes. For example, the literature demonstrates that targeting ALK, EGFR, and BRAF gene mutations have resulted in remarkable responses in critical patients [11–14].
The two cases we present demonstrate that PARP inhibition can result in dramatic responses in patients with homologous recombination DNA repair aberrations beyond germline BRCA. Patient A had a pathogenic gPALB2 mutation with an additional somatic mutation likely representing biallelic inactivation, and patient B had sBRCA2 and sPIK3CA mutations. Like BRCA1/2, the PALB2 gene is involved in HR DNA repair, and PALB2 mutations are associated with an increased risk of breast cancer and reduced survival [15, 16]. Patients with PALB2 mutations may also benefit from treatment with PARPi’s [8, 17–19]. TBCRC048 is currently one of the only prospective trials demonstrating response to olaparib in patients with breast cancer and sBRCA1/2 mutations and gPALB2 mutations [8]. Another single institution phase II trial that investigated the PARPi talazoparib in patients with advanced HER2/neu-negative breast cancer and HR pathway gene mutations found that all five patients with a gPALB2 mutation experienced reduction in target lesions and that three patients achieved a RECIST response [18]. A follow-up study to this phase II trial, focused on evaluating the ORR of talazoparib monotherapy specifically in patients with g/sPALB2 mutation-associated advanced breast cancer, is currently ongoing [20]. A retrospective, real-world analysis of the Flatiron Health-Foundation Medicine Clinico-Genomic Database also demonstrated benefit with olaparib in four patients with gPALB2 mutations and nine patients with sBRCA mutations [21].
TBCRC048 required participants to have an ECOG PS of 0–1 and adequate organ function, while the talazoparib trials required an ECOG PS of 0–2 [8]. Furthermore, the pharmacokinetics of olaparib have only been studied with mild and moderate hepatic impairment [22]. In our report, Patient A was treated with olaparib for 9 months, despite her initial PS of 3–4 and evidence of hepatic failure. Although Patient B had an initial PS of 3–4, multiple comorbidities, and poor prognosis, he responded to olaparib for 1 year. Despite the poor performance status of these two patients at the time of treatment initiation, both tolerated olaparib well with limited side effects and demonstrated improvement in performance status with treatment response. These cases illustrate the therapeutic potential in targeting beyond gBRCA1/2 mutations using PARPi’s, despite clinically critical situations. Future research is also needed to identify other patient populations that may benefit from PARPi, but also in overcoming PARPi resistance, and developing optimal combination strategies.
Conclusion
Patients with a poor ECOG PS that is being driven by their cancer burden still have the potential to have meaningful clinical responses to cancer treatments targeting oncogenic drivers, such as using PARPi’s to target gPALB2 and g/sBRCA mutations. Clinical trials have supported the efficacy of PARP inhibition in treating patients with g/sBRCA1/2 and gPALB2 mutations and a PS of 0–2. We have described two cases of patients with a PS of 3–4 and significant visceral disease who demonstrated remarkable clinical responses to treatment with PARPi’s. While not all patients in such oncologic crisis may benefit from PARPi’s, more studies evaluating PARPi’s beyond gBRCA1/2 mutations and in sub-optimal PS would help identify patients who may benefit from these therapies.
Abbreviations
- HR
Homologous recombination
- PARP
Poly(adenosine diphosphate-ribose) polymerase
- PARPi
PARP inhibitor/inhibition
- PFS
Progression-free survival
- HER2/neu
Human epidermal growth factor receptor 2
- ECOG
Eastern Cooperative Oncology Group
- PS
Performance status
- ER
Estrogen receptor
- PR
Progesterone receptor
- XRT
Radiation therapy
- NGS
Tumor next-generation sequencing
- TMB
Tumor mutational burden
- LFT
Liver function test
- ALP
Alkaline phosphatase
- AST
Aspartate transferase
- ALT
Alanine transaminase
- INR
International normalized ratio
- RECIST
Response Evaluation Criteria in Solid Tumors
- ASCO
American Society of Clinical Oncology
- CDK4/6
Cyclin-dependent kinase 4/6
- RB
Retinoblastoma
- TNBC
Triple negative breast cancer
Author contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by JMC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Competing interests
Cesar A. Santa-Maria, MD MSCI disclosures: Research funding: Astra Zeneca, Pfizer, Merck, BMS, Genentech, GSK/Tesaro. Advisory board (paid): Seattle Genetics, Genomic Health, Athenex. Advisory board (not paid): BMS, Merck. Joyce M. Cheng, Jenna Canzoniero, Seoho Lee, Sudeep Soni, Neha Mangini has no financial or non-financial disclosures.
Ethical approval
This is a case report of two patients. The Johns Hopkins University Institutional Review Board has confirmed that ethical approval is not required for retrospective analyses of up to three clinical cases.
Consent to participate
The Johns Hopkins University Institutional Review Board does not classify this case report as human subjects research; therefore, informed consent was not required.
Consent to publish
The Johns Hopkins University Institutional Review Board does not classify this case report as human subjects research; therefore, informed consent was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3(6):257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litton JK, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo MZ, Marrone KA, Spira A, Scott SC. Poly(ADP-ribose) polymerase inhibition in small cell lung cancer: a review. Cancer J. 2021;27(6):476–481. doi: 10.1097/PPO.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 5.Robson M, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 6.Robson ME, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutt ANJ, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tung NM, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 9.Schnipper LE, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 10.West H, Jin JO. Performance status in patients with cancer. JAMA Oncol. 2015;1(7):998–998. doi: 10.1001/jamaoncol.2015.3113. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, Chen C, Zeng Y-Y, Zhu J-J, Huang J-A, Zhu Y-H. Rapid response in a critical lung adenocarcinoma presenting as large airway stenoses after receiving stent implantation and sequential rebiopsy guided ALK inhibitor therapy: a case report. J Thorac Dis. 2017 doi: 10.21037/jtd.2017.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz J, Schlette E, Kurzrock R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J Clin Oncol. 2013;31(20):e351–e352. doi: 10.1200/JCO.2012.45.7739. [DOI] [PubMed] [Google Scholar]
- 13.Koba T, et al. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation. Medicine (Baltimore) 2017;96(6):e6087. doi: 10.1097/MD.0000000000006087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C, et al. Rapid and durable response to fifth-line lorlatinib plus olaparib in an ALK-rearranged, BRCA2-mutated metastatic lung adenocarcinoma patient with critical tracheal stenosis: a case report. Anticancer Drugs. 2022;33(7):696–700. doi: 10.1097/CAD.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 15.Rahman N, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007 doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cybulski C, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol. 2015;16(6):638–644. doi: 10.1016/S1470-2045(15)70142-7. [DOI] [PubMed] [Google Scholar]
- 17.Grellety T, et al. Dramatic response to PARP inhibition in a PALB2-mutated breast cancer: moving beyond BRCA. Ann Oncol. 2020;31(6):822–823. doi: 10.1016/j.annonc.2020.03.283. [DOI] [PubMed] [Google Scholar]
- 18.Gruber JJ, et al. Talazoparib beyond BRCA: a phase II trial of talazoparib monotherapy in BRCA1 and BRCA2 wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in homologous recombination (HR) pathway genes. J Clin Oncol. 2019;37(15_suppl):3006–3006. doi: 10.1200/JCO.2019.37.15_suppl.3006. [DOI] [Google Scholar]
- 19.Kuemmel S, et al. Olaparib for metastatic breast cancer in a patient with a germline PALB2 variant. npj Breast Cancer. 2020 doi: 10.1038/s41523-020-00174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber JJ, Gross W, McMillan A, Ford JM, Telli ML. A phase II clinical trial of talazoparib monotherapy for PALB2 mutation-associated advanced breast cancer. J Clin Oncol. 2021;39(15_suppl):TPS1109. doi: 10.1200/JCO.2021.39.15_suppl.TPS1109. [DOI] [Google Scholar]
- 21.Batalini F, et al. Analysis of real-world (RW) data for metastatic breast cancer (mBC) patients (pts) with somatic BRCA1/2 (sBRCA) or other homologous recombination (HR)-pathway gene mutations (muts) treated with PARP inhibitors (PARPi) J Clin Oncol. 2021;39(15_suppl):10512. doi: 10.1200/JCO.2021.39.15_suppl.10512. [DOI] [Google Scholar]
- 22.Rolfo C, et al. Pharmacokinetics and safety of olaparib in patients with advanced solid tumours and mild or moderate hepatic impairment. Br J Clin Pharmacol. 2020;86(9):1807–1818. doi: 10.1111/bcp.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.