Abstract
Background
Enhanced recovery programs (ERPs) improve outcomes, but over 20 % of patients fail ERP and the contribution of social vulnerability is unknown. This study aimed to characterize the association between social vulnerability and ERP adherence and failure.
Methods
This was a retrospective cohort study of colorectal surgery patients between 2015 and 2020 utilizing ACS-NSQIP data. Patients who failed ERP (LOS > 6 days) were compared to patients not failing ERP. The CDC's social vulnerability index (SVI) was used to assess social vulnerability.
Result
273 of 1191 patients (22.9 %) failed ERP. SVI was a significant predictor of ERP failure (OR 4.6, 95 % CI 1.3–16.8) among those with >70 % ERP component adherence. SVI scores were significantly higher among patients non-adherent with 3 key ERP components: preoperative block (0.58 vs. 0.51, p < 0.01), early diet (0.57 vs. 0.52, p = 0.04) and early foley removal (0.55 vs. 0.50, p < 0.01).
Conclusions
Higher social vulnerability was associated with non-adherence to 3 key ERP components as well as ERP failure among those who were adherent with >70 % of ERP components. Social vulnerability needs to be recognized, addressed, and included in efforts to further improve ERPs.
Key message
Social vulnerability is associated with non-adherence to enhanced recovery components and ERP failure among those with high ERP adherence. Social vulnerability needs to be addressed in efforts to improve ERPs.
Keywords: Enhanced recovery, Social vulnerability, Adherence
Highlights
-
•
Social vulnerability is associated with non-adherence to enhanced recovery.
-
•
Social vulnerability is associated with ERP failure among those with high ERP adherence.
-
•
Social vulnerability needs to be addressed in efforts to improve ERPs.
Introduction
Since the 1990s, enhanced recovery programs (ERP) have proliferated across surgical specialties. The benefits of ERPs are well-documented and include reduced postoperative lengths of stay, complications, and pain in addition to earlier mobilization and faster return of bowel function [[1], [2], [3], [4], [5], [6]]. ERPs have also been shown to reduce racial and socioeconomic disparities in surgical outcomes such as length-of-stay (LOS) [1,2,[4], [5], [6], [7], [8], [9]]. Given these many benefits of ERPs, it is important to understand who still ‘fails’ ERPs and to identify the driving forces behind these failures [10].
Previous work has demonstrated that preoperative factors (age, gender, anxiety, chronic pain, etc.), intraoperative factors (operative duration, blood loss, anastomosis type, stoma formation, open approach, etc.), and postoperative factors (complications, reoperation, ERP adherence, etc.) are associated with ERP failure and prolonged LOS [[11], [12], [13], [14], [15], [16], [17], [18]]. Studies have also observed racial and socioeconomic disparities in adherence to specific aspects of ERPs in colorectal surgery patients [19]. Few studies, however, have focused on understanding the mechanism(s) driving these observations - specifically how social determinants of health (SDOH) contribute to ERP adherence and ERP outcomes. This knowledge gap is important to fill as increasing evidence has implicated SDOH as key, and often neglected, contributors to health outcomes and health disparities [[20], [21], [22], [23], [24], [25]].
This gap is further compounded by the challenge in measuring SDOHs. Recently, however, social vulnerability has been recognized as a measurable predictor of adverse surgical outcomes such as complications, increased LOS, low adherence to postoperative recovery instructions, and receipt of surgery for cancer [[26], [27], [28], [29], [30]]. To measure social vulnerability, the Social Vulnerability Index (SVI) was originally developed by the Centers for Disease Control and Prevention to assess the vulnerability of populations to environmental stressors [31]. The SVI captures key components of patients' socioecological environment including socioeconomic status, household composition and disability, minority status and language, and housing and transportation. As such, it allows for a comprehensive area-level assessment of specific SDOHs that may affect surgical outcomes and disparities. Given the role ERPs play in improving surgical outcomes and reducing disparities, understanding the role of social vulnerability in determining ERP component adherence and failure has important implications in further improving ERPs.
Therefore, the goal of this study was to characterize the role SVI plays in determining ERP failure and ERP component adherence among colorectal surgery patients. This knowledge may provide targets to enhance the quality of current ERPs, reduce ERP failure rates, and further optimize outcomes for all patients. We hypothesized that increasing SVI (meaning more socially vulnerable patients) would be associated with lower ERP component adherence. Additionally, given the known impact of ERP component adherence on ERP success, we hypothesized that increasing SVI would also be associated with increased rates of ERP failure among both ERP adherent and non-adherent patients.
Methods
Study design
This study is a retrospective cohort analysis of colorectal surgery patients at The University of Alabama at Birmingham (UAB), a tertiary referral center serving a diverse patient population. The study protocol was reviewed and approved by the UAB Institutional Review Board (IRB -140,304,007). The ERP pathway was implemented at UAB in December of 2014 and is consistent with the ERP Society Guidelines for colon and rectal surgery [32]. Adherence with 10 individual ERP components (Table 2) is also collected prospectively at the institution, in tandem with traditional demographic and clinical variables used by the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP).
Table 2.
ERP component adherence, ERP failure status among component adherent population and mean SVI among components adherent population.
| ERP components | Overall component adherence (n, %) | Adherence rates by ERP failure status |
SVI by adherence status |
||||
|---|---|---|---|---|---|---|---|
| ERP non-failure (n, %) | ERP failure (n, %) | p value | Mean (SD) SVI among adherent | Mean (SD) SVI among nonadherent | p value | ||
| Preoperative education | 245 (20.6) | 201 (21.9) | 44 (16.1) | 0.04 | 0.50 (0.28) | 0.53 (0.3) | 0.10 |
| Preoperative bowel prep | 1013 (85.1) | 794 (86.5) | 219 (80.2) | 0.01 | 0.53 (0.3) | 0.52 (0.3) | 0.82 |
| Preoperative block | 945 (79.4) | 744 (81.1) | 201 (73.6) | <0.01 | 0.51 (0.3) | 0.58 (0.2) | <0.01 |
| Regular diet by post-op day (POD) 1 | 994 (83.5) | 811 (88.3) | 183 (67.0) | <0.01 | 0.52 (0.3) | 0.57 (0.3) | 0.04 |
| Early (POD 1) mobilization | 471 (39.6) | 395 (43.1) | 76 (27.9) | <0.01 | 0.51 (0.3) | 0.53 (0.3) | 0.17 |
| Foley removal by POD 2 | 668 (56.1) | 562 (61.2) | 106 (38.8) | <0.01 | 0.50 (0.3) | 0.55 (0.3) | <0.01 |
| Any DVT ppx by POD 1 (mechanical or chemical) | 1164 (97.7) | 902 (98.3) | 262 (95.9) | 0.03 | 0.53 (0.3) | 0.52 (0.3) | 0.89 |
| Receipt of multimodal pain meds | 944 (79.3) | 735 (80.1) | 209 (76.6) | 0.21 | 0.52 (0.3) | 0.54 (0.3) | 0.32 |
| Maintenance of normothermia | 1069 (89.8) | 842 (91.7) | 227 (83.2) | <0.01 | 0.53 (0.3) | 0.52 (0.3) | 0.95 |
| Chemical DVT prophylaxis by POD 1 | 965 (81.0) | 760 (82.8) | 205 (75.1) | <0.01 | 0.52 (0.3) | 0.53 (0.3) | 0.99 |
| >70 % ERP component adherence | 967 (81.2) | 836 (91.1) | 131 (48.0) | <0.01 | 0.51 (0.3) | 0.59 (0.3) | <0.01 |
| Mean % ERP component adherence (SD) | 78.7 % (16.4) | 83.1 % (13.3) | 63.9 % (17.4) | <0.01 | NA | NA | NA |
Bold font represents significant p values <0.05.
Patient population and data sources
The study population included all patients undergoing colorectal surgery with ERP at UAB between 2015 and 2020 and captured in a prospectively maintained institutional ACS-NSQIP database. All patients were older than 18 years of age and underwent major elective colorectal operations as identified by CPT codes. Due to a small sample size (n = 21), patients with races other than white or black (i.e. American Indian/Alaska native n = 5, Asian n = 11, and unknown n = 5) were excluded. Patient- and procedure-level variables were obtained from the UAB ACS-NSQIP colorectal registry and included all ACS-NSQIP recommended variables (e.g. preoperative wound infection noted either preoperatively or intraoperatively at intended surgical site).
The 2018 SVI is a composite score constructed from 15 variables in the 2014–2018 American Community Survey aggregated to Census tracts and grouped into 4 domains: Socioeconomic Status, Household Composition and Disability, Minority Status and Language, and Housing and Transportation. Variables are ranked across U.S. Census tracts, and a percentile rank is calculated for each Census tract. The resulting index ranges from 0 to 1, with higher values indicating higher vulnerability. The full methodologic details of the creation of the SVI are available elsewhere [33]. We used the 2018 SVI dataset in our analysis as it represented a midpoint of our study population and had not undergone significant changes to census tract geography (as with the 2020 version).
Participant addresses were matched to Census tract codes using a 2018 address to census tract geocoding tool provided by the Census Bureau [34]. Records were then linked by Census tract to the 2018 Social Vulnerability Index (SVI) scores obtained from a publicly available dataset [35]. The median overall and subtheme vulnerability measures of the census tracts linked to each patient's address were utilized for the described analyses. Patients with missing or PO box addresses (N = 199) were excluded from the adjusted analysis as no census tract was able to be assigned to these patients.
Measures and analysis
The primary outcome was ERP failure defined as hospital length-of-stay in the top quartile of all patients undergoing colorectal surgery with ERP at UAB (>6 days in this population). This definition was based on previous literature definitions related to prolonged length-of-stay [10,[13], [14], [15],17,18] and ERP failure. The secondary outcome was ERP component adherence which was defined as adherence with >70 % (>7/10) of the recorded ERP components (Table 2). The primary exposure was overall vulnerability score (SVI) used as a continuous variable.
Bivariate comparisons were made using Chi-square, t-tests, and Wilcoxon Rank Sums tests where appropriate. Multivariable logistic regression models for the primary outcome (ERP failure) were constructed starting with all covariates found significantly different between the ERP failure groups on bivariate analysis. The covariates considered for initial model inclusion included demographic variables (age, gender, race), comorbidities (preoperative dyspnea, dialysis dependence, preoperative wound infection, steroid use, and preoperative weight loss), procedure type, operative approach (open vs. minimally invasive), overall ERP adherence, and overall SVI score.
After removing covariates with significant missing values as well as those not statistically significant or clinically relevant, final covariates included in the model were age, race, gender, preoperative wound infection, dialysis dependence, dyspnea, steroid use, procedure type, operative approach, ERP adherence >70 %, and overall SVI score. We also chose to include an interaction factor between ERP adherence >70 % and SVI in the model to determine whether the effect on SVI on length-of-stay varied by ERP adherence. Additionally, we created separate models stratified by ERP adherence. Preoperative weight loss was not included in the model due to a significant amount of missing data (40 %) and limited overall incidence (n = 22, 3.1 %). Robust standard errors were used in modeling to account for clustering of participants by census tract.
As a sensitivity analysis, and to account for any potential confounding between our exposure of interest (SVI) and outcome (ERP failure) due to complication status and type of procedure (i.e. open or APR/exenteration), a similar multivariate logistic regression model was constructed using only patients with no postoperative complications (n = 939) and patients with only minimally invasive extended or partial colectomies (n = 761) (Table 4). Finally, the secondary outcome of ERP adherence was analyzed using multivariate logistic regression models with the outcome of >70 % ERP component adherence. For all models, only patients with complete clinical data were included. Data was analyzed using R [36] and SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was assessed at p-value <0.05.
Table 4.
Sensitivity analysis: multivariable logistic regression models for ERP failure in patients with no complications (n = 939) and no open or APR procedures (n = 761): Overall and stratified by ERP adherence.
| Outcome: ERP failure | Overall |
Stratified models |
|
|---|---|---|---|
| ERP adherent |
ERP non-adherent |
||
| OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | |
| Overall model: SVI score (per 1 unit increase) | 1.3 (0.5–3.6) | 4.6 (1.3–16.8) | 1.1 (0.4–3.0) |
| n | 819 | 532 | 280 |
| Model with no complicationsa: SVI score (per 1 unit increase) | 1.1 (0.3–4.3) | 2.7 (0.6–11.9) | 0.8 (0.2–3.5) |
| n | 651 | 458 | 186 |
| Model with no open or APR proceduresb: SVI score (per 1 unit increase) | 0.6 (0.2–2.1) | 3.2 (0.8–14.9) | 0.6 (0.2–2.2) |
| n | 640 | 455 | 185 |
Bold font indicates covariate significantly associated with increase (or decrease) in odds of ERP failure.
No complications model adjusted for age, gender, race, dyspnea, dialysis, steroids, procedure type, operative approach, and ERP adherence status.
No APR or open procedures model adjusted for age, gender, race, dyspnea, steroid, ERP adherence.
Results
The analytic sample included 1191 patients with a median age of 58 (IQR 47.5–68.5) years, 55 % females, 24.7 % Black race, and 80 % of the sample undergoing partial or extended colectomy. Median overall SVI score for the sample was 0.5 (IQR 0.2–0.7), with higher vulnerability scores in the Household Composition and Disability theme (median 0.6, IQR 0.3–0.8) and lower vulnerability scores in the Minority Status and Language (median 0.4, IQR 0.2–0.6) and Housing and Transportation themes (median 0.4, IQR 0.2–0.7). Participant characteristics and procedure types, overall and by ERP status, are presented in Table 1. As defined a priori, nearly a quarter of the sample (n = 273, 22.9 %) failed ERP and had the highest quartile length-of-stay.
Table 1.
Participant characteristics, procedure types and outcomes, by ERP status (N = 1191).
| Characteristics | Overall (N = 1191) | ERP non-failure (N = 918) | ERP failure (N = 273) | p-Value |
|---|---|---|---|---|
| Age, median (Q1, Q3) | 58.4 (47.5–68.5) | 58.4 (47.6–68.3) | 58.8 (46.7–69.1) | 0.75 |
| Gender, n (%) | <0.01 | |||
| Female | 663 (55.7) | 530 (57.7) | 133 (48.7) | |
| Male | 528 (44.3) | 388 (42.3) | 140 (51.3) | |
| Race, n (%) | <0.01 | |||
| Black | 294 (24.7) | 202 (22.0) | 92 (33.7) | |
| White | 897 (75.3) | 716 (78.0) | 181 (66.3) | |
| SVI, median (Q1, Q3) | ||||
| Overall score | 0.5 (0.2–0.7) | 0.5 (0.2–0.7) | 0.6 (0.3–0.8) | <0.01 |
| Theme 1: Socioeconomic status | 0.6 (0.3–0.8) | 0.5 (0.2–0.8) | 0.7 (0.3–0.8) | <0.01 |
| Theme 2: Household composition and disability | 0.6 (0.3–0.8) | 0.6 (0.3–0.8) | 0.7 (0.4–0.9) | 0.01 |
| Theme 3: Minority status and language | 0.4 (0.2–0.6) | 0.4 (0.2–0.6) | 0.4 (0.2–0.6) | 0.29 |
| Subtheme: limited English proficiency, mean (SD) | 0.3 (0.24) | 0.27 (0.24) | 0.26 (0.25) | 0.71 |
| Theme 4: Housing and transportation | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.5 (0.3–0.7) | 0.01 |
| Independent functional health status (n, %) | 1185 (99.5) | 915 (99.7) | 270 (98.9) | 0.11 |
| COPD (n, %) | 40 (3.4) | 27 (2.9) | 13 (4.8) | 0.14 |
| Preop wound infection (n, %) | 9 (0.8) | 2 (0.2) | 7 (2.6) | <0.01 |
| Diabetes mellitus, n (%) | 170 (14.3) | 119 (13.0) | 51 (18.6) | 0.06 |
| Smoking, n (%) | 192 (16.1) | 139 (15.1) | 53 (19.4) | 0.09 |
| Dyspnea on moderate exertion, n (%) | 123 (10.3) | 76 (8.3) | 47 (17.2) | <0.01 |
| CHF, n (%) | 5 (0.4) | 2 (0.2) | 3 (1.1) | 0.05 |
| Dialysis dependent, n (%) | 11 (0.9) | 1 (0.1) | 10 (3.7) | <0.01 |
| Cancer, n (%) | 55 (4.6) | 42 (4.6) | 13 (4.8) | 0.9 |
| Steroid Use, n (%) | 203 (17.0) | 145 (15.8) | 58 (21.2) | 0.04 |
| Preop loss of >10 % body weight, n (%) | 22 (3.1) | 9 (1.7) | 13 (7.5) | <0.01 |
| Bleeding disorder, n (%) | 30 (2.5) | 19 (2.1) | 11 (4.0) | 0.07 |
| Operative approach, n (%) | <0.01 | |||
| Open | 280 (28.4) | 167 (22.1) | 113 (48.9) | |
| Minimally invasive (laparoscopic or robotic) | 705 (71.6) | 587 (77.9) | 118 (51.1) | |
| Procedure, n (%) | <0.01 | |||
| APR/exenteration | 187 (15.7) | 122 (13.3) | 65 (23.8) | |
| Extended colectomy | 508 (42.7) | 402 (43.8) | 106 (38.8) | |
| Partial colectomy | 450 (37.8) | 349 (38.0) | 101 (37.0) | |
| Other | 46 (3.9) | 45 (4.9) | 1 (0.4) | |
| HLOS, median (Q1, Q3) | 4 (3.0–6.0) | 3 (2.0–4.0) | 10 (8.0–16.0) | <0.01 |
| Readmission, n (%) | 152 (12.8) | 108 (11.8) | 44 (16.1) | 0.06 |
| Any complication, n (%) | 252 (21.2) | 121 (13.2) | 131 (47.9) | <0.01 |
Bold font represents significant p values <0.05.
Demographic factors associated with ERP failure included male gender (51.3 % vs. 42.3 % in the ERP non-failure group, p < 0.01) and Black race (33.7 % vs. 22.1 % in the ERP non-failure group, p < 0.01). Preoperative clinical characteristics associated with ERP failure included dyspnea (17.2 % vs. 8.3 % in the ERP non-failure group, p < 0.01), dialysis dependence (3.1 % vs. 0.1 % in the ERP non-failure group, p < 0.01), steroid use (21.3 % vs. 15.8 % in the ERP non-failure group, p = 0.04), and preoperative weight loss >10 % of total body weight (7.5 % vs. 1.7 % in the ERP non-failure group, p < 0.01). In terms of procedures, the ERP failure group had more open approaches (49 % vs. 22 % in the ERP non-failure group, p < 0.01), APR/exenteration (23.8 % vs. 13.3 % in the ERP non-failure group, p < 0.01) and fewer extended colectomy procedures (38.8 % vs. 43.8 % in the ERP non-failure group, p < 0.01) (Table 1).
Table 1 and Appendix 1 present postoperative clinical characteristics of the sample by ERP status. As defined, median hospital length-of-stay was 10 days (IQR 8–16) in the ERP failure group and 3 days (IQR 2–4) ERP non-failure group. Postoperative complications were more frequent within the ERP failure group (47 % vs. 13 %, p < 0.01). Specific complications higher in the ERP failure group included readmission (16 % vs. 11 %, p = 0.06), renal failure (1.1 % vs. 0.1 %, p = 0.01), acute kidney injury (2.6 % vs. 0.4 % p < 0.01), organ space infection (15.8 % vs. 3.8 %, p < 0.01), superficial infection (6.6 % vs. 2.5 %, p < 0.01), sepsis (3.7 % vs. 1.2 %, p < 0.01), transfusion requirement (17.6 % vs. 4.0 %, p < 0.01), and venous thromboembolism (2.6 % vs. 0.2 %, p < 0.01).
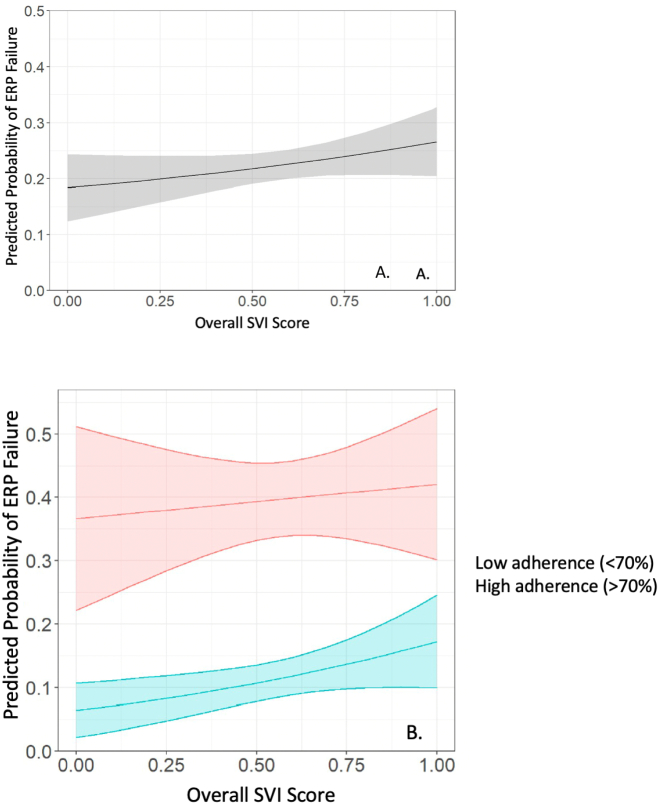
There were significant differences in ERP failure overall by social vulnerability. The median overall SVI score (0.6 vs. 0.5, p < 0.01) and three theme scores (socioeconomic status median 0.7 vs. 0.5, household composition and disability median 0.7 vs. 0.6, and housing and transportation median 0.5 vs. 0.4, all p0.01) were all significantly higher in the ERP failure group compared to the non-failure group (Table 1). The predicted probability of ERP failure by overall SVI scores is shown in Fig. 1. When stratifying by ERP adherence, we observed that among those who were adherent with ERP, higher SVI score was associated with increased odds of ERP failure (SVI 1.0 vs. 0.0: OR 4.6, 95 % CI 1.3–16.8). This relationship was not seen among those who were non-adherent with ERP (SVI 1.0 vs. 0.0: OR 1.1, 95 % CI 0.4–3.0) with non-adherent patients experiencing higher odds of ERP failure unaffected by SVI status.
Fig. 1.
Predicted probability of ERP failure by SVI score overall (A) and by SVI score and ERP adherence status (B).
ERP component adherence was assessed for 10 individual ERP components (Table 2). Mean ERP adherence rate was 78.7 %, with 81.2 %% of the cohort achieving >70 % ERP component adherence. Compared to the ERP non-failure group, adherence rates in the ERP failure group were significantly lower for 9 of the 10 measured ERP components. ERP component adherence of >70 % was also significantly lower in the ERP failure group compared to the ERP non-failure group (48.0 % vs. 91.1 %, p < 0.01). There were significant differences in social vulnerability by ERP adherence. Specifically, overall SVI scores were higher among those who were not compliant with preoperative block (p < 0.01), foley catheterization removal by POD 2 (p < 0.01) and early diet initiation (p = 0.04). Additionally, higher SVI was seen among those adherent with <70 % of individual ERP components compared to those adherent with >70 % ERP components (p < 0.01).
Multivariable logistic regression models were constructed to assess factors associated with SVI and with ERP failure (Table 3). In the overall model, we found that SVI was not associated with ERP failure. Factors associated with increased odds of ERP failure included Black race, preoperative dialysis, preoperative steroid use, open operative approach, and APR/Exenteration procedures. When models were stratified by ERP component adherence >70 %, we found that SVI was associated with ERP failure (OR 4.6, 95 % CI 1.3–16.8) for the ERP adherent group but was not associated with ERP failure in the ERP non-adherent ground (OR 1.1, 95 % CI 0.4–3.0). Alternatively, Black race did not remain a significant predictor of ERP failure among those who were ERP adherent (OR 1.0, 95 % CI 0.5–2.1), but was associated with increased odds of ERP failure among those non-adherent to ERP (OR 2.0, 95 % CI 1.1–3.6).
Table 3.
Multivariable logistic regression models for ERP failure: Overall and stratified by ERP adherence.
| Outcome: ERP failure | Overall |
Stratified models |
|
|---|---|---|---|
| ERP adherent |
ERP non-adherent |
||
| OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | |
| Age | 1.0 (0.99–1.0) | 0.98 (0.9–1.0) | 1.02 (1.0–1.04) |
| Male gender | 1.2 (0.8–1.8) | 1.2 (0.7–2.3) | 1.2 (0.7–2.0) |
| Black race | 1.6 (1.0–2.5) | 1.0 (0.5–2.1) | 2.0 (1.1–3.6) |
| Preop wound infection | 5.9 (0.7–144) | NA | NA |
| Preop dyspnea with moderate exertion | 1.6 (0.9–2.9) | 2.3 (0.8–5.8) | 1.4 (0.7–2.9) |
| Preop dialysis | 14.9 (2.0–318) | NA | 7.7 (1.1–156.7) |
| Preop steroids | 1.8 (1.0–3.0) | 1.8 (0.8–3.8) | 1.7 (0.9–3.5) |
| Procedure | |||
| Other vs. partial colectomy | 0.1 (0.0–0.6) | 0.4 (0.02–2.8) | NA |
| Extended vs. partial colectomy | 1.1 (0.7–1.7) | 1.2 (0.6–2.4) | 1.0 (0.6–1.9) |
| APR/exenteration vs. partial colectomy | 1.8 (1.0–3.1) | 2.3 (0.9–5.5) | 1.6 (0.8–3.2) |
| Open operative approach (vs. MIS) | 2.5 (1.7–3.7) | 2.7 (1.4–5.2) | 2.7 (1.6–4.5) |
| SVI score (per 1 unit increase) | 1.3 (0.5–3.6) | 4.6 (1.3–16.8) | 1.1 (0.4–3.0) |
| ERP adherent | 0.1 (0.04–0.3) | – | – |
| SVI × ERP adherence interaction | 2.6 (0.6–11.8) | – | – |
| n | 819 | 532 | 280 |
Bold font indicates covariate significantly associated with increase (or decrease) in odds of ERP failure.
Additionally, a model was constructed to assess factors associated with ERP failure only among those with no postoperative complications (n = 939, Table 4) and those undergoing only minimally invasive partial or extended colectomies (n = 761, Table 4). Among those with no complications, SVI was not significantly associated with ERP failure (OR 1.1, 95 % CI 0.3–34.3), and on stratification by ERP adherence status, increasing SVI did not appear to have any effect on ERP failure rates in either the ERP adherent or non-adherent groups (Table 4). Additionally, after excluding those undergoing open surgeries or APR/exenteration procedures increasing SVI was not significantly associated with odds of ERP failure either overall (OR 0.6, 95 % CI 0.2–2.1) or when stratified by ERP adherence status (Table 4).
Finally, a model was constructed assessing factors associated with the outcome of >70 % ERP component adherence (or adherence to >7/10 measured ERP components). Factors significantly associated with decreased odds of >70 % ERP component adherence included preoperative dyspnea with moderate exertion (OR 0.5, 95 % CI 0.3–0.9), preoperative dialysis (OR 0.1, 95 % CI 0.00–0.54), APR/Exenteration procedure (OR 0.5, 95 % CI 0.3–0.8), open operative approach (OR 0.4, 95 % CI 0.3–0.5). Black race (OR 0.7, 95 % CI 0.5–0.98) and increasing SVI (OR 0.5, 95 % CI 0.3–0.95) (Table 5).
Table 5.
Multivariable logistic regression models for >70 % ERP component adherence.
| Outcome: >70 % ERP component adherence | Overall |
|---|---|
| OR (95 % CI) | |
| Age | 1.0 (0.98–1.0) |
| Male gender | 0.9 (0.6–1.2) |
| Black race | 0.7 (0.5–0.9) |
| Preop dyspnea with moderate exertion | 0.6 (0.4–0.9) |
| Preop dialysis | 0.1 (0.00–0.5) |
| Preop steroids | 0.6 (0.4–1.0) |
| Procedure | |
| Other vs. partial colectomy | 2.1 (0.8–6.2) |
| \Extended vs. partial colectomy | 1.0 (0.7–1.4) |
| APR/Exenteration vs. partial colectomy | 0.5 (0.3–0.8) |
| Open operative approach (vs. MIS) | 0.4 (0.3–0.5) |
| SVI score (per 1 unit increase) | 0.5 (0.3–0.99) |
| Preoperative wound infection | 0.3 (0.01–2.0) |
| n | 819 |
Bold font indicates covariate significantly associated with increase (or decrease) in odds of ERP failure.
Discussion
This study demonstrated that higher social vulnerability was associated with non-adherence to three key elements of ERPs (preoperative block, early foley removal, and early diet initiation) and that even among those who achieved >70 % ERP adherence, higher social vulnerability was associated with increased odds of ERP failure. Increasing SVI not only impacted ERP failure among those expected to benefit the most from ERPs, but also was associated with the mechanism whereby ERP benefits surgical patients, ERP component adherence. This study represents one of the first attempts to establish the role that social determinants of health, including social vulnerability, play in ERPs and demonstrates a novel association between social vulnerability and ERP adherence and failure.
Previous studies have shown that a range of traditional pre, intra, and post-operative factors are associated with increased risk of ERP failure [10]. Our results are similar to previous investigations showing that surgical complications [[11], [12], [13],18] and procedures like an APR (a technically complicated case with longer operative duration [11,13,15,17]) are associated with increased risk of ERP failure. Many studies have also reported that increased blood loss [13,[15], [16], [17], [18]] is associated with increased risk of ERP failure. Operative blood loss was not collected in this analysis; however, our findings demonstrated that the need for postoperative transfusion is associated with increased risk of ERP failure. Additionally, preoperative dyspnea and weight loss were found to be predictors of ERP failure, a finding in line with the study done by Renz et al. who documented that lower preoperative albumin is associated with increased ERP failure [16]. These findings underscore the need for attention to preoperative functional and nutritional status, and optimization as necessary, as recommended by ERAS society guidelines [32]. However, the question of who fails ERP also needs to move beyond traditional clinical factors. Social determinants of health play a known role in health outcomes [[26], [27], [28], [29]] and as our study demonstrates, also appear to have a previously unrecognized and important role in ERP adherence and failure.
Previous literature has demonstrated that the benefits of ERP are dependent on adherence to individual components, and these adherence rates vary considerably [37,38]. Additionally, our study showed that SVI was associated with overall >70 % ERP component adherence, and vulnerable patients were more likely to be non-adherent with key ERP components such as receipt of a preoperative block, early foley removal, and early diet initiation. Therefore, a portion of the effect of social vulnerability on ERP failure may relate to ERP process adherence. As such, a multidisciplinary, individualized preoperative social vulnerability assessment using the SVI could guide attention toward patients of greatest risk and could serve to link vulnerable patients with existing social services. This approach could be tailored to disparity populations (i.e. pairing a patient with high vulnerability in the transportation sub theme with transportation assistance programs etc.) to reduce health disparities by improving ERP component adherence, ERP success, and surgical outcomes in those most vulnerable.
In this study, we also demonstrated a significant and novel association between social vulnerability and ERP failure rates. Specifically, those with high vulnerability in areas of Socioeconomic Status, Household Composition and Disability, and Housing and Transportation had higher rates of ERP failure. Furthermore, higher social vulnerability was specifically associated with increased odds of ERP failure among those adherent with >70 % ERP components. Conversely, increasing social vulnerability did not affect the odds of ERP failure among those non-adherent to ERP processes. This finding highlights the importance of ERP component adherence and the impact non-adherence has on length-of-stay and ERP success, regardless of social vulnerability. This suggests that while improving ERP adherence is important to improve outcomes in socially vulnerable populations, additional efforts to improve outcomes beyond ERP adherence are necessary.
Finally, while previous work has demonstrated reductions in racial disparities following implementation of ERP [[1], [2], [3], [4], [5],7] findings from this study add an important caveat to this body of work. Namely, the association of race on ERP failure may depend on ERP process adherence, and that among those adherent to >70 % of individual ERP components, no racial disparities in odds of ERP failure were observed. However, among those non-adherent to ERP components racial disparities remained with the odds of ERP failure significantly impacted by patient race. Additionally, while the effects of SVI on ERP failure were most pronounced among those adherent to ERP, this effect was diminished among those patients with no complications and those undergoing minimally invasive partial or extended colectomies. This also points to a potentially important interaction between social vulnerability, postoperative complications, and procedure type that should be the subject of future research work.
Our study adds to the growing body of work showing that social vulnerability is associated with worse surgical outcomes [27], including in the field of colorectal surgery [26,30]. While no previous studies have focused on the relationship between SVI and ERP component adherence, prior studies have linked lower socioeconomic status and minority race with decreased ERP component adherence [19,38]. Previous studies have also demonstrated important reductions in healthcare disparities following ERP implementation [1,2,4,5,7], however our findings suggest that there is an additional need for ERPs to be improved to have a greater impact on these disparities. To advance our understanding of this association between SVI and ERP adherence and failure, further work is necessary to characterize a range of SDOH at multiple socioecological levels (i.e. individual, interpersonal, community, organizational and policy levels) using comprehensive tools.
This study has several limitations. First, no intraoperative data was captured or included in this analysis. However, procedure type and postoperative transfusion requirement were included, and both were associated with increased risk of ERP failure. These factors, while not exact, would be expected to approximate the variables of operative time and blood loss which have previously been shown to be associated with increased risk of ERP failure. Second, using length-of-stay as the primary outcome and defining measure of ERP failure does not account for many patient reported factors that are important in surgical care. However, it has been used routinely in previous literature in this field and represents a strong composite measure of outcomes that are important to patients, physicians, and hospitals. Third, rates of preoperative education were relatively low in this group (<25 % documented as receiving preoperative education). These findings have led to an institutional review of both the processes of delivering and recording preoperative education with a resulting increase in the percentage of patients having documented preoperative education. This work will be highlighted in future studies by this group.
Fourth, this study only included Black or white patients and did not specifically assess patients' English-speaking ability. While there were no significant differences by ERP failure status in levels of English proficiency as recorded by the SVI, these populations deserve future dedicated study to optimize ERP use among these potentially vulnerable patients. Fifth, 17 % of SVI data were not available, due to both missing patient address data and the inability to assign a Census tract to a PO box address. While this has the potential to introduce selection bias, the distribution of obtained SVI values was representative of the area served by the institution, with approximately 30 % of patients classified as living in a high vulnerability Census tract. Finally, SVI represents the average vulnerability of the area within which a patient lives and may not accurately capture social vulnerability at the individual patient level. Further work is needed to develop and administer instruments to collect comprehensive measures of social determinants of health at an individual level within this population.
Conclusion
Social vulnerability may affect both ERP process adherence, as well as clinical outcomes among those who are adherent to ERPs. Social vulnerability needs to be recognized, included, and targeted in efforts to further improve ERPs. A preoperative assessment of an individual's social vulnerability within each domain could lead to targeted interventions aimed at modifying these specific social determinants of health and reducing their impact on surgical care and recovery. For example, identifying low cost or free transportation for those with high vulnerability in the transportation subtheme, facilitating easy access to necessary translator services for those with high vulnerability in the language subtheme, or even proactively pairing patients with high vulnerability in the socioeconomic status subtheme with necessary low cost or free prescription medication or home medical equipment may prove beneficial in the care of high vulnerability ERP patients. Finally, unmeasured social determinants of health likely impact ERP adherence and success as well, and focused, prospective assessment of these (largely unstudied) domains will be vital in understanding their impact on colorectal surgical care and disparities.
Ethical approval statement
The study protocol was reviewed and approved by the UAB Institutional Review Board (IRB -140304007).
Funding sources
DIC supported in part by K12 HS023009 (2017–2019), K23 MD013903 (2019–2022), and R01 MD013858 (2020–2025). BPS supported in part by UAB Surgical Oncology T32 (T32 CA229102) and 2020–2022 ACS Resident Research Scholarship.
CRediT authorship contribution statement
Burkely P. Smith: Conceptualization, Investigation, Writing – original draft, Project administration. Robert H. Hollis: Conceptualization, Writing – review & editing. Connie Shao: Conceptualization, Writing – review & editing. Lauren Gleason: Conceptualization, Writing – review & editing. Lauren Wood: Formal analysis, Data curation, Methodology, Writing – review & editing. Marshall C. McLeod: Formal analysis, Data curation, Methodology, Writing – review & editing. Danielle I. Kay: Conceptualization, Writing – review & editing. Gabriela R. Oates: Conceptualization, Writing – review & editing. Maria Pisu: Conceptualization, Writing – review & editing. Daniel I. Chu: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing.
Conflicts of interest
The authors have no additional conflict of interest to disclose other than the funding sources listed below.
Appendix 1. Patient postoperative characteristics, by ERP status (N = 1191)
| Postoperative variables | Overall (N = 1191) | ERP non-failure (N = 918) | ERP failure (N = 273) | p value |
|---|---|---|---|---|
| HLOS, median (Q1, Q3) | 4 (3.0–6.0) | 3 (2.0–4.0) | 10 (8.0–16.0) | <0.01 |
| Readmission, n (%) | 152 (12.8) | 108 (11.8) | 44 (16.1) | 0.06 |
| Any complication, n (%) | 252 (21.2) | 121 (13.2) | 131 (47.9) | <0.01 |
| Renal failure, n (%) | 4 (0.3) | 1 (0.1) | 3 (1.1) | 0.01 |
| Clostridium difficile infection, n (%) | 8 (0.7) | 4 (0.4) | 4 (1.5) | 0.07 |
| Deep SSI, n (%) | 12 (1.0) | 7 (0.8) | 5 (1.8) | 0.12 |
| Myocardial infarction, n (%) | 4 (0.3) | 2 (0.2) | 2 (0.7) | 0.2 |
| Postop ventilator requirement, n (%) | 7 (0.6) | 3 (0.3) | 4 (1.5) | 0.03 |
| Organ space infection, n (%) | 78 (6.6) | 35 (3.8) | 43 (15.8) | <0.01 |
| Postop pneumonia, n (%) | 8 (0.7) | 0 (0) | 8 (2.9) | <0.01 |
| Postop acute kidney injury (AKI), n (%) | 11 (0.9) | 4 (0.4) | 7 (2.6) | <0.01 |
| Pulmonary thromboembolism (PTE), n (%) | 2 (0.2) | 2 (0.2) | 0 (0) | 0.44 |
| Postop sepsis, n (%) | 21 (1.8) | 11 (1.2) | 10 (3.7) | <0.01 |
| Superficial SSI, n (%) | 41 (3.4) | 23 (2.5) | 18 (6.6) | <0.01 |
| Postop transfusion, n (%) | 85 (7.1) | 37 (4.0) | 48 (17.6) | <0.01 |
| Intubation, n (%) | 9 (0.8) | 2 (0.2) | 7 (2.6) | <0.01 |
| Urinary tract infection (UTI), n (%) | 15 (1.3) | 11 (1.2) | 4 (1.5) | 0.73 |
| Deep venous thrombosis (DVT), n (%) | 9 (0.8) | 2 (0.2) | 7 (2.6) | <0.01 |
Bold font represents significant p values <0.05.
References:
- 1.Wahl T.S., et al. Enhanced recovery after surgery (ERAS) eliminates racial disparities in postoperative length of stay after colorectal surgery. Ann Surg. 2018;268(6):1026–1035. doi: 10.1097/SLA.0000000000002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marques I.C., Wahl T.S., Chu D.I. enhanced recovery after surgery and surgical disparities. Surg Clin North Am. 2018;98(6):1223–1232. doi: 10.1016/j.suc.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Theiss L.M., Wood T., McLeod M.C., Shao C., Santos Marques I.D., Bajpai S., Lopez E., Duong A.M., Hollis R., Morris M.S., Chu D.I. The association of health literacy and postoperative complications after colorectal surgery: a cohort study. Am J Surg. 2022;223(6):1047–1052. doi: 10.1016/j.amjsurg.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss L.E., et al. Achieving health equity in surgery through enhanced recovery after surgery (ERAS): the elimination of racial disparities in post-operative length-of-stay is sustained long-term. Clin Nutr ESPEN. 2018;25:178–179. [Google Scholar]
- 5.Rozental O., White R.S., Weinberg R. Role of adherence to enhanced recovery after surgery programs in mitigating health care disparities. JAMA Surg. 2020;155(1):91–92. doi: 10.1001/jamasurg.2019.3486. [DOI] [PubMed] [Google Scholar]
- 6.Giglia M.D., et al. Racial disparities in length-of-stay persist even with no postoperative complications. J Surg Res. 2017;214:14–22. doi: 10.1016/j.jss.2017.02.063. [DOI] [PubMed] [Google Scholar]
- 7.Theiss L.M., Chu D.I. An overview of the evidence for enhanced recovery. Semin Colon Rectal Surg. 2021;32(3) [Google Scholar]
- 8.Akinyemiju T., Meng Q., Vin-Raviv N. Race/ethnicity and socio-economic differences in colorectal cancer surgery outcomes: analysis of the nationwide inpatient sample. BMC Cancer. 2016;16(1):1–10. doi: 10.1186/s12885-016-2738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damle R.N., et al. Examination of racial disparities in the receipt of minimally invasive surgery among a national cohort of adult patients undergoing colorectal surgery. Dis Colon Rectum. 2016;59(11):1055–1062. doi: 10.1097/DCR.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 10.Sun S.-D., et al. Failure of enhanced recovery after surgery in laparoscopic colorectal surgery: a systematic review. Int J Colorectal Dis. 2020;35(6):1007–1014. doi: 10.1007/s00384-020-03600-3. [DOI] [PubMed] [Google Scholar]
- 11.Chen C.-C., et al. Is it appropriate to apply the enhanced recovery program to patients undergoing laparoscopic rectal surgery? Surg Endosc. 2011;25(5):1477–1483. doi: 10.1007/s00464-010-1417-z. [DOI] [PubMed] [Google Scholar]
- 12.Kariv Y., et al. Clinical outcomes and cost analysis of a “fast track” postoperative care pathway for ileal pouch-anal anastomosis. A case control study. Dis Colon Rectum. 2007;50(2):137–146. doi: 10.1007/s10350-006-0760-6. [DOI] [PubMed] [Google Scholar]
- 13.Keller D.S., et al. Predicting delayed discharge in a multimodal Enhanced Recovery Pathway. Am J Surg. 2017;214(4):604–609. doi: 10.1016/j.amjsurg.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Keller D.S., et al. Predicting who will fail early discharge after laparoscopic colorectal surgery with an established enhanced recovery pathway. Surg Endosc. 2014;28(1):74–79. doi: 10.1007/s00464-013-3158-2. [DOI] [PubMed] [Google Scholar]
- 15.Oh H.-K., et al. Factors associated with failure of enhanced recovery programs after laparoscopic colon cancer surgery: a single-center retrospective study. Surg Endosc. 2016;30(3):1086–1093. doi: 10.1007/s00464-015-4302-y. [DOI] [PubMed] [Google Scholar]
- 16.Renz B., et al. The CR-POSSUM risk calculator predicts failure of enhanced recovery after colorectal surgery. Acta Chir Belg. 2015;115(1):20–26. doi: 10.1080/00015458.2015.11681062. [DOI] [PubMed] [Google Scholar]
- 17.Smart N.J., et al. Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis. 2012;14(10):e727–e734. doi: 10.1111/j.1463-1318.2012.03096.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., et al. Factors associated with failure of Enhanced Recovery After Surgery (ERAS) in colorectal and gastric surgery. Scand J Gastroenterol. 2019;54(9):1124–1131. doi: 10.1080/00365521.2019.1657176. [DOI] [PubMed] [Google Scholar]
- 19.Leeds I.L., et al. Racial and socioeconomic differences manifest in process measure adherence for enhanced recovery after surgery pathway. Dis Colon Rectum. 2017;60(10):1092–1101. doi: 10.1097/DCR.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen-Bohlman L., Panzer A., Kindig D., Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine . 2004. Health literacy: a prescription to end confusion. [Google Scholar]
- 21.Paasche-Orlow M.K., Wolf M.S. The causal pathways linking health literacy to health outcomes. Am J Health Behav. 2007;31(1):S19–S26. doi: 10.5555/ajhb.2007.31.supp.S19. [DOI] [PubMed] [Google Scholar]
- 22.Pandit A.U., et al. Education, literacy, and health: mediating effects on hypertension knowledge and control. Patient Educ Couns. 2009;75(3):381–385. doi: 10.1016/j.pec.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Schillinger D., et al. Association of health literacy with diabetes outcomes. Jama. 2002;288(4):475–482. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]
- 24.Smith P.C., Brice J.H., Lee J. The relationship between functional health literacy and adherence to emergency department discharge instructions among Spanish-speaking patients. J Natl Med Assoc. 2012;104(11–12):521–527. doi: 10.1016/s0027-9684(15)30218-2. [DOI] [PubMed] [Google Scholar]
- 25.Wright J.P., et al. Association of health literacy with postoperative outcomes in patients undergoing major abdominal surgery. JAMA Surg. 2018;153(2):137–142. doi: 10.1001/jamasurg.2017.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz A., et al. County-level social vulnerability is associated with worse surgical outcomes especially among minority patients. Ann Surg. 2021;274(6):881–891. doi: 10.1097/SLA.0000000000004691. [DOI] [PubMed] [Google Scholar]
- 27.Hyer J.M., et al. High social vulnerability and “textbook outcomes” after cancer operation. J Am Coll Surg. 2021;232(4):351–359. doi: 10.1016/j.jamcollsurg.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Diaz A., et al. Intersection of social vulnerability and residential diversity: postoperative outcomes following resection of lung and colon cancer. J Surg Oncol. 2021;124(5):886–893. doi: 10.1002/jso.26588. [DOI] [PubMed] [Google Scholar]
- 29.Azap R.A., et al. Association of county-level vulnerability, patient-level race/ethnicity, and receipt of surgery for early-stage Hepatocellular Carcinoma. JAMA Surg. 2021;156(2):197–199. doi: 10.1001/jamasurg.2020.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmichael H., et al. Social vulnerability is associated with increased morbidity following colorectal surgery. Am J Surg. 2022;224(1, Part A):100–105. doi: 10.1016/j.amjsurg.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 31.ATDSR CDC SVI Documentation 2018. 2022. https://www.atsdr.cdc.gov/placeandhealth/svi/documentation/SVI_documentation_2018.html Available from: [Web Page 2/10/2022, cited 2022 5/3/2022]
- 32.Gustafsson U.O., et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2019;43(3):659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 33.CDC Social Vulnerability Index (SVI) data and documentation download. 2020. https://svi.cdc.gov/data-and-tools-download.html Available from:
- 34.Census Bureau, U.S 2022. https://geocoding.geo.census.gov/geocoder/geographies/addressbatch?form Available from: [cited 2022 5/30/2022]
- 35.Flanagan B.E., et al. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention's Social Vulnerability Index. J Environ Health. 2018;80(10):34–36. [PMC free article] [PubMed] [Google Scholar]
- 36.R: A language and environment for statistical computing. R Core Team; Vienna, Austria: 2020. [computer program] [Google Scholar]
- 37.Gustafsson U.O., et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146(5):571–577. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- 38.Ripollés-Melchor J., et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg. 2019;154(8):725–736. doi: 10.1001/jamasurg.2019.0995. [DOI] [PMC free article] [PubMed] [Google Scholar]