As the major effector cells for protecting the host from pathogens, T cells are required to meet massive demands of proteins to expand themselves quickly upon activation. In a recent paper published in Nature Immunology, Liu et al. reported that the m1A modification of transfer RNA can promote CD4+ T cell proliferation by enhancing the translation efficiency of a specific group of messenger RNAs, providing a new mechanism of translational control of T cell expansion.
CD4+ T cells are the major players of adaptive immune response for fighting against pathogens and maintaining immune homeostasis. Upon antigen recognition, naive CD4+ T cells are activated and exit from quiescence into rapid activation, clonal expansion and differentiation developing effector function. The area has been actively investigated to understand the mechanisms whereby T cell homeostasis is regulated, laying the foundation for new therapeutic strategies for cancer and autoimmune diseases.1 A known mechanism of driving activated T cells to undergo mitosis is to produce more messenger RNAs (mRNAs) and thereby augment protein production for effector functions in activated T cells.2 However, this transcriptional mechanism is not sufficient to propel T cells to enter cell cycle, as activated T cells need massive numbers of proteins for rapid proliferation in a short time window. As a key component of the translation machinery, transfer RNAs (tRNAs) carry amino acids to the ribosome and facilitate mRNAs to translate into corresponding proteins. tRNA modifications can influence protein biogenesis by utilizing the existing mRNA pool, suggesting that rapid changes in tRNA modifications might be a potential translational mechanism by which protein synthesis is accelerated to drive the massive clonal expansion of T cells.
A recent study by Liu et al. in Nature Immunology reported that N1-methyladenosine (m1A) -modified tRNAs enhance the translation efficiency of MYC protein, promoting T cell proliferation after activation.3 Using RNA sequencing and tRNA sequencing, Liu et al. observed an increased level of genes related to translation and tRNA modification during early T cell activation in vitro, suggesting that tRNA-mediated protein translation is an essential player in this process, which is consistent with a recent report.4 Technical advances that enable analysis of base modifications across tRNAs have shed new light on the roles of these modifications. Using tRNA methylation sequencing, the authors found that the level of m1A at position 58 of tRNA (tRNA-m1A58) was much higher than other tRNA methylations after T cell activation. Given the significance of tRNA-m1A58 methylation in regulating translation efficiency,5,6 the authors focused on tRNA-m1A58 ‘writer’ genes (encoding tRNA methyltransferases TRMT61A and TRMT6), and they found that both genes were among the most upregulated translation-related genes upon T cell activation. After the characterization of abundance and modification of tRNAs, Liu et al. investigated the function of tRNA-m1A58 in vivo by generating CD4+ T cell-specific knockout (KO) mice for Trmt61a and Trmt6. They revealed disrupted immune function of Trmt61a-deficient CD4+ T cells using an adoptive T cell transfer colitis model. To understand the underlying cellular mechanism, the authors evaluated the effects of TRMT61A on T cell activation, proliferation, apoptosis, and differentiation separately, and they found that TRMT61A can maintain the proliferation ability of CD4+ T cells. The authors successfully rescued the phenotype of the defective proliferation of Trmt61a-KO T cells by the reintroduction of WT Trmt61a gene rather than m1A58 catalytic-dead Trmt61a gene, confirming that the proliferation defect of Trmt61a-deficient CD4+ T cells was caused by the reduction of tRNA-m1A58 modification.
The transcription factor MYC can promote cellular proliferation. Given that MYC-regulated targets were among the most downregulated genes after Trmt61a deletion, the authors revealed that MYC protein level, rather than Myc mRNA level, was reduced in Trmt61a-KO cells, leading to cell cycle arrest after T cell activation. They further revealed that tRNA-m1A58 is essential for Myc mRNA translation rather than MYC protein degradation. Translation efficiency can be precisely controlled by the abundance of tRNA species pairing with codons at specific positions of the mRNA. Further mechanistic studies revealed that m1A58 modification levels on tRNAs decoding serine and leucine were largely reduced in Trmt61a-KO cells. Given that serine and leucine were two of the most frequently used codons by Myc mRNA, the authors engineered a mutant Myc plasmid with replaced serine and leucine codons. Expression of this mutant Myc mRNA was sufficient to restore the defective MYC protein expression in KO cells and rescue the defective phenotype of T cell proliferation in vivo. These findings confirm that TRMT61A-mediated tRNA-m1A58 modification directly enhances the translation efficiency of Myc mRNA through codon decoding, and thereby promotes MYC-dependent proliferation after T cell activation (Fig. 1). The authors also raised a question of whether the translation of other proteins besides MYC might be affected after Trmt61a deletion. To address this question, the authors examined the translation efficiency of all mRNAs using RiboTag RNA sequencing. They found that global reduction in tRNA-m1A58 specifically reduced the translation efficiency of certain pre-cell-cycling proteins that are required for rapid proliferation upon T cell activation.
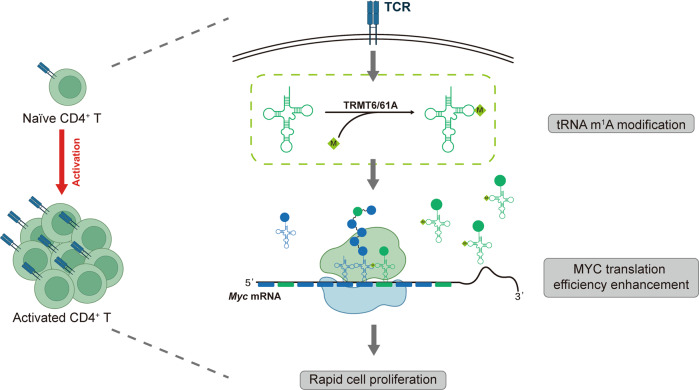
Fig. 1. Proposed model for tRNA-m1A modification as a translational checkpoint for T cell proliferation.
Upon T cell activation, tRNAs and tRNA-m1A58 “writer” proteins (TRMT61A and TRMT6) are upregulated, resulting in an increased level of tRNA-m1A58 modification that facilitates MYC protein synthesis and thus promotes MYC-dependent cell proliferation.
The current study by Liu et al. indicated that, for the first time, TRMT61A-mediated tRNA-m158A methylation is a translational checkpoint that functions as a “gas pedal” for driving T cells to enter cell cycle. The findings of Liu et al. raised new questions for future studies. Upon activation, T cells undergo metabolic reprogramming before they enter the cell cycling phase.7 Several studies reported that tRNA modifications are regulated by the cellular metabolic status.8,9 It would be very interesting to explore how tRNA modification is metabolically controlled at the early stage of T cell activation. In the tRNA pool, chemical modifications are diverse, generating a huge population of tRNA species.5,10 Given that m1A methylation is only one of the modification types of tRNAs, their findings provided a strong motivation to discover other tRNA modifications that might also control cell proliferation via the efficient synthesis of certain proteins. Clinically, tens of human diseases are related to mutations that affect tRNA modification enzymes, indicating the significant role of tRNA modifications in human diseases10 that can be potentially targeted. Liu et al. provided a novel therapeutic target, tRNA-m158A methylation, that might be manipulated to alleviate T cell-mediated diseases such as colitis.
Contributor Information
Weike Pei, Email: peiweike@westlake.edu.cn.
Vijay K. Kuchroo, Email: vkuchroo@evergrande.hms.harvard.edu
References
- 1.Krovi SH, Kuchroo VK. Immunol. Rev. 2022;307:161–190. doi: 10.1111/imr.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan H, et al. Immunity. 2017;46:488–503. doi: 10.1016/j.immuni.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, et al. Nat. Immunol. 2022;23:1433–1444. doi: 10.1038/s41590-022-01301-3. [DOI] [PubMed] [Google Scholar]
- 4.Rak R, et al. Proc. Natl. Acad. Sci. USA. 2021;118:e2106556118. doi: 10.1073/pnas.2106556118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schimmel P. Nat. Rev. Mol. Cell Biol. 2018;19:45–58. doi: 10.1038/nrm.2017.77. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, et al. Cell. 2016;167:816–828. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalek RD, Rathmell JC. Immunol. Rev. 2010;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asano K, et al. Nucleic Acids Res. 2018;46:1565–1583. doi: 10.1093/nar/gky068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H, et al. Nat. Commun. 2018;9:1875. doi: 10.1038/s41467-018-04250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki S. Nat. Rev. Mol. Cell Biol. 2021;22:375–392. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]