Novel Teaching Points.
-
•
Several options are available for alternative access for TAVR.
-
•
Transaxillary TAVR with a previous David procedure has not been described.
-
•
In patients with previous aortic root surgery, TAVR is a safe option.
-
•
Consideration of aortic graft size and type is required for proper TAVR sizing.
-
•
Coronary height must be considered for valve selection and placement.
Transcatheter aortic valve replacement (TAVR) is a suitable alternative to surgical aortic valve replacement in specific patient populations. The transfemoral approach is the one most commonly used in TAVR, although several alternatives are possible when the transfemoral approach is contraindicated. TAVR is infrequently used in patients with previous valve-sparing aortic root replacements, and the use of alternate access in these patients is rarely described, given the increased procedural complexity. We describe the case of a 75-year-old with a previous David procedure undergoing transaxillary TAVR. This report demonstrates that TAVR is safe and effective even in patients with previous valve-sparing aortic root surgery.
TAVR has been demonstrated to be a safe and suitable alternative to surgical aortic valve replacement in specific patient populations. TAVR has recently been classified as a class Ia indication in patients with severe, symptomatic aortic stenosis (AS) aged between 65 and 80 years.1 The most commonly utilized approach in TAVR is the transfemoral (TF) approach. However, several alternatives are available for cases in which the TF approach is contraindicated—as it is in up to 20% of TAVR patients, owing to peripheral arterial disease or small iliofemoral arteries. These alternatives include the transapical, transaortic, transcarotid, transcaval, transsubclavian, and transaxillary approaches.2, 3, 4, 5, 6 Although alternative approaches are increasingly common, they still account for only a fraction of the total number of TAVR cases. Additionally, description of alternative TAVR approaches in the context of previous aortic surgery is limited. Herein, we describe the case of a 75-year-old man with a previous David procedure who was undergoing transaxillary TAVR. To our knowledge, this report is the first of this procedure being performed in a patient with previous valve-sparing root surgery.
Case Presentation
A 75-year-old man with a David procedure 22 years prior presented to the hospital for melena and underwent urgent endoscopy. This procedure was complicated by hypoxemia and congestive heart failure. The patient continued to experience shortness of breath, and after stabilization, the patient underwent further investigation and was subsequently diagnosed with severe AS, to which his symptoms and heart failure were attributed.
Past medical history
The patient’s pertinent past medical history included a history of atrial fibrillation, hypertension, dyslipidemia, previous cerebrovascular accident, an upper gastrointestinal bleed, and chronic obstructive pulmonary disease. His surgical history included a valve-sparing aortic root replacement (David procedure) and appendectomy. At the time of transaxillary TAVR, the patient was on dual antiplatelet therapy with aspirin and clopidogrel, in addition to amlodipine, perindopril/indapamide, rosuvastatin, and several other medications for unrelated conditions.
Investigations
Severe AS was diagnosed on echocardiography, based on an aortic valve area of 0.9 cm2, a peak gradient of 65.9 mm Hg, and a mean gradient of 31.4 mm Hg. Echocardiography also demonstrated trivial aortic valve regurgitation, mild concentric left ventricular hypertrophy with normal function, and otherwise normal heart structure and function. Preoperative coronary angiography demonstrated no significant lesions. Electrocardiography found sinus rhythm with supraventricular bigeminy.
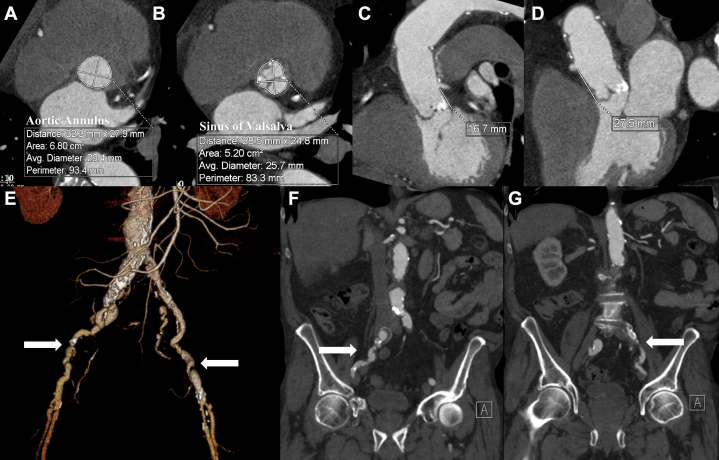
Preoperative computed tomography (CT) demonstrated the following: calcified AS; a 26-mm prosthetic straight graft in the ascending aorta; reimplanted coronary arteries with the left coronary 16.7 mm, and the right coronary 27.5 mm, above the annulus; aortic annulus measuring 32.3 x 27.9 mm; sinus of Valsalva measuring 28.5 x 24.8 mm; extensive iliac artery atherosclerosis with severe external iliac artery stenoses bilaterally; minimal symmetrical iliac artery tortuosity; and focal ectasia right common iliac artery (Fig. 1, A-G). These results precluded TF TAVR, as the iliac artery anatomy was not suitable for a TF approach. Review of the preoperative CT identified an annulus that would accommodate a 29-mm valve, but the patient’s straight aortic graft measured 26 mm. To prevent oversizing the valve for the graft, a 26-mm Edwards Sapien S3 (Edwards Lifesciences, Irvine, CA) was chosen; additional reasons for this choice were experience at our centre, lower paravalvular leak risk, and previous procedural success.
Figure 1.
Preoperative computerized tomography scans with measurements taken at (A) the aortic annulus, at 32.3 x 27.9 mm, and a valve area of 6.80 cm2; (B) the sinus of Valsalva within the aortic graft at 28.5 x 24.8 mm and an area of 5.20 cm2; (C) left coronary height at 16.7 mm; (D) right coronary height at 27.5 mm; and (E-G) bilateral iliac stenosis and tortuosity. Measurements of the peripheral vessels at the time of the scans were as follows: 5 mm, left and right common iliac; 2 mm, left and right external iliac; and 7 mm, left and right common femoral arteries.
Management
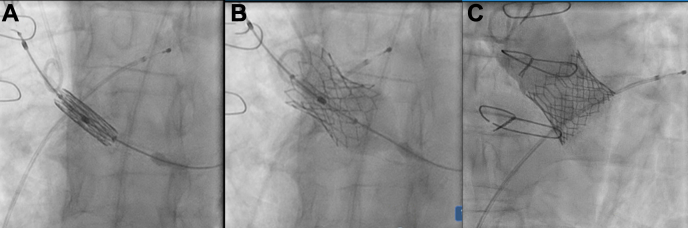
Due to the significant surgical risk, based on the patient’s age and previous aortic surgery, the patient opted for TAVR. As preoperative CT contraindicated TF TAVR, transaxillary TAVR was chosen due to its previously demonstrated safety and efficacy. Under conscious sedation, the right radial artery was accessed with a 6-F sheath, and the right femoral vein was accessed with a 7-F sheath. The left axillary artery was accessed with direct cutdown. In this procedure, the incision was first landmarked using the distal third of the clavicle, where a transverse infraclavicular incision was made approximately 2 cm below the clavicle, medial and parallel to the deltopectoral groove. The lateral border of the pectoralis major muscle was identified and moved medially; the cephalic vein was identified and pushed laterally. Dissection continues until the identification of pectoralis minor muscle that was divided, and the tissue posterior to the pectoralis minor was dissected until the brachial plexus was identified. Depending on the location of the brachial plexus, it is dissected and pulled either inferiorly or superiorly. In this case, it was pulled inferiorly. Care must be taken during dissection to identify and avoid injuring the lateral, medial, and posterior cords of the brachial plexus. Once the axillary artery was identified and exposed, proximal and distal control could be gained, and a purse-string was then placed with a 5-0 prolene suture. With the Seldinger technique, the artery was accessed with a short 0.035-inch wire, followed by a 6-F Avanti sheath (Cordis, Miami Lakes, FL), followed by the placement of an exchange length 0.035-J tipped catheter from the axillary artery into the aortic root. An AL1 catheter (Medtronic, Dublin, Ireland) was then placed over the 0.035-wire, and the exchange length 0.035-J wire was substituted with the manually preshaped 0.035-inch Lunderquist Super-Stiff wire (Cook Medical, Bloomington, IN) to cross the stenotic aortic valve. Once it is crossed with the straight wire, the AL1 wire was crossed into the left ventricle, the straight wire is removed, and an exchange length Lunderquist extra stiff, double curl wire (Cook Medical, Bloomington, IN) was placed into the left ventricle. A 14-F sheath (Edwards Lifesciences, Irvine, CA) was positioned across the left axillary artery into the ascending aorta. A 26-mm Edwards Sapien S3 valve was advanced into position and was visualized using the 3-nadir view. Care was taken to ensure appropriate alignment of the TAVR and native valve. Under fluoroscopic guidance, with right ventricular pacing at 160 beats per minute, the valve was deployed (Fig. 2). Valve position was confirmed with angiography with no paravalvular leak. The Perclose (Abbott Laboratories, Chicago, IL) sutures placed in the femoral vein were deployed, and left-sided sheaths were left in situ. Hemostasis was achieved, and the patient was transferred to the cardiac care unit in a hemodynamically stable state.
Figure 2.
Intraoperative x-ray images showing the valve stent positioned in the aortic root (A) before deployment, (B) during expansion of the valve, and (C) seated in the aortic annulus post-deployment.
Follow-up
Postoperative echocardiography demonstrated successful TAVR with mild paravalvular regurgitation and a mean gradient of 8.5mm Hg. The patient had significant bruising around the lateral left chest and arm and was held for an additional night, owing to an acute kidney injury, improving the following day. The patient was discharged on postoperative day 2 in stable condition at baseline. Follow-up was organized with the family physician and TAVR team. Three months postoperatively, the patient is alive and well at home, demonstrating the safety of the transaxillary approach, even among patients with previous aortic root surgery.
Discussion
TAVR has become increasingly common in recent years and has even become the standard of care for certain populations with AS. TF TAVR is the most common approach, although up to 20% of the population has contraindications to the TF approach.2 Given the relatively high rates of comorbidities and the increased rates of peripheral arterial disease among patients undergoing TAVR, alternative approaches are becoming increasingly common, and the transaxillary approach has been demonstrated to be safe and effective.
The use of transaxillary TAVR in patients with previous aortic surgery is relatively underdocumented. To our knowledge, this case is the first of transaxillary TAVR in a patient with a previous David procedure. Transaxillary TAVR requires careful consideration and preoperative planning. Patency of the upper branch vessels must be evaluated and is of paramount importance, given that patients being considered for alternate TAVR approaches often have some form of peripheral arterial disease. Additionally, transaxillary TAVR often requires cutdown to the axillary artery, dissection or division of the pectoralis minor muscle, and dissection near the brachial plexus, which requires an understanding of anatomy to avoid complications and significant postoperative morbidity. The limited literature describing alternate-access TAVR in higher-risk patients or those with previous cardiac surgery may result in slow uptake of alternative approaches and limited TAVR use in these cases.
In the case of patients who have had previous aortic root surgery, several factors must be considered before TAVR is undertaken. First, the graft size and shape must be considered. In this case, a 26-mm aortic straight graft was present, limiting the size of the TAVR valve that could be implanted safely. A Valsalva graft may reduce size restriction due to aortic graft size. Coronary height is of great importance, given that the coronaries are reimplanted during an aortic root replacement. In this case, the coronary heights were sufficiently far from the annulus to reduce concern about this issue. When coronary heights are low, options to prevent coronary obstruction include the following: attempting commissural alignment with the CoreValve Evolut valve (Medtronic, Dublin, Ireland); using a valve with a lower frame height, such as the Sapien S3; using TAVR valves with larger stent cell sizes; lower implantation of the valve; and consideration of surgical aortic valve replacement.7
Identification of suitable alternatives for a variety of potential cases is imperative given the increasing prevalence of TAVR and aortic valve disease, especially in patients with previous cardiac surgery. Patients with previous aortic root surgery of are particular interest, given the limited literature demonstrating the safety of TAVR in this patient population. This case report demonstrates that TAVR is safe and effective, even in patients with previous valve-sparing aortic root surgery, and should be considered in cases in which surgical replacement is considered high risk.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The subject of this report provided written informed consent to publish his case.
See page 236 for disclosure information.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Overtchouk P., Modine T. Alternate access for TAVI: Stay clear of the chest. Interven Cardiol. 2018;13:145–150. doi: 10.15420/icr.2018.22.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bapat V., Tang G.H.L. Axillary/subclavian transcatheter aortic valve replacement. JACC Cardiovasc Interven. 2019;12:670–672. doi: 10.1016/j.jcin.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Dahle T.G., Castro N.J., Stegman B.M., et al. Supraclavicular subclavian access for Sapien transcatheter aortic valve replacement—a novel approach. J Cardiothorac Surg. 2018;13:16. doi: 10.1186/s13019-018-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damluji A.A., Murman M., Byun S., et al. Alternative access for transcatheter aortic valve replacement in older adults: a collaborative study from France and United States. Catheter Cardiovasc Interv. 2018;92:1182. doi: 10.1002/ccd.27690. [DOI] [PubMed] [Google Scholar]
- 6.Madigan M., Atoui R. Non-transfemoral access sites for transcatheter aortic valve replacement. J Thorac Dis. 2018;10:4505–4515. doi: 10.21037/jtd.2018.06.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arshi A., Yakubov S.J., Stiver K.L., et al. Overcoming the transcatheter aortic valve replacement Achilles heel: coronary re-access. Ann Cardiothorac Surg. 2020;9:468–477. doi: 10.21037/acs-2020-av-38. [DOI] [PMC free article] [PubMed] [Google Scholar]