Abstract
The insulin-like growth factor (IGF) axis plays important roles in cancer development and metastasis. The type 1 IGF receptor (IGF-1R) is a key member in the IGF axis and has long been recognized for its oncogenic role in multiple cancer lineages. Here we review the occurrence of IGF-1R aberrations and activation mechanisms in cancers, which justify the development of anti-IGF-1R therapies. We describe the therapeutic agents available for IGF-1R inhibition, with focuses on the recent or ongoing pre-clinical and clinical studies. These include antisense oligonucleotide, tyrosine kinase inhibitors and monoclonal antibodies which may be conjugated with cytotoxic drug. Remarkably, simultaneous targeting of IGF-1R and several other oncogenic vulnerabilities has shown early promise, highlighting the potential benefits of combination therapy. Further, we discuss the challenges in targeting IGF-1R so far and new concepts to improve therapeutic efficacy such as blockage of the nuclear translocation of IGF-1R.
Keywords: Cancer, IGF-1R, Targeted therapy, Metastasis, Combination therapy
Introduction
Targeted cancer therapies are designed to harness the specific molecular abnormalities that drive tumorigenesis. Because of the specificity, targeted therapies are expected to achieve higher efficacy with lower toxicity compared with the traditional approaches such as chemotherapy. Next generation sequencing has facilitated the discovery of genomic abnormalities that underlie the development and progression of cancer.1 These abnormalities represent potential therapeutic targets.
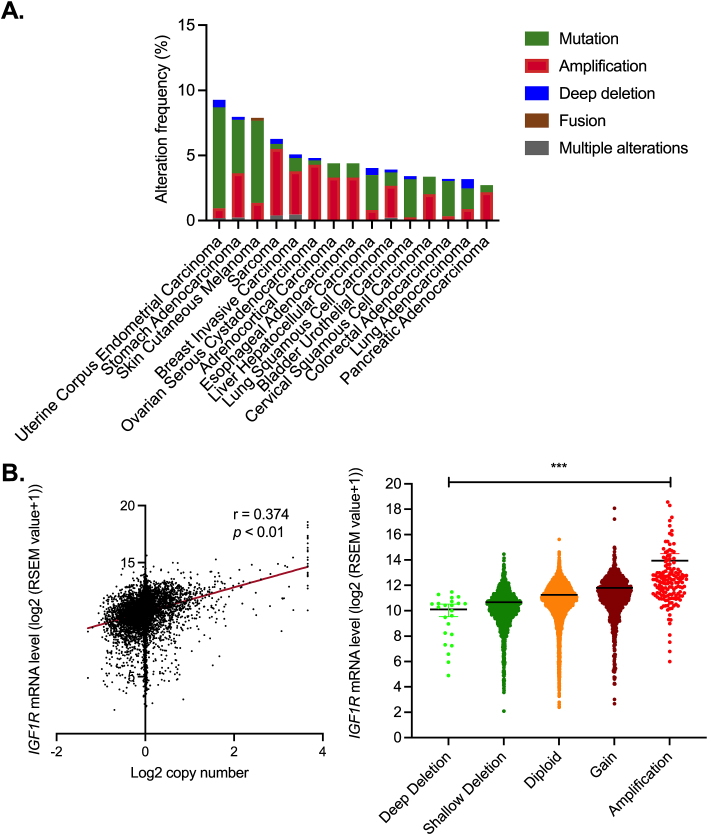
Gene amplification of IGF1R, which encodes insulin-like growth factor 1 receptor, is a frequent event across cancer types (Fig. 1A). IGF-1R is a key member of the insulin-like growth factor (IGF) axis, which involves in cancer development, metastasis and cancer therapeutic resistance.2, 3, 4 The IGF axis consists of several ligands (insulin, IGF-1, IGF-2) and cell surface receptors, including IGF-1R, IGF-2 receptor (IGF-2R) and insulin receptor (InsR).5 Additionally, the levels of circulating IGF ligands are regulated by seven soluble IGF binding proteins (IGFBP1–7) and IGFBP proteases.5,6 IGFBPs act as the serum reservoirs of IGF-1 and IGF-2 by regulating their stability and their release at target tissues, thereby modulating the bioavailability of these two ligands to the receptors temporally and spatially.7, 8, 9 Among the molecules of the IGF axis, IGF-1R is recognized as a promising target of cancer therapy because of (i) its frequent amplification and overexpression in cancers2; (ii) its established functions in transmitting tumorigenic signals10,11; and (iii) its role as the primary receptor of the IGFs.5,8
Figure 1.
Amplification of IGF1R copy number is frequent in several common types of human cancer. (A) The top 15 most frequent cancer types that have been reported to have IGF1R mutations or copy number variations (TCGA; n = 10,953). (B) Correlation of IGF1R copy number and mRNA levels in TCGA cancer patients (n = 9,889).
The mature IGF-1R is a heterotetramer comprised of two extracellular ligand binding α subunits and two transmembrane β subunits that contain the intracellular kinase domain.12 Upon ligand binding, the tyrosine residues in the intracellular kinase domain of IGF-1R are autophosphorylated. This activation leads to the binding of the phosphorylated IGF-1R to docking proteins, including insulin receptor substrates 1 to 4 (IRS-1 to IRS-4) as well as Src homolog and collagen domain protein (Shc).13 Recruitment of these docking proteins not only promotes the membrane retention of IGF-1R, but also mediates the activation of downstream signaling pathways of the IGF axis, which are primarily the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR and RAS/RAF/mitogen-activated protein kinase (MAPK) pathways.14 In addition, IGF-1R is highly homologous to InsR (60% overall sequence homology and 80% homology in their kinase domains). IGF-1R and InsR (InsR-A or InsR-B) can heterodimerize to form IGF-1R/InsR hybrid,15 potentially resulting in crosstalk between IGF-1R and insulin signaling which may have impacts on glucose uptake and other cellular functions.16 Here, we review the recent pre-clinical and clinical evidence demonstrating the therapeutic potential of targeting IGF-1R in cancers. We also discuss the challenges and the emerging opportunities to inhibit IGF-1R in combinational therapies that can overcome therapeutic resistance and maximize the efficacy of cancer treatment.
Overexpression and activation of IGF-1R in human cancers
Mechanistically, activated IGF-1R promotes an array of cancer phenotypes such as growth and metastasis.17,18 The activity of IGF-1R can be regulated through multiple mechanisms described below.
-
(i)
IGF-1R overexpression caused by IGF1R gene aberrations
Overexpression of IGF-1R was reported in multiple human cancer lineages and was associated with poor prognosis of cancer patients.19, 20, 21 In prostate cancer (n = 136), protein levels of IGF-1R were significantly higher in malignant epithelia compared with benign glands from the same biopsies.19 In a head and neck squamous cell cancer cohort (n = 346), patients with high IGF-1R levels had significantly reduced overall survival (OS) and disease-specific survival.20 At least 50% of breast cancer cases were found to express activated IGF-1R.21 These studies showed strong IGF-1R staining in the membrane and/or cytoplasm and nucleus. Cytoplasmic localization of IGF-1R is thought to be an indicator of receptor activation which subsequently leads to receptor internalization, whereas nuclear IGF-1R has also been reported to transmit tumorigenic signal.22 Interestingly, a breast cancer study (n = 50) revealed that estrogen receptor (ER)-positive breast cancer was significantly correlated with membranous and mixed IGF-1R expression whereas HER2-positive cases showed less membranous staining but mostly cytoplasmic and mixed patterns.23 While these patterns have to be validated in larger independent cohorts, crosstalk between IGF-1R and ER or HER2 to stimulate oncogenic signal has been reported.17,24,25
Notably, alterations in IGF1R gene copy number have been found in 384 out of 10,953 (3.5%) pan-cancer patient samples from The Cancer Genome Altas (TCGA). A positive correlation between IGF1R copy numbers and its mRNA levels could be observed (Fig. 1B). Single nucleotides mutations in IGF1R are also common. The majority of these mutations remain uncharacterized, therefore their functional significance and therapeutic implications are largely uncertain. Correlation analyses using cancer samples of the breast, colon, pancreas and skin have demonstrated significantly positive association between single nucleotide polymorphism (SNP) of IGF1R and an increased cancer risk.26, 27, 28, 29 Interestingly, there are also SNPs which were found to associate with protective effect on breast cancer or melanoma.29,30 The molecular consequences driven by all these reported SNPs of IGF1R remain elusive. It would be interesting to examine the expression levels of IGF-1R in the presence of these IGF1R SNPs.
-
(ii)
Regulation of IGF-1R expression by modifier genes
Intriguingly, IGF-1R protein may act as an auto-activator by translocating to nucleus. Nuclear IGF-1R binds directly to its own promoter to upregulate its expression level.31 In addition, increase of IGF-1R expression in cancer cells can be attributed to deregulation of modifier genes.32, 33, 34, 35, 36 Examples of tumor suppressors that regulate IGF1R promoter are VHL, TP53 and BRCA1,32,37,38 whereas oncogenes that promotes IGF1R transcription include MYC and EWS-WT1 gene fusion.35,36 Lastly, the epidermal growth factor receptor (EGFR) may stabilize the IGF-1R protein by preventing IGF-1R proteasomal degradation through a mechanism that remains to be unraveled.39
-
(iii)
Increased levels of ligands
Apart from upregulation of IGF-1R expression, increased levels of its ligands are expected to activate the signaling axis. Accordingly, high circulating IGF-1 level has been found to correlate with increased risk of developing cancers including that of breast, ovary and prostate.40, 41, 42 Recently, an interesting study has demonstrated that IGF-2 secreted by cancer-associated fibroblasts in the stroma induced paracrine IGF1-R/InsR axis and downstream PI3K/Akt pathway of colon cancer cells.43 The findings provide evidence that IGF-1R signaling can be regulated by extrinsic modulators in the tumor microenvironment.43
-
(iv)
Transactivation by other membrane receptors
There is accumulating evidence that IGF-1R can be activated by other membrane receptors, in particular integrins and G-protein-coupled receptor (GPCR). Integrins, which are heterodimers formed by α and β chains, mediate cell–cell and cell-extracellular matrix (ECM) interactions upon stimulation by ECM ligands or cell surface ligands.44 Integrins ανβ3 and α6β4 have been shown to interact with IGF-1 and IGF-2 45, 46, 47. Integrin-binding defective IGF-1 or IGF-2 mutants could not activate IGF-1R.45, 46, 47 This indicates that apart from binding to IGF-1R, direct binding of IGF-1/-2 to integrins is crucial to trigger IGF-1R signaling. Intriguingly, IGF-1 induces the formation of integrin/IGF-1/IGF-1R protein complex. All these observations together put forward a hypothetical model in which integrins (at least ανβ3 and α6β4) are common co-receptors for IGF-1 and IGF-2 signaling.
GABAB receptor (a GPCR activated by the neurotransmitter γ-aminobutyric acid) has been demonstrated to activate IGF-1R.48 Mechanistically, GABAB receptor and IGF-1R form a signaling complex mediated by focal adhesion kinase (FAK). The heterotrimeric G proteins are composed of three subunits: α (4 subfamilies: Gi/o, Gs, Gq/11, or G12/13), β, and γ (these two subunits bind to form Gβγ).49 The involvement of the different subunits in IGF-1R activation has been investigated.48,50,51 Inhibition of Gi/o by pertussis toxin prevented IGF-1R transactivation and recruitment of FAK to the complex, indicating the critical role of Gi/o.48 While the Gi/o heterotrimer is constitutively associated with IGF-IR, ligand stimulation by IGF-I induces the release of Gβγ subunits from the IGF-IR complex.50 Another study showed that the Gβγ subunit mediates the activation of MAPK by IGF-1 because the activation was impaired by Gβγ subunit antagonist.52
Therapeutic strategies for targeting IGF-1R
-
(1)
Downregulation of IGF-1R expression by antisense oligonucleotide (AS-ODN) and small interfering RNA (siRNA)
Oligonucleotide therapy has been a subject of intensive research for novel cancer therapeutic modality. The primary effect of this strategy is depletion of the target gene which is theoretically an oncogene. Apart from this primary effect, much evaluations are directed to AS-ODN with immunostimulatory activity. Cancer immunotherapy has emerged as a promising cancer therapeutics. Autologous cell vaccine, which is a type of personalized immunotherapy, capitalizes on the patient's own tumor cells to induce cytotoxic T-cell response against endogenous tumor antigens and thereby tumor cell death. Multiple in vitro and in vivo studies using breast cancer and glioblastoma multiforme (GBM) models have provided evidence of the ability of AS-ODN targeting IGF1R to induce anti-tumor immune responses.53,54 The immune stimulation effect of AS-ODN is attributed to the presence of unmethylated CpG motif within the sequence, which is recognized by Toll-like receptor 9 (TLR9) as pathogen-associated molecule. This reaction stimulates a cascade of innate and adaptive immune responses. In this regard, inhibition of IGF-1R signaling in breast cancer cells stimulated the secretion of pro-inflammatory cytokines (TNF-α and IFN-γ)55 and was dependent on CD8+ cell-mediated cytotoxicity via activation of the Fas/Fas ligand (FasL) death pathway.53 In GBM, B cells and tumor-specific antibody production are thought to be the primary contribution to the immunity.54
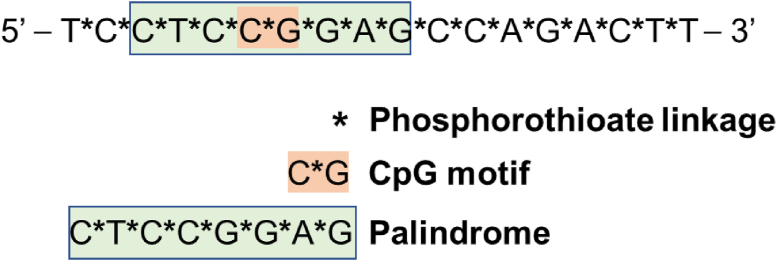
Indeed, IGF1R/AS-ODN has a long history. Back in 2001, a pilot clinical study demonstrated encouraging therapeutic effects of IGF1R/AS-ODN in glioma patients (n = 12).56 Sustained radiographic improvement was observed in 8 patients, who displayed tumor regression after treatment. In this study, an 18-mer phosphorothioate oligode-oxynucleotide (5′-GGACCCTCCTCCGGAGCC-3′) which started 6 nucleotides downstream from the start codon was used. A recent study published in 2021 reported immune activation with enhanced overall survival of GBM patients treated with IGV-001, which is an autologous cell vaccine with autologous tumor cells integrated with an IGF1R/AS-ODN named IMV-001 57. IMV-001 is an optimized phosphorothioate-linked AS-ODN complementary to the codons 2 to 7 of IGF1R mRNA (Fig. 2) with immunostimulatory properties to stimulate antigen presentation.54 The IGV-001 was encapsulated in an bio-diffusion chamber which was then implanted to the abdomen of patients,56 thereby inducing antigenicity of malignant cells and the innate immune response of patients to further promote immunogenic cell death.54,58 In this phase Ib clinical trial (n = 33), the median progression-free survival (PFS) of patients receiving IGV-001 was significantly longer than that of patients receiving standard of care (radiation and temozolomide) (9.8 months vs. 6.5 months; P = 0.0003).57
Figure 2.
The sequence and the corresponding immunostimulatory functions of motifs within IMV-001, which is complementary to IGF1R. The sequence contains a CpG motif and a palindromic sequence, which stimulate innate immune responses upon recognized by toll-like receptor 9. Phosphorothioate modification promotes nuclease resistance and binding of AS-ODN to plasma proteins, thereby decreasing renal loss and enhancing tissue delivery. The modification may also induce immune activities.
Silencing IGF1R using siRNA is another molecular therapeutic strategy to inhibit gene expression. Short RNA duplexes of 20–25 base pairs bind to IGF1R mRNA specifically and induce mRNA degradation, thereby reducing the abundance of IGF-1R protein. To increase the stability of siRNA upon in vivo delivery, the siRNA was chemically modified by replacing the alternate 2′-hydroxy groups of the ribose sugar ring with 2′-O-Me.59 Xenograft models of renal cancer cells showed induction of apoptosis and reduced cell survival upon IGF1R depletion.59 Another in vivo study using murine breast cancer cell line supported the anit-tumor effect of IGF1R siRNA.55 Interestingly, the suppression of tumor growth was accompanied with an induction of antitumor immune response and increased secretion of proinflammatory cytokines.55 A new nanocarrier for IGF1R siRNA has recently been reported.60 This lipid-polymer hybrid nanoparticles (LPHNP) using dimethyldioctadecylammonium bromide-methoxy poly(ethylene glycol)-poly (ε-caprolactone) (DDAB-mPEG-PCL) copolymer is suggested to be more stable, more biocompatible and less toxic with better cellular uptake. So far, there has been no siRNA clinical trials designed for IGF1R.
-
(2)
Disrupting the binding between IGF-1R and its ligands
Inhibiting the binding of IGF ligands to the receptor can inactivate IGF-1R. This therapeutic strategy of IGF-1R inhibition has been reviewed previously.61,62 We focus here on the monoclonal antibodies that entered late-stage clinical studies.
Monoclonal antibodies targeting IGF-1R
Anti-IGF-1R monoclonal antibody is among the first major therapeutic agents for IGF-1R inhibition. These antibodies inhibit the binding between IGF-1R and its ligands, thereby blocking the activation of IGF-1R homodimer and IGF-1R/InsR hybrid, but not the InsR homodimer.63, 64, 65 A number of fully human monoclonal antibodies, which target the α subunit of IGF-1R, have demonstrated anti-tumor activities in pre-clinical studies. After completion of early phase clinical trials, three of the IGF-1R antibodies (figitumumab, ganitumab, and dalotuzumab) were tested in phase III clinical trials.66, 67, 68
In a trial with advanced non-small-cell lung cancer (NSCLC) patients (n = 671), combination of figitumumab with chemotherapy did not improve PFS nor OS (4.7 months with chemotherapy plus figitumumab vs. 4.6 months with chemotherapy alone for median PFS; 8.6 months vs. 9.8 months for OS).67 Adverse events including pneumonia, dehydration, asthenia and hyperglycemia were observed in a number of patients.67 Similarly, the other antibody dalotuzumab did not show therapeutic benefit in a randomized trial involving KRAS wild-type metastatic colon cancer patients (n = 344).68 The median PFS was 3.9 months and 5.4 months in patients provided with dalotuzumab weekly or once every two weeks respectively, whereas the PFS of placebo-treated patients was 5.6 months. Due to the lack of clear clinical benefit in these phase III trials, further clinical research on figitumumab and dalotuzumab is not being pursued.
The low efficacy of these IGF-1R antibodies might be explained by two reasons. First, although the antibodies are thought to prevent the ligand–receptor interaction and therefore IGF-1R kinase activity, it has been suggested that the IGF-1R-targeting antibody can act as “biased” IGF-1R agonist to promote IGF-1R/β-arrestin1 association.69 This association results in ERK1/2 signaling pathway activation, despite the depletion of IGF-1R kinase activity. Whether this “biased” agonism also occurs via the IGF-1R/InsR hybrid receptor is unclear. In this regard, in vitro analysis has demonstrated that figitumumab can also recognize and downregulate the hybrid receptor.70 Second, pro-tumorigenic activities of nuclear IGF-1R has been documented.71 This nuclear pool of IGF-1R cannot be neutralized by the antibodies.
Although it appears that dalotuzumab might not be effective in these patients, exploratory biomarker analyses in the study revealed an intriguing observation that IGF1 mRNA level could be a predictive marker of response. Among patients administered with weekly dalotuzumab, those tumors with high IGF1 mRNA had better PFS (5.6 vs. 3.6 months, HR = 0.59, 95% CI = 0.28 to 1.23, P = 0.16) and OS (17.9 vs. 9.4 months, HR = 0.67, 95% CI = 0.31 to 1.45, P = 0.31) compared with those having high IGF1 mRNA tumors in placebo group.68 In contrast, high IGF1 mRNA level predicted significantly lower response rate (17.6% vs. 37.3%, P = 0.04), shorter PFS (3.6 vs. 6.6 months, HR = 2.15, 95% CI = 1.15 to 4.02, P = 0.02), and shorter OS (9.4 vs. 15.5 months, HR = 2.42, 95% CI = 1.21 to 4.82, P = 0.01) than low IGF1 mRNA level in placebo group. These together highlight the potential of the ligand expression level as stratifying marker to increase response rate. In addition to IGF1 level, inspired by another study which showed a partial response (PR) to dalotuzumab of a patient with Ewing's sarcoma,72 the EWS/FLI1 fusion may represent another determinant of response. The EWS/FLI1 fusion results from t(11; 22) (q24; q12) translocation and can be detected in ∼90% of Ewing's sarcoma cases. The fusion protein is a transcription factor that drives the development of the disease. Because IGF-IR contributes to the transformation capacity of EWS/FLI1,36,73 the anti-tumor effect of dalotuzumab in Ewing's sarcoma patient is most likely due to a direct functional interference of the fusion driver by blocking the interaction between IGF-1R and EWS/FLI1.
At present, ganitumab is the only IGF-1R monoclonal antibody with ongoing clinical trials. Ganitumab demonstrated notable clinical activity with acceptable toxicity in several phase I or II clinical trials of different cancer types.74,75 Antitumor activity upon receipt of ganitumab was observed in patients with advanced solid malignancies or non-Hodgkin's lymphoma (n = 53). Two patients with Ewing/primitive neuroectodermal tumors (PNET) exhibited durable complete responses (CR) and unconfirmed PR respectively, whereas two patients with neuroendocrine tumors experienced PR or a minor response.76 Notably, the patient with Ewing/PNET, who experienced a CR, remained disease free after 28 months of treatment.76 In 2017, FDA granted an orphan drug designation to ganitumab for the treatment of patients with Ewing sarcoma.
There have been attempts to combine ganitumab with other therapies. A combination of ganitumab and a chemotherapeutic agent gemcitabine improved OS in patients with metastatic pancreatic cancer compared with that of placebo arm in a phase II study (8.7 months vs. 5.9 months; n = 125).77 However, a subsequent phase III study demonstrated that such drug combination did not improve OS versus gemcitabine alone in an independent metastatic pancreatic cancer cohort (n = 800).66 Apart from the difference in therapeutic outcomes, the results of biomarker assessment are different in these two studies. While analysis of the phase II trial data revealed that higher circulating levels of IGF-1, IGF-2, or IGFBP-3 associated with better response to the drug combination,78 stratifying the patients in the phase III trial based on the levels of these IGFs did not result in a treatment effect on survival by ganitumab.66 It is possible that dichotomization simply by median level is not robust. Two rational clinical trials involving ganitumab are on-going. These include a phase II study on the combination of ganitumab and palbociclib (small molecular cyclin-dependent kinase) in patients with Ewing's sarcoma (NCT04129151). A selection criterion is the presence of gene translocation involving EWSR1 or FUS with ETS family in the tumor. Another phase I/II trial (NCT03041701) will study the outcome of ganitumab and dasatinib (Src family kinase inhibitor) in patients with embryonal and alveolar rhabdomyosarcoma which has been suggested to be driven by Src.79,80
Bispecific antibodies can simultaneously bind two separate antigens and thus carry dual-specificity. A phase II study in which metastatic pancreatic cancer patients were treated with istiratumab (MM-141), which is a fully human tetravalent bispecific antibody that binds to and co-inhibits IGF-1R and HER3, has released its results in 2020.81 Although istiratumab was shown to be effective in preclinical studies with ovarian cancer or pancreatic cancer cells,82,83 the clinical trial demonstrated a lack of benefit of istiratumab (including patients with high levels of IGF-1 and the HER3 ligand heregulin).81
IGF neutralizing antibodies
Several other therapeutic agents were designed to disrupt the binding between the ligands and IGF-1R. These include IGF neutralizing antibodies, IGF ligand TRAPs which are engineered molecules that bind to the ligands at high affinity, recombinant IGFBPs which represent naturally occurring IGF inhibitors, and inhibitors of pregnancy-associated plasma protein-A (PAPP-A) which is a metalloprotease that enhances IGF bioactivity by proteolytic cleavage of IGFBPs. Among these strategies, IGF ligand TRAPs, recombinant IGFBPs and PAPP-A inhibitors were shown to be effective in inhibiting the IGF-1R axis in pre-clinical studies. While further investigations are warranted to examine the clinical effects of these agents, two IGF neutralizing antibodies (dusigitumab and xentuzumab) had entered clinical trials. In contrast to dusigitumab which did not show prominent activity on PFS in a phase Ib/II trial for patients with hormone receptor-positive breast cancer (NCT01446159), xentuzumab showed anti-tumor activity in a phase I study in which 2 patients with advanced solid cancer had partial responses.84 Xentuzumab is an IgG1 monoclonal antibody that has high affinity to IGF-1 and IGF-2, thereby outcompeting the binding between these ligands and IGF-1R or InsR-A.84,85 Because xentuzumab does not interfere with the metabolic effects of InsR-B or insulin, the reported incidence of hyperglycaemia with xentuzumab was low.
IGF-1R decoy receptor
Soluble IGF-1R truncated mutants that correspond to ligand-binding portion of the α subunits (aa 1–524; dnIGF1Rα) and the α subunit-interacting portion of the β subunits (aa 741–936; dnIGF1Rβ) have been developed.86 These decoy receptors effectively blocked IGF-1R activity in a dominant-negative inhibition manner and suppressed tumorigenicity of osteosarcoma cells in vitro and in vivo.86 These mutants, particularly dnIGF1Rβ, may allow for more specific interactions with the IGF ligands and cause less interference with insulin signaling.
Anti-idiotypic antibody
Anti-idiotypic antibody may represent an alternative immunotherapeutic treatment strategy. Anti-idiotypic antibody directed against another anti-IGF-1 antibody functionally mimic the 3D structure of the original IGF-1 antigen. An IGF-1 anti-idiotypic monoclonal antibody (named B003-2A) that can specifically bind to IGF-1R has been identified.87 B003-2A mimics an epitope on IGF-1 and competes with IGF-1 for receptor binding. IGF-1R-mediated signaling was inhibited by B003-2A, accompanied by downregulation of IGF-1R protein and ovarian tumor growth inhibition in xenograft.87
-
(3)
Tyrosine Kinase Inhibitors (TKIs)
Several small molecule TKIs have been developed for inactivating IGF-1R. These TKIs are categorized according to their ATP or non-ATP competitive activities. IGF-1R and InsR are 80% identical in the kinase domains. The ATP-binding domains in the two receptors are 100% identical. Given the roles of InsR in glucose metabolism, the majority of the ATP-competitive IGF-1R TKIs have been reported to cause hyperinsulinemia and hyperglycemia due to the simultaneous inhibition of InsR.88,89 Therefore, it is reasonable to deduce that non-ATP competitive IGF-1R TKIs may result in less toxicities associated with disrupting glucose homeostasis.
Linsitinib (OSI-906) is an ATP-competitive TKI against both IGF-1R and InsR. Preclinical study has reported the synergistic anti-tumor effect of linsitinib in combination with two validated approaches to treat multiple myeloma (proteasome inhibitors bortezomib and dexamethasone).90 Building on the preclinical data, a phase I trial was initiated to assess the effect of this drug combination in patients with multiple myeloma (n = 19).91 Because of the predicted disturbance of glucose metabolism by this dual IGF-1R/InsR TKI, patients were required to have good glycemic control during the course of the study. This study showed that the drug combination was safe and well tolerated. Hyperglycemia was detected in only 11% (2/19) patients, at grades 1 and 3 severity respectively. The drug combination did not significantly improve overall responses when compared with historical controls of bortezomib and dexamethasone alone. However, 4 of 5 bortezomib-refractory patients showed PR or stable disease, suggesting that a subset of patients may be responsive to such combinatorial regimen.
AXL-1717 is a non-ATP competitive IGF-1R TKI. It demonstrated promising anti-tumor efficacy as a single agent and acceptable safety profile in two phase I trials, including 15 heavily pretreated NSCLC patients92 and patients with recurrent or progressive malignant astrocytomas (n = 9).93 AXL-1717 has been granted orphan drug designation for the treatment of patients with relapsed malignant astrocytomas. The optimal dose, tolerability and efficacy of AXL-1717 in treating recurrent malignant astrocytomas will be further examined in a phase II clinical trial (NCT01721577).
NT157 is a non-ATP competitive IGF1R-IRS1/2 inhibitor that has been shown to effectively target chronic myeloid leukemia (CML) cells and to overcome BCR-ABL1 TKI resistance. Imatinib is a frontline TKI in CML treatment. Common mechanism of imatinib resistance includes T315I mutation in the kinase domain of BCR-ABL1. NT157 allosterically binds to IRS1/2 which are the common cytoplasmic effectors of IGF-1R and BCR-ABL1, thereby inhibiting these two signaling axes.94 In preclinical models of CML, NT157 caused apoptosis independent of the mutation status of BCR-ABL1, indicating the potential of NT157 in overcoming TKI resistance driven by T315I mutation.94 Of note, the combination of NT157 and imatinib produced an additive anti-tumor effect.94
-
(4)
Cytotoxic drug conjugate
Antibody–drug conjugate (ADC) represents another major focus in oncology therapeutics. About 80 ADCs are currently being tested in clinical trials and 4 ADCs have been approved by the European Medicine Agency and FDA for the treatment of metastatic cancers.95,96 ADC has been exploited to treat IGF-1R overexpressing tumors. W0101, which tethers a monoclonal antibody (hz208F2-4) to a cytotoxic derivative of auristatin via a chemical linker (noncleavable maleimidocaproyl linker), is another new ADC. W0101 demonstrates high specific binding to IGF-1R but not InsR. Upon binding of W0101 to the membranous IGF-1R, the receptor readily undergoes internalization and auristatin can then be released in the tumor cells. Auristatin blocks cell division by repressing the polymerization of tubulin in the cytoplasm.97 The effective delivery of this ADC to the intracellular compartment is acquired by catabolism of ADC in the lysosomes, which release the free cytotoxic drug to cytoplasm.97 In IGF-1R-overexpressing cancer cell line models MCF-7 (breast) and NCI–H2122 (lung), W0101 induced a potent cytotoxic activity.97 A phase I/II clinical trial is currently recruiting to assess the clinical safety and efficacy of W0101 in advanced or metastatic solid tumors (NCT03316638).
Apart from antibody–drug conjugate, peptide–drug conjugate that targets IGF-1R-overexpressing tumors has been explored. IGF-1 is the primary ligand of the IGF-1R. However, when IGF-1 is bound to IGFBPs, IGF-1 cannot bind to IGF-1R.7,9 To enhance the chance of binding to IGF-1R, an IGF-1 variant (765IGF) which has reduced affinity for IGFBPs has been engineered. IGF-methotrexate (IGF-MTX) is a conjugate of 765IGF and an antifolate drug methotrexate (MTX; a chemotherapeutic and immunosuppressant agent). The conjugate can selectively enter cancer cells through binding to IGF-1R.98 The high binding affinity of IGF-MTX to IGF-1R contributes to its promising anti-tumor activities in LNCaP and MCF7 xenograft compared with free MTX.98,99 It has been suggested that low doses of IGF-MTX are sufficient to induce anti-tumor effects and cause less side effects as well as systemic toxicity.99 The smaller size of peptide–drug conjugate compared with antibody–drug conjugate may also allow for efficient cellular uptake. Two phase I clinical trials in patients with advance solid and hematologic malignancy (NCT02045368) or high-grade myelodysplastic syndromes (n = 2, NCT03175978) have suggested promising efficacy of IGF-MTX at tolerable dose and desirable toxicity profile.98,100 Stable disease could be observed in 20% of patients (n = 3 out of 15) with refractory solid or hematologic malignancies98 and in patients (n = 2 out of 2) with myelodysplastic syndromes.100
To further improve the therapeutic efficacy of ADC exploiting IGF-1R, approach to advance the conjugated drug by chemical modification has been explored.101 For example, maytansine (DM1) kills cancer cells by inhibiting microtubule assembly. Anti-IGF-1R antibody cixutumumab conjugated to PEGylated DM1 may gain potency because modifying DM1 through PEGylation renders DM1 the ability to evade from drug efflux pumps.101
Combinational therapy involving IGF-1R inhibition
Overexpression of IGF-1R may confer resistance towards radiotherapy, chemotherapy and targeted therapy in multiple cancer types.102 The therapeutic resistance is mainly mediated through two mechanisms: (i) crosstalk with other signaling pathways resulting in network rewiring and compensatory survival signal; and (ii) promotion of homologous and non-homologous end-joining recombination to repair DNA double-strand breaks induced by radio- and chemotherapeutic agents.103, 104, 105 Intriguingly, recent pre-clinical studies have also highlighted the role of IGF-1R in the nucleus.71,106 In non-small cell lung and cervical cancer cell lines, nuclear IGF-1R might upregulate the promoter activities of target genes involved in the WNT pathway, for examples CCND1 (encoding cyclin D1) and AXIN2 (encoding Axin 2).107 As a result, proliferation of cancer stem cells could be maintained via the sustained activation of WNT signaling. This potential role of nuclear IGF-1R in maintaining cancer stemness may present another mechanism of drug resistance.
Activation of IGF-1R was found in prostate cancer cells resistant to an EGFR inhibitor gefitinib.108 Along this line, co-treatment of gefitinib and an IGF-1R inhibitor AG1024 resulted in reduced cell proliferation and invasion capability compared with single treatment in these cells.108 Treatment of EGFR-mutated NSCLC with osimertinib (a third-generation EGFR-TKI selectively inhibiting EGFR with exon 19 deletion, L858R, and T790M) combined with IGF-1R TKI linsitinib (OSI-906) led to tumor eradication in xenograft and PDX models.109 Although a phase I study (n = 95) has demonstrated the potential efficacy of combined EGFR and IGF-1R inhibition (by erlotinib and linsitinib, respectively) in multiple advanced solid tumor types,110 such combination did not lead to better therapeutic outcome compared with erlotinib alone in a phase II clinical trial involving NSCLC patients (n = 88).111
HER2 and ER signaling are major drivers of breast cancer. Circulating IGF-2 levels in HER2-positive breast cancer patients with poor response to trastuzumab (Herceptin; anti-HER2 monoclonal antibody) were significantly higher than that of the patients with better response.112 Enhanced anti-proliferative effects of trastuzumab in combination with IGF-1R TKIs BMS-536924 or NVP-AEW541 were observed in HER2-positive breast cancer cell lines.113 Indeed, mechanistically, formation of IGF-1R/HER-2 heterodimer was observed in trastuzumab-resistant breast cancer cells.25 Moreover, trastuzumab resistance in HER2-overexpressing breast cancer cells is attributed to IGF-1R-activated FAK-Src signalling and can be reversed by an IGF-1R monoclonal antibody (α-IR3).17 It has been shown that IGF-1R could induce the phosphorylation and activation of ER through the PI3K/AKT and Ras/Raf/MAPK signaling pathways.114 As a result, combinatory inhibition of IGF-IR (by a monoclonal antibody αIGF-1R scFv-Fc) and ER (by tamoxifen) resulted in enhanced therapeutic effect in breast cancer cell line.115 Co-targeting IGF-1R (by TKIs NVP-AEW541 or BMS-536924 or AG1024) and ER (by tamoxifen or fulvestrant) significantly enhanced cell cycle arrest at G1 phase or apoptosis in the breast cancer cell lines.116,117
Another combination strategy shown to be effective in vitro and in vivo involves combined inhibition of IGF-1R (by linsitinib or figitumumab) and MEK 1/2 (by U0126 or selumetinib). These combinations caused synergistic anti-proliferative effects in a panel of colorectal cancer cell lines and xenograft118 as well as in small cell lung cancer cell line.119 Interestingly, building on the facts that IGF-1R inhibitor may induce hyperglycemia and that metformin has both hypo-glycemic and anti-cancer effects, the effects of combining metformin and an anti-IGF-1R monoclonal antibody figitumumab or BMS-754807 (an IGF-1R inhibitor) were examined in SCLC or breast cancer cell line respectively.119,120 The combination resulted in superior therapeutic effects in both studies. However, a study suggested that BMS-754807 might inhibit additional protein kinase targets apart from IGF-1R and its anti-tumorigenic activity may be independent of IGF-1R inhibition.121
The regulation of the IGFR-1R signaling by the tumor microenvironment might be exploited in the design of combination therapy. Upon treatment with an IGF-1R monoclonal antibody cixutumumab, STAT3 was activated to upregulate the transcription of IGF-2 which then acts on IGF-2R on the stromal cells.122 The infiltrated stromal cells produce pro-angiogenic cytokines, thereby recruiting vascular endothelial cells and enhancing tumor angiogenesis.122 In light of these data, co-targeting IGF-1R and STAT3 may inhibit tumor metastasis and overcome STAT3-mediated resistance to anti-IGF-1R therapy.
Conclusions and future prospective
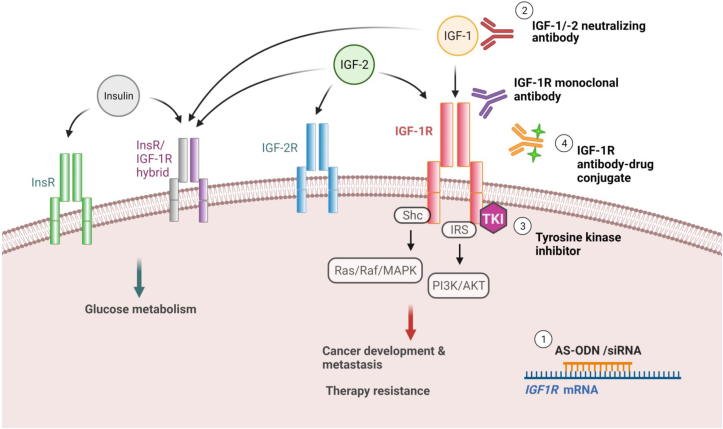
IGF-1R remains an attractive druggable target of cancer. Multiple therapeutic strategies revolving around this receptor have been developed and investigated (Fig. 3). Although the outcomes of clinical trials so far are conflicting, these studies have generated new and important insights into strategies that target IGF-1R. First, the lack of patient stratification could be a leading cause of the conflicting treatment outcomes. Investigation on the molecular profiles of responsive and non-responsive patients will shed light on potential biomarkers of responses. Second, rational combinational targeted approaches that inhibit IGF-1R signaling and the linked networks have demonstrated additive anti-tumor activities. Elucidating the signaling network and drug resistant mechanism associated with IGF-1R will uncover more potential drug combination to improve therapeutic outcomes. Third, the hyperglycemic side effect associated with simultaneous inhibition of InsR might be circumvented by several approaches. These include the development of new inhibitors that spare InsR. Interfering with the IRS-1 and IRS-2 proteins, which are the signal adaptors of IGF-1R, may be effective in patients with high expressions of IGF-1R. Inhibiting IRS-1/2 may suppress tumor growth without affecting the normal glucose homeostasis mediated by the other adapter proteins in insulin target organs. Alternatively, the incorporation of metformin, which induces hypoglycemic and anti-cancer effects, with IGF-1R inhibition may reduce the occurrence of hyperglycaemia. Study that examines the structural different between InsR and IGF-1R may also help reveal novel class of specific allosteric inhibitor.123
Figure 3.
Overview of the therapeutic strategies developed to inhibit IGF-1R signaling. The schematic shows the key components of the IGF axis and the four major approaches for IGF-1R inhibition: (1) by suppressing IGF-1R expression using antisense oligonucleotide (AS-ODN) or siRNA; (2) by blocking the binding of IGF-1R with ligands (IGF-1R monoclonal antibodies or IGF-1/-2 neutralizing antibodies); (3) by repressing IGF-1R kinase activity using tyrosine kinase inhibitor (TKI); and (4) by conjugating cytotoxic drug to IGF-1R antibody.
Further, insights from biological research will continue to foster the development of effective anti-IGF-1R therapy. Accumulating evidence has highlighted the functional significance of nuclear IGF-1R in therapeutic resistance.71 It has been found in colorectal and prostate cancers that high abundance of nuclear IGF-1R promoted tumorigenesis and hindered the effectiveness of chemotherapy and targeted therapies (inhibition of IGF-1R by ganitumab or NVP-AEW541; inhibition of Src by dasatinib).124,125 A poor OS has been reported in colorectal cancer patients with high levels of nuclear phosphorylated IGF-1R compared with low/negative levels (median 16.7 months vs. 24.6 months; P < 0.01).124 Building on these findings, inhibiting the nuclear sequestration event of IGF-1R may re-sensitize cancer cells to anti-IGF-1R therapy. Strikingly, a preclinical study using prostate cancer cells has revealed a novel function of IGF-1R TKI (AZ12253801) in the downregulation of the total and phosphorylated IGF-1R protein in the nucleus, shedding light on the possibility to reposition IGF-1R inhibitors in future combinational drug studies.106 Nuclear translocation of IGF-1R is mediated by clathrin-dependent endocytosis and SUMOlyation.106,126,127 Thorough understanding of the nuclear translocation process may lead to viable strategy that abrogates the translocation.
Conflict of interests
The authors declare no conflict of interests.
Funding
This research was supported by funding from the National Natural Science Foundation of China (No. 82022078).
Acknowledgements
The authors thank Dr Amy YT Lau for brainstorming.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Weinstein J.N., Collisson E.A., Mills G.B., et al. The cancer Genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denduluri S.K., Idowu O., Wang Z., et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2(1):13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y., Wang Y., James M., et al. Inhibition of IGF1R signaling abrogates resistance to afatinib (BIBW2992) in EGFR T790M mutant lung cancer cells. Mol Carcinog. 2016;55(5):991–1001. doi: 10.1002/mc.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne B.C., Crown J., Venkatesan N., et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol. 2011;22(1):68–73. doi: 10.1093/annonc/mdq349. [DOI] [PubMed] [Google Scholar]
- 5.Brahmkhatri V.P., Prasanna C., Atreya H.S. Insulin-like growth factor system in cancer: novel targeted therapies. BioMed Res Int. 2015;2015:538019. doi: 10.1155/2015/538019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evdokimova V., Tognon C.E., Benatar T., et al. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5(255):ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- 7.Bach L.A. IGF-binding proteins. J Mol Endocrinol. 2018;61(1):T11–T28. doi: 10.1530/JME-17-0254. [DOI] [PubMed] [Google Scholar]
- 8.Chitnis M.M., Yuen J.S.P., Protheroe A.S., et al. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14(20):6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 9.Baxter R.C. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14(5):329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 10.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 11.Tognon C.E., Sorensen P.H. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16(1):33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- 12.Adams T.E., Epa V.C., Garrett T.P., et al. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57(7):1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcaro A. Targeting the insulin-like growth factor-1 receptor in human cancer. Front Pharmacol. 2013;4:30. doi: 10.3389/fphar.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancarella C., Morrione A., Scotlandi K. Novel regulators of the IGF system in cancer. Biomolecules. 2021;11(2):273. doi: 10.3390/biom11020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandini G., Frasca F., Mineo R., et al. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277(42):39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 16.Rozengurt E., Sinnett-Smith J., Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16(9):2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanabria-Figueroa E., Donnelly S.M., Foy K.C., et al. Insulin-like growth factor-1 receptor signaling increases the invasive potential of human epidermal growth factor receptor 2-overexpressing breast cancer cells via Src-focal adhesion kinase and forkhead box protein M1. Mol Pharmacol. 2015;87(2):150–161. doi: 10.1124/mol.114.095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevenini A., Orrù S., Mancini A., et al. Molecular signatures of the insulin-like growth factor 1-mediated epithelial-mesenchymal transition in breast, lung and gastric cancers. Int J Mol Sci. 2018;19(8):E2411. doi: 10.3390/ijms19082411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aleksic T., Verrill C., Bryant R.J., et al. IGF-1R associates with adverse outcomes after radical radiotherapy for prostate cancer. Br J Cancer. 2017;117(11):1600–1606. doi: 10.1038/bjc.2017.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale O.T., Aleksic T., Shah K.A., et al. IGF-1R expression is associated with HPV-negative status and adverse survival in head and neck squamous cell cancer. Carcinogenesis. 2015;36(6):648–655. doi: 10.1093/carcin/bgv053. [DOI] [PubMed] [Google Scholar]
- 21.Farabaugh S.M., Boone D.N., Lee A.V. Role of IGF1R in breast cancer subtypes, stemness, and lineage differentiation. Front Endocrinol. 2015;6:59. doi: 10.3389/fendo.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon-Zemler R., Sarfstein R., Werner H. Nuclear insulin-like growth factor-1 receptor (IGF1R) displays proliferative and regulatory activities in non-malignant cells. PLoS One. 2017;12(9):e0185164. doi: 10.1371/journal.pone.0185164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkhayyal N., Talaat I., Vinodnadat A., et al. Correlation of insulin-like growth factor 1 receptor expression with different molecular subtypes of breast cancer in the UAE. Anticancer Res. 2020;40(3):1555–1561. doi: 10.21873/anticanres.14102. [DOI] [PubMed] [Google Scholar]
- 24.Ekyalongo R.C., Yee D. Revisiting the IGF-1R as a breast cancer target. NPJ Precis Oncol. 2017;1:14. doi: 10.1038/s41698-017-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahta R., Yuan L.X., Zhang B., et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 26.Kang H.S., Ahn S.H., Mishra S.K., et al. Association of polymorphisms and haplotypes in the insulin-like growth factor 1 receptor (IGF1R) gene with the risk of breast cancer in Korean women. PLoS One. 2014;9(1):e84532. doi: 10.1371/journal.pone.0084532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanilov N.S., Karakolev I.A., Deliysky T.S., et al. Association of insulin-like growth factor-I receptor polymorphism with colorectal cancer development. Mol Biol Rep. 2014;41(12):8099–8106. doi: 10.1007/s11033-014-3708-2. [DOI] [PubMed] [Google Scholar]
- 28.Dong X., Javle M., Hess K.R., et al. Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology. 2010;139(2):464–473. doi: 10.1053/j.gastro.2010.04.042. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan T.A., Yourk V., Farhat A., et al. A possible link of genetic variations in ER/IGF1R pathway and risk of melanoma. Int J Mol Sci. 2020;21(5):E1776. doi: 10.3390/ijms21051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell M.J., Dufault S.M., Henry J.E., et al. Pregnancy hypertension and a commonly inherited IGF1R variant (rs2016347) reduce breast cancer risk by enhancing mammary gland involution. JAMA Oncol. 2019;2019:6018432. doi: 10.1155/2019/6018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarfstein R., Pasmanik-Chor M., Yeheskel A., et al. Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells. J Biol Chem. 2012;287(4):2766–2776. doi: 10.1074/jbc.M111.281782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuen J.S., Cockman M.E., Sullivan M., et al. The VHL tumor suppressor inhibits expression of the IGF1R and its loss induces IGF1R upregulation in human clear cell renal carcinoma. Oncogene. 2007;26(45):6499–6508. doi: 10.1038/sj.onc.1210474. [DOI] [PubMed] [Google Scholar]
- 33.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31(22):2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 34.Schayek H., Bentov I., Rotem I., et al. Transcription factor E2F1 is a potent transactivator of the insulin-like growth factor-I receptor (IGF-IR) gene. Growth Hormone IGF Res. 2010;20(1):68–72. doi: 10.1016/j.ghir.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Sun J., Lu Z., Deng Y., et al. Up-regulation of INSR/IGF1R by C-myc promotes TSCC tumorigenesis and metastasis through the NF-κB pathway. Biochim Biophys Acta BBA Mol Basis Dis. 2018;1864(5):1873–1882. doi: 10.1016/j.bbadis.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Werner H., Meisel-Sharon S., Bruchim I. Oncogenic fusion proteins adopt the insulin-like growth factor signaling pathway. Mol Cancer. 2018;17(1):28. doi: 10.1186/s12943-018-0807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idelman G., Glaser T., Roberts C.T., Jr., et al. WT1-p53 interactions in insulin-like growth factor-I receptor gene regulation. J Biol Chem. 2003;278(5):3474–3482. doi: 10.1074/jbc.M211606200. [DOI] [PubMed] [Google Scholar]
- 38.Abramovitch S., Glaser T., Ouchi T., et al. BRCA1-Sp1 interactions in transcriptional regulation of the IGF-IR gene. FEBS Lett. 2003;541(1–3):149–154. doi: 10.1016/s0014-5793(03)00315-6. [DOI] [PubMed] [Google Scholar]
- 39.Riedemann J., Takiguchi M., Sohail M., et al. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun. 2007;355(3):707–714. doi: 10.1016/j.bbrc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Lukanova A., Lundin E., Toniolo P., et al. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int J Cancer. 2002;101(6):549–554. doi: 10.1002/ijc.10613. [DOI] [PubMed] [Google Scholar]
- 41.Roddam A.W., Allen N.E., Appleby P., et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149(7):461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. W83-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian F., Huo D. Circulating insulin-like growth factor-1 and risk of total and 19 site-specific cancers: cohort study analyses from the UK biobank. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2332–2342. doi: 10.1158/1055-9965.EPI-20-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unger C., Kramer N., Unterleuthner D., et al. Stromal-derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms. Oncogene. 2017;36(38):5341–5355. doi: 10.1038/onc.2017.116. [DOI] [PubMed] [Google Scholar]
- 44.Bianconi D., Unseld M., Prager G.W. Integrins in the spotlight of cancer. Int J Mol Sci. 2016;17(12):E2037. doi: 10.3390/ijms17122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takada Y., Takada Y.K., Fujita M. Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1. Cytokine Growth Factor Rev. 2017;34:67–72. doi: 10.1016/j.cytogfr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita M., Ieguchi K., Davari P., et al. Cross-talk between integrin alpha6beta4 and insulin-like growth factor-1 receptor (IGF1R) through direct alpha6beta4 binding to IGF1 and subsequent alpha6beta4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions. J Biol Chem. 2012;287(15):12491–12500. doi: 10.1074/jbc.M111.304170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cedano Prieto D.M., Cheng Y., Chang C.C., et al. Direct integrin binding to insulin-like growth factor-2 through the C-domain is required for insulin-like growth factor receptor type 1 (IGF1R) signaling. PLoS One. 2017;12(9):e0184285. doi: 10.1371/journal.pone.0184285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X., Li X., Jiang M., et al. An activity-based probe reveals dynamic protein-protein interactions mediating IGF-1R transactivation by the GABA(B) receptor. Biochem J. 2012;443(3):627–634. doi: 10.1042/BJ20120188. [DOI] [PubMed] [Google Scholar]
- 49.Arang N., Gutkind J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 2020;594(24):4201–4232. doi: 10.1002/1873-3468.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallak H., Seiler A.E., Green J.S., et al. Association of heterotrimeric G(i) with the insulin-like growth factor-I receptor. Release of G(betagamma) subunits upon receptor activation. J Biol Chem. 2000;275(4):2255–2258. doi: 10.1074/jbc.275.4.2255. [DOI] [PubMed] [Google Scholar]
- 51.Dalle S., Ricketts W., Imamura T., et al. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276(19):15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- 52.Luttrell L.M., van Biesen T., Hawes B.E., et al. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270(28):16495–16498. doi: 10.1074/jbc.270.28.16495. [DOI] [PubMed] [Google Scholar]
- 53.Schillaci R., Salatino M., Cassataro J., et al. Immunization with murine breast cancer cells treated with antisense oligodeoxynucleotides to type I insulin-like growth factor receptor induced an antitumoral effect mediated by a CD8+ response involving Fas/Fas ligand cytotoxic pathway. J Immunol. 2006;176(6):3426–3437. doi: 10.4049/jimmunol.176.6.3426. [DOI] [PubMed] [Google Scholar]
- 54.Morin-Brureau M., Hooper K.M., Prosniak M., et al. Enhancement of glioma-specific immunity in mice by “NOBEL”, an insulin-like growth factor 1 receptor antisense oligodeoxynucleotide. Cancer Immunol Immunother. 2015;64(4):447–457. doi: 10.1007/s00262-015-1654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durfort T., Tkach M., Meschaninova M.I., et al. Small interfering RNA targeted to IGF-IR delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. PLoS One. 2012;7(1):e29213. doi: 10.1371/journal.pone.0029213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews D.W., Resnicoff M., Flanders A.E., et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19(8):2189–2200. doi: 10.1200/JCO.2001.19.8.2189. [DOI] [PubMed] [Google Scholar]
- 57.Andrews D.W., Judy K.D., Scott C.B., et al. Phase Ib clinical trial of IGV-001 for patients with newly diagnosed glioblastoma. Clin Cancer Res. 2021;27(7):1912–1922. doi: 10.1158/1078-0432.CCR-20-3805. [DOI] [PubMed] [Google Scholar]
- 58.Harshyne L.A., Hooper K.M., Andrews E.G., et al. Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol Immunother. 2015;64(3):299–309. doi: 10.1007/s00262-014-1622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuen J.S.P., Akkaya E., Wang Y., et al. Validation of the type 1 insulin-like growth factor receptor as a therapeutic target in renal cancer. Mol Cancer Therapeut. 2009;8(6):1448–1459. doi: 10.1158/1535-7163.MCT-09-0101. [DOI] [PubMed] [Google Scholar]
- 60.Khodaei M., Rostamizadeh K., Taromchi A.H., et al. DDAB cationic lipid-mPEG, PCL copolymer hybrid nano-carrier synthesis and application for delivery of siRNA targeting IGF-1R into breast cancer cells. Clin Transl Oncol. 2021;23(6):1167–1178. doi: 10.1007/s12094-020-02507-3. [DOI] [PubMed] [Google Scholar]
- 61.Gualberto A., Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28(34):3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 62.Olmos D., Tan D.S., Jones R.L., et al. Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: twenty years from the bench to the bedside. Cancer J. 2010;16(3):183–194. doi: 10.1097/PPO.0b013e3181dbebf9. [DOI] [PubMed] [Google Scholar]
- 63.Wang W., Zhang Y., Lv M., et al. Anti-IGF-1R monoclonal antibody inhibits the carcinogenicity activity of acquired trastuzumab-resistant SKOV3. J Ovarian Res. 2014;7:103. doi: 10.1186/s13048-014-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broussas M., Dupont J., Gonzalez A., et al. Molecular mechanisms involved in activity of h7C10, a humanized monoclonal antibody, to IGF-1 receptor. Int J Cancer. 2009;124(10):2281–2293. doi: 10.1002/ijc.24186. [DOI] [PubMed] [Google Scholar]
- 65.Calzone F.J., Cajulis E., Chung Y.A., et al. Epitope-specific mechanisms of IGF1R inhibition by ganitumab. PLoS One. 2013;8(2):e55135. doi: 10.1371/journal.pone.0055135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchs C.S., Azevedo S., Okusaka T., et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26(5):921–927. doi: 10.1093/annonc/mdv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langer C.J., Novello S., Park K., et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2014;32(19):2059–2066. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sclafani F., Kim T.Y., Cunningham D., et al. A randomized phase II/III study of dalotuzumab in combination with cetuximab and irinotecan in chemorefractory, KRAS wild-type, metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(12):djv258. doi: 10.1093/jnci/djv258. [DOI] [PubMed] [Google Scholar]
- 69.Zheng H., Shen H., Oprea I., et al. Β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing's sarcoma. Proc Natl Acad Sci USA. 2012;109(50):20620–20625. doi: 10.1073/pnas.1216348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen B.D., Baker D.A., Soderstrom C., et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751, 871. Clin Cancer Res. 2005;11(5):2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 71.Chughtai S. The nuclear translocation of insulin-like growth factor receptor and its significance in cancer cell survival. Cell Biochem Funct. 2020;38(4):347–351. doi: 10.1002/cbf.3479. [DOI] [PubMed] [Google Scholar]
- 72.Frappaz D., Federico S.M., Pearson A.D.J., et al. Phase 1 study of dalotuzumab monotherapy and ridaforolimus-dalotuzumab combination therapy in paediatric patients with advanced solid tumours. Eur J Cancer. 2016;62:9–17. doi: 10.1016/j.ejca.2016.03.084. [DOI] [PubMed] [Google Scholar]
- 73.Huang H.J., Angelo L.S., Rodon J., et al. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing’s sarcoma: convergence at the IGF/IGFR/Akt axis. PLoS One. 2011;6(10):e26060. doi: 10.1371/journal.pone.0026060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vlahovic G., Meadows K.L., Hatch A.J., et al. A phase I trial of the IGF-1R antibody ganitumab (AMG 479) in combination with everolimus (RAD001) and panitumumab in patients with advanced cancer. Oncol. 2018;23(7):782–790. doi: 10.1634/theoncologist.2016-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tap W.D., Demetri G., Barnette P., et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 2012;30(15):1849–1856. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 76.Tolcher A.W., Sarantopoulos J., Patnaik A., et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 77.Kindler H.L., Richards D.A., Garbo L.E., et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23(11):2834–2842. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 78.McCaffery I., Tudor Y., Deng H., et al. Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clin Cancer Res. 2013;19(15):4282–4289. doi: 10.1158/1078-0432.CCR-12-1840. [DOI] [PubMed] [Google Scholar]
- 79.Cen L., Arnoczky K.J., Hsieh F.C., et al. Phosphorylation profiles of protein kinases in alveolar and embryonal rhabdomyosarcoma. Mod Pathol. 2007;20(9):936–946. doi: 10.1038/modpathol.3800834. [DOI] [PubMed] [Google Scholar]
- 80.Abraham J., Chua Y.X., Glover J.M., et al. An adaptive Src-PDGFRA-Raf axis in rhabdomyosarcoma. Biochem Biophys Res Commun. 2012;426(3):363–368. doi: 10.1016/j.bbrc.2012.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kundranda M., Gracian A.C., Zafar S.F., et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE) Ann Oncol. 2020;31(1):79–87. doi: 10.1016/j.annonc.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Camblin A.J., Pace E.A., Adams S., et al. Dual inhibition of IGF-1R and ErbB3 enhances the activity of gemcitabine and nab-paclitaxel in preclinical models of pancreatic cancer. Clin Cancer Res. 2018;24(12):2873–2885. doi: 10.1158/1078-0432.CCR-17-2262. [DOI] [PubMed] [Google Scholar]
- 83.Camblin A.J., Tan G., Curley M.D., et al. Dual targeting of IGF-1R and ErbB3 as a potential therapeutic regimen for ovarian cancer. Sci Rep. 2019;9(1):16832. doi: 10.1038/s41598-019-53322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Bono J., Lin C.C., Chen L.T., et al. Two first-in-human studies of xentuzumab, a humanised insulin-like growth factor (IGF)-neutralising antibody, in patients with advanced solid tumours. Br J Cancer. 2020;122(9):1324–1332. doi: 10.1038/s41416-020-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedbichler K., Hofmann M.H., Kroez M., et al. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol Cancer Therapeut. 2014;13(2):399–409. doi: 10.1158/1535-7163.MCT-13-0598. [DOI] [PubMed] [Google Scholar]
- 86.Cao D., Lei Y., Ye Z., et al. Blockade of IGF/IGF-1R signaling axis with soluble IGF-1R mutants suppresses the cell proliferation and tumor growth of human osteosarcoma. Am J Cancer Res. 2020;10(10):3248–3266. [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang W., Xie Y., Wang W., et al. IGF-1R anti-idiotypic antibody antagonist exhibited anti-ovarian cancer bioactivity and reduced cisplatin resistance. Hum Cell. 2021;34(4):1197–1214. doi: 10.1007/s13577-021-00535-x. [DOI] [PubMed] [Google Scholar]
- 88.King H., Aleksic T., Haluska P., et al. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev. 2014;40(9):1096–1105. doi: 10.1016/j.ctrv.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carboni J.M., Wittman M., Yang Z., et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Therapeut. 2009;8(12):3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 90.Kuhn D.J., Berkova Z., Jones R.J., et al. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood. 2012;120(16):3260–3270. doi: 10.1182/blood-2011-10-386789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khan S., LeBlanc R., Gyger M., et al. A phase-1 trial of linsitinib (OSI-906) in combination with bortezomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Leuk Lymphoma. 2021;62(7):1721–1729. doi: 10.1080/10428194.2021.1876864. [DOI] [PubMed] [Google Scholar]
- 92.Ekman S., Harmenberg J., Frödin J.E., et al. A novel oral insulin-like growth factor-1 receptor pathway modulator and its implications for patients with non-small cell lung carcinoma: a phase I clinical trial. Acta Oncol. 2016;55(2):140–148. doi: 10.3109/0284186X.2015.1049290. [DOI] [PubMed] [Google Scholar]
- 93.Aiken R., Axelson M., Harmenberg J., et al. Phase I clinical trial of AXL1717 for treatment of relapsed malignant astrocytomas: analysis of dose and response. Oncotarget. 2017;8(46):81501–81510. doi: 10.18632/oncotarget.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scopim-Ribeiro R., Machado-Neto J.A., Eide C.A., et al. NT157, an IGF1R-IRS1/2 inhibitor, exhibits antineoplastic effects in pre-clinical models of chronic myeloid leukemia. Invest N Drugs. 2021;39(3):736–746. doi: 10.1007/s10637-020-01028-8. [DOI] [PubMed] [Google Scholar]
- 95.Chalouni C., Doll S. Fate of antibody-drug conjugates in cancer cells. J Exp Clin Cancer Res. 2018;37(1):20. doi: 10.1186/s13046-017-0667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hafeez U., Parakh S., Gan H.K., et al. Antibody-drug conjugates for cancer therapy. Molecules. 2020;25(20):E4764. doi: 10.3390/molecules25204764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akla B., Broussas M., Loukili N., et al. Efficacy of the antibody-drug conjugate W0101 in preclinical models of IGF-1 receptor overexpressing solid tumors. Mol Cancer Therapeut. 2020;19(1):168–177. doi: 10.1158/1535-7163.MCT-19-0219. [DOI] [PubMed] [Google Scholar]
- 98.Venepalli N.K., Emmadi R., Danciu O.C., et al. Phase I study of IGF-methotrexate conjugate in the treatment of advanced tumors expressing IGF-1R. Am J Clin Oncol. 2019;42(11):862–869. doi: 10.1097/COC.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 99.McTavish H., Griffin R.J., Terai K., et al. Novel insulin-like growth factor-methotrexate covalent conjugate inhibits tumor growth in vivo at lower dosage than methotrexate alone. Transl Res. 2009;153(6):275–282. doi: 10.1016/j.trsl.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Alkhateeb H.B., Patnaik M.M., Al-Kali A., et al. Phase 1b study of IGF-methotrexate conjugate in the treatment of high-grade myelodysplastic syndromes. Anticancer Res. 2020;40(7):3883–3888. doi: 10.21873/anticanres.14378. [DOI] [PubMed] [Google Scholar]
- 101.Solomon V.R., Alizadeh E., Bernhard W., et al. Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer. Sci Rep. 2020;10(1):18549. doi: 10.1038/s41598-020-75279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hua H., Kong Q., Yin J., et al. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol. 2020;13(1):64. doi: 10.1186/s13045-020-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chitnis M.M., Lodhia K.A., Aleksic T., et al. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene. 2014;33(45):5262–5273. doi: 10.1038/onc.2013.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramcharan R., Aleksic T., Kamdoum W.P., et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget. 2015;6(37):39877–39890. doi: 10.18632/oncotarget.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh R.K., Gaikwad S.M., Jinager A., et al. IGF-1R inhibition potentiates cytotoxic effects of chemotherapeutic agents in early stages of chemoresistant ovarian cancer cells. Cancer Lett. 2014;354(2):254–262. doi: 10.1016/j.canlet.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 106.Aleksic T., Chitnis M.M., Perestenko O.V., et al. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70(16):6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warsito D., Sjöström S., Andersson S., et al. Nuclear IGF1R is a transcriptional co-activator of LEF1/TCF. EMBO Rep. 2012;13(3):244–250. doi: 10.1038/embor.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones H.E., Goddard L., Gee J.M., et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11(4):793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 109.Wang R., Yamada T., Kita K., et al. Transient IGF-1R inhibition combined with osimertinib eradicates AXL-low expressing EGFR mutated lung cancer. Nat Commun. 2020;11(1):4607. doi: 10.1038/s41467-020-18442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacAulay V.M., Middleton M.R., Eckhardt S.G., et al. Phase I dose-escalation study of Linsitinib (OSI-906) and Erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2016;22(12):2897–2907. doi: 10.1158/1078-0432.CCR-15-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leighl N.B., Rizvi N.A., de Lima L.G., Jr., et al. Phase 2 study of Erlotinib in combination with Linsitinib (OSI-906) or placebo in chemotherapy-naive patients with non-small-cell lung cancer and activating epidermal growth factor receptor mutations. Clin Lung Cancer. 2017;18(1):34–42.e2. doi: 10.1016/j.cllc.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo L., Zhang Z., Qiu N., et al. Disruption of FOXO3a-miRNA feedback inhibition of IGF2/IGF-1R/IRS1 signaling confers Herceptin resistance in HER2-positive breast cancer. Nat Commun. 2021;12(1):2699. doi: 10.1038/s41467-021-23052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Browne B.C., Eustace A.J., Kennedy S., et al. Evaluation of IGF1R and phosphorylated IGF1R as targets in HER2-positive breast cancer cell lines and tumours. Breast Cancer Res Treat. 2012;136(3):717–727. doi: 10.1007/s10549-012-2260-9. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Wester L., He J., et al. IGF1R signaling drives antiestrogen resistance through PAK2/PIX activation in luminal breast cancer. Oncogene. 2018;37(14):1869–1884. doi: 10.1038/s41388-017-0027-9. [DOI] [PubMed] [Google Scholar]
- 115.Ye J.J., Liang S.J., Guo N., et al. Combined effects of tamoxifen and a chimeric humanized single chain antibody against the type I IGF receptor on breast tumor growth in vivo. Horm Metab Res. 2003;35(11–12):836–842. doi: 10.1055/s-2004-814145. [DOI] [PubMed] [Google Scholar]
- 116.Chakraborty A.K., Welsh A., Digiovanna M.P. Co-targeting the insulin-like growth factor I receptor enhances growth-inhibitory and pro-apoptotic effects of anti-estrogens in human breast cancer cell lines. Breast Cancer Res Treat. 2010;120(2):327–335. doi: 10.1007/s10549-009-0382-5. [DOI] [PubMed] [Google Scholar]
- 117.McDermott M.S.J., Canonici A., Ivers L., et al. Dual inhibition of IGF1R and ER enhances response to trastuzumab in HER2 positive breast cancer cells. Int J Oncol. 2017;50(6):2221–2228. doi: 10.3892/ijo.2017.3976. [DOI] [PubMed] [Google Scholar]
- 118.Flanigan S.A., Pitts T.M., Newton T.P., et al. Overcoming IGF1R/IR resistance through inhibition of MEK signaling in colorectal cancer models. Clin Cancer Res. 2013;19(22):6219–6229. doi: 10.1158/1078-0432.CCR-13-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao H., Dong W., Shen H., et al. Combinational therapy enhances the effects of anti-IGF-1R mAb figitumumab to target small cell lung cancer. PLoS One. 2015;10(8):e0135844. doi: 10.1371/journal.pone.0135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue L., Chen F., Yue F., et al. Metformin and an insulin/IGF-1 receptor inhibitor are synergistic in blocking growth of triple-negative breast cancer. Breast Cancer Res Treat. 2021;185(1):73–84. doi: 10.1007/s10549-020-05927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuentes-Baile M., Ventero M.P., Encinar J.A., et al. Differential effects of IGF-1R small molecule tyrosine kinase inhibitors BMS-754807 and OSI-906 on human cancer cell lines. Cancers. 2020;12(12):E3717. doi: 10.3390/cancers12123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J.S., Kang J.H., Boo H.J., et al. STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nat Commun. 2015;6:8499. doi: 10.1038/ncomms9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bano N., Hossain M.M., Bhat A.Q., et al. Analyzing structural differences between insulin receptor (IR) and IGF1R for designing small molecule allosteric inhibitors of IGF1R as novel anti-cancer agents. Growth Hormone IGF Res. 2020;55:101343. doi: 10.1016/j.ghir.2020.101343. [DOI] [PubMed] [Google Scholar]
- 124.Codony-Servat J., Cuatrecasas M., Asensio E., et al. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br J Cancer. 2017;117(12):1777–1786. doi: 10.1038/bjc.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aleksic T., Gray N., Wu X., et al. Nuclear IGF1R interacts with regulatory regions of chromatin to promote RNA polymerase II recruitment and gene expression associated with advanced tumor stage. Cancer Res. 2018;78(13):3497–3509. doi: 10.1158/0008-5472.CAN-17-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sehat B., Tofigh A., Lin Y., et al. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. 2010;3(108):ra10. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- 127.Deng H., Lin Y., Badin M., et al. Over-accumulation of nuclear IGF-1 receptor in tumor cells requires elevated expression of the receptor and the SUMO-conjugating enzyme Ubc9. Biochem Biophys Res Commun. 2011;404(2):667–671. doi: 10.1016/j.bbrc.2010.12.038. [DOI] [PubMed] [Google Scholar]