Abstract
Left bronchial compression is a rarely reported, postoperative complication of the arterial switch operation with the LeCompte maneuver for transposition of the great arteries. Postoperative neopulmonary root dilatation and the anterior-posterior, anatomical relationship of the great vessels may cause this condition. Hypoxic pulmonary vasoconstriction may mask the condition even if the left bronchus has been severely obstructed. The apparent inconsistency between the abnormally decreased pulmonary blood flow and the absence of any irregularities in the vascular structure that might account for it suggested hypoxic pulmonary vasoconstriction to be the cause. We present herein a case of left bronchial compression presenting malacia after an arterial switch operation with the LeCompte maneuver and also present a review of seven, other, reported cases.
Learning objectives
Left bronchial compression is a rare complication of the arterial switch operation with the LeCompte maneuver for transposition of the great arteries and is possibly caused by root dilatation and the anatomical relationship of the great vessels. Hypoxic pulmonary vasoconstriction may mask the condition.
Keywords: Arterial switch operation with the LeCompte maneuver, Hypoxic pulmonary vasoconstriction, Left bronchial compression, Transposition of the great arteries
Introduction
The arterial switch operation with the LeCompte maneuver (ASO/L) is the most widely performed type of surgery for the treatment of transposition of the great arteries (TGA). Pulmonary stenosis, semilunar valve dysfunction, neoaortic or neopulmonary root dilatation, and coronary artery lesions are well-known, postoperative complications of ASO/L. However, respiratory problems are rarely reported as a complication. We herein report a case of left bronchial compression presenting malacia after ASO/L and discuss its etiology and key clues to its diagnosis.
Case report
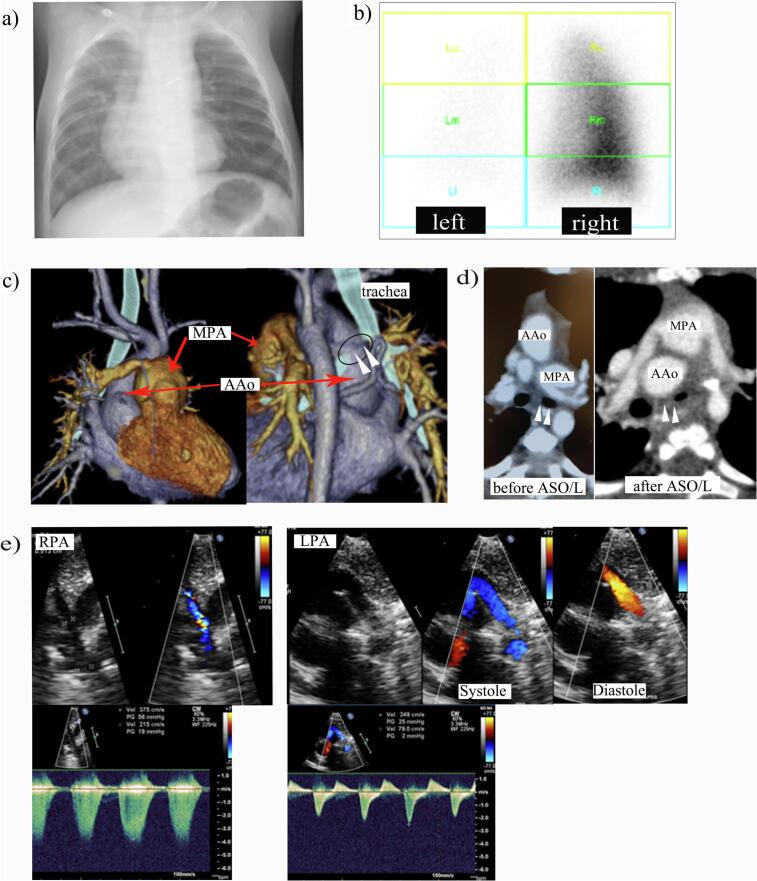
A 4-month old, male infant presented with severe desaturation at anesthetic induction for cardiac catheterization after ASO/L for TGA. He was delivered at a local obstetric clinic at 39 weeks' gestation via normal vaginal delivery with a birthweight of 2754 g. He exhibited cyanosis and was transferred to our hospital, where TGA with intact ventricular septum was diagnosed. He also had a restrictive patent foramen ovale, for which an emergency balloon atrial septostomy was performed. ASO/L was performed at postpartum day 8. Negative pressure wound therapy for a deep, sternal, wound infection was performed for one month. Chest X-ray findings at postoperative month 3 indicated decreased pulmonary blood flow in the left lung, which was confirmed by pulmonary perfusion scintigraphy (Fig. 1a, b). Meanwhile, transthoracic echocardiography led to the suspicion of right branch pulmonary artery stenosis. No stenosis was observed in the left branch. Cardiac catheterization was scheduled for postoperative month 4. The patient became desaturated after anesthesia induction with sevoflurane, nitrous oxide, and intravenous pethidine and midazolam. Bronchoscopy revealed severe left bronchial stenosis with brochomalacia caused by anterior pulsatile compression. Prominent main pulmonary artery dilatation with right branch pulmonary artery stenosis was confirmed on pulmonary arteriography. The mean left pulmonary artery pressure was 33 mmHg, while the left pulmonary artery wedge pressure was 3 mmHg (Table 1). Left bronchial compression by the ascending aorta, which appeared to have an anterior, concave curve owing to compression caused by dilatation of the main pulmonary artery, was observed on computed tomography (Fig. 1c, d). Main pulmonary artery excision and right pulmonary arterioplasty using an anterior main pulmonary artery flap with anterior aortopexy was performed at month 5 after the previous operation. The pulmonary artery configuration seemed good, and the pulmonary artery pressure was normal at cardiac catheterization two years after the resurgery. Bronchoscopy performed at the time demonstrated preservation of bronchial patency. The patient's postoperative course was uneventful, and he is now 5 years old and has no respiratory symptoms.
Fig. 1.
Diagnostic images of the present case before and after ASO/L. (a) Chest X-ray image at postoperative month 3. The vascularity of the left lung is markedly reduced. (b) Pulmonary perfusion scintigraphy of the posterior projection at postoperative month 4. The perfusion distribution was 93 % in the right lung and 7 % in the left lung. (c) Three-dimensional volume rendering with computed tomography image reconstruction at postoperative month 4. Note the prominent main pulmonary artery dilatation with branch pulmonary artery stenosis on the right side. Left bronchial compression by the ascending aorta (arrow head), which appears to curve concave anteriorly due to compression by the dilated main pulmonary artery, was observed. (d) Axial computed tomography of the present case before and after ASO/L. Note the left bronchus (arrow head) is completely patent before ASO/L but almost entirely occluded after the procedure. (e) Echocardiographic image at postoperative month 4. Note the ‘to and fro’ flow of the left pulmonary artery (found on retrospective inspection of the imaging findings after re-operation), which was not seen on the right side.
AAo, ascending aorta; ASO/L, arterial switch operation with the LeCompte maneuver; LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery.
Table 1.
Summary of the hemodynamic values measured by cardiac catheterization at pre and post re-operation in the present case.
| Pressures (mmHg) |
||
|---|---|---|
| Pre-reoperation | Post-reoperation | |
| MPA (sys. / dia. / mean) | 54 / 14 / 30 | 33 / 9 / 18 |
| RPA (sys. / dia. / mean) | 25 / 17 / 21 | 19 / 8 / 14 |
| Right PAW | 9 | 8 |
| LPA (sys. / dia. / mean) | 57/ 14 / 33 | 21 / 11/ 15 |
| Left PAW | 3 | 9 |
| RV (sys. / ED) | 66 / 11 | 35 / 7 |
| LV (sys. / ED) | 61 / 5 | 71 / 10 |
| PVRI (WU·m2) | ||
| Pre-reoperation | Post-reoperation | |
| RPA | 4.1 | 3.7 |
| LPA | 137 | 4.3 |
dia, diastolic; ED, end diastolic; LPA, left pulmonary artery; MPA, main pulmonary artery; PAW, pulmonary arterial wedge; PVRI, pulmonary vascular resistance index; RPA, right pulmonary artery; sys, systolic; WU, wood unit.
Discussion
Left bronchial compression after ASO/L for TGA is rarely reported and may account for about 1 % of all postoperative complications [1], [2]. Table 2 shows a summary of eight cases of left bronchial compression after ASO/L for TGA reported in detail to date, including the present case [3], [4], [5], [6], [7]. The median age of respiratory symptom onset was 6.8 weeks after ASO/L. None of the patients experienced symptoms in the early postoperative period. Half the patients had a severe respiratory problem and were intubated at symptom onset. All the patients required intervention.
Table 2.
Summary of the eight cases of left bronchial compression after ASO/L for TGA reported in detail to date.
| Pt. No | Sex | Diagn-osis | Age at ASO/L | Age at respiratory symptoms onset | Respiratory symptom at onset | Treatment | Age at treatment | Ref. No |
|---|---|---|---|---|---|---|---|---|
| 1 | NM | TGA + VSD | 4 d | 3 mos | Stridor and delay of air entry | Anterior and posterior aortopexies | 16 mos | [3] |
| 2 | NM | TGA + IVS | 11 d | 5 wks | Respiratory arrest → intubated | Dissection | 8 wks | [4] |
| 3 | M | TGA + IVS | 5 d | 16 wks | Respiratory arrest → intubated | Dissection | 13 mos | [4] |
| 4 | M | TGA + IVS | 9 d | 6 wks | Stridor and wheezing | Dissection with anterior and posterior aortopexies | 14 wks | [4] |
| 5 | M | TGA + IVS | 10 d | 3 mos | Stridor and wheezing | Planning for dissection and aortopexy | NM | [5] |
| 6 | M | TGA + VSD | 9 d | 1 mo | Severe dyspnea → intubated | MPA excision and reconstruction with bovine pericardium | 2 mos | [6] |
| 7 | M | TGA + IVS | 6 d | 1 mo | Respiratory arrest → intubated | SI for trachea and left bronchus (#) | 1 mo | [7] |
| Present case | M | TGA + IVS | 8 d | 4 mos | Poor ventilation at anesthesia | MPA excision and reconstruction, RPA reconstruction, anterior aortopexy | 5 mos |
ASO/L, arterial switch operation with the LeCompte maneuver; d, days; IVS, intact ventricular septum; M, male; mo, month; MPA, main pulmonary artery; NM, not mentioned; RPA, right pulmonary artery; SI, stent insertion; TGA, transposition of the great arteries; VSD, ventricular septal defect; wk., week.
#. Extrinsic tracheal compression due to innominate artery was also present. Innominate artery suspension was attempted but resulted in a hemorrhage during dissection of adhesions. Further surgical attempts were avoided.
The anatomical relationship between the great vessels and neopulmonary or neoaortic root dilatation may be important clues of the cause of left bronchial compression. Some authors have stated that posterior displacement of the ascending aorta behind the neopulmonary root during the LeCompte maneuver may impinge upon the left main bronchus and lead to stenosis [3], [4]. Other authors have stated that aneurysmal dilatation of the neopulmonary root compresses the aorta backward, compressing the left main bronchus [6]. These authors stated that neopulmonary root dilatation may become exacerbated through using a fresh pericardial patch to reconstruct the root after harvesting the coronary arteries. There are several, possible reasons which may explain the compression of the left main bronchus in the present case. First, the right branch pulmonary artery stenosis has increased the pressure in the neopulmonary root, resulting in aneurysmal dilatation. Second, the pressure in the main pulmonary artery was comparable to that in the aorta. Third, the anterior-posterior relationship of the great vessels may have limited the space for their projection, thereby affecting the posterior left main bronchus. Fourth, the main pulmonary artery was possibly short from the beginning, pulling the neopulmonary root more posteriorly than usual after translocation. The combination of these mechanisms might have led to the compression of the left main bronchus.
The discrepancy between the results of the quantitative evaluation of the pulmonary flow and pulmonary vasculature suggested the involvement of hypoxic pulmonary vasoconstriction (HPV), which raised the index of suspicion for unilateral lobar hypoventilation. HPV is an important mechanism which matches alveolar ventilation and perfusion via vascular smooth muscle contraction in alveolar areas with less ventilation and decreased partial pressure of oxygen. If this compensatory mechanism fails to operate, the desaturated pulmonary flow enters the circulatory system without receiving oxygenation via the alveoli, thus causing a drop in the level of arterial oxygen saturation. Inhaled anesthetic agent attenuates this reflex, as might have been the case in the present patient [8]. Chest X-rays and pulmonary perfusion scintigraphy indicated the presence of hypoperfusion while the echocardiographic and angiographic findings denied stenosis on the ipsilateral side. The apparent inconsistency between these features was caused by HPV. Moreover, retrospective inspection of the echocardiographic images confirmed to and fro pulmonary artery flow on the affected side but not on the contralateral side possibly as a result of high vascular resistance induced by HPV, itself caused by hypoventilation (Fig. 1e).
In conclusion, neopulmonary root dilatation after ASO/L and the anterior-posterior anatomical relationship of the great vessels may be major, contributory factors of left bronchial compression in TGA. The lack of congruence between the pulmonary hypoperfusion and the normal ipsilateral vascular structure with a to and fro pulmonary artery flow suggested the involvement of HPV and raised suspicion of ipsilateral hypoventilation. More data from future studies are needed to verify the conclusions of this report.
Acknowledgments
Acknowledgment
The authors thank Mr. James R. Valera for his assistance with editing.
Conflict of interest
The authors have indicated they have no potential conflicts of interest to disclose.
Author's contributions
The first draft of the manuscript was written by NF, and all the authors commented on its previous versions. All the authors have read and approved the final manuscript.
Ethics approval
The present report was approved by the ethics committee of Tokyo Metropolitan Children's Medical Center.
Consent to publication
The patient's parents have consented to the publication of the details of this case.
References
- 1.Chiu I.S., Lee M.L., Huang S.C., Chang C.I., Chen Y.S., Wu M.H., Anderson R.H. The concept of the arch window in the spiral switch of the great arteries. Pediatr Cardiol. 2016;37:1153–1161. doi: 10.1007/s00246-016-1412-9. [DOI] [PubMed] [Google Scholar]
- 2.Haas F., Wottke M., Poppert H., Meisner H. Long-term survival and functional follow-up in patients after the arterial switch operation. Ann Thorac Surg. 1999;68:1692–1697. doi: 10.1016/s0003-4975(99)01039-5. [DOI] [PubMed] [Google Scholar]
- 3.Worsey J., Pham S.M., Newman B., Park S.C., del Nido P.J. Left main bronchus compression after arterial switch for transposition. Ann Thorac Surg. 1994;57:1320–1322. doi: 10.1016/0003-4975(94)91384-6. [DOI] [PubMed] [Google Scholar]
- 4.Robotin M.C., Bruniaux J., Serraf A., Uva M.S., Roussin R., Lacour-Gayet F., Planché C. Unusual forms of tracheobronchial compression in infants with congenital heart disease. J Thorac Cardiovasc Surg. 1996;112:415–423. doi: 10.1016/S0022-5223(96)70269-6. [DOI] [PubMed] [Google Scholar]
- 5.Toker A., Tireli E., Bostanci K., Ozcan V., Dayioğlu E. Uncommon complication of arterial switch operation: tracheobronchial compression. Ann Thorac Surg. 2000;69:927–929. doi: 10.1016/s0003-4975(99)01400-9. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Y., Su W., Li Y., Dong N. Pulmonary artery aneurysm compressing the tracheobronchial tree following an arterial switch operation. J Card Surg. 2016;31:106–109. doi: 10.1111/jocs.12686. [DOI] [PubMed] [Google Scholar]
- 7.Bugmann P., Rouge J.C., Berner M., Friedli B., Le Coultre C. Use of gianturco Z stents in the treatment of vascular compression of the tracheobronchial tree in childhood. A feasible solution when surgery fails. Chest. 1994;106:1580–1582. doi: 10.1378/chest.106.5.1580. [DOI] [PubMed] [Google Scholar]
- 8.Lumb A.B., Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122:932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]