The functional exhaustion of CD8+ T cells represents a fundamental hallmark of chronic viral infection and cancer and, in both scenarios, is driven by prolonged exposure to persistent cognate antigens in the context of an immunoinhibitory microenvironment. Exhausted CD8+ T cells upregulate the expression of a wide diversity of coinhibitory immunoreceptors (also referred to as immune checkpoint receptors), such as PD-1, Tim-3, LAG-3, and TIGIT. Concomitantly, exhausted CD8+ T cells lose their potential to differentiate into functional memory cells and are characterized by hierarchical loss of effector function, leading to compromised tumor control and viral eradication [1, 2].
Exhausted CD8+ T cells in the tumor microenvironment (TME) are highly heterogeneous, mainly consisting of subsets of TCF-1-expressing precursors of exhausted T (TPEX) cells and Tim-3-expressing terminally differentiated exhausted CD8+ T (TEX) cells [3, 4]. Immune checkpoint receptor blockade (ICB) therapies, such as those that block the PD-1/PD-L1 pathway, result in remarkable remission in a subset of cancer patients, with these effects generally attributed to the reversal of CD8+ T-cell exhaustion in the TME [5–7]. However, accumulating evidence highlights the potential role of systemic CD8+ T-cell responses in the control of tumor progression upon PD-1/PD-L1 ICB treatment, especially in tumor-draining lymph nodes (TdLNs) [8–14]. Recently, we reported on tumor-specific memory CD8+ T cells in TdLNs (TdLN-TTSM) of both mice tumor models and human hepatocellular carcinoma patients and showed that TdLN-TTSM cells serve as primary responders to PD-1/PD-L1 ICB and exhibit superior tumor repression to that of TCF-1+ TPEX cells [15].
TdLN-TTSM cells were comparable to conventional memory T cells (TMEM) generated during acute viral infection in several aspects, including antigen-independent self-renewal and proliferation burst upon antigen reencounter. TTSM cells express the lymphoid homing molecule L-selectin (CD62L) and C-C chemokine receptor 7 (CCR7). Moreover, we noticed that genes associated with T-cell extravasation and chemotaxis were less enriched in TdLN-derived P14 cells than in TMEM P14 cells, indicating the potentially distinct circulating features between these two subsets [15]. TMEM cells can patrol between lymphoid organs, blood and peripheral tissues, while during chronic viral infection, TPEX cells are reported to largely reside in lymphoid tissues, with a very limited population in infected peripheral tissues [16]. Importantly, the migratory pattern of TTSM cells derived from TdLNs during tumorigenesis has not yet been elucidated. Thus, herein, we sought to dissect the migratory pattern of TTSM cells.
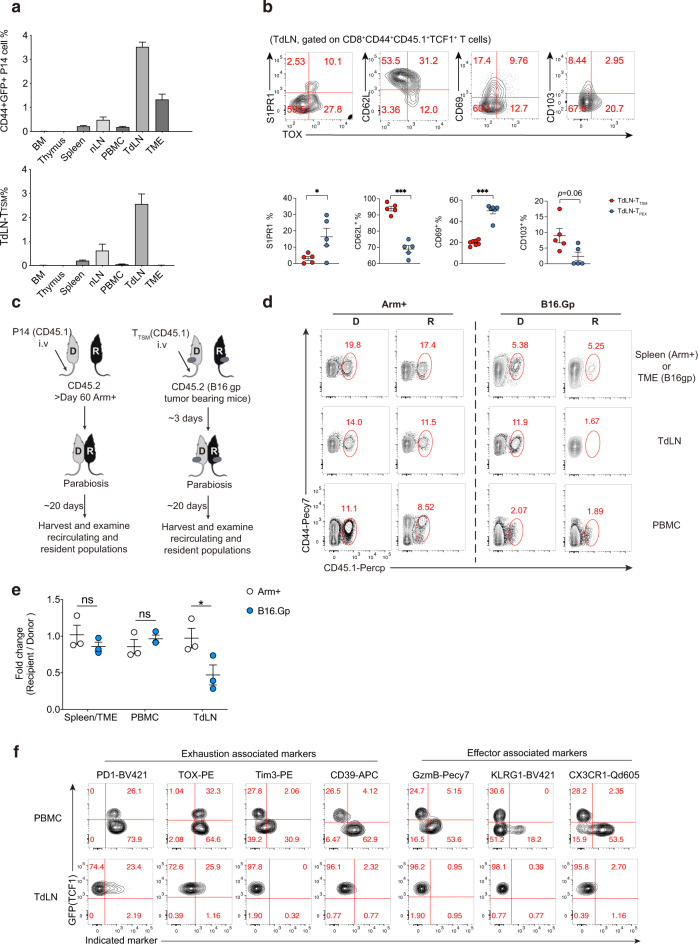
First, to precisely trace the immune response of TdLN-TTSM cells during tumorigenesis, C57BL/6 mice (hereafter referred to as B6 mice) were first adoptively transferred with naive Tcf7 (encoding TCF-1 protein)-GFP P14 cells (CD44−GFP+, GFP indicating TCF-1 expression) harboring transgenic TCRs specific to the H-2Db Gp33-41 epitope from Tcf7-GFP knock-in reporter P14 mice, and then these B6 mice were subcutaneously inoculated with B16.F10 melanoma cells expressing the LCMV glycoprotein as a surrogate neoantigen (hereafter referred to as B16.Gp) as we previously reported [15]. Fourteen days later, TdLN-TTSM P14 cells were sorted and retransferred into tumor-bearing recipients at Day 8 post B16.Gp inoculation (Supplementary Fig. S1a, b). Then, activated P14 cells derived from different tissues were analyzed. Eight days later, we noticed that CD44+P14 cells were more abundant in TdLNs than in other compartments of tumor-bearing mice (Fig. 1a, upper panel). Furthermore, we found that TCF1+TOX− TTSM P14 cells were more enriched in TdLNs than in other tissues, including those of the TME (Fig. 1a, lower panel). In addition, we noted that the majority of TTSM cells in TdLNs were CD62L positive but negative for sphingosine-1-phosphate receptor-1 (S1PR1) expression; notably, S1PR1 mediates lymphocyte egress from LNs [17]. A fraction of TTSM cells also expressed CD69 and CD103 (Fig. 1b), which are known markers for tissue resident memory T cells [18]. The unique expression pattern of these molecules may guarantee their retention within dLNs. Thus, we hypothesized that TTSM cells are likely resident in TdLNs.
Fig. 1.
The in vivo distribution and migration pattern of tumor-specific memory CD8+ T cells during tumorigenesis. a Statistical summary of the proportions of CD45.1+CD44+ P14 cells (upper panel) and CD45.1+CD44+TCF1+TOX− TTSM cells (lower panel) in gated live lymphocytes from each compartment. BM bone marrow, nLNs nondraining lymph nodes, including axillary lymph nodes and submaxillary lymph nodes, TdLNs tumor-draining lymph nodes. n ≥ 4/group. b Flow cytometry analyses of TOX expression versus the indicated markers in CD45.1+CD44+TCF1+ donor P14 cells in TdLNs. c Experimental design of the parabiotic system. d Representative flow-cytometry plots of donor TTSM-derived CD44 + P14 cells in the TME, TdLNs, and PBMCs of the donor (D) and host/recipient (R) tumor-bearing parabionts (right panel). Donor-derived CD44+ P14 cells in the spleens, TdLNs, and PBMCs of donor- and recipient-infected parabionts are listed in the left panel. Numbers are frequencies. e The ratio of donor-derived CD44+ P14 cells from each indicated compartment in the recipient relative to the donor is summarized. f Flow-cytometry analyses of GFP (indicating TCF-1 expression) versus indicated exhaustion and effector cell-associated marker expression in CD45.1+CD44+ donor P14 cells from different compartments. n = 3/group. *p < 0.05 versus control, n.s. stands for not significant, paired two-tailed Student’s t test (d). Data are representative of 2 independent experiments (mean ± SEM)
To test this hypothesis, we next investigated the in vivo migratory properties of TTSM cells by using a parabiotic system. TTSM cells (CD45.1+CD45.2−CD44+PD-1lowGFP+) were first sorted as previously reported [15] and transferred into tumor-bearing B6 recipients (CD45.1−CD45.2+). After resting for 3 days, the vasculature of B16.Gp tumor-bearing mice (adoptively transferred with TTSM P14 cells on Day 6 post tumor implantation) were conjoined to those of tumor-matched mice (without P14 cell adoptive transfer) via parabiosis surgery. As a control, we performed the same surgery using LCMV-Arm+-acutely infected mice (P14 cells adoptively transferred) in which acute infection (>60 days) had cleared. Then, we tested whether donor P14 cells equilibrated between the parabionts 20 days later in the peripheral blood (PBMC), spleen (Arm+ infection), TME, and inguinal draining lymph node (Fig. 1c). As expected, virus-specific memory P14 cells established equilibrium between the two acutely infected conjoined parabionts in the spleen, peripheral blood, and inguinal lymph nodes, consistent with a previous report [16].
Notably, for tumor-bearing parabionts, donor (D)-derived P14 cells, including the TTSM subset, were nearly undetectable in the TdLNs of recipient mice (R) (Fig. 1d, e). In contrast, donor-derived P14 cells reached equilibrium between parabionts in the tumor mass. Furthermore, we noticed that only a small fraction of donor-derived antigen-specific CD8+ T cells were recovered from peripheral blood, although the proportions seemed comparable between parabionts (Fig. 1d, e). This small population of antigen-specific CD8+ T cells in peripheral blood reminded us that during chronic LCMV infection (Cl13 infection), the frequency of tetramer-positive CD8+ T cells in the blood is very low compared to that in the spleen, and the majority of these circulating virus-specific CD8+ T cells in chronically infected mice were CD101−Tim-3+ CX3CR1+ transitory cells [16, 19–22]. To further characterize the progeny cells in the blood, we compared the phenotype of PBMC- and tumor-derived CD44+P14 cells from the host parabiont with those from the TdLNs of donor mice. Consistent with published data [15], antigen-specific CD8+ T cells in TdLNs consisted of TCF1+TOX− TTSM cells and a small proportion of TCF1+TOX+ TPEX cells. In contrast, in PBMCs, a substantial proportion (~70%) of TTSM-derived P14 cells differentiated into TCF1-negative cells and upregulated the expression of exhaustion-associated markers, including PD-1, TOX, and CD39 (Fig. 1f). Furthermore, TTSM-derived P14 cells in PBMCs had highly increased expression levels of the chemokine receptor CX3CR1 and effector molecules KLRG1 and granzyme B, while these markers were barely expressed by TCF-1-expressing TTSM cells in the draining lymph nodes (Fig. 1f), suggesting that antigen-specific CD8+ T cells in the peripheral blood were heterogeneous and in a transitory differentiation stage between progenitor and terminal exhausted CD8+ T cells. Additionally, TTSM-derived P14 cells primarily differentiated into TCF1-negative cells in the TME, with high levels of PD-1, TOX, Tim3, and CD39 expression (Supplementary Fig. S1c).
Collectively, our study delineated the unique tissue distribution and different migratory patterns of TTSM and TMEM cells. TdLN-TTSM cells were predominantly found in TdLNs and seemed to be resident at these sites. Importantly, this study indicates that TdLNs might provide a unique niche in facilitating the differentiation and residency of TdLN-TTSM cells. However, more efforts are needed to further explore this possibility and examine how such lymphoid niches are generated and operated during tumorigenesis.
Supplementary information
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (no. 2021YFC-2300602 to LY), the Key Program of the National Natural Science Foundation of China (no. 32030041 to LY), the National Science Foundation for Outstanding Young Scholars of China (no. 82122028 to LX), the National Natural Science Foundation of China (no. 82173094 to LX, no. 31900643 to QH) and the Chongqing Postdoctoral Science Foundation Project (no. cstc2021jcyj-bshX0232 to QL).
Author contributions
QH, LX and LY designed the research. QL, LR and ZY performed the experiments. XS, LW, SW, SL, XY, YZ, JH, QW, JT, ZL, and LH assisted in the experiments. BZ assisted in the data interpretation. QL, LX and QH analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: In this article the affiliation details were incorrectly given.
These authors contributed equally: Qiao Liu, Ling Ran, Zhengliang Yue.
Change history
5/25/2023
A Correction to this paper has been published: 10.1038/s41423-023-01025-w
Contributor Information
Lifan Xu, Email: xlftofu@sina.com.
Lilin Ye, Email: yelilinlcmv@tmmu.edu.cn.
Qizhao Huang, Email: huangqizhao1988@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-023-00986-2.
References
- 1.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;24:041015–55318. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 2.Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2021;22:209–23. doi: 10.1038/s41577-021-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol. 2020;20:128–36. doi: 10.1038/s41577-019-0223-7. [DOI] [PubMed] [Google Scholar]
- 4.Philip M, Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr Opin Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–18. doi: 10.1146/annurev-med-012017-43208.. [DOI] [PubMed] [Google Scholar]
- 8.Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci USA. 2017;114:E761–E70. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med. 2020;26:1125–34. doi: 10.1038/s41591-020-0892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502.e15. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25:1251–9. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579:274–8. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 13.Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. 2021;6:eabg7836. doi: 10.1126/sciimmunol.abg7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenkel JM, Herbst RH, Canner D, Li A, Hillman M, Shanahan S-L, et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+CD8+ T cells in tumor-draining lymph nodes. Immunity. 2021;54:2338–53.e6. doi: 10.1016/j.immuni.2021.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu Y, et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell. 2022;185:4049–66.e25. doi: 10.1016/j.cell.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc Natl Acad Sci USA. 2020;117:4292–9. doi: 10.1073/pnas.1917298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 19.Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity. 2019;51:1043–58.e4. doi: 10.1016/j.immuni.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Wang L, Lin H, Wang Z, Wu J, Guo J, et al. Tumor-specific CD4(+) T cells restrain established metastatic melanoma by developing into cytotoxic CD4(-) T cells. Front Immunol. 2022;13:875718. doi: 10.3389/fimmu.2022.875718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Hu J, Li Y, Xiao M, Wang H, Tian Q, et al. The transcription factor TCF1 preserves the effector function of exhausted CD8 T cells during chronic viral infection. Front Immunol. 2019;10:169. doi: 10.3389/fimmu.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.