Abstract
Physical activity and cognitive functioning are strongly intertwined. However, the causal relationships underlying this association are still unclear. Physical activity can enhance brain functions, but healthy cognition may also promote engagement in physical activity. Here, we assessed the bidirectional relationships between physical activity and general cognitive functioning using Latent Heritable Confounder Mendelian Randomization (LHC-MR). Association data were drawn from two large-scale genome-wide association studies (UK Biobank and COGENT) on accelerometer-measured moderate, vigorous, and average physical activity (N = 91,084) and cognitive functioning (N = 257,841). After Bonferroni correction, we observed significant LHC-MR associations suggesting that increased fraction of both moderate (b = 0.32, CI95% = [0.17,0.47], P = 2.89e − 05) and vigorous physical activity (b = 0.22, CI95% = [0.06,0.37], P = 0.007) lead to increased cognitive functioning. In contrast, we found no evidence of a causal effect of average physical activity on cognitive functioning, and no evidence of a reverse causal effect (cognitive functioning on any physical activity measures). These findings provide new evidence supporting a beneficial role of moderate and vigorous physical activity (MVPA) on cognitive functioning.
Subject terms: Genetics, Neuroscience, Psychology, Risk factors
Introduction
Multiple cross-sectional and longitudinal studies have shown that physical activity and cognitive functioning are strongly intertwined and decline through the course of life1–5. However, the evidence of causality of this relationship remains unclear. Previous results have shown that physical activity can improve cognitive functioning6–12, but recent studies have also suggested that well-functioning cognitive skills can influence engagement in physical activity1,13–20.
Several mechanisms could explain how physical activity, especially at moderate intensities, enhances general cognitive functioning12,21–27. For example, physical activity can increase brain plasticity, angiogenesis, synaptogenesis, and neurogenesis primarily through the upregulation of growth factors (e.g., brain-derived neurotrophic factor; BDNF)23,24,26. In addition, the repetitive activation of higher-order brain functions (e.g., planning, inhibition, and reasoning) required to engage in physical activity may contribute to the improvement of these functions27,28. In turn, other mechanisms could explain how cognitive functioning may affect physical activity. For example, cognitive functioning may be required to counteract the automatic attraction to effort minimization and thereby influence a person’s ability to engage in physically active behavior20,29–31. Of note, these mechanisms are not mutually exclusive and could therefore lead to bidirectionally reinforcing relationships (i.e., positive feedback loop) between physical activity and cognitive functioning32. Thus, there is a mechanistic explanation theoretically supporting the associations between moderate physical activity and cognitive function.
Although these studies point to a potential mutually beneficial interplay between physical activity and cognitive functioning across the lifespan, these findings mainly stem from observational designs and analytical methods that cannot fully rule out the influence of social, behavioral, and genetic confounders32. While randomized controlled trials minimizing these potential confounds have been conducted33, they were typically based on small sample sizes (n < 100) that can bias the estimations33. Critically, these trials only investigated the effect of physical activity on cognitive functioning, not the opposite. Accordingly, current evidence on the causal association between physical activity and cognitive functioning and on whether this association is one or two-way could be considered weak. Because Mendelian Randomization (MR) is less vulnerable to confounding or reverse causation than conventional approaches in observational studies34,35, this method is particularly appropriate to address this knowledge gap.
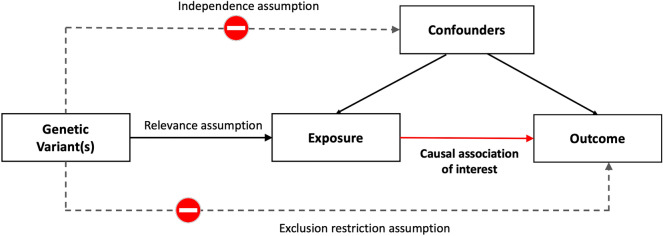
MR is an epidemiological method in which the randomized inheritance of genetic variation is considered as a natural experiment to estimate the potential causal effect of a modifiable risk factor (exposure) on health-related outcomes in an observational design34,35. MR draws on the assumption that genetic variants associated with the exposure, because they are randomly allocated at conception, are less associated with other risk factors that may be confounders of the association between the exposure and the outcome, and are immune to reverse causality since diseases or health-related outcomes have no reverse effect on genetic variants. Accordingly, if an exposure (e.g., physical activity) causally affects an outcome (e.g., cognitive function), the genetic variants that influence this exposure is expected to affect the outcome to a proportional degree if no separate pathway exists by which these genetic variants can affect the outcome32. In other words, genetic variants associated with an exposure of interest can serve as instruments (or proxies) for estimating the causal association with an outcome (see Fig. 1 for the conceptual illustration of the MR method).
Figure 1.
Conceptual illustration of the Mendelian Randomization (MR) method. The causal association of interest is between the exposure (e.g., physical activity) and the outcome (e.g., cognitive function). Relevance assumption states that the genetic instruments are strongly associated with the exposure but are not associated with the confounders. The exclusion restriction assumption states that the genetic instruments are only indirectly associated with the outcome via the exposure. Thus, the solid paths are expected to exist, while the dashed paths are expected to be nonsignificant according to the core MR assumptions.
We used a newly-developed MR method showing improved power to simultaneously estimate the bidirectional causal effects between physical activity and cognitive functioning36. In a two-sample MR design, genetic instruments can be obtained from summary statistics of nonoverlapping large-scale genome-wide association studies (GWAS). That is, the genetic instruments for the exposure and the genetic instruments for the outcome can be obtained from separate studies37. This is an outstanding advantage for estimating the causal relationships between two traits (e.g., cognitive functioning and physical activity) because a trait does not necessarily need to be assessed in both samples37. Here, the causal estimates were modeled based on recently available summary statistics from large-scale GWAS of accelerometer-measured physical activity38, and general cognitive functioning39,40.
The current study focused on general cognitive functioning estimated from a battery of neuropsychological tests (e.g., N-Back working memory task, Stroop Test, Wechsler Adult Intelligence Scale)41,42. Although the influence of physical activity on different types of cognitive functions may differ, cognitive tests measuring these different functions yield highly correlated results in a given individual, making the assessment of general cognitive functioning highly relevant40.
Since it has been suggested that the intensity of physical activity can be an important consideration, with moderate intensity having greater beneficial effects than vigorous intensity43–47, we assessed whether the causal effect estimates on cognitive functioning were dependent on physical activity intensity (i.e., moderate vs. vigorous vs. average). However, if a stronger effect on cognitive function could be expected for moderate physical activity, recent studies showed that high-intensity exercise can also impact the above-mentioned mechanisms such as increased BDNF48–50. Here, consistent with existing literature using UK Biobank data38,51, the fraction of accelerations > 100 milli-gravities (mg) and < 425 mg was used to estimate moderate physical activity, and the fraction of accelerations 425 mg was used to estimate vigorous physical activity. Of note, as existing literature suggests reciprocal associations between physical activity and cognitive function, we applied bidirectional MR to examine the causal link from physical activity to cognitive function and from cognitive function to physical activity.
Methods
Data sources and instruments
This study used de-identified GWAS summary statistics from original studies that were approved by relevant ethics committees. The current study was approved by the Ethics Committee of Geneva Canton, Switzerland (CCER-2019–00,065). The available summary-level data were based on 257,841 samples for general cognitive functioning and 91,084 samples for accelerometer-based physical activity. Participants’ age ranged from 40 to 69 years in the UK Biobank and from 8 to 96 years in the COGENT consortium.
Physical activity
Accelerometer-measured physical activity was assessed based on summary statistics from a recent GWAS38, analyzing accelerometer-based physical activity data from the UK Biobank. In the UK Biobank, about 100,000 participants wore a wrist-worn triaxial accelerometer (Axivity AX3) that was set up to record data for seven days. Individuals with less than 3 days (72 h) of data or not having data in each 1-h period of the 24-h cycle or for whom the accelerometer could not be calibrated were excluded. Data for non-wear segments, defined as consecutive stationary episodes 60 min where all three axes had a standard deviation < 13 mg, were imputed. The details of data collection and processing can be found elsewhere52. We examined three measures derived from the three to seven days of accelerometer wear: the average acceleration in mg that includes acceleration > 0 mg, the fraction of accelerations > 100 mg and < 425 mg to estimate moderate physical activity, and the fraction of accelerations 425 mg to estimate vigorous physical activity38. As previous reported51, 425 mg cut-off was chosen because it corresponds to vigorous intensity (6 METS). The GWAS for average physical activity (nmax = 91,084) identified 2 independent genome-wide significant SNPs (P < 5e − 09), with an SNP-based heritability of ~ 14%.
As for the other two physical activity measures, the fractions of accelerations corresponding to moderate and vigorous physical activity were obtained by running new GWAS on the decomposed acceleration data from UK Biobank using the BGENIE software53. The phenotype for moderate physical activity was limited to acceleration magnitudes ranging from 100 to < 425 mg, whereas vigorous physical activity was limited to acceleration magnitudes ranging from 425 to 2000 mg. These acceleration fractions were adjusted for age, sex, and the first 40 principal components (PC), and the analyzed individuals were restricted to unrelated white-British. The two datasets of average physical activity summary statistics, alongside the moderate and vigorous physical activity summary statistics, were used in Latent Heritable Confounder Mendelian Randomization (LHC-MR) to investigate the possible bidirectional effect that exists between these physical activity traits and cognitive functioning.
General cognitive functioning
General cognitive functioning was assessed based on summary statistics from a recent GWAS combining cognitive and genetic data from the UK Biobank and the COGENT consortium (N = 257,841)39. The phenotypes of these cohorts are well-suited to meta-analysis because their pairwise genetic correlation has been shown to be high40. In the UK Biobank (nmax = 222,543) participants were asked to complete 13 multiple-choice questions that assessed verbal and numerical reasoning. For verbal reasoning, a typical question was “bud is to flower what child is to …?”, and possible answers presented to the participants are “Grow”, “Develop”, “Improve”, “Adult”, or “Old”. For numerical reasoning, a typical question was “150…137…125…114…104… what comes next?” with possible answers being “96”, “95”, “94”, “93”, or “92”39. The verbal and numerical reasoning score was based on the number of questions answered correctly within a two-minute time limit. Each respondent took the test up to four times. This test was designed as a measure of fluid intelligence. The phenotype consists of the mean of the standardized score across the measurement occasions for a given participant. In the COGENT consortium (nmax = 35,298), general cognitive function is statistically derived from a principal components analysis of individual scores on a neuropsychological test battery, such as the Verbal or spatial N-Back working memory task, Stroop Test, the Trail Making Test, or the Wechsler Adult Intelligence Scale41. Details on the test battery are available in the supplementary material of Davies et al.42. Of note, Davies et al.42 demonstrated that two general cognitive function components extracted from different sets of cognitive tests on the same participants exhibit a high correlation, addressing the fact that different cohorts relied on different cognitive tests. Thus, the phenotype estimates overall cognitive functioning and is relatively invariant to the battery used and specific cognitive abilities assessed54,55. These COGENT data used to assess general cognitive functioning were also used in another GWAS study40. The GWAS identified 226 independent genome-wide significant SNPs, with a SNP-based heritability of ~ 20%.
Statistical analysis
MR is a statistical approach for causal inference that can overcome the weaknesses of traditional observational studies34,35. MR-based effect estimates rely on three main assumptions56, stating that genetic instruments (i) are strongly associated with the exposure (relevance assumption), (ii) are independent of confounding factors of the exposure-outcome relationship (independence assumption), and (iii) are not associated to the outcome conditional on the exposure and potential confounders (exclusion restriction assumption). Well-powered GWAS offer multiple genetic instruments that are strongly associated with exposures of interest (cognitive functioning or physical activity in our case), which validates the relevance assumption. Each of these genetic variants (instruments) provides a causal effect estimate of the exposure on the outcome, which can be in turn combined through meta-analysis using inverse-variance weighting (IVW) to obtain an overall estimate. The second and third assumptions are less easily validated and can be violated in the case of a heritable confounder affecting the exposure-outcome relationship and biasing the causal estimate. Such confounders can give rise to instruments with proportional effects on the exposure and outcome, hence violating the Instrument Strength Independent of Direct Effect (InSIDE) assumption requiring the independence of the exposure and direct outcome effects. There have been several extensions to the common IVW method of MR analysis, including MR-Egger, which allows for directional pleiotropy of the instruments and attempts to correct the causal regression estimate. Other extensions, such as the median and mode-based estimators, assume that at least half of or the most “frequent” genetic instruments are valid/non-pleiotropic. However, despite these extensions and relaxed assumptions, all these classical MR methods are notably underpowered and still suffer from two major limitations. First, they only use a subset of markers as instruments (genome-wide significant markers), which often dilutes the true relationship between traits. Second, they ignore the presence of a potential latent heritable confounder of the exposure-outcome relationship (e.g., body mass index, educational attainment, level of physical activity at work, or material deprivation).
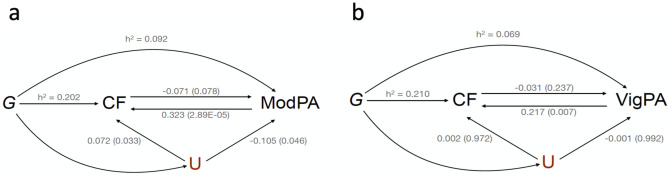
LHC-MR also uses GWAS summary statistics 36, but importantly, this new method appropriately uses genome-wide genetic markers to estimate bidirectional causal effects, direct heritability, and confounder effects while accounting for sample overlap. LHC-MR can be viewed as an extension of the linkage disequilibrium score regression (LDSC) 57, designed to estimate trait heritability, in that it models all genetic marker effects as random, but additionally estimates bidirectional causal effect, as well as other parameters. LHC-MR extends the standard two-sample MR by modeling a latent (unmeasured) heritable confounder that has an effect on the exposure and outcome traits. This allows LHC-MR to differentiate SNPs based on their co-association to a pair of traits and distinguish heritable confounding that leads to genetic correlation from actual causation. Thus, the unbiased bidirectional causal effect between these two traits are estimated simultaneously along with the confounder effect on each trait (Fig. 2a, b). The LHC-MR framework, with its multiple pathways through which SNPs can have an effect on the traits, as well as its allowance for null effects, make LHC-MR more precise at estimating causal effects compared to standard MR methods (i.e., MR egger, weighted median, inverse variance weighted, simple mode, and weighted mode).
Figure 2.
Visual representation of the model in Latent Heritable Confounder Mendelian Randomization (LHC-MR). G = Genetic instruments; CF = general cognitive functioning; (a) For moderate physical activity (ModPA); (b) For vigorous physical activity (VigPA); U = Latent heritable confounder; h2 = direct heritability. Each figure includes the bidirectional causal effects between the two traits as well as the confounder effects on each of them. Coefficients are beta values. P-values are indicated in brackets. The models were adjusted for age, sex, genotyping chip, first ten genomic principal components, center, and season (month) of wearing an accelerometer.
The likelihood function for LHC-MR, which is derived from the mixture of different pathways through which the genome-wide SNPs can have an effect (acting on either the exposure, the outcome, the confounder, or the combinations of these three), is then optimized given random starting values for the parameters it can estimate. The optimization of the likelihood function then yields the maximum likelihood estimate (MLE) value for a set of estimated parameters, including the bidirectional causal effect between the exposure and the outcome as well as the strength of the confounder effect on each of those two traits. The standard errors of each of the parameters estimated using LHC-MR were obtained by implementing a block jackknife procedure where the SNP effects are split into blocks, and the MLE is computed again in a leave-one-block-out fashion. The variance of the estimates can then be computed from the results of the various MLE optimizations. Furthermore, the causal estimates obtained from LHC-MR are on the scale of 1 standard deviation (SD) outcome difference upon a 1 SD exposure change due to the use of standardized summary statistics for the two traits.
A sensitivity analysis in which the model was further adjusted for baseline self-reported level of physical activity at work, walking or standing at work, and the Townsend Deprivation Index was conducted.
Ethical approval
This study was approved by the Ethics Committee of Geneva Canton, Switzerland (CCER-2019–00,065).
Results
Three measures derived from accelerometer wear were used as a proxy for physical activity: average, moderate, and vigorous physical activity. These three measures were used in LHC-MR to investigate the possible bidirectional causal effects between them and cognitive functioning. The model tested was adjusted for age, sex, genotyping chip, first ten genomic principal components (PC), center, and season (month) of wearing accelerometer. The Bonferroni correction was used to control for familywise error rates, yielding an α = 0.05 / (2 directions × 3 tests) = 0.008.
Average physical activity and general cognitive functioning
LHC-MR applied to summary statistics belonging to model 1 showed no evidence for a potential causal effect of average physical activity on cognitive functioning (b = 0.245, CI95% = [− 0.01,0.50], P = 0.065) (Table 1, Fig. 3) and no evidence for the reverse causal effect (b = − 0.145, CI95%. = [− 0.26,− 0.03], P = 0.013 [α = 0.008]). Similarly, standard MR methods such as IVW, MR Egger, weighted median, simple mode, and weighted mode yielded non-significant causal estimates in either direction (Table 2), using 129 genome-wide significant single nucleotide polymorphisms (SNPs) as instruments for cognitive functioning and 6 SNPs for average acceleration.
Table 1.
Latent Heritable Confounder Mendelian Randomization (LHC-MR) results for the association between accelerometer-measured physical activity and general cognitive functioning.
| Parameter | Cognitive functioning | Physical activity | Cognitive functioning → Physical activity |
Physical activity → Cognitive functioning |
||
|---|---|---|---|---|---|---|
| Heritability | t | Heritability | t | |||
| Average accelerometer-measured physical activity (fraction of acceleration > 0 mg) | ||||||
| Estimate | 0.207 | 0.033 | 0.123 | − 0.011 | − 0.145 | 0.245 |
| P value | 2.67E − 115 | 0.612 | 4.41E − 28 | 0.816 | 0.013 | 0.065 |
| Moderate accelerometer-measured physical activity (fraction of acceleration > 100 mg and < 425 mg) | ||||||
| Estimate | 0.202 | 0.072 | 0.092 | − 0.105 | − 0.071 | 0.323 |
| P value | 1.13E − 165 | 0.032 | 5.98E − 29 | 0.046 | 0.078 | 2.89e − 05 |
| Vigorous accelerometer-measured physical activity (fraction of acceleration 425 mg and < 2000 mg) | ||||||
| Estimate | 0.210 | 0.002 | 0.069 | − 0.001 | − 0.031 | 0.212 |
| P value | 6.75E − 157 | 0.972 | 3.95E − 25 | 0.992 | 0.237 | 0.007 |
Notes. Parameters estimates and their P-values were obtained from the LHC-MR optimized model with the maximum likelihood. Bidirectional associations from cognitive functioning to physical activity and from physical activity to cognitive functioning are reported. t = effect of the confounder. Bonferroni corrected α = 0.008.
Figure 3.
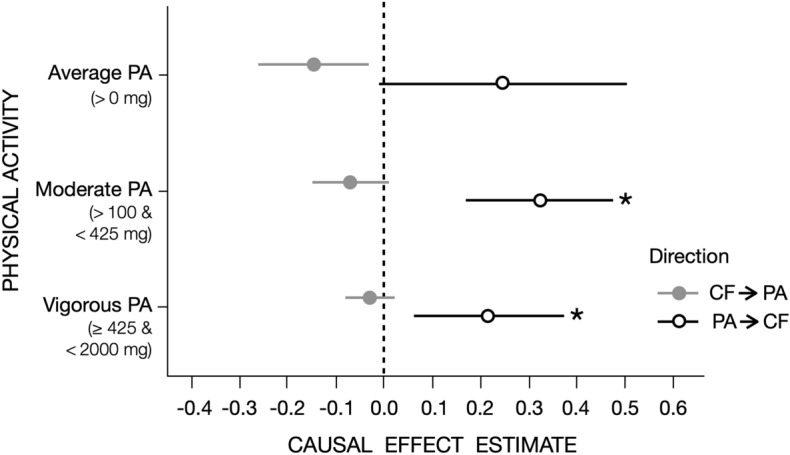
LHC-MR plots for the association between accelerometer-based physical activity and general cognitive functioning, Notes, This modified dot-and-whisker plot reports the causal estimate between general cognitive functioning (CF) as exposure and varying physical activity (PA)-related traits as outcomes. The forward (CF → PA) and reverse (PA → CF) causal estimates are shown in two different colors as dots (grey and white) with 95% CI whiskers (grey and black). Average PA = average of overall accelerations > 0 mg. Moderate PA = fraction of acceleration corresponding to moderate physical activity (> 100 mg and < 425 mg). Vigorous PA = fraction of acceleration corresponding to vigorous physical activity (≥ 425 mg and < 2000 mg). The models were adjusted for age, sex, genotyping chip, first ten genomic principal components (PC), center, and season (month) of wearing accelerometer. * = significant effect after Boneferroni correction (i.e., P-value < .008).
Table 2.
Standard Mendelian Randomization (MR) results for the association between accelerometer-based physical activity and general cognitive functioning.
| Exposure | Outcome | MR method | Valid SNPs | Causal estimate | SE | P value |
|---|---|---|---|---|---|---|
| Average accelerometer-based physical activity (fraction of acceleration > 0 mg) | ||||||
| Cognitive functioning | Physical activity | MR Egger | 129 | 0.015 | 0.185 | 0.935 |
| Weighted median | 129 | − 0.027 | 0.036 | 0.440 | ||
| Inverse variance weighted | 129 | − 0.011 | 0.032 | 0.723 | ||
| Simple mode | 129 | − 0.102 | 0.116 | 0.376 | ||
| Weighted mode | 129 | − 0.084 | 0.111 | 0.452 | ||
| Physical activity | Cognitive functioning | MR Egger | 4 | − 2.833 | 1.148 | 0.069 |
| Weighted median | 4 | 0.017 | 0.062 | 0.782 | ||
| Inverse variance weighted | 4 | − 0.088 | 0.127 | 0.488 | ||
| Simple mode | 4 | 0.020 | 0.076 | 0.801 | ||
| Weighted mode | 4 | 0.023 | 0.074 | 0.770 | ||
| Moderate accelerometer-based physical activity (fraction of acceleration > 100 mg and < 425 mg) | ||||||
| Cognitive functioning | Physical activity | MR Egger | 129 | − 0.054 | 0.181 | 0.766 |
| Weighted median | 129 | − 0.032 | 0.037 | 0.389 | ||
| Inverse variance weighted | 129 | − 0.012 | 0.032 | 0.710 | ||
| Simple mode | 129 | − 0.059 | 0.106 | 0.575 | ||
| Weighted mode | 129 | − 0.031 | 0.091 | 0.729 | ||
| Physical activity | Cognitive functioning | MR Egger | 106 | 0.325 | 0.319 | 0.310 |
| Weighted median | 106 | − 0.001 | 0.021 | 0.981 | ||
| Inverse variance weighted | 106 | 0.023 | 0.022 | 0.309 | ||
| Simple mode | 106 | − 0.017 | 0.057 | 0.767 | ||
| Weighted mode | 106 | − 0.010 | 0.050 | 0.837 | ||
| Vigorous accelerometer-based physical activity (fraction of acceleration 425 mg) | ||||||
| Cognitive FUNCTIONING | Physical activity | MR Egger | 129 | 0.009 | 0.149 | 0.952 |
| Weighted median | 129 | 0.018 | 0.036 | 0.623 | ||
| Inverse variance weighted | 129 | 0.002 | 0.026 | 0.939 | ||
| Simple mode | 129 | 0.021 | 0.097 | 0.829 | ||
| Weighted mode | 129 | 0.021 | 0.088 | 0.812 | ||
| Physical activity | Cognitive functioning | MR Egger | 88 | 0.151 | 0.335 | 0.653 |
| Weighted median | 88 | − 0.035 | 0.022 | 0.108 | ||
| Inverse variance weighted | 88 | − 0.016 | 0.020 | 0.432 | ||
| Simple mode | 88 | − 0.065 | 0.060 | 0.286 | ||
| Weighted mode | 88 | − 0.059 | 0.052 | 0.257 | ||
Causal estimates from 5 standard Mendelian Randomization (MR) methods on alternating exposure and outcome traits. For both moderate and vigorous physical activity as exposure, the cutoff was decreased to 6.33e − 5 because of the low number of genome wide significant single nucleotide polymorphisms (SNPs) to use as instruments. Corrected α = 0.008.
Moderate physical activity and general cognitive functioning
LHC-MR applied to the fraction of accelerations corresponding to moderate physical activity showed a potential positive causal effect of moderate physical activity on greater cognitive functioning (b = 0.32, CI95% = [0.17,0.47], P = 2.89e − 05) (Table 1, Fig. 3). We found no evidence for the reverse causal effect (b = − 0.071, CI95%. = [− 0.15, 0.01], P = 0.078 [α = 0.008]). As was found with average physical activity, there was no evidence for the presence of a heritable confounder. Standard MR methods yielded non-significant causal estimates in both directions (Table 2).
Vigorous physical activity and general cognitive functioning
LHC-MR applied to the fraction of accelerations corresponding to vigorous physical activity on cognitive functioning showed a potential positive causal effect of vigorous physical activity on greater cognitive functioning (b = 0.22, CI95% = [0.06,0.37], P = 0.007) (Table 1, Fig. 3). We found no evidence for the reverse causal effect (b = − 0.031, CI95%. = [-0.08, 0.02], P = 0.237 [α = 0.008]). As was found with average and moderate physical activity, there was no evidence for the presence of a heritable confounder. Of note, the coefficient of this causal effect was qualitatively weaker than of the causal effect of moderate physical activity on cognitive functioning (b = 0.22 vs. b = 0.32). Standard MR methods yielded non-significant causal estimates in both directions (Table 2).
Sensitivity analyses
We tested another model where an extra adjustment had been done for the baseline self-reported level of physical activity at work, walking or standing at work, and the Townsend Deprivation Index. LHC-MR applied to summary statistics emerging from this second model showed consistent results with that of the first model (b = 0.22, CI95% = [− 0.05,0.50], P = 0.111 and b = − 0.090, CI95% = [− 0.23,0.05], P = 0.200, respectively). Both models showed no evidence for the presence of a heritable confounder. Due to the similarity in results between these models, we did not conduct this second model on moderate and vigoruous physical activity.
Discussion
Main findings
This study used a genetically informed method that provides evidence of putative causal relations to investigate the bidirectional associations between accelerometer-based physical activity and general cognitive functioning. Drawing on large-scale GWAS, we found evidence for potential causal effects, suggesting that higher levels of moderate and vigorous physical activity lead to increased cognitive functioning. In the opposite direction, we did not observe evidence of a causal effect of cognitive functioning on physical activity. Hence, our study suggests a favorable effect of moderate and vigorous physical activity on cognitive functioning, but does not provide evidence that increased cognitive functioning promotes engagement in more physical activity.
Comparison with previous studies
Previous reviews and meta-analyses of observational studies showed a beneficial effect of physical activity on cognitive functioning 6,9,10,27. However, the evidence arising from intervention studies was inconclusive 11,12,14–16,58. It has been argued that these inconsistencies may primarily be attributed to the design-specific tools used to assess physical activity 14. Specifically, many observational studies rely on self-reported measures of physical activity, whereas intervention studies often rely on accelerometer-measured physical activity, or have people exercising under monitored conditions. In other words, evidence of a favorable effect of physical activity on cognitive functioning may have emerged in observational studies because of the self-reported nature of the measures they typically used. Yet, in our study, results are based on accelerometer-assessed physical activity, thereby partially ruling out this explanation. Therefore, our findings further support the literature that demonstrated a protective role of physical activity on cognitive functioning and extend it by doing so using an accelerometer-based measure.
Of note, results obtained from LHC-MR differed from those obtained with standard MR methods. At least three key differences in the methods can explain this divergence: i) standard MR uses only genome-wide significant markers, ii) standard MR is biased in case of sample overlap (as is the case in this study) and hence their estimate may be biased towards the observational correlation, and iii) LHC-MR explicitly models correlated pleiotropy unlike standard MR. Accordingly, our results obtained from LHC-MR are expected to be more robust than those obtained from standard MR. Since LHC-MR could not find evidence for the presence of a heritable confounder, correlated pleiotropy is less likely, or there might be multiple confounders with opposite effects canceling each other out. This finding highlights that the main reason for the difference between LHC-MR and classical MR methods is statistical power. For testing the reverse causal effect (cognition on physical activity), we had numerous instruments available, ensuring that all MR-methods are well-powered and yielding the same (null effect) conclusion. The forward effect (physical activity on cognition) relied on only a few (weak) instruments, rendering classical MR methods notably underpowered. This is the type of situation in which methods such as LHC-MR, which leverage genome-wide genetic markers, are crucial to facilitate discovery. It is important to point out that while the statistical conclusion from classical and LHC-MR methods differ, their effect estimates are not significantly different, suggesting that there is no discrepancy in the results, but that they have different precision. Finally, we acknowledge that LHC-MR assumptions may be violated and results should thus still be considered cautiously. Yet, while the assumptions of LHC-MR may not hold, the assumptions of the other five methods are known not to hold because of insufficient genome-wide significant instruments.
To the best of our knowledge, our study is the first to investigate the potential causal relationship between physical activity and cognitive functioning using a genetically informed method. We are aware of only two other, non-genetic studies that examined the potential bidirectional associations between physical activity and cognitive functioning 1,13. In contrast to the present study, those two studies observed a positive influence of cognitive functioning on physical activity. At least two factors can explain the differences in the results observed. First, both those studies are based on longitudinal assessment of the two traits, while our approach is based on a genetically instrumented causal inference technique (LHC-MR). Second, these studies draw on self-reported physical activity rather than accelerometer-measured physical activity, which may not accurately reflect the objective level of physical activity.
Our results obtained with recently-improved genetically-informed analyses (LHC-MR) highlight the potential critical role of physical activity, specifically of moderate and vigorous intensity, on cognitive functioning. However, it should be noted that the estimated effect of moderate physical activity on cognitive functioning was about 1.5 times stronger in magnitude than the effect of vigorous physical activity. To the best of our knowledge, this study is the first to assess and compare the causal relationships of moderate and vigorous physical activity with cognitive functioning using a genetically-informed method based on large-scale datasets. Alhough additional evidence is needed, this study confirms the importance to examine the extent to which the intensity of physical activity moderates the effects observed on cognitive functioning 43.
The LHC-MR method revealed two causal relations that are consistent with each other. Importantly, these findings are consistent with theoretical and experimental work explaining the mechanisms underlying the association between the physical activity and cognitive functioning. Results obtained with both the LHC and standard MR methods showed no evidence of an effect of average physical activity on cognitive functioning. This finding can likely be explained by physical activities of low intensity (i.e., < 100 mg) that are part of the average physical activity, which further suggests that physical activity should be of moderate-to-vigorous intensity to benefit cognitive functioning.
The absence of evidence for a reverse causal effect of cognitive functioning on physical activity may be partly explained by the lower power of this analysis due to smaller sample size of the GWAS of physical activity (n = 91,084) compared to the sample size of the GWAS of cognitive functioning (n = 257,841). This absence of evidence contrasts with other studies arguing that cognitive functioning is critical for supporting engagement in physical activity 20,29,30. This difference could be explained in at least two ways. Firstly, previous studies examining the positive effect of cognitive functions on physical activity relied on self-reported physical activity, which can bias the observed associations 1,17,20. Secondly, our study relied on general cognitive functioning, whereas previous results highlight the specific importance of inhibition resources that may be required to counteract an automatic tendency for effort minimization 20,29–31,59,60. Therefore, future studies should investigate the specific relationships between motor inhibition and physical activity when such data is available.
Strengths and limitations
Among the strengths of the current study are the use of large-scale datasets, the reliance on instruments derived from objective measures of physical activity, and the application of a robust genetically informed method that can estimate causal effects. However, this study has several features that limit the conclusions that can be drawn. First, the measure of cognitive functioning spans multiple performance domains, which reduced the specificity of the cognitive functioning that was assessed. This feature limits our ability to evaluate the putative causal effects between specific cognitive functioning, such as motor inhibition, and physical activity. Second, MR analysis is designed to elucidate a life-long exposure effect on a life-long outcome (except in special cases when genetic factors have time-dependent effects), thus it is not suited to explore temporal aspects of these causal relationships. Third, 2-sample MR methods require that SNP effects on the exposure are homogeneous between the two samples. Here, because our two samples differ in age, we rely on the assumption that these genetic effects do not change depending on age. This assumption often turns out to be true, although there are rare exceptions 61. It is therefore still possible that genetic variants related to physical activity and cognitive function may differ across the life course. For example, genetic variants related to cognitive development, maintenance and decline may strongly differ. Likewise, the genetic variance predicting physical activity engagement in early-life may differ from those predicting engagement in adulthood or late life. Accordingly, as the age range between the sample is not equivalent (40 to 60 years for the UK Biobank vs. 8 to 96 years in the COGENT consortium) and, most importantly, as physical activity was only assessed in the UK biobank that provides the narrowest age range, the potential differences in the genetic variants depending on individual’s age may have bias the current findings. Testing to which extent age may influence the genetic variants associated with physical activity and cognitive functioning traits is thus warranted in future studies. Fourth, LHC-MR can be limited by the low heritability of traits, potentially causing bimodal/unreliable estimates. Fifth, LHC-MR assumes a single confounder (or several ones with similar effects), but a limitation exists when multiple confounders are present with similar but opposing effect directions on the traits of interest, resulting in a higher misdetection rate. Sixth, although the coefficients estimated with LHC-MR did not statistically differ from the coefficents estimated with classical MR, it is important to acknowledge that no classical MR was unable to find a significant association between physical activity and cognitive function. Accordongly, even if we can be rather confident in the estimation provided by the newly developed methods, it seems more reasonable to consider that the current findings are provisional and need to be replicated. Finally, it is worth noting that the genetic instruments were developed on a primarily white population of European ancestry, limiting the generalizability of the results.
Conclusion and policy implications
Our findings provide preliminary support for a unidirectional relation whereby higher levels of moderate and vigorous physical activity lead to improved cognitive functioning. These results underline the essential role of moderate and vigorous physical activity in maintaining or improving general cognitive functioning. Therefore, health policies and interventions that promote moderate and vigorous physical activity are relevant to improve cognitive functioning or to delay its decline.
Acknowledgements
B.C. is supported by an Ambizione grant (PZ00P1_180040) from the Swiss National Science Foundation (SNSF). M.P.B. is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2021-03153), the Canada Foundation for Innovation (CFI), and the Banting Research Foundation. Y.C.K. is supported by the National Institute of Health (R01 HL136528). The authors are thankful to Gail Davies for providing useful comments on the manuscript.
Author contributions
B.C., M.P.B. conceived and designed the study. L.D., Z.K. analyzed the data. B.C., M.P.B., L.D, Z.K. drafted the manuscript. All authors critically appraised the manuscript, worked on its content, and approved its submitted version.
Data availability
The datasets used for the analysis are openly available from the Neale Lab GWAS results at http://www.nealelab.is/uk-biobank and from the Social Science Genetic Association Consortium Downloads at https://www.thessgac.org/data. Only the new GWAS dataset created for the fractions of physical activity are available with permission from the UK Biobank https://www.ukbiobank.ac.uk/. The LHC-MR code is available at https://github.com/LizaDarrous/lhcMR.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Boris Cheval and Liza Darrous.
These authors jointly supervised this work: Zoltán Kutalik and Matthieu P. Boisgontier.
Contributor Information
Boris Cheval, Email: boris.cheval@unige.ch.
Liza Darrous, Email: liza.darrous@unil.ch.
Zoltán Kutalik, Email: zoltan.kutalik@unil.ch.
Matthieu P. Boisgontier, Email: matthieu.boisgontier@uOttawa.ca
References
- 1.Cheval B, et al. Relationship between decline in cognitive resources and physical activity. Health Psychol. 2020;39:519–528. doi: 10.1037/hea0000857. [DOI] [PubMed] [Google Scholar]
- 2.Cheval B, et al. Effect of early-and adult-life socioeconomic circumstances on physical inactivity. Med. Sci. Sports Exerc. 2018;50:476–485. doi: 10.1249/MSS.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 3.DiPietro L. Physical activity in aging: Changes in patterns and their relationship to health and function. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:13–22. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 4.Levy R. Aging-associated cognitive decline. Int. Psychogeriatr. 1994;6:63–68. doi: 10.1017/S1041610294001626. [DOI] [PubMed] [Google Scholar]
- 5.Sebastiani P, et al. Patterns of multi-domain cognitive aging in participants of the long life family study. Geroscience. 2020;42:1335–1350. doi: 10.1007/s11357-020-00202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgart M, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer M, Terrera GM, Demakakos P. Physical activity and trajectories in cognitive function: English Longitudinal Study of Ageing. J. Epidemiol. Community Health. 2018;72:477–483. doi: 10.1136/jech-2017-210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan GS, et al. Physical activity in middle-age and dementia in later life: Findings from a prospective cohort of men in Caerphilly, South Wales and a meta-analysis. J. Alzheimers Dis. 2012;31:569–580. doi: 10.3233/JAD-2012-112171. [DOI] [PubMed] [Google Scholar]
- 10.Sofi F, et al. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 11.Angevaren M, Aufdemkampe G, Verhaar H, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008;3:CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 13.Daly M, McMinn D, Allan JL. A bidirectional relationship between physical activity and executive function in older adults. Front. Hum. Neurosci. 2015;8:1044. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabia S, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. Brit. Med. J. 2017;357:j2709. doi: 10.1136/bmj.j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snowden M, et al. Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. J. Am. Geriatr. Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- 16.Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2015;4:CD005381. doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindwall M, et al. Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. J. Aging Res. 2012;2012:793598. doi: 10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheval B, et al. Higher inhibitory control is required to escape the innate attraction to effort minimization. Psychol. Sport Exerc. 2020;51:101781. doi: 10.1016/j.psychsport.2020.101781. [DOI] [Google Scholar]
- 19.Cheval B, et al. Cognitive functions and physical activity in aging when energy is lacking. Eur. J. Ageing. 2022;19:533–544. doi: 10.1007/s10433-021-00654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheval B, et al. Cognitive resources moderate the adverse impact of poor neighborhood conditions on physical activity. Prev Med. 2019;126:105741. doi: 10.1016/j.ypmed.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Colzato LS, Kramer AF, Bherer L. Editorial special topic: Enhancing brain and cognition via physical exercise. J. Cogn. Enhanc. 2018;2:135–136. doi: 10.1007/s41465-018-0084-1. [DOI] [Google Scholar]
- 22.Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013;37:1645–1666. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 24.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 25.Lisanne F, Hsu CL, Best JR, Barha CK, Liu-Ambrose T. Increased aerobic fitness is associated with cortical thickness in older adults with mild vascular cognitive impairment. J. Cogn. Enhanc. 2018;2:157–169. doi: 10.1007/s41465-018-0077-0. [DOI] [Google Scholar]
- 26.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Raichlen DA, Alexander GE. Adaptive capacity: An evolutionary neuroscience model linking exercise, cognition, and brain health. Trends Neurosci. 2017;40:408–421. doi: 10.1016/j.tins.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frith E, Loprinzi P. Physical activity and individual cognitive function parameters: Unique exercise-induced mechanisms. JCBPR. 2018;7:92–106. doi: 10.5455/JCBPR.284071. [DOI] [Google Scholar]
- 29.Cheval B, et al. Behavioral and neural evidence of the rewarding value of exercise behaviors: A systematic review. Sports Med. 2018;48:1389–1404. doi: 10.1007/s40279-018-0898-0. [DOI] [PubMed] [Google Scholar]
- 30.Cheval B, et al. Avoiding sedentary behaviors requires more cortical resources than avoiding physical activity: An EEG study. Neuropsychologia. 2018;119:68–80. doi: 10.1016/j.neuropsychologia.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Cheval B, et al. Higher inhibitory control is required to escape the innate attraction to effort minimization. Psychol. Sport Exerc. 2020;51:101781. doi: 10.1016/j.psychsport.2020.101781. [DOI] [Google Scholar]
- 32.Choi KW, et al. Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample mendelian randomization study. JAMA Psychiatry. 2019;76:399–408. doi: 10.1001/jamapsychiatry.2018.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018;52:154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 34.Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne EM, Yang J, Wray NR. Inference in psychiatry via 2-sample mendelian randomization—from association to causal pathway? JAMA Psychiatry. 2017;74:1191–1192. doi: 10.1001/jamapsychiatry.2017.3162. [DOI] [PubMed] [Google Scholar]
- 36.Darrous L, Mounier N, Kutalik Z. Simultaneous estimation of bi-directional causal effects and heritable confounding from GWAS summary statistics. Nat. Commun. 2021;12:1–15. doi: 10.1038/s41467-021-26970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemani G, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klimentidis YC, et al. Genome-wide association study of habitual physical activity in over 377,000 UK biobank participants identifies multiple variants including CADM2 and APOE. Int. J. Obes. 2018;42:1161–1176. doi: 10.1038/s41366-018-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JJ, et al. Gene discovery and polygenic prediction from a 1.1-million-person GWAS of educational attainment. Nat. Genet. 2018;50:1112. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies G, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trampush JW, et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: A report from the COGENT consortium. Mol. Psychiatry. 2017;22:336–345. doi: 10.1038/mp.2016.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies G, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N= 112 151) Mol. Psychiatry. 2016;21:758–767. doi: 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatry Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch BM, Bringard A, Ferretti G, Schwartz S, Iglói K. Effect of cerebral vasomotion during physical exercise on associative memory, a near-infrared spectroscopy study. Neurophotonics. 2017;4:041404. doi: 10.1117/1.NPh.4.4.041404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang Y-K, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 46.Suwabe K, et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. PNAS. 2018;115:10487–10492. doi: 10.1073/pnas.1805668115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosch BM, et al. A single session of moderate intensity exercise influences memory, endocannabinoids and brain derived neurotrophic factor levels in men. Sci. Rep. 2021;11:14371. doi: 10.1038/s41598-021-93813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antunes BM, Rossi FE, Teixeira AM, Lira FS. Short-time high-intensity exercise increases peripheral BDNF in a physical fitness-dependent way in healthy men. Eur. J. Sport. Sci. 2020;20:43–50. doi: 10.1080/17461391.2019.1611929. [DOI] [PubMed] [Google Scholar]
- 49.Piepmeier AT, et al. A preliminary investigation of acute exercise intensity on memory and BDNF isoform concentrations. Eur. J. Sport. Sci. 2020;20:819–830. doi: 10.1080/17461391.2019.1660726. [DOI] [PubMed] [Google Scholar]
- 50.Rentería I, et al. Short-term high-Intensity interval training increases systemic brain-derived neurotrophic factor (BDNF) in healthy women. Eur. J. Sport Sci. 2020;20:516–524. doi: 10.1080/17461391.2019.1650120. [DOI] [PubMed] [Google Scholar]
- 51.Hildebrand M, Van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist-and hip-worn monitors. Med. Sci. Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 52.Doherty A, et al. Large scale population assessment of physical activity using wrist worn accelerometers: The UK biobank study. PLoS One. 2017;12:e0169649. doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson W, te Nijenhuis J, Bouchard TJ., Jr Still just 1 g: Consistent results from five test batteries. Intelligence. 2008;36:81–95. doi: 10.1016/j.intell.2007.06.001. [DOI] [Google Scholar]
- 55.Panizzon MS, et al. Genetic and environmental influences on general cognitive ability: Is ga valid latent construct? Intelligence. 2014;43:65–76. doi: 10.1016/j.intell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 57.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erickson KI, et al. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheval B, et al. Inhibitory control elicited by physical activity and inactivity stimuli: An EEG study. Motiv. Sci. 2021;7:386–389. doi: 10.1037/mot0000236. [DOI] [Google Scholar]
- 60.Cheval B, Boisgontier MP. The theory of effort minimization in physical activity. Exerc. Sport Sci. Rev. 2021;49:168–178. doi: 10.1249/JES.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler TW, et al. The influence of age and sex on genetic associations with adult body size and shape: A large-scale genome-wide interaction study. PLoS Genet. 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the analysis are openly available from the Neale Lab GWAS results at http://www.nealelab.is/uk-biobank and from the Social Science Genetic Association Consortium Downloads at https://www.thessgac.org/data. Only the new GWAS dataset created for the fractions of physical activity are available with permission from the UK Biobank https://www.ukbiobank.ac.uk/. The LHC-MR code is available at https://github.com/LizaDarrous/lhcMR.