Abstract
Immune tolerance deletes or suppresses autoreactive lymphocytes and is established at multiple levels during the development, activation and effector phases of T and B cells. These mechanisms are cell-intrinsically programmed and critical in preventing autoimmune diseases. We have witnessed the existence of another type of immune tolerance mechanism that is shaped by lifestyle choices, such as diet, microbiome and microbial metabolites. Short-chain fatty acids (SCFAs) are the most abundant microbial metabolites in the colonic lumen and are mainly produced by the microbial fermentation of prebiotics, such as dietary fiber. This review focuses on the preventive and immunomodulatory effects of SCFAs on autoimmunity. The tissue- and disease-specific effects of dietary fiber, SCFAs and SCFA-producing microbes on major types of autoimmune diseases, including type I diabetes, multiple sclerosis, rheumatoid arthritis and lupus, are discussed. Additionally, their key regulatory mechanisms for lymphocyte development, tissue barrier function, host metabolism, immunity, autoantibody production, and inflammatory effector and regulatory lymphocytes are discussed. The shared and differential effects of SCFAs on different types and stages of autoimmune diseases are discussed.
Keywords: Short-chain fatty acids, Immune tolerance, Diabetes mellitus, Rheumatoid arthritis, Lupus, Multiple sclerosis
Subject terms: Autoimmunity, Lymphocytes
Introduction
Autoimmune diseases include more than one hundred different chronic and often life-threatening illnesses and affect 3–7% of human populations in developed countries [1]. Autoimmune diseases target cells expressing specific antigens, and the most prevalent diseases include type 1 diabetes mellitus (T1DM), rheumatoid arthritis (RA), multiple sclerosis (MS), lupus, myasthenia gravis, and celiac disease [2]. Autoimmune diseases are caused, in part, by the loss of immune tolerance, which leads to the deletion of autoreactive T and B cells. The selective deletion of developing lymphocytes expressing autoreactive antigen receptors, such as T-cell receptor (TCR) and B-cell receptor (BCR), is the first line of immune tolerance, but some autoreactive lymphocytes appear to escape this mechanism [3, 4]. Luckily, autoreactive T and B cells that escape central tolerance mechanisms are controlled in the periphery by inhibitory signals and regulatory immune cells, so they do not damage tissues. Moreover, autoreactive lymphocytes frequently become regulatory, rather than effector, lymphocytes to contradictorily contribute to immune tolerance [5]. An emerging group of immune tolerance mechanisms that further rein in autoreactive lymphocytes are influenced by our lifestyle choices that affect diet, the microbiome and microbial metabolites in the gut [6–8].
The adult human colon harbors large numbers (1013–1014) of microbial cells at a fairly high diversity (400–1000 operational taxonomic units); these microbes are dominated by Firmicutes, Actinobacteria, and Bacteroidetes [9–11]. The human gut microbiome changes with age. Actinobacteria is the most abundant microbe after weaning, and the abundance of Proteobacteria is considerably increased in seniors over 70 years of age [12]. Healthy individuals have balanced compositions of gut microbes, but patients with autoimmune diseases often have dysbiosis or altered microbial composition in the colon [13]. Even subtle changes in the microbiome without overt dysbiosis may contribute to autoimmunity in humans, as exemplified by the distinct effects of different lipopolysaccharide-producing microbes on T1DM [14].
In recent years, a plethora of research findings on the functions of the gut microbiota and its metabolites in regulating autoimmune diseases have been reported [15]. Major factors that determine the composition of the gut microbial community include diet and host condition. In general, high microbial diversity with high ratios of protective to pathogenic taxa is beneficial, and these characteristics are promoted by a high prebiotic content in the diet. Aging is a risk factor for autoimmunity that is also linked to disturbances in the balanced gut microbial community [16]. Short-chain fatty acids (SCFAs) are produced from carbohydrates and certain amino acids that reach the colon for bacterial fermentation. SCFAs have important functions in maintaining the gut barrier, host metabolism, immune tolerance and immunity functions (Fig. 1). In autoimmune-associated dysbiosis, the production of SCFAs is frequently altered, which has important ramifications in the immune regulation and pathogenesis of various autoimmune diseases. The functions of SCFAs in supporting immune tolerance and modulating major autoimmune diseases are discussed below.
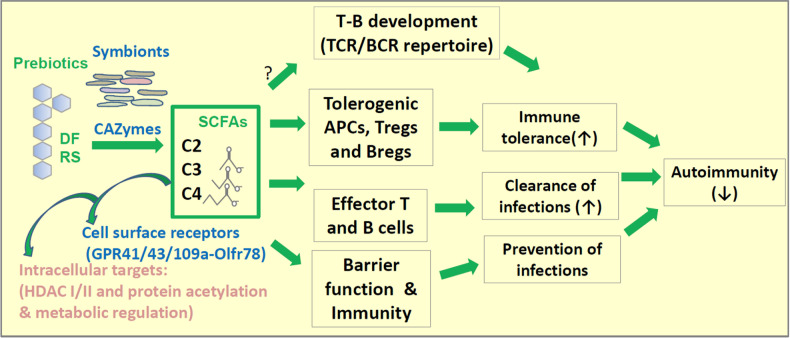
Fig. 1.
Regulation of immune tolerance by SCFAs. Dietary fiber (DF) and resistant starch (RS) are prebiotics that are processed by microbes to produce SCFAs. Intestinal SCFAs are best produced by certain symbionts expressing CAZymes and/or active SCFA production pathways. By triggering GPR signaling via GPR43, GPR41, GPR109A, and Olfr78, SCFAs affect distinct groups of cells in the body. Intracellular SCFAs, particularly propionate (C3) and butyrate (C4), function as natural HDAC inhibitors. It is expected that certain cell types with high expression of SCFA-transporting solute transporters can effectively concentrate SCFAs within cells for HDAC inhibition. HDAC inhibition triggers elevated gene expression and cell activation to boost tissue barrier functions and the activity of T and B cells. Optimal barrier functions and immune functions are important for preventing infections. HDAC inhibition by SCFAs also generates Tregs, Bregs, tolerogenic antigen presenting cells (APCs such as DCs), and IL-10-producing macrophages, all of which function to suppress inflammatory responses. Microbiota and SCFAs have the potential to shape the antigen receptor repertoire in developing lymphocytes to prevent autoimmunity
Production, receptors and intracellular targets of SCFAs
Major SCFAs, such as acetate (C2), propionate (C3) and butyrate (C4) [17], reach concentrations exceeding 0.1 mol per kg of luminal content in the human colon [18]. They are produced from various prebiotics by fermentation processes such as Wood–Ljungdahl (C2), carbon dioxide fixation (C3), and acetyl-S coenzyme A condensation (C4) processes [18, 19]. Prebiotics include digestion-resistant oligosaccharides (oligofructose), dietary fiber (DF) (e.g., inulin, pectin, and arabinoxylan), and resistant starch (RS) from various plant sources. The structure and composition of DFs vary greatly, depending on the type, content level and plant source [20]. The effects of these prebiotics on SCFA production and gut microbes can differ [20]. Bacterial species that utilize prebiotics express DF-degrading carbohydrate-active enzymes (CAZymes) [21]. Diets rich in DF increase the expression level of microbiome-encoded CAZymes, which have diverse substrate specificity depending on the microbes [22]. Therefore, the level of SCFA production in the colon is largely determined by microbial composition and the types and amount of DF in the diet. Amino acids, excluding branched chain amino acids such as valine, leucine, and isoleucine, can also be metabolized by microbes to produce C2, C3 and C4 [23]. Threonine, for example, can be metabolized to the three major SCFAs. Microbes that are efficient in producing SCFAs are generally considered beneficial and are enriched in the gut of healthy hosts with sufficient levels of DF in their diet [24–26]. In contrast, people consuming low levels of DF or having chronic health conditions, such as autoimmune diseases, are variably deficient in SCFA-producing microbes [27, 28]. In addition to SCFA production and microbial changes, DFs have stool bulking and transit time-shortening effects [29, 30]. Therefore, the effects of DF and SCFAs are not necessarily equal.
SCFAs produced in the gut lumen are absorbed into colonocytes and further transported into the blood circulation by the functions of several solute transporters (SLC5a8, SLC16a1, SLC16a3 and SLC5a12), which are distinctly expressed by different cell types [31]. SCFAs have several different ways to regulate host cells. Some of the functions of SCFAs are mediated through four G-protein-coupled receptors (GPRs): GPR41, GPR43, GPR109A and Olfr78 [32–34]. C4 appears to also activate peroxisome proliferator-activated receptor γ (PPARγ) and Ahr [35–37]. These receptors are selectively expressed by certain cell types in the body. For example, GPR41 is expressed by intestinal epithelial cells, adipocytes, thymic medullary epithelial cells, follicular B cells, germinal center B cells, B1b cells and DCs, whereas GPR43 is expressed by Group 3 innate lymphoid cells (ILC3s), neutrophils, marginal zone B cells, Pre-T cells, DCs, Group 2 innate lymphoid cells (ILC2s), some macrophages and intestinal epithelial cells) [31]. This allows the selective regulation of certain cell types by SCFAs. SCFAs also regulate host cells in several GPR-independent manners. SCFAs that are absorbed into cells can suppress the activity of Class I and II histone deacetylases (HDACs). HDACs deacetylate many proteins, such as histones and signaling molecules, thereby changing their activity [38, 39]. In addition, SCFAs decrease the expression of Type III HDACs, such as sirtuin 1 [36, 37]. Thus, SCFAs increase protein acetylation and therefore affect gene expression and cell signaling. For example, SCFAs increase the acetylation of signaling molecules such as p70 S6 kinase [40]. SCFA-induced acetylation appears to be important for the generation of IL-10-producing B cells [41]. Intracellular SCFAs have metabolic effects in cells. SCFAs are converted into acetyl coenzyme A (acetyl-CoA) to feed into the citric acid cycle for energy production and mTOR activation [40, 42, 43]. Acetyl-CoA that is converted from SCFAs can also increase fatty acid synthesis, which can boost cellular differentiation and functions [42]. The effects of SCFAs on particular cell types appear to be determined by combinations of intracellular functions and cell surface GPR signaling. Of course, different cell types greatly vary in their expression of cell surface GPRs, SCFA transporters, and intracellular targets of SCFAs as a part of cell-specific gene expression programs, leading to diverse and cell type-specific responses to SCFAs.
Regulation of immune cells and immune tolerance by SCFAs
Developing lymphocytes express antigen receptors randomly selected from recombined DNA sequences in the thymus for T cells and in the bone marrow for B cells. Therefore, they would inherently express autoreactive antigen receptors. Positive and negative selections in the thymus and bone marrow select for lymphocytes with functional receptors that are not autoreactive [44, 45]. Some lymphocytes escape the central selection processes but are regulated by various peripheral tolerance mechanisms [4]. These mechanisms include self-peptide-MHC complex-induced apoptosis, tolerogenic dendritic cells (DCs), and negative costimulatory receptors, such as cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4/CD152) and programmed death-1 (PD-1/CD279). MHC alleles can promote or suppress autoimmune diseases because they control T cells by interacting with TCRs. In this regard, certain MHC alleles affect the gut microbiome and prevent experimental T1DM [46]. The microbiome is important for the development of Treg and non-Treg CD4 T cells and affects their TCR repertoire [47]. Abnormal T-cell development occurs in germ-free mice or pathogenic conditions such as preeclampsia, and this defect was rescued by C2 feeding [48], implying a role for SCFAs in thymic T-cell development. SCFAs and other microbial signals regulate the transition of intestinal DCs to tolerogenic antigen-presenting cells [49]. These DCs, including CX3CR1+ DCs, may migrate from the intestine to the thymus to mediate positive selection [50].
SCFAs, particularly C4 and C3, are natural HDAC inhibitors. SCFAs increase IL-10 expression in lymphocytes, and this process appears to be mediated by their HDAC inhibition activity. In this regard, SCFAs promote IL-10+ regulatory T-cell activity [51, 52]. SCFAs also promote the production of IL-10 from macrophages. While it is debated whether SCFAs directly induce the generation of FoxP3+ T cells from naïve CD4 T cells in the periphery, it is generally accepted that SCFAs enhance the suppressive activity of Tregs [40, 53, 54]. Retinoic acid is a key factor for intestinal immunity and immune tolerance that acts by generating gut-homing effector and regulatory T cells [55–57]. It has also been reported that SCFAs increase the production of retinoic acid from retinaldehyde dehydrogenase (RALDH)-expressing DCs [49, 58]. SCFAs promote tolerogenic macrophages by activating GPRs and inhibiting HDACs [49]. These functions dampen inflammatory responses and suppress autoimmune diseases (Fig. 2).
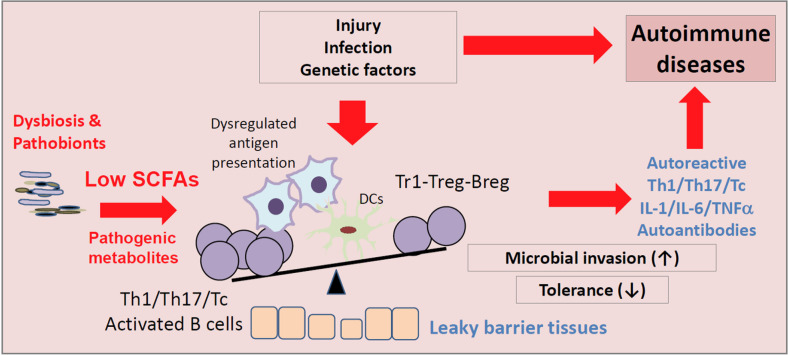
Fig. 2.
Regulation of immune cells important for immune tolerance by SCFAs. In diseased states following infection or injury, microbial dysbiosis and prolonged inflammatory responses work together to break immune tolerance. Microbial dysbiosis can lead to altered production of microbial metabolites, including decreased SCFAs. SCFA production deficiency may change the activities of APCs, T cells and B cells to generate autoreactive effector lymphocytes. In microbial dysbiosis, pathogenic metabolites, such as certain bile acid derivatives, formate, trimethylamine N-oxide, polyamines, and hydrogen sulfide, are produced instead of SCFAs. These metabolites damage tissues and increase the inflammatory activities of immune cells
Certain infections are linked to autoimmune diseases [59]. For example, infection with viruses (rubella, B4 Coxsackievirus, and cytomegalovirus), parasitic protozoa (Trypanosoma cruzi), and bacteria (Borrelia burgdorfii) are associated with multiple diseases such as T1DM, myocarditis, Chagas disease, multiple sclerosis, and/or Lyme arthritis [60, 61]. Molecular mimicry between pathogens and host antigens and the adjuvant effects of chronic infection are thought to break immune tolerance, leading to autoimmune diseases [61]. Therefore, clearance of pathogens at early stages before they reach chronic states is key to preventing autoimmune diseases. SCFAs are known to boost the functions of Th1 and Th17 cells, which prevent and fight infection [62]. SCFAs increase the production of the effector cytokines IL-22 and IL-17 during active immune responses [63, 64], which are key to preventing infection and fighting pathogens in the intestinal barrier. These functions have the potential to clear infections to prevent chronic inflammatory responses.
B cells can produce autoantibodies, which induce many autoimmune diseases, including RA, systemic lupus erythematosus, autoimmune hepatitis, immune thrombocytopenic purpura, and Guillain‒Barre syndrome [65–67]. SCFAs can serve as nutrients for B cells and enhance B-cell activation during active immune responses [42]. SCFAs are converted into acetyl-CoA, which is used to make long-chain fatty acids needed for plasma B-cell differentiation. SCFAs inhibit HDACs and increase mitochondrial oxidative phosphorylation and glycolysis in B cells, all of which are required for optimal generation of plasma B cells. These functions of SCFAs increase IgG and IgA production to boost humoral immunity. SCFAs increase the numbers of IgA-coated bacteria in the gut, and this is potentially important for the regulation of gut microbes and the prevention dysbiosis [42]. In this regard, SCFAs suppress microbial invasion and leaky gut, which are common in autoimmune diseases [68]. Another function of SCFAs in B cells is to increase the activity of Bregs or B cells that produce IL-10 [41].
The gut is a conducive environment for Tregs that suppress inflammatory responses to microbiota, which may be important for preventing inflammatory and autoimmune responses throughout the body. Important roles of ILCs in promoting intestinal immune tolerance have been reported [69]. It has been reported that MHC II-expressing ILC3s induce Tregs that are selective to microbiota [69]. Another group reported that RORγt+ antigen-presenting cells that resemble thymic epithelial cells and/or DCs, but not ILC3s, are required to induce microbiota-specific Tregs in the gut [70, 71]. Independent of their controversial function in inducing Tregs, ILCs produce key effector cytokines of T cells and therefore have the potential to coregulate autoimmune and inflammatory diseases induced by T cells and antibodies. Importantly, SCFAs regulate the activity of ILCs. They increase the proliferation and activity of ILC3s by GPR43 activation but suppress those of ILC2s by HDAC inhibition [31, 72, 73]. These functions of SCFAs are important to maintain barrier immunity and prevent infection and inflammatory diseases.
Leaky barrier functions in the gut, skin and brain are common in autoimmune diseases. These conditions increase infection, tissue damage and antigen exposure, leading to inadvertent activation of the immune system [74–76]. SCFAs maintain the health and barrier function of intestinal epithelial cells. Intestinal epithelial cells highly express SCFA receptors, such as GPR43 and GPR41. Once triggered by SCFAs, GPR43 and GPR41 generate functionally important GPR signaling, leading to mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated kinase (ERK) activation [77]. GPR activation by SCFAs induces the expression of chemokines, cytokines and antimicrobial peptides, which are necessary for preventing and fighting infection. The HDAC inhibition function of SCFAs enhances the expression of tight junction proteins on intestinal epithelial cells [78, 79]. SCFAs are used by colonocytes as an energy source, supporting the maintenance and growth of intestinal epithelial cells [80]. During microbial invasion, SCFAs facilitate the change in transient permeability in the intestinal barrier to facilitate immune surveillance and activation [77]. SCFAs induce the expression of NLRP3 (NLR Family Pyrin Domain Containing 3) and the inflammasome product IL-18 in a GPR109a- and GPR43-dependent manner [81, 82]. Thus, SCFAs can not only strengthen epithelial barrier function but also boost epithelial innate immune responses to fight pathogens. C4 also acts as a PPARγ ligand and limits the bioavailability of oxygen and nitrate in the colonic lumen. Oxygen and nitrate serve as respiratory electron acceptors, and therefore, this function limits the expansion of potentially pathogenic bacteria in the colonic lumen [83]. Additionally, C4 can induce the expression of tolerogenic TGF-β cytokines in intestinal epithelial cells via HDAC inhibition and transcriptional regulation [84]. Thus, SCFAs have the capacity to promote both epithelial immunity and immune tolerance (Fig. 1).
Dysbiosis, decreased SCFA production, and leaky gut in T1DM
T1DM is caused by autoimmune destruction of insulin-producing pancreatic islet β-cells. Breakdown of peripheral tolerance to β-cell antigens due to genetic, epigenetic, molecular, and/or environmental issues is thought to cause T1DM [3]. The gut microbiota and SCFAs have the potential to prevent T1DM by promoting immune tolerance through various mechanisms that are described in the previous section. For example, they influence the thymic development of T cells to affect the TCR repertoire and Treg generation [48]. They regulate B cells for effective humoral immune responses and generate Bregs [42, 85]. They also induce tolerogenic antigen-presenting cells [49].
It is generally accepted that microbial composition is altered with decreased levels of the SCFA-producing intestinal microbiome in T1DM. Decreased abundance of Bifidobacterium and Lachnospiraceae and the overabundance of the genera Blautia, Rikenellaceae, Ruminococcus and Bacteroides ovatus are observed in T1DM patients [86–88]. In a similar cohort, children who progressed to T1DM had dysbiosis with an altered Bacteroidetes/Firmicutes ratio and SCFA production [89]. Considerable differences in the levels of intestinal SCFAs were detected between T1DM children and the healthy control group, with lower concentrations of fecal C2 and C4 [90]. In this study, an increased abundance of Ruminococcaceae and Coprococcus but decreased abundance of Roseburia and Megamonas was observed in the stool samples from children with diabetes. In another study, decreased levels of blood C2 but not C4 in T1DM subjects were observed [91]. Children with a recent-onset of diabetes had gut microbiome dysbiosis and increased intestinal permeability [92]. Subtle microbial changes were also detected in children with anti-islet cell autoantibodies [93].
SCFAs, mainly by their HDAC inhibition functions, protect pancreatic islet cells from inflammatory responses. C4 improved the islet dysfunction induced by oxidative mitochondrial stress [94, 95]. C4 suppressed nuclear factor kappa B (NF-κB) and inflammatory cytokine production by β cells [96]. Moreover, C4 had positive effects on the differentiation of pancreatic islet cells to β cells in vitro and promoted insulin secretion from pancreatic islets [97, 98]. In nonobese diabetic (NOD) mice, long-term antibiotic treatment depleted SCFA-producing bacteria, accelerated disease activity and increased the diabetogenic intestinal microbiome [99]. DF (i.e., inulin) feeding ameliorated streptozotocin-induced diabetes in mice, in part by increasing SCFA production and IL-22 expression [100]. Similarly, an SCFA-producing diet had protective effects in this model with decreased levels of autoreactive T cells and IL-21 but an increased number of Tregs [101]. Protective effects of SCFAs were also observed in NOD mice by suppressing autoimmune T cells but increasing Tregs to suppress pancreatic islet β-cell destruction [90, 101].
Despite the anti-inflammatory and other beneficial effects of SCFAs, β cell loss in advanced T1DM is often permanent, and therefore, it is unlikely that short-term treatment with prebiotics or SCFAs restores β cell functions. This explains why it is difficult to restore β cell function in advanced T1DM with SCFAs and prebiotics. For example, the glucose levels and insulin requirements were unchanged in individuals with diabetes who were treated with SCFA-producing starch (high-amylose maize-RS chemically conjugated with acetate and butyrate) [102]. However, those who had greater changes in SCFA production exhibited elevated levels of glycemic control with increased levels of regulatory T and B cells [102].
Independent of their immune regulatory functions in T1DM, SCFAs regulate general energy metabolism through various mechanisms. This includes the GPR activation-induced secretion of metabolic hormones, which act on the brain, pancreas, adipocytes, muscle, and liver. GPR43 is highly expressed by enteroendocrine L cells [103, 104]. SCFAs and GPR43 agonists increase the secretion of the gut hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from L cells [105–107]. In this regard, the effects of SCFAs in suppressing metabolic syndrome and type II diabetes (T2DM) have been well established [108, 109]. These hormones decrease appetite and gastric emptying but increase insulin production, urinary excretion of sodium, and urine production [110]. Additionally, SCFAs increase leptin secretion and insulin sensitivity [111, 112]. Because of these beneficial functions, GLP-1 receptor agonists are used to treat patients with diabetes [113, 114]. While the function of SCFAs in increasing GLP-1 production would metabolically benefit T2DM patients, GLP-1 also has neuroprotective and anti-inflammatory functions [115]. This anti-inflammatory function would also benefit T1DM patients [114]. Thus, SCFAs have the potential to control both T1DM and T2DM by immunological and metabolic regulation (Fig. 3).
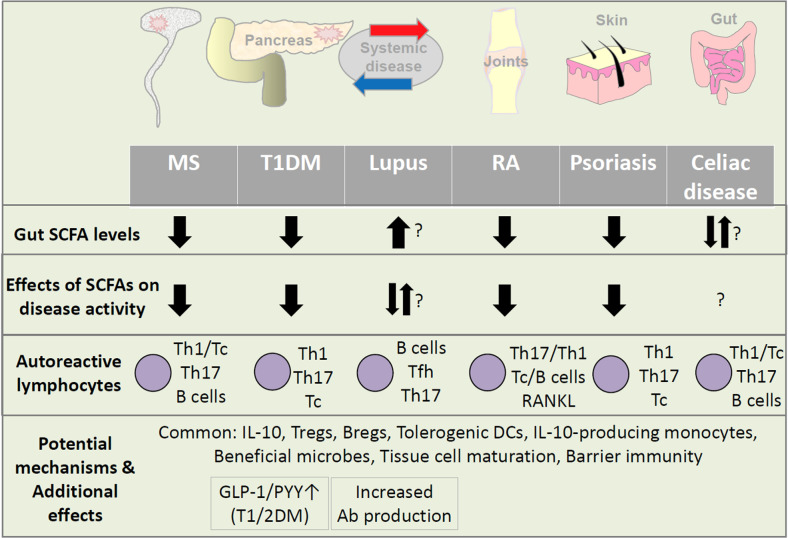
Fig. 3.
Shared and distinct effects of SCFAs on autoimmune diseases. While there are shared effects of SCFAs on most autoimmune diseases, the effects of SCFAs on the selected autoimmune diseases are heterogeneous. SCFA levels and microbiome diversity are decreased in most autoimmune diseases. Interestingly, SCFA levels are abnormally upregulated in a subset of lupus patients, indicating the presence of disease-specific dysbiosis and/or host metabolism in these subjects. SCFAs are known to activate B cells and induce AID expression to boost class-switched antibody production. Therefore, certain functions of SCFAs have the potential to exacerbate lupus and other autoimmune diseases. Common functions of SCFAs in promoting regulatory lymphocytes, such as Tregs and Bregs, and tolerant antigen-presenting cells appear to operate in most autoimmune diseases. Moreover, microbiota and SCFAs have the potential to shape the antigen receptor repertoire in developing lymphocytes. These functions would prevent autoimmunity in general and decrease ongoing autoimmune responses. In addition, SCFAs have metabolic effects, such as the induction of gut hormone (GLP-1 and PYY) secretion to decrease food intake and inflammatory responses, which could benefit both Type I and II diabetes patients. In contrast, the adverse effect of SCFAs on autoantibody production in lupus and other diseases is a potentially important problem. Despite the progress, it remains still unclear whether SCFAs have significant regulatory effects in many other autoimmune diseases. This is due to either a lack of information or contradictory functions of SCFAs in promoting both the effector and regulatory arms of the immune system
Potentially divergent effects of SCFAs on rheumatoid arthritis and lupus
RA involves chronic inflammation in the joints, leading to destruction of bone and cartilage [116]. RA manifests as symmetric invasive and extra-articular inflammation in multiple joints, and rheumatoid factor and anti-citrullinated protein antibodies are present in the serum of many RA patients. Immune cells and cytokines, such as tumor necrosis factor (TNF), interleukin-1 (IL-1), interleukin-6 (IL-6) and interleukin-18 (IL-18), mediate chronic inflammatory processes [117]. Chronic inflammation increases osteoclastic bone resorption but decreases osteoblastic bone formation for gradual bone and cartilage loss in the joints. Increased Th17 cell responses are frequently involved in RA. These responses lead to the activation of fibroblasts, which increase receptor activator of nuclear factor kappa-B ligand (RANKL) expression and bone resorption [118].
Intestinal and oral dysbiosis in RA patients has been observed with overrepresented intestinal gram-positive bacteria and oral Porphyromonas gingivalis and Lactobacillus salivarius [119, 120]. It has been reported that levels of stool C3 and C4 were decreased in RA patients [85]. Similar changes in fecal C4 have been observed in animal models, and oral administration of C4 ameliorated experimental RA in mice. This effect may be mediated in part by the function of SCFAs in increasing IL-10-producing B cells. More specifically, the number of AhR+ Bregs and the expression of IL-10 by AhR+ Bregs were increased by C4. C4 treatment in a mouse model of methylated albumin-induced arthritis decreased inflammation and increased the level of the tryptophan metabolite 5-hydroxyindoleacetic acid (5-HIAA). 5-HIAA triggers the activation of aryl-hydrocarbon receptor (AhR) in B cells, and this increases IL-10 expression [85]. A diet rich in RS had a preventive effect on collagen-induced arthritis (CIA) [121]. RS increased SCFA levels and altered the gut microbial composition. This decreased the abundance of CIA-associated Lactobacillus and Lachnoclostridium genera but increased the abundance of the Lachnospiraceae_NK4A136_group and Bacteroidales_S24-7_group genera. Similarly, a high-DF diet increased IL-10 levels but decreased the production of inflammatory cytokines, alleviating disease severity. C3 administration in the drinking water increased Treg cells and IL-10 production [121]. Thus, SCFAs and prebiotics appear to selectively increase the activity of regulatory lymphocytes, such as Tregs and Bregs, over inflammatory effector cells, such as Th17 cells and autoantibody-producing B cells. These immune regulatory effects of prebiotics and SCFAs are consistent with their tolerance maintenance and preventive effects.
In line with the evidence from animal studies, high DF supplementation in RA patients for 4 weeks increased circulating Treg numbers and Th1/Th17 ratios and decreased markers of bone erosion [122]. In another study [123], a high-fiber dietary intervention resulted in a shift in the Firmicutes to Bacteroidetes ratio and increased levels of SCFAs. However, it decreased proarthritic cytokine concentrations. Thus, prebiotics that increase SCFAs are beneficial for RA patients. Prebiotics support the anti-inflammatory effects of SCFAs and increase beneficial microbes that produce other products that also suppress inflammatory responses.
We have witnessed significant levels of controversy regarding the roles of SCFAs in regulating the pathogenesis of lupus. Autoimmune antibodies are the major pathogenic factors for lupus [124]. Low DF intake deteriorated disease progression in lupus-prone NZB/WF1 mice [28]. DF insufficiency exacerbated immune dysregulation and autoantibody production. However, microbiota suppression by antibiotics or direct SCFA feeding did not affect lupus-like disease in this model. DF insufficiency increased fat mass, gut barrier leakage, and low-grade inflammation. A similar beneficial role of RS in suppressing lupus-like pathogenesis in Toll-like receptor 7 (TLR7)-dependent mouse models of systemic lupus erythematosus (SLE) has been reported [125]. Tissue infiltration of L. reuteri is associated with the pathogenesis of lupus in this model, and this effect was suppressed by SCFA feeding. This is in line with the effect of DF in preventing dysbiosis and associated inflammatory responses.
DF and SCFAs have strong positive effects on B cells, supporting their activation and differentiation into plasma B cells [42]. This function of SCFAs in promoting humoral immunity could be a potential problem in the pathogenesis of lupus because SCFAs can also increase autoantibody production. In this regard, the levels of C2, C3, and C4 were all increased in fecal samples from lupus patients [126]. A negative association between fecal SCFA levels and the Firmicutes to Bacteroidetes ratio was observed in healthy control individuals, but this was lost in lupus patients. Thus, microbial dysbiosis with increased SCFA production occurs in lupus patients. The positive effect of SCFAs in boosting antibody production is in line with the positive correlation between SCFAs and disease activity in lupus [42].
SCFAs may also regulate the emergence of autoimmune B cells by increasing central and peripheral tolerance mechanisms. Pathogenic autoantibodies in lupus patients are mostly class-switched and hypermutated. Activation-induced deaminase (AID) is required for antibody somatic hypermutation and class switch recombination in B cells, and its expression is regulated by HDAC inhibition [42, 127, 128]. SCFAs are natural HDAC inhibitors and have the potential to regulate AID expression. Indeed, in normal mouse B cells that are activated by BCR activation or during infection by bacterial pathogens, SCFAs increase AID expression potentially through direct epigenetic changes in the Aicda gene [42]. Interestingly, SCFAs also increase the expression of several miRNAs that suppress AID expression [129]. Moreover, estrogen, which increases autoimmune antibody production, appears to counteract the SCFA-mediated inhibition of AID expression and CSR [129]. The administration of a high dose of C4 in drinking water modestly suppressed the autoantibody response in autoimmune MRL/Faslpr/lpr mice [129]. While more in-depth studies are needed, the available body of information suggests that autoantibody production may be bidirectionally regulated depending on SCFA levels, B-cell types and the context of immune responses.
Regulation of MS by microbes and SCFAs
MS pathogenesis involves the demyelination of the central nervous system (CNS), which is caused by chronic immune activation as well as nonimmune mechanisms [130]. There are several types of MS depending on the mode of clinical activity and pathogenesis, including clinically isolated syndrome (CIS), primary progressive MS (PPMS), relapsing-remitting MS (RRMS), and secondary progressive MS (SPMS) [131]. The immune responses mediated by T cells, myeloid cells, and B cells are implicated in MS pathogenesis. Autoimmune T cells target CNS myelin antigens for demyelination. Autoimmune T cells can be activated by viral infection and environmental factors in individuals with certain MHC alleles, such as the human leukocyte antigen (HLA) DRB1*1501 [130]. B cells can bidirectionally regulate MS by presenting autoantigens and producing IL-10 and other immunosuppressive molecules [132]. Microbial dysbiosis occurs in human MS and rodent experimental encephalomyelitis (EAE) models [133, 134]. The abundances of certain microbial species, such as segmented filamentous bacteria (SFB), P. heparinolytica, and S. aureus, are increased in the gut of hosts with CNS inflammation [135–139]. These microbes increase Th17 activity, which mediates inflammatory responses in the CNS [140, 141]. In contrast, other microbes, such as Clostridium tyrobutyricum, which produce SCFAs and other beneficial microbial products, such as polysaccharide A and poly-γ-glutamic acid, are associated with decreased CNS inflammation [142].
The levels of SCFAs in the blood and feces are generally decreased in MS patients [143–147]. MS patients have reduced levels of all major SCFAs (C2, C3, and C4) in the blood [145]. SCFAs increase the number of regulatory T cells and decrease clinical activities in MS patients. C3 ameliorates disease severity in MS patients [148]. However, it has also been reported that C2 was increased in MS patients with severe disability [149, 150]. A caveat is that C2 can be produced by host cells, and the increase may be due to altered host metabolism rather than increased production by gut microbiota [151].
The suppressive effect of SCFAs on experimental CNS inflammation has been observed [152, 153]. This protective effect may be due, in part, to the strengthening effect of SCFAs on the blood brain barrier (BBB) [154–156]. SCFAs also promote microglial cell and oligodendrocyte maturation in a GPR43-dependent manner [155, 156]. Valerate (C5) is another SCFA produced from branched chain amino acids and can increase Tregs and Bregs in a manner potentially dependent on HDAC inhibition [157]. While SCFAs have protective effects, the effect of their precursor, soluble DF, is not entirely clear [158–160]. Soluble DFs, such as pectin and inulin, failed to significantly protect mice from EAE [145]. Highly artificial zero-fiber diets that contained no DF at all, whether soluble or insoluble, exacerbated CNS inflammation compared with a cellulose-containing diet [134, 153]. This finding suggests that most forms of DF, even insoluble cellulose, appear to be beneficial. The beneficial effect of cellulose is likely to be mediated by increased microbial diversity in the gut rather than changes in SCFA production [160]. Surprisingly, the EAE responses of GPR43- or GPR41-deficient mice were lower than those of WT control mice, suggesting that the SCFA effect mediated by their cell surface receptors can even be inflammatory [145]. In human MS patients, elevated levels of DF consumption increased the numbers of Tregs and tolerogenic monocytes in the blood. Coincidentally, the abundance of the Lachnospiraceae phylum also increased. However, a high-DF diet failed to change clinical activity in MS patients [161].
Overall, it is controversial whether SCFAs are effective microbial metabolites that suppress MS pathogenesis. This is not due to the lack of regulatory effects of SCFAs on the CNS immune system. Rather, SCFAs can promote both regulatory and effector responses, and therefore, various outcomes would occur depending on host conditions. What appears clear is that microbial dysbiosis and decreased SCFA production generally occur in MS patients and have the potential to exacerbate ongoing CNS inflammation (Fig. 3).
Impact on other diseases and closing remarks
The effects of microbially produced SCFAs on immune tolerance and major autoimmune diseases have been discussed in this review. Dysbiosis and inadequate SCFA production have the potential to compromise central and peripheral immune tolerance. The combined information from the four major autoimmune diseases suggests the presence of inflammation-associated dysbiosis and disease-induced lifestyle changes in autoimmune diseases, including inadequate levels of prebiotic consumption. These changes can alter microbial metabolite production, leading to decreased production of SCFAs. However, SCFA production, particularly that of C2, can be upregulated in autoimmune diseases. Consumption of prebiotics at sufficient levels appears beneficial in preventing certain autoimmune diseases. This is mediated by the increased diversity of gut microbes and their metabolites, including SCFAs. SCFAs have profound and complex regulatory effects on diverse types of immune cells. For example, SCFAs can affect host cells via cell surface GPRs, which are differentially expressed by myeloid cells and ILCs. These GPRs are also expressed by metabolically important adipocytes and enterocytes. SCFAs function as natural HDAC inhibitors and promote gene expression in most cell types, leading to balanced activities of immune cells as well as increased tissue integrity. Furthermore, SCFAs serve as nutrients that generate acetyl-CoA and cellular energy. These functions are indeed important to strengthening barrier tissue integrity and preventing autoimmune diseases. It is expected that the functions of SCFAs may vary, depending on the organs that are affected and the type and stage of autoimmune diseases. Because SCFAs support the functions of innate and adaptive immune effector cells, it is likely that certain effects of SCFAs can also exacerbate autoimmune responses during active immune responses [153, 162]. Moreover, late-stage autoimmune diseases are irreversible due to permanent cell loss in affected tissues. While ongoing inflammatory responses may be regulated by SCFA-based approaches, it is unlikely that these approaches can restore lost tissue functions in certain autoimmune diseases, such as T1DM.
Despite the progress thus far in this field, more in-depth studies on the impact of dysbiosis, DFs, and SCFAs on immune tolerance and autoimmune diseases are required. We still do not have enough information regarding the regulatory functions of SCFAs in cells in diverse organs that are targets of autoimmune responses. The study of other autoimmune diseases, including celiac disease for example, is in its infancy. Altered metabolic activity of the intestinal microbial flora in children with celiac disease has been observed [163]. However, there have been mixed reports on SCFA levels in patients with celiac disease. Overall, it is debated whether SCFA levels are significantly altered in celiac disease. Because SCFA levels are altered in many autoimmune diseases and SCFAs can often be anti-inflammatory, the SCFA system composed of SCFAs, their receptors, prebiotics, and SCFA-producing microbes may be targeted to alter the pathogenic course of certain autoimmune diseases. A major caveat is the multifaceted functions of the SCFA system in regulating immune cells and tissue cells, which could make such therapies ineffective and may potentially exacerbate diseases. In this regard, prevention of autoimmune diseases by maintaining optimal microbial diversity and SCFA production by healthy lifestyle choices (e.g., diet, exercise, and sleep cycle) would be more effective.
Acknowledgements
CHK is the Judy and Kenneth Betz Endowed Professor in the Mary H Weiser Food Allergy Center.
Author contributions
C Kim wrote this review.
Funding
This study was supported, in part, by the NIH (R01AI121302, R21AI14889801, R01AI074745, and R01AI080769 to CHK).
Competing interests
The authors declare no competing interests.
References
- 1.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–95. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–25. doi: 10.1016/S1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 3.Jeker LT, Bour-Jordan H, Bluestone JA. Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb Perspect Med. 2012;2:a007807. doi: 10.1101/cshperspect.a007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 5.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 6.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012;245:164–76. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154:220–9. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephen-Victor E, Crestani E, Chatila TA. Dietary and Microbial Determinants in Food Allergy. Immunity. 2020;53:277–89. doi: 10.1016/j.immuni.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajilic-Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costea PI, Hildebrand F, Arumugam M, Backhed F, Blaser MJ, Bushman FD, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3:8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WH, Zegarra-Ruiz DF, Diehl GE. Intestinal Microbes in Autoimmune and Inflammatory Disease. Front Immunol. 2020;11:597966. doi: 10.3389/fimmu.2020.597966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 15.Brown EM, Kenny DJ, Xavier RJ. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu Rev Immunol. 2019;37:599–624. doi: 10.1146/annurev-immunol-042718-041841. [DOI] [PubMed] [Google Scholar]
- 16.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe. 2018;23:570. doi: 10.1016/j.chom.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 19.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 20.Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe. 2020;27:389–404.e386. doi: 10.1016/j.chom.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Wardman JF, Bains RK, Rahfeld P, Withers SG. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat Rev Microbiol. 2022;20:542–56. doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- 22.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–4153.e4114. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker HA. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- 24.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 25.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winiarska-Mieczan A, Tomaszewska E, Donaldson J, Jachimowicz K. The Role of Nutritional Factors in the Modulation of the Composition of the Gut Microbiota in People with Autoimmune Diabetes. Nutrients. 2022;14:2498. doi: 10.3390/nu14122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfer AL, Eichhorst A, Hentze C, Kraemer AN, Amend A, Sprenger D, et al. Low Dietary Fiber Intake Links Development of Obesity and Lupus Pathogenesis. Front Immunol. 2021;12:696810. doi: 10.3389/fimmu.2021.696810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephen AM, Cummings JH. Water-holding by dietary fibre in vitro and its relationship to faecal output in man. Gut. 1979;20:722–9. doi: 10.1136/gut.20.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makki K, Deehan EC, Walter J, Backhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23:705–15. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol. 2021;18:1161–71. doi: 10.1038/s41423-020-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–64. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 33.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep. 2017;19:25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–32. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinelli L, Martin-Gallausiaux C, Bourhis JM, Béguet-Crespel F, Blottière HM, Lapaque N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep. 2019;9:643. doi: 10.1038/s41598-018-37019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Shahir AM, Sha J, Feng Z, Eapen B, Nithianantham S, et al. Short-chain fatty acids from periodontal pathogens suppress histone deacetylases, EZH2, and SUV39H1 to promote Kaposi’s sarcoma-associated herpesvirus replication. J Virol. 2014;88:4466–79. doi: 10.1128/JVI.03326-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–21. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017;486:499–505. doi: 10.1016/j.bbrc.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 40.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daien CI, Tan J, Audo R, Mielle J, Quek LE, Krycer JR, et al. Gut-derived acetate promotes B10 cells with antiinflammatory effects. JCI Insight. 2021;6:e144156. doi: 10.1172/jci.insight.144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M, Qie Y, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20:202–14. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–54. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JW, Schickel JN, Tsakiris N, Sng J, Arbogast F, Bouis D, et al. Positive and negative selection shape the human naive B cell repertoire. J Clin Invest. 2022;132:e150985. doi: 10.1172/JCI150985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15:815–23. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman M, Kua L, Tanca A, Pala M, Palomba A, Tanes C, et al. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc Natl Acad Sci. 2017;114:9671–6. doi: 10.1073/pnas.1712280114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–62. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun. 2019;10:3031. doi: 10.1038/s41467-019-10703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goverse G, Molenaar R, Macia L, Tan J, Erkelens MN, Konijn T, et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells To Induce Mucosal Tolerogenic Dendritic Cells. J Immunol. 2017;198:2172–81. doi: 10.4049/jimmunol.1600165. [DOI] [PubMed] [Google Scholar]
- 50.Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu WJH, Saldana-Morales FB, et al. Thymic development of gut-microbiota-specific T cells. Nature. 2021;594:413–7. doi: 10.1038/s41586-021-03531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saemann MD, Bohmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C, et al. Anti‐inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL‐12 and up‐regulation of IL‐10 production. FASEB J. 2000;14:2380–2. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 52.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu M, Alashkar Alhamwe B, Santner-Nanan B, Miethe S, Harb H, Renz H, et al. Short-Chain Fatty Acids Augment Differentiation and Function of Human Induced Regulatory T Cells. Int J Mol Sci. 2022;23:5740. doi: 10.3390/ijms23105740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 56.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–33. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 57.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016;15:2809–24. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 59.Kordonouri O, Cuthbertson D, Belteky M, Aschemeier-Fuchs B, White NH, Cummings E, et al. Infections in the first year of life and development of beta cell autoimmunity and clinical type 1 diabetes in high-risk individuals: the TRIGR cohort. Diabetologia. 2022;65:2098–107. doi: 10.1007/s00125-022-05786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- 61.Quaratino S, Thorpe CJ, Travers PJ, Londei M. Similar antigenic surfaces, rather than sequence homology, dictate T-cell epitope molecular mimicry. Proc Natl Acad Sci. 1995;92:10398–402. doi: 10.1073/pnas.92.22.10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–88. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–4. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 64.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 65.Kitazawa K, Tagawa Y, Honda A, Yuki N. Guillain-Barre syndrome associated with IgG anti-GM1b antibody subsequent to Mycoplasma pneumoniae infection. J Neurol Sci. 1998;156:99–101. doi: 10.1016/S0022-510X(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 66.Bai Y, Wang Z, Bai X, Yu Z, Cao L, Zhang W, et al. Cross-reaction of antibody against Helicobacter pylori urease B with platelet glycoprotein IIIa and its significance in the pathogenesis of immune thrombocytopenic purpura. Int J Hematol. 2009;89:142–9. doi: 10.1007/s12185-008-0247-4. [DOI] [PubMed] [Google Scholar]
- 67.Rojas M, Restrepo-Jimenez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramirez-Santana C, et al. Molecular mimicry and autoimmunity. J Autoimmun. 2018;95:100–23. doi: 10.1016/j.jaut.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Christovich A, Luo XM. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front Immunol. 2022;13:946248. doi: 10.3389/fimmu.2022.946248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. 2022;610:744–51. doi: 10.1038/s41586-022-05141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORgammat(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. 2022;610:737–43. doi: 10.1038/s41586-022-05089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Deep D, Parisotto YF, et al. Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature. 2022;610:752–60. doi: 10.1038/s41586-022-05309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sepahi A, Liu Q, Friesen L, Kim CH. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal Immunol. 2021;14:317–30. doi: 10.1038/s41385-020-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, et al. Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity. Immunity. 2019;51:871–884.e876. doi: 10.1016/j.immuni.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi SC, Brown J, Gong M, Ge Y, Zadeh M, Li W, et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci Transl Med. 2020;12:eaax2220. doi: 10.1126/scitranslmed.aax2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Luciano R, et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci. 2019;116:15140–9. doi: 10.1073/pnas.1814558116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e1-10.. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 78.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–35. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 79.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–25. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br J Nutr. 1987;58:95–103. doi: 10.1079/BJN19870073. [DOI] [PubMed] [Google Scholar]
- 81.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 83.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–5. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, et al. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep. 2018;8:9742. doi: 10.1038/s41598-018-28048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosser EC, Piper C, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020;31:837–851.e810. doi: 10.1016/j.cmet.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–94. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–73. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samuelsson U, Ludvigsson J. The concentrations of short-chain fatty acids and other microflora-associated characteristics in faeces from children with newly diagnosed Type 1 diabetes and control children and their family members. Diabet Med. 2004;21:64–67. doi: 10.1046/j.1464-5491.2003.01066.x. [DOI] [PubMed] [Google Scholar]
- 90.Huang J, Pearson JA, Peng J, Hu Y, Sha S, Xing Y, et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight. 2020;5:e135718. doi: 10.1172/jci.insight.135718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Groot PF, Belzer C, Aydin Ö, Levin E, Levels JH, Aalvink S, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One. 2017;12:e0188475. doi: 10.1371/journal.pone.0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harbison JE, Roth-Schulze AJ, Giles LC, Tran CD, Ngui KM, Penno MA, et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr Diabetes. 2019;20:574–83. doi: 10.1111/pedi.12865. [DOI] [PubMed] [Google Scholar]
- 93.Endesfelder D, zu Castell W, Ardissone A, Davis-Richardson AG, Achenbach P, Hagen M, et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. 2014;63:2006–14. doi: 10.2337/db13-1676. [DOI] [PubMed] [Google Scholar]
- 94.de Groot PF, Nikolic T, Imangaliyev S, Bekkering S, Duinkerken G, Keij FM, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia. 2020;63:597–610. doi: 10.1007/s00125-019-05073-8. [DOI] [PubMed] [Google Scholar]
- 95.Hu S, Kuwabara R, de Haan BJ, Smink AM, de Vos P. Acetate and Butyrate Improve beta-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int J Mol Sci. 2020;21:1542. doi: 10.3390/ijms21041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedersen SS, Prause M, Williams K, Barres R, Billestrup N. Butyrate inhibits IL-1beta-induced inflammatory gene expression by suppression of NF-kappaB activity in pancreatic beta cells. J Biol Chem. 2022;298:102312. doi: 10.1016/j.jbc.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Lei Y, Honarpisheh M, Kemter E, Wolf E, Seissler J. Butyrate and Class I Histone Deacetylase Inhibitors Promote Differentiation of Neonatal Porcine Islet Cells into Beta Cells. Cells. 2021;10:3249. doi: 10.3390/cells10113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S, Yuan M, Zhang L, Zhu K, Sheng C, Zhou F, et al. Sodium butyrate potentiates insulin secretion from rat islets at the expense of compromised expression of beta cell identity genes. Cell Death Dis. 2022;13:67. doi: 10.1038/s41419-022-04517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10:321–32. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou J, Reddivari L, Shi Z, Li S, Wang Y, Bretin A, et al. Inulin Fermentable Fiber Ameliorates Type I Diabetes via IL22 and Short-Chain Fatty Acids in Experimental Models. Cell Mol Gastroenterol Hepatol. 2021;12:983–1000. doi: 10.1016/j.jcmgh.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18:552–62. doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 102.Bell KJ, Saad S, Tillett BJ, McGuire HM, Bordbar S, Yap YA, et al. Metabolite-based dietary supplementation in human type 1 diabetes is associated with microbiota and immune modulation. Microbiome. 2022;10:9. doi: 10.1186/s40168-021-01193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–60. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 104.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 105.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park BO, Kim SH, Kong GY, Kim DH, Kwon MS, Lee SU, et al. Selective novel inverse agonists for human GPR43 augment GLP-1 secretion. Eur J Pharm. 2016;771:1–9. doi: 10.1016/j.ejphar.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 107.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600–5. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–6. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 110.Holst JJ, Madsbad S, Bojsen-Møller KN, Svane MS, Jørgensen NB, Dirksen C, et al. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14:708–14. doi: 10.1016/j.soard.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci. 2004;101:1045–50. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–91. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 113.Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharm Res. 2022;182:106320. doi: 10.1016/j.phrs.2022.106320. [DOI] [PubMed] [Google Scholar]
- 114.Dejgaard TF, von Scholten BJ, Christiansen E, Kreiner FF, Bardtrum L, von Herrath M, et al. Efficacy and safety of liraglutide in type 1 diabetes by baseline characteristics in the ADJUNCT ONE and ADJUNCT TWO randomized controlled trials. Diabetes Obes Metab. 2021;23:2752–62. doi: 10.1111/dom.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y, Glotfelty EJ, Karlsson T, Fortuno LV, Harvey BK, Greig NH. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J Neurochem. 2021;159:867–86. doi: 10.1111/jnc.15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 117.Alippe Y, Mbalaviele G. Omnipresence of inflammasome activities in inflammatory bone diseases. Semin Immunopathol. 2019;41:607–18. doi: 10.1007/s00281-019-00753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komatsu N, Takayanagi H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat Rev Rheumatol. 2022;18:415–29. doi: 10.1038/s41584-022-00793-5. [DOI] [PubMed] [Google Scholar]
- 119.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–94. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 121.Bai Y, Li Y, Marion T, Tong Y, Zaiss MM, Tang Z, et al. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J Autoimmun. 2021;116:102564. doi: 10.1016/j.jaut.2020.102564. [DOI] [PubMed] [Google Scholar]
- 122.Hager J, Bang H, Hagen M, Frech M, Trager P, Sokolova MV, et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients. 2019;11:2392. doi: 10.3390/nu11102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Durholz K, Hofmann J, Iljazovic A, Hager J, Lucas S, Sarter K, et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients. 2020;12:3207. doi: 10.3390/nu12103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lou H, Ling GS, Cao X. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. J Autoimmun. 2022;132:102861. doi: 10.1016/j.jaut.2022.102861. [DOI] [PubMed] [Google Scholar]
- 125.Zegarra-Ruiz DF, El Beidaq A, Iñiguez AJ, Lubrano Di Ricco M, Manfredo Vieira S, Ruff WE, et al. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell Host Microbe. 2019;25:113–127.e116. doi: 10.1016/j.chom.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodríguez-Carrio J, López P, Sánchez B, González S, Gueimonde M, Margolles A, et al. Intestinal Dysbiosis Is Associated with Altered Short-Chain Fatty Acids and Serum-Free Fatty Acids in Systemic Lupus Erythematosus. Front Immunol. 2017;8:23. doi: 10.3389/fimmu.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, et al. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11:60. doi: 10.1038/s41467-019-13603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–6. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 129.Casali P, Shen T, Xu Y, Qiu Z, Chupp DP, Im J, et al. Estrogen Reverses HDAC Inhibitor-Mediated Repression of Aicda and Class-Switching in Antibody and Autoantibody Responses by Downregulation of miR-26a. Front Immunol. 2020;11:491. doi: 10.3389/fimmu.2020.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron. 2018;97:742–68. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 131.Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 2021;20:470–83. doi: 10.1016/S1474-4422(21)00063-6. [DOI] [PubMed] [Google Scholar]
- 132.Wanleenuwat P, Iwanowski P. Role of B cells and antibodies in multiple sclerosis. Mult Scler Relat Disord. 2019;36:101416. doi: 10.1016/j.msard.2019.101416. [DOI] [PubMed] [Google Scholar]
- 133.Johanson DM, Goertz JE, Marin IA, Costello J, Overall CC, Gaultier A. Experimental autoimmune encephalomyelitis is associated with changes of the microbiota composition in the gastrointestinal tract. Sci Rep. 2020;10:1–14. doi: 10.1038/s41598-020-72197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berer K, Martínez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep. 2018;8:10431. doi: 10.1038/s41598-018-28839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benedek G, Zhang J, Nguyen H, Kent G, Seifert HA, Davin S, et al. Estrogen protection against EAE modulates the microbiota and mucosal-associated regulatory cells. J Neuroimmunol. 2017;310:51–9. doi: 10.1016/j.jneuroim.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci. 2017;114:10713–8. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Visser L, Jan de Heer H, Boven LA, van Riel D, van Meurs M, Melief M, et al. Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol. 2005;174:808–16. doi: 10.4049/jimmunol.174.2.808. [DOI] [PubMed] [Google Scholar]
- 138.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calcinotto A, Brevi A, Chesi M, Ferrarese R, Garcia Perez L, Grioni M, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9:4832. doi: 10.1038/s41467-018-07305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci. 2011;108:4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–50. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 142.Chen H, Ma X, Liu Y, Ma L, Chen Z, Lin X, et al. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front Immunol. 2019;10:1662. doi: 10.3389/fimmu.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front Immunol. 2020;11:1390. doi: 10.3389/fimmu.2020.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park J, Wang Q, Wu Q, Mao-Draayer Y, Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep. 2019;9:8837. doi: 10.1038/s41598-019-45311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng Q, Junli Gong JG, Liu X, Chen C, Sun X, Li H, et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int. 2019;129:104468. doi: 10.1016/j.neuint.2019.104468. [DOI] [PubMed] [Google Scholar]
- 147.Takewaki D, Suda W, Sato W, Takayasu L, Kumar N, Kimura K, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci USA. 2020;117:22402–12. doi: 10.1073/pnas.2011703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell. 2020;180:1067–1080.e1016. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 149.Perez-Perez S, Dominguez-Mozo MI, Alonso-Gomez A, Medina S, Villarrubia N, Fernandez-Velasco JI, et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ. 2020;8:e10220. doi: 10.7717/peerj.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moussallieh FM, Elbayed K, Chanson JB, Rudolf G, Piotto M, De Seze J, et al. Serum analysis by 1H nuclear magnetic resonance spectroscopy: a new tool for distinguishing neuromyelitis optica from multiple sclerosis. Mult Scler. 2014;20:558–65. doi: 10.1177/1352458513504638. [DOI] [PubMed] [Google Scholar]
- 151.Crabtree B, Souter MJ, Anderson SE. Evidence that the production of acetate in rat hepatocytes is a predominantly cytoplasmic process. Biochem J. 1989;257:673–8. doi: 10.1042/bj2570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2016;44:951–3. doi: 10.1016/j.immuni.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 153.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. 2019;16:165. doi: 10.1186/s12974-019-1552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schneeman BO, Gallaher D. Changes in Small Intestinal Digestive Enzyme Activity and Bile Acids with Dietary Cellulose in Rats. J Nutr. 1980;110:584–90. doi: 10.1093/jn/110.3.584. [DOI] [PubMed] [Google Scholar]
- 159.Levrat M-A, Behr SR, Rémésy C, Demigné C. Effects of Soybean Fiber on Cecal Digestion in Rats Previously Adapted to a Fiber-Free Diet. J Nutr. 1991;121:672–8. doi: 10.1093/jn/121.5.672. [DOI] [PubMed] [Google Scholar]
- 160.Fischer F, Romero R, Hellhund A, Linne U, Bertrams W, Pinkenburg O, et al. Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota. Gut Microbes. 2020;12:1–17. doi: 10.1080/19490976.2020.1829962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saresella M, Mendozzi L, Rossi V, Mazzali F, Piancone F, LaRosa F, et al. Immunological and Clinical Effect of Diet Modulation of the Gut Microbiome in Multiple Sclerosis Patients: A Pilot Study. Front Immunol. 2017;8:1391. doi: 10.3389/fimmu.2017.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically Elevated Levels of Short-Chain Fatty Acids Induce T Cell-Mediated Ureteritis and Hydronephrosis. J Immunol. 2016;196:2388–2400. doi: 10.4049/jimmunol.1502046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tjellstrom B, Stenhammar L, Hogberg L, Falth-Magnusson K, Magnusson KE, Midtvedt T, et al. Gut microflora associated characteristics in children with celiac disease. Am J Gastroenterol. 2005;100:2784–8. doi: 10.1111/j.1572-0241.2005.00313.x. [DOI] [PubMed] [Google Scholar]