Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a crucial ion channel for transport of chloride and bicarbonate anions. Functional roles of CFTR have been identified in a broad range of cell types including epithelial, endothelial, immune and structural cells. While CFTR has been investigated largely in the context of inborn dysfunction in cystic fibrosis, recent evidence shows that CFTR is also affected by acquired dysfunction in COPD. In patients with COPD and smokers, CFTR impairment has been demonstrated in the upper and lower airways, sweat glands and intestines, suggesting both pulmonary and systemic defects. Cigarette smoke, a key factor in COPD development, is the major cause of acquired CFTR dysfunction. Inflammation, bacterial byproducts and reactive oxygen species can further impair CFTR expression and function. CFTR dysfunction could contribute directly to disease manifestation and progression of COPD including disturbed airway surface liquid homeostasis, airway mucus obstruction, pathogen colonisation and inflammation. Mucus plugging and neutrophilic inflammation contribute to tissue destruction, development of dysfunction at the level of the small airways and COPD progression. Acquired CFTR dysfunction in extrapulmonary organs could add to common comorbidities and the disease burden. This review explores how CFTR dysfunction may be acquired and its potential effects on patients with COPD, particularly those with chronic bronchitis. The development of CFTR potentiators and the probable benefits of CFTR potentiation to improve tissue homeostasis, reduce inflammation, improve host defence and potentially reduce remodelling in the lungs will be discussed.
Short abstract
Acquired CFTR dysfunction may increase COPD pathogenesis through CFTR impairment across multiple cell types, including epithelial, immune and structural cells. CFTR potentiation may lead to an overall improvement in lung health in patients with COPD. http://bit.ly/3VlR7S5
Introduction
The causative role of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction in cystic fibrosis (CF) is well established, but its broad biological effects may also impact patients with COPD, a chronic disease that presents with features of chronic neutrophilic inflammation and small airways mucus obstruction similar to CF [1]. Evidence of CFTR dysfunction has been observed in patients with COPD [2–7]. Moreover, carriers of certain CFTR mutations have been shown to have increased risk of chronic bronchitis and bronchiectasis (1.3-fold and 1.9-fold, respectively) [8], challenging the idea that heterozygotes for CFTR mutations are asymptomatic [9].
The CFTR gene exhibits substantial natural variability, with >2000 variants reported, including >1000 pathogenic mutations [10], and at least six mutation classes defined by the nature of the molecular defect [11]. There is evidence from CF for a logarithmic relationship between CFTR function and clinical manifestation across the spectrum of disease [12]. Disease-causing variants often have more than one molecular defect [11] and ultimately lead to CFTR channel dysfunction [13, 14]. CFTR dysfunction can be acquired; for example, as discussed in detail later, as a consequence of cigarette smoking, the primary cause of COPD [6, 7, 15, 16], which can lead to reduced CFTR protein/mRNA expression, reduced CFTR cell surface expression and even direct modifications to the channel itself [17]. Furthermore, proteases such as neutrophil elastase, released as part of the inflammatory responses seen in chronic bronchitis, also degrade CFTR protein [18, 19]. In this review, we discuss CFTR, the effects of CFTR dysfunction, how such dysfunction could be acquired in patients with COPD and the potential impact it may have on patients based on clinical and pre-clinical data.
The role of CFTR in epithelial cells
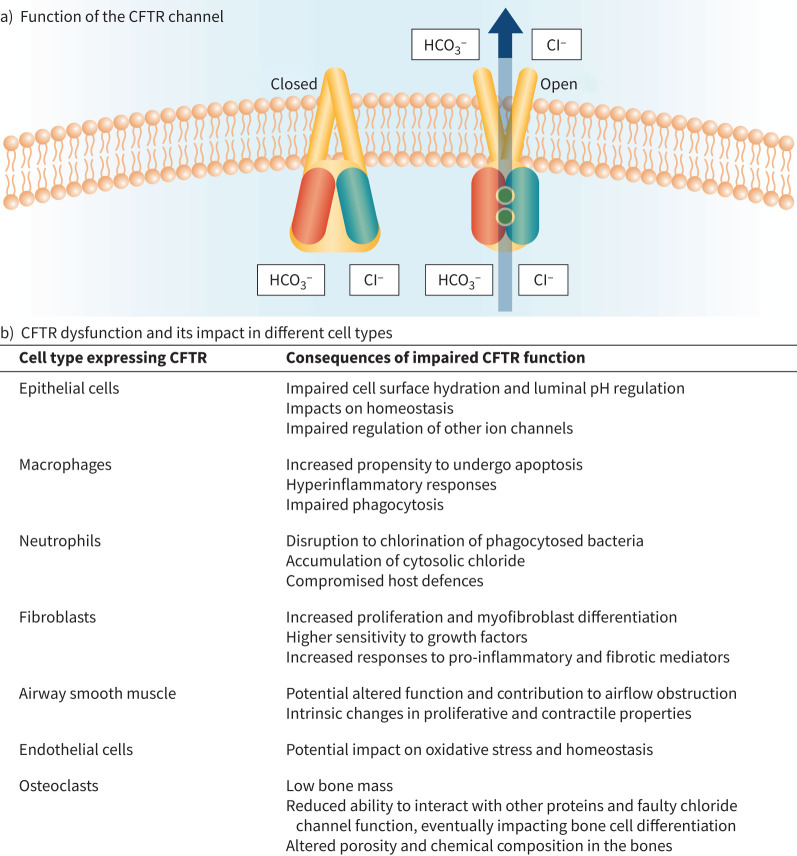
The CFTR protein resides in the apical membrane of epithelial cells [2]. It belongs to the ATP-binding cassette transporter superfamily and functions as a crucial chloride and bicarbonate anion channel involved in salt and water transport across epithelial surfaces, requiring energy to fulfil its role [20–22]. Based on total expression levels, secretory and basal cells are responsible for the majority of CFTR expression in the superficial airway epithelium [23], whereas the rare pulmonary ionocyte cell type expresses the highest levels of CFTR per cell in the conducting airway epithelium [24, 25]. Mucus is produced by airway gland cells with CFTR-expressing serous cells producing most of the fluid component of gland secretions [26]. Mucus cells themselves do not express CFTR [27], but dysfunction in serous cell fluid secretion ultimately affects mucin secretion and maturation, leading to mucus plugging within the glands [26, 28]. Overall, CFTR regulates many mechanisms in epithelial physiology, including maintenance of epithelial surface hydration and luminal pH regulation [26], making it a key contributor to lung homeostasis [29]. Figure 1a illustrates a functional CFTR channel, contributing to epithelial surface fluid regulation and bicarbonate-dependent pH regulation. The function of CFTR in epithelial cells has been discussed in detail elsewhere [17]; the aim of this review is to look beyond epithelial cells to the other potential roles of CFTR in nonepithelial cells to broaden the understanding of the impacts of acquired CFTR dysfunction.
FIGURE 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) function and expression in a variety of cell types in multiple organ systems. a) Function of the CFTR channel; b) CFTR dysfunction and its impact in different cell types. Cl−: chloride; HCO3−: bicarbonate.
The role of CFTR in in nonepithelial cells
CFTR is expressed in many cell types of nonepithelial origin [30], including immune cells, fibroblasts, keratinocytes, airway smooth muscle cells, endothelial cells and bone cells. Figure 1b summarises the different cell types in which CFTR is functionally expressed and the consequences of CFTR impairment.
Immune cells
CFTR is expressed in macrophages [31–33]. Analysis of CFTR expression, apoptosis, polarisation, phagocytosis, bacterial killing and cytokine production in human CF and non-CF peripheral blood monocyte-derived macrophages (MDMs) found that CF MDMs display decreased CFTR expression, increased apoptosis and decreased phagocytosis compared with non-CF MDMs [34]. A study in CFTR-deficient mice of the effects of CFTR-proficient haematopoietic stem cell transfer on the outcome of severe pulmonary bacterial infection provided evidence for the role of CFTR in macrophage defence: CFTR-proficient CD68+ macrophage-like cells migrated into the lungs and replaced the recipients’ CFTR-deficient macrophages, leading to a milder clinical course and higher survival following infection with Pseudomonas aeruginosa [35]. CFTR expression is associated with reduction of apoptosis; increases in CFTR expression correlate with decreased cellular apoptosis and CFTR knockout led to an increased propensity for macrophages to undergo apoptosis [36]. Interestingly, ex vivo treatment of macrophages from CF patients with CFTR modulators was able to augment phagocytosis of P. aeruginosa and reduce inflammatory status of the cells, demonstrating that macrophages are directly responsive to CFTR modulators [37]. In addition, a lack of CFTR has been found to be associated with hyperinflammatory responses [38], and treatment with CFTR modulators is associated with downregulation of inflammation [39, 40]. Macrophages are implicated in COPD pathogenesis directly through production of pro-inflammatory mediators and proteases and indirectly through defective phagocytosis of bacteria and reduced clearance of apoptotic cells [41, 42].
CFTR is expressed in neutrophils [43–45], with reduced expression observed in cells of patients with CF [43]. Neutrophils play a role in chlorination of phagocytosed bacteria, which is disrupted by CFTR channel dysfunction [44]. Additionally, suppression of CFTR function resulted in accumulation of cytosolic chloride in healthy neutrophils [43]. Activated neutrophils regulate CFTR distribution by mobilising it to the subcellular sites of activation, while neutrophils with the most common CFTR mutation, F508del, do not achieve this targeting and thus compromise host defence function [45]. In patients with CF with residual CFTR function, CFTR modulator therapy has been shown to bring about phenotypic changes in neutrophils, consistent with neutrophils becoming less activated [46]. Polymorphonuclear neutrophil (PMN)-dominated airway inflammation is a feature of CF. Compared with healthy controls, PMN counts are higher and PMN apoptosis is delayed in both those who are homozygous for CFTR mutations and heterozygotes, although it is unclear how this is modulated by CFTR [47]. Neutrophils have been implicated in many of the pathogenic effects of COPD features including emphysema and mucus hypersecretion [48], and airway neutrophilia is observed in COPD irrespective of clinical phenotype or disease severity [49].
CFTR is also expressed in B- and T-lymphocytes [50], which are a key part of lymphoid follicles frequently seen in CF [51, 52]. The role of lymphoid follicles in COPD is unclear, but association was found between the progression of COPD and the frequency of airways containing lymphoid follicles [53]; formation of lymphoid follicles is part of the molecular signature of emphysema [54].
Fibroblasts and keratinocytes
Fibroblasts are involved in the maintenance of extracellular matrix in the lungs; inappropriate recruitment and activation of fibroblasts is implicated in airway fibrosis in multiple chronic lung diseases [55]. Fibroblasts from CF mice display an altered phenotype compared with wild-type littermates, having increased proliferation and myofibroblast differentiation, higher sensitivity to growth factors and increased responses to pro-inflammatory and fibrotic mediators [56]. A CF stromal lung model based on fibroblasts from patients with CF found that fibroblasts maintain an activated pro-fibrotic state in vitro and a high proliferation rate. Furthermore, this model showed enhanced pro-fibrotic markers and upregulation of genes involved in epithelial function and inflammatory response [57]. In patients with COPD, fibroblasts demonstrate decreased capacity for sustaining tissue repair [58]. Keratinocytes impact wound healing [59–61] and are a source of matrix metalloproteinase (MMP)-9, which plays a role in matrix remodelling [62]. CFTR knockdown in mice led to delayed cutaneous wound healing with inflammation, increased keratinocyte proliferation and aberrant differentiation [60], while knockdown of CFTR in cultured human keratinocytes promoted cell migration, but inhibited differentiation. Suppressed migration with enhanced differentiation was associated with overexpression of CFTR, suggesting that CFTR helps regulate keratinocyte behaviour [59].
Airway smooth muscle
Airway smooth muscle (ASM) displays a hyperproliferative phenotype in COPD [63], and airway wall remodelling in patients with COPD features increased thickness of the ASM. Patients with CF often have symptoms typical of asthma and bronchial hyperresponsiveness, suggesting that CFTR may play a role in ASM function. Loss of CFTR alters porcine ASM function and may contribute to the airflow obstruction phenotype observed in human CF [64]. A study of ASM cells isolated from healthy donor and CF transplant lungs found that impaired CFTR function in ASM cells causes intrinsic changes in proliferative and contractile properties [65]. The CFTR modulator ivacaftor may relax smooth muscle in patients with CF, suggesting that CFTR dysfunction could cause increased smooth muscle tone in people with CF [66]. CFTR expression is greatly decreased in pulmonary artery smooth muscle in human and animal models of pulmonary hypertension [67], and radiographic evidence of pulmonary hypertension is associated with abnormal sweat chloride [68]. CFTR plays a key role in hypoxic pulmonary vasoconstriction, modulating the response to sphingolipids, which are important signal mediators of this physiological response [69].
Endothelial cells
Endothelial cell apoptosis and senescence are features of COPD [70]. Significant loss of markers of healthy endothelium has been observed in lung tissue isolated from patients with COPD. Furthermore, loss of several endothelial markers was found to correlate with reduced pulmonary function and increased emphysema [71]. CFTR is expressed by endothelial cells. Functional endothelial CFTR limits oxidative stress and contributes to the normal anti-inflammatory state of human umbilical vein endothelial cells (HUVEC) [72]. Studies on HUVEC suggest a role for CFTR in maintenance of endothelial cell homeostasis [73], while other studies suggest CFTR upregulation protects against palmitate-induced endothelial dysfunction by improving autophagic flux [74]. Although CFTR impairment downregulates endothelial cell proliferation, migration and autophagy, defective CFTR function leads to endothelial cell activation and a persisting pro-inflammatory endothelium state with increased leukocyte adhesion. CFTR modulators partially reduce the increase in endothelial cell activation markers and leukocyte adhesion shown in CF patient-derived endothelial cells [75]. Endothelial cells in human and animal models of pulmonary hypertension decrease CFTR expression [67].
Bone cells
Osteoporosis is a frequent comorbidity in patients with COPD [76]. CFTR is expressed in osteoblasts and osteoclasts at lower levels than in epithelial cells [77]. Genetic inactivation of CFTR in osteoblasts has been found to contribute to low bone mass [78]. The main effects of CFTR mutations are diminished ability to interact with other proteins and faulty chloride channel function, which ultimately influence bone cell differentiation [79]. CFTR deficiency alters porosity and chemical composition in the bones of newborn pigs [80], as well as reducing bone length, growth plate thickness, bone content and insulin-like growth factor-I in CFTR-knockout rats [81]. In patients with CF, CFTR mutation may impact NF-κB and Wnt signalling, resulting in disruption to osteoblast differentiation as well as decreased bone formation and increased bone resorption [79, 82]. Cartilage may also be affected, as ultrasounds of human tracheas showed that those in patients with CF had a greater width and were less circular in shape compared with those without CF [83].
Mechanisms of acquired CFTR dysfunction
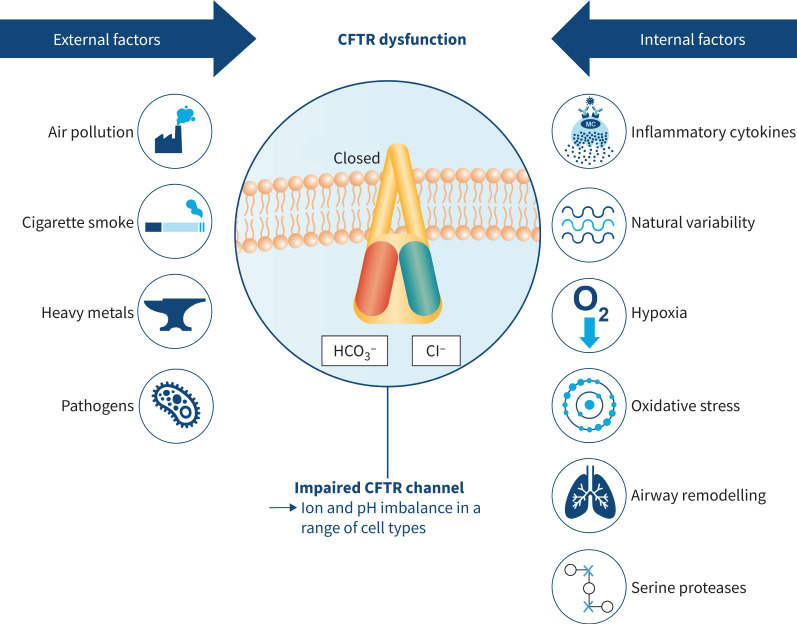
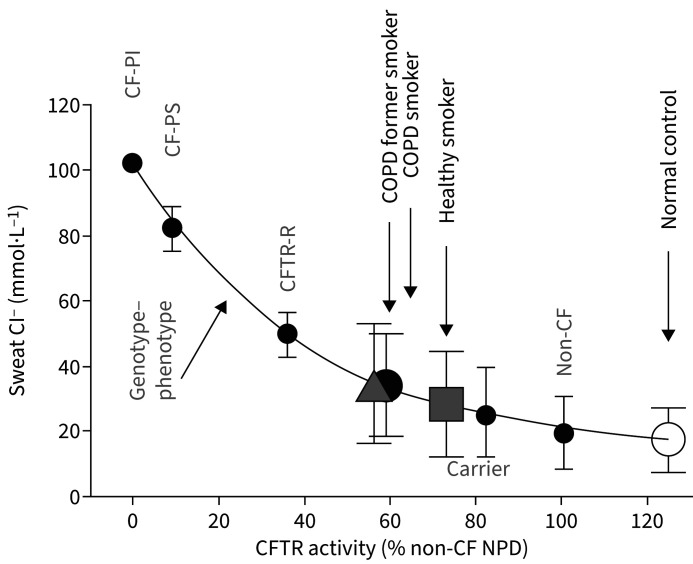
Multiple internal and external factors may lead to acquired CFTR dysfunction (figure 2) through multiple direct and indirect effects [17]. In persons without CF, there are several means by which CFTR dysfunction may develop outside of inherited genetic mutations, with cigarette smoking being the most documented [3, 5, 6, 15, 16, 84, 85]. Thus, the predominant cause of COPD is also the most common cause of acquired CFTR dysfunction. Cigarette smoke decreases the expression of the CFTR gene, thus influencing mRNA levels, protein and function in vitro. Compared with nonsmokers, acquired CFTR deficiency was observed in the nasal respiratory epithelium of cigarette smokers [15]. Cigarette smoke can directly modify specific amino acid residues in CFTR, as can acrolein, an aldehyde contained in tobacco smoke [86]. CFTR dysfunction occurs in smokers who use e-cigarettes (which produce acrolein when heated) [87]. Cigarette smoking reduces CFTR function at extrapulmonary sites, removed from direct exposure, including the intestinal epithelia (mice), sweat glands and distal rectum (humans) suggesting that acquired CFTR dysfunction is a systemic phenomenon [7]. CFTR dysfunction can be transmitted to the fetus of smoke-exposed animals [88]. Acquired CFTR dysfunction in patients with COPD persists in the lungs following smoking cessation [3]. The level of CFTR activity in patients with COPD who smoke is roughly halfway between levels of activity in control individuals who have neither COPD nor CF and patients with CF [7] (figure 3). The relationship between protein levels and function is nonlinear. Even when individuals have “half-normal” CFTR function alongside acquired dysfunction, they still have substantially worse CFTR activity than individuals who are genetic heterozygotes [89, 90]. The extent to which individuals who are heterozygotes for CFTR mutations are impacted by cigarette smoke is debated. A Danish study showed reduced fecundity in F508del heterozygote smokers [91] and it has been suggested that this could be explained by cigarette smoke inducing a further decrease in CFTR function in reproductive tissues of F508del heterozygotes [15]. In contrast, in vitro and in vivo studies have shown that CFTR mutation heterozygosity does not affect the magnitude of reduction in CFTR activity induced by cigarette smoke [92].
FIGURE 2.
Multiple internal and external factors can lead to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. O2: oxygen; Cl−: chloride; HCO3−: bicarbonate.
FIGURE 3.
Level of cystic fibrosis transmembrane conductance regulator (CFTR) function in cigarette smokers with and without COPD compared with that of patients with cystic fibrosis (CF) with various severities of CFTR mutations grouped by clinical phenotype. PI: pancreatic-insufficient; PS: pancreatic-sufficient; CFTR-R: CFTR-related disorders; Cl−: chloride; NPD: nasal potential difference. Reproduced from [7] with permission.
In addition to cigarette smoke, other air pollutants and occupational exposure to certain vapours, dusts, gases and fumes have detrimental effects in patients with COPD [93–95]. Such environmental factors can also impact CFTR. Exposure to cadmium, a component of cigarette smoke and byproduct of smelters and therefore a prevalent air pollutant, inhibits CFTR protein expression in the lungs of COPD patients [96]. Pollutants such as cigarette smoke and cadmium can upregulate certain microRNAs such as miR-101 [97], which suppress CFTR expression [98]. Exposure to environmental arsenic can induce CFTR dysfunction and bronchitis symptoms [99]. Ozone downregulates the expression and function of CFTR [100, 101]. There is conflicting evidence for the effects of treatments such as corticosteroids on CFTR function [102]: glucocorticoids in particular may reduce CFTR expression and channel activity, as shown in rats [103, 104], while cell culture studies showed that dexamethasone can rescue mutated CFTR expression through inhibition of a ubiquitin ligase [105]. It has been shown in human cell culture that dexamethasone regulates wild-type CFTR protein post-transcriptionally, enhancing maturation and expression [106], and induces SGK1, which selectively increases wild-type CFTR in the plasma membrane by inhibiting its endocytic retrieval from the membrane [107]. Additionally, dexamethasone stimulates CFTR activity through nongenomic signalling in rats [108].
Internal factors that influence CFTR function include inflammatory cytokines such as interferon-γ, which negatively affects the expression and activity of CFTR [109]. In vitro and in vivo, the protease neutrophil elastase induces CFTR degradation through the activation of intracellular calpains [18]. Localised acquired CFTR deficiency and subsequent abnormalities of fluid and electrolytes within the sinuses may be created by an oxygen-restricted environment in patients with chronic rhinosinusitis [84].
There is substantial variability of endogenous CFTR function. Variations in natural processes such as post-translational modifications (PTMs) can influence CFTR expression. PTMs and functional crosstalk between different PTMs regulate protein activity in addition to influencing protein turnover, conformation and localisation, as well as interactions with other proteins [110]. The folding state of CFTR determines how the channel is trafficked intracellularly: misfolding of CFTR prevents intracellular recycling and facilitates its degradation [111]. CFTR can also be post-transcriptionally regulated by microRNAs [112]. Some microRNAs are induced by inflammation and remodelling, perpetuating dysfunction [113, 114]. CFTR expression and biogenesis is downregulated by transforming growth factor-β1 exposure [115], potentially mediated through miR-145 [116]. There are variable degrees of CFTR function in healthy individuals without variants/mutations in the CFTR gene. This is evidenced by the large variability in CFTR function detected by in vivo measurements of nasal potential difference in healthy individuals (which partially overlaps with CF patients) [117] and studies of CFTR function in mice from different genetic backgrounds suggesting that genetic modifiers may play an important role in influencing wild-type CFTR activity [118]. This suggests potential for an interaction between host and gene-modifier environment, whereby cigarette smoke and environmental factors may have detrimental effects on non-CF individuals with low endogenous CFTR activity.
Certain aspects of COPD pathogenesis itself may contribute to CFTR dysfunction. Hypoxia has been shown to alter CFTR mRNA, protein and function in human cells, and the expression of CFTR in airways, gastrointestinal tissues and the liver was repressed in mice subjected to hypoxia in vivo [119]. Chronic infection is indelibly linked to chronic bronchitis and plays an important role in the initiation and elaboration of CFTR dysfunction. Hypoxia-inducible factor-1α is a transcriptional regulator of cellular responses to hypoxia that can upregulate the platelet-activating factor receptor (PAFR) on the airway epithelial surface, thereby facilitating infection by PAFR-dependent bacteria such as Streptococcus pneumoniae, Haemophilus influenzae and P. aeruginosa [120]. Infection of the respiratory tract may lead to CFTR dysfunction; the P. aeruginosa virulence factor Cif negatively affects CFTR expression [121]. P. aeruginosa infection or exposure to P. aeruginosa exoproducts impair CFTR proteins in non-CF airway epithelial cells [122, 123]. Treatment of P. aeruginosa cultures with a quorum-sensing inhibitor prevents the negative effect of P. aeruginosa exoproducts on wild-type CFTR [124]. Oxidative stress by pyocyanin, a redox-active virulence factor produced by P. aeruginosa, impairs CFTR chloride ion transport [125]. Viral infection influences CFTR activity, e.g. influenza matrix protein 2 decreases CFTR activity by increasing secretory organelle pH [126]. Similarly, influenza infection reduces CFTR function in murine respiratory and alveolar epithelia, which persists beyond the infection period [127]. CFTR expression is repressed by prolonged exposure to oxidative stress in human bronchial cells [128]. Dysfunctional CFTR leads to reduced transport of the natural antioxidants glutathione and thiocyanate, and can therefore trigger an imbalance between antioxidants and reactive oxygen species [129]. Oxidative stress may be a common mechanism through which many of these factors (tobacco smoke, particulate pollutants, cytokines, hypoxia) mediate loss of cell-surface CFTR. This can then become a circular feed-forward loop: the initial CFTR dysfunction leads to mucus stasis and infection with inflammation, which worsen the dysfunction, especially at the local level. Interrupting this cycle could have important implications for patients with acquired CFTR dysfunction. CFTR dysfunction may also affect lipid metabolism: abnormal essential fatty acid profiles have been observed in CF [130]. A pro-inflammatory fatty acid imbalance could further exacerbate CFTR dysfunction [129].
Role of acquired CFTR dysfunction in COPD airways
COPD is characterised by persistent airflow limitation, frequently taking the form of chronic bronchitis, where small airway obstruction and tissue damage accelerates loss of lung function and mortality [131–133]. Small airway mucus plugging is an issue for patients with the emphysema phenotype of COPD [53, 134] and contributes to the development of emphysema in patients with chronic bronchitis. The chronic bronchitis phenotype of COPD has many phenotypical features in common with CF including chronic neutrophilic airway inflammation, goblet cell metaplasia and mucin hypersecretion, mucus obstruction of the small airways and chronic bacterial infection [3, 4, 135]. The clinical consequences for the chronic bronchitis phenotype of COPD encompasses a predisposition to lower respiratory tract infection, higher exacerbation frequency, additional comorbidities and worse overall mortality [136]. Patients with COPD and chronic bronchitis have a greater exacerbation frequency, are more likely to have gastro-oesophageal reflux, allergic ocular and nasal symptoms and poor lung function than those without chronic bronchitis [137, 138]. Current smoking and increased airway wall thickness are associated with chronic bronchitis [138], a finding recapitulated in a ferret model of acquired CFTR dysfunction [139].
CFTR dysfunction leads to defective chloride and bicarbonate ion transport into the airway lumen, which results in dehydration and acidification of airway surface liquid (ASL), and production of highly viscoelastic and acidic secretions [26, 27]. Bicarbonate ion secretion helps regulate local pH [26, 140, 141] and allows mucins to unfold [142], and supports innate immunity [2]. These processes are disrupted by the lack of chloride and bicarbonate ion secretion when CFTR is dysfunctional. Moreover, as the epithelial sodium channel (ENaC) is inhibited by CFTR, and its activity is increased by exposure to cigarette smoke [143], CFTR dysfunction (and the key factor leading to its acquisition) results in increased sodium absorption via ENaC, aggravating ASL dehydration [144].
The resultant abnormal airway milieu, with impaired innate host defences, favours chronic airway infection and inflammation, leading to increased lung injury [2]. Chronic bacterial colonisation and persistent neutrophilic inflammation are features of COPD. These processes may be amplified by CFTR impairment in macrophages and neutrophils, leading to reduced bacterial killing and increased inflammatory responses [145]. In addition, debris from pathogens and extracellular DNA from apoptotic cells can entangle with mucins, causing further mucus obstruction and potentially plugging [146].
Dysfunction in CFTR contributes to disruption of the microbiome. Healthy ASL is a primary innate defence mechanism of the lungs, and the disruption of ASL homeostasis provides a suitable environment for bacterial colonisation and dysbiosis [147–149]. A heterogeneous environment is created by the changing oxygen and pH gradients; these environmental changes favour coexistence and proliferation of a wide range of microbial species [148, 150]. Proteobacteria and Actinobacteria are over-represented in the CF pulmonary microbiome [151]. There is some overlap with the sputum microbiota of patients with COPD, where Proteobacteria dominance is associated with disease severity [152]. Indeed, P. aeruginosa infections are associated with exacerbations and high mortality in patients with COPD [153]. Dysbiosis has been observed in infants and children with CF compared with those without CF, characterised by increased relative abundances of Proteobacteria, especially Escherichia coli, and decreased Clostridiales [154, 155]. Overall, the airway microbiome is less diverse in patients with CF compared with healthy individuals and the reduction in diversity is associated with increasing age, antibiotic use, lung function decline and disease progression, often leading to dominance of typical CF pathogens such as P. aeruginosa or Staphylococcus aureus [156–159]. It has been demonstrated in germ-free mice that mutated CFTR alone is sufficient to alter the microbiome [160] and there is variety in microbial representation in faecal microbiota depending on CFTR variants [161]. It has been shown that pharmacological restoration of CFTR activity led to rapid decreases in the sputum abundance of bacteria, in particular P. aeruginosa [162]. A possible link between CFTR mutations and allergic bronchopulmonary aspergillosis (ABPA), a pulmonary disorder characterised by a hypersensitivity response to the allergens of the fungus Aspergillus fumigatus, has been proposed [163], suggesting that acquired CFTR dysfunction in patients with COPD could make them more susceptible to this condition. Augmented CFTR activity in CF reduces the prevalence of Aspergillus infection [164, 165]. ABPA is increasingly recognised in COPD [166]. Aspergillus sensitisation in COPD is associated with frequent exacerbations, poor lung function and bronchiectasis [167–169].
Increased inflammation is associated with CFTR dysfunction in a probable circular fashion, with CFTR dysfunction leading to inflammation that results in worsening of the CFTR dysfunction. In patients with CF, airway inflammation is dominated by neutrophils, which produce reactive oxygen species, neutrophil extracellular traps, proteases such as neutrophil elastase, and pro-inflammatory mediators, leading to progressive tissue damage, immune cell recruitment and inflammation [145, 170]. Increased mucus viscosity affects neutrophil motility, impairing phagocytosis and consequently supporting chronic bacterial infection [132, 171]. Patients with COPD, particularly those with chronic bronchitis, already exhibit impaired airway clearance, chronic bacterial colonisation and persistent neutrophilic inflammation like patients with CF, which may be exacerbated by acquired CFTR dysfunction [3, 19, 137, 172]. Small airway disease (SAD), a key feature of COPD, is a major cause of airflow obstruction and is characterised by mucus plugging, airway remodelling and neutrophilic inflammation [173–175]. In turn, these changes may result in increased COPD exacerbations and bacterial colonisation, which may further contribute to COPD progression through increased SAD and emphysema development [175]. Patients with COPD and SAD have poorer health status, worse spirometry results and more severe lung hyperinflation than those without SAD [174, 176]. In the small airways of patients with COPD who experience frequent exacerbations, measures of SAD were associated strongly with neutrophilic inflammation [177]. Disease progression in COPD is heterogeneous and can take the form of small airway dysfunction and emphysema preceding large airway wall abnormalities or large airway wall abnormalities may precede emphysema and small airway dysfunction [178]. Acquired CFTR dysfunction may contribute to COPD pathogenesis via chronic airway inflammation, mucus hypersecretion and recurrent infections and SAD. ASM plays a pivotal role in airway remodelling in both CF [65] and COPD [179]. Airway epithelial remodelling in COPD may induce an abnormal localisation and expression of ion channel proteins such as CFTR [180]. As COPD progresses, large areas of the conducting airway are replaced by squamous epithelium with little to no CFTR expression. Cellular injury and inflammation in COPD is diverse and complex; recent single-cell RNA sequencing profiles of explanted lung tissue from patients with advanced COPD showed distinct changes in epithelial cell populations and associated CFTR expression [181]. Abnormal expression and distribution of CFTR protein has been observed in non-CF inflamed and/or remodelled airway tissues [182].
CFTR dysfunction may have important consequences for disease initiation and progression in COPD, including destruction of airway and lung parenchyma epithelial cells causing tissue remodelling, reduction of gas exchange area, impairment of lung function [183], damage to the pulmonary matrix through neutrophil elastase and matrix metalloproteins [184], and effects on contractility of ASM [185]. Additionally, dehydrated and acidified ASL and airway inflammation contribute to mucociliary dysfunction and ultimately support increased bacterial colonisation and locally amplified neutrophilic inflammation. Many of these pathways have the potential to further perpetuate CFTR dysfunction.
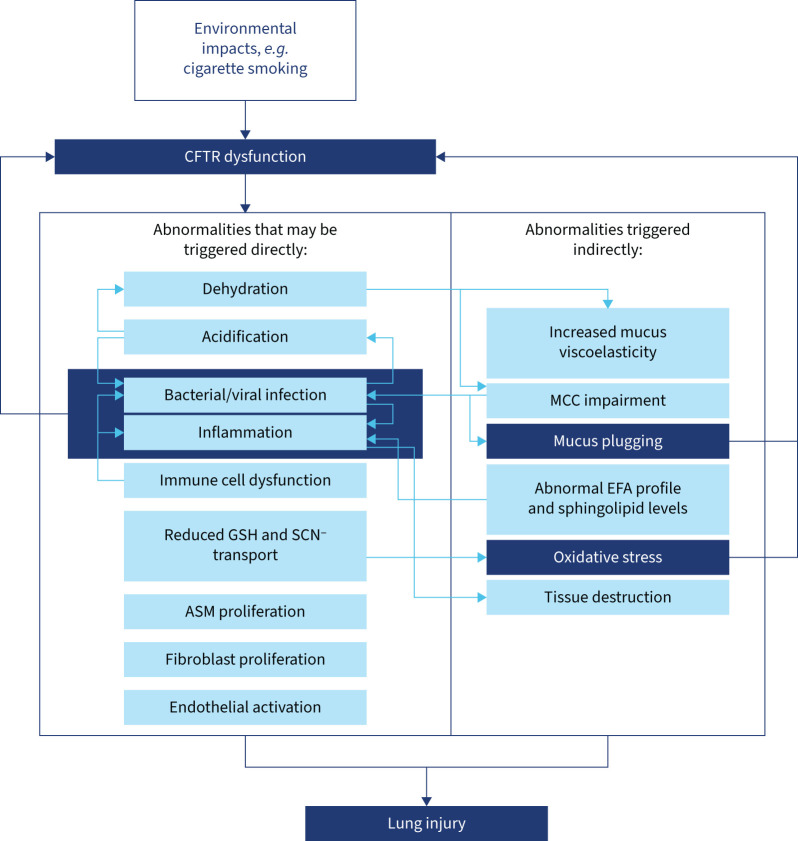
Figure 4 illustrates different aspects of CFTR dysfunction in the lungs. The ASL is acidified through reduced CFTR-mediated bicarbonate ion transport, which leads to altered mucus properties and attracts pathogen colonisation. In addition, dehydration of the ASL impairs mucociliary clearance. Furthermore, CFTR impairment in immune cells involved in pathogen defence leads to reduced bacterial killing, increased apoptosis and inflammatory responses [145]. Together, these effects lead to increased pathogen colonisation and subsequent immunological effects (increased inflammasome activity). The pathogen metabolism can further acidify the ASL, which feeds back into reduced bacterial clearance. Table 1 summarises the multiple consequences of CFTR impairment in the body.
FIGURE 4.
Interplay of multiple impacts of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction in the lungs. GSH: glutathione; SCN–: thiocyanate; ASM: airway smooth muscle; MCC: mucociliary clearance; EFA: essential fatty acid.
TABLE 1.
Multifaceted roles of cystic fibrosis transmembrane conductance regulator (CFTR) impairment in the body
| Epithelial homeostasis | CFTR dysfunction acidifies ASL by limiting bicarbonate secretion [26] CFTR dysfunction dehydrates ASL by reduced anion secretion and increased sodium absorption via ENaC [144] ASL acidification and dehydration contributes to impaired MCC and consequential mucus overload and plugging [17] Suboptimal pH for antimicrobial proteins leads to weakened host defence [140] |
| Bacterial infection | CFTR dysfunction in phagocytes impairs bacterial defence [34] pH alteration fuels bacterial colonisation [140] Impaired MCC facilitates accumulation of bacteria and biofilm formation [148] Imbalance of microbiome [151, 160] |
| Inflammatory status | CFTR dysfunction in macrophages leads to increased release of inflammatory cytokines (e.g. IL-6 and IL-8) [42] Impaired CFTR affects neutrophil function, and can lead to overexuberant pro-inflammatory responses to acute insults such as LPS [145] Bacterial exoproducts trigger inflammasome activation and release of IL-8 [145] |
| Lung remodelling | Direct effects of CFTR dysfunction on specific cells and downstream effects of airway dehydration and mucus accumulation [65] Remodelling itself may impact CFTR expression [180] |
| Extrapulmonary comorbidities | CFTR dysfunction is systemic in smokers [7] CFTR dysfunction has been associated with multisystem morbidities, which worsen COPD outcomes, such as GORD, sinusitis, diabetes and bone fractures [208] |
ASL: airway surface liquid; ENaC: epithelial sodium channel; MCC: mucociliary clearance; IL: interleukin; LPS: lipopolysaccharide; GORD: gastro-oesophageal reflux disease.
Development of CFTR potentiators for COPD with chronic bronchitis
Development of CFTR modulator therapies to improve CFTR function systemically represents a significant step in the treatment of patients with CF [10, 144, 186–188]. These new therapies include CFTR potentiators, which increase the flow of ions through activated CFTR channels on the cell surface [189]. Potentiators augment CFTR activity in the presence of endogenous stimuli, in contrast to CFTR activators which stimulate CFTR function irrespective of the surrounding physiologic situation [189]. Potentiators act on multiple mutant forms of CFTR with channel gating defects [190] and can also increase wild-type CFTR activity [16, 189]. The benefits of CFTR potentiation include augmented airway surface hydration, improved mucus rheology, accelerated mucus clearance, reduced inflammatory burden and improved bacterial clearance; CFTR potentiators have good safety profiles and are well tolerated [86, 169, 191, 192]. Furthermore, there is evidence that the systemic impacts of CF are ameliorated by CFTR potentiators in the gastrointestinal tract [193], pancreas [194, 195], sweat ducts [196] and bones [197, 198]. CFTR activity is augmented by increasing cAMP-mediated activation using the phosphodiesterase-4 inhibitor roflumilast. Roflumilast improves ion transport in cell culture studies and smoke-exposed mice, and consequently increases ASL depth in epithelial cells [192, 199]; this may also explain why roflumilast has been shown to be helpful in COPD patients who specifically exhibit chronic bronchitis [200]. Taken together, CFTR potentiators or other CFTR activators may therefore represent a viable therapeutic option for patients with COPD of both chronic bronchitis and emphysema phenotypes. Improving CFTR function in patients with COPD has the potential to improve lung health, facilitate mucus clearance and reduce bacterial colonisation in patients with COPD. Patients with COPD with predominant chronic bronchitis (i.e. cough and sputum overproduction) who continue to experience exacerbations and symptoms despite inhaled therapies are expected to benefit the most with CFTR potentiation. CFTR potentiation has been shown to be effective and well tolerated in CF patients, even with chronic use [196]. Given the potential role of CFTR dysfunction in other airway diseases such as asthma, bronchiectasis and ABPA, CFTR modulators could also prove to be a therapy option beyond CF and COPD [201].
Ivacaftor is approved for treatment of patients with CF with specific CFTR mutations [202]. It is a potentiator of CFTR chloride channel function, facilitating increased chloride transport by potentiating channel-open probability (or gating) of the surface-localised CFTR protein [189]. Ivacaftor potentiates mutant CFTR forms with defects in channel gating caused by CFTR gating (class III) mutations in addition to normal CFTR and F508del-CFTR in in vitro studies [203]. It increases channel opening time by stabilising the open state of the CFTR [204]. Ivacaftor restores CFTR-dependent chloride transport in cigarette smoke exposed epithelial cells and improves mucociliary transport, ASL depth and ciliary beating in vitro, demonstrating the potential of CFTR modulators to address pathological airway physiology evident in acquired CFTR dysfunction [16, 86]. In a pilot study, ivacaftor augmented measures of CFTR function and clinical symptoms in patients with COPD and chronic bronchitis [202]. Ivacaftor improves extrapulmonary disease manifestations in patients with CF with gating mutations, suggesting potential systemic benefits to CFTR potentiation [165]. Furthermore, in a ferret model of COPD with chronic bronchitis, the CFTR potentiator GLPG2196 reversed CFTR dysfunction and pathological chronic bronchitis features [205].
Icenticaftor is an oral, small-molecule CFTR potentiator currently in development. In patients with CF, icenticaftor demonstrated clinically meaningful changes in lung function and sweat chloride level across a broad array of mutations [206]. In a phase II safety, tolerability and efficacy study (www.clinicaltrials.gov identifier NCT02449018) conducted in 92 patients with moderate-to-severe COPD, icenticaftor significantly increased forced expiratory volume in 1 s (≥50 mL) at 28 days. Moreover, icenticaftor reduced systemic inflammation, with a trend towards reduced sputum bacterial colonisation in patients with COPD; however, it was not observed to improve lung clearance index [207]. An ongoing phase II study (www.clinicaltrials.gov identifier NCT0426882) aims to assess the mode of action of icenticaftor in an estimated 100 patients with COPD. Dose selection for icenticaftor is being assessed in a phase II dose-range finding study in 974 patients (www.clinicaltrials.gov identifier NCT04072887).
Conclusions
Smoking is the most prevalent cause of both COPD and acquired CFTR dysfunction, which affects both epithelial and nonepithelial cells in pulmonary and extrapulmonary systems. Certain aspects of COPD pathogenesis can amplify CFTR dysfunction, and CFTR dysfunction can persist even after smoking cessation. Acquired CFTR dysfunction may contribute to COPD pathogenesis through CFTR impairment in a range of different cell types, including epithelial, immune and structural cells. The consequence of CFTR dysfunction could be amplifying effects resulting in chronic airway inflammation, recurrent infections and sputum overload. These pathological manifestations have been linked to chronic bronchitis, small airway disease, structural alterations and development of emphysema. In addition, the demonstrated systemic impairment of CFTR in smokers and its functional roles in organs beyond the lungs suggests that CFTR impairment may contribute to the systemic burden of COPD. Therefore, systemic CFTR potentiation for these patients may be beneficial, leading to an overall improvement in lung health through supporting epithelial homeostasis, controlling infection and inflammation and reducing lung remodelling.
Shareable PDF
Acknowledgements
Financial support for medical editorial assistance was provided by Novartis Pharma AG, Basel, Switzerland. We thank Sorcha McGinty and Ian Wright (Novartis Medical and Knowledge Solutions CONEXTS, Dublin, Ireland) for their medical editorial assistance with this manuscript. Authors had full control of the content and made the final decision for all aspects of this article.
Footnotes
Conflict of interest: M.A. Mall declares editorial support from Novartis Pharma AG since the initial planning of the work, and declares grants from German Ministry for Education and Research and the German Research Foundation, consulting fees from Abbvie, Antabio, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pieris Pharmaceuticals, Santhera, Sterna Biologicals and Vertex Paharmaceuticals, lecture fees from Arrowhead Pharmaceuticals, Boehringer Ingelheim and Vertex Pharmaceuticals, travel reimbursement from Boehringer Ingelheim and Vertex Pharmaceuticals, and personal fees for participation on advisory boards from Boehringer Ingelheim, Arrowhead Pharmaceuticals, Vertex Pharmaceuticals, Santhera, Enterprise Therapeutics, Antabio, Kither Biotech and Abbvie; until 2020, M.A. Mall was also an elected unpaid member of the ECFS board. G.J. Criner has no declarations. In the past 36 months, M. Miravitlles has received consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Inhibrx, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, ONO Pharma, pH Pharma, Palobiofarma SL, Takeda, Novartis, Sanofi and Grifols, speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, support for attending meetings/travel from Novartis, Boehringer Ingelheim and Menarini, research grants from Grifols, and has participated on a data safety monitoring board for Mereo. S.M. Rowe declares grant support for clinical trials conducted through university grants/contracts, consulting services on the design and conduct of clinical trials and support for travel to attend meetings from Novartis; support for clinical trials conduct through university grants/contracts, consulting services on the design and conduct of clinical trials, support for travel to attend meetings, co-chair of the Next Generation Steering Committee and providing research product for investigator initiated research from Vertex; grant support for clinical trials conducted through university grants/contracts, consulting services on the design and conduct of clinical trials, material transfer agreements (MTAs) for investigator-initiated and externally funded research efforts from Galapagos/Abbvie since the initial planning of the work; grant support for clinical trials conducted through university grants/contracts from Bayer, TranslateBio and Proteostasis; research grants through university grants/contracts from Synedgen/Synspira; research contract through university grants/contracts from Celtaxsys, Arrowhead, Ionis and AstraZeneca; research contract through university grants/contracts from Eloxx; declares consulting services on the design and conduct of clinical trials from Bayer, Renovion, Arrowhead, Ionis, Cystetic Medicines, Arcturus and Synedgen/Synspira, including stock options for Synedgen/Synspira within the past 36 months; receipt of research products for investigator initiated research from Renovion; MTA agreements for research efforts from Proteostasis; MTAs for investigator-initiated and externally funded research efforts from Ionis, Galapagos/Abbvie and Synedgen/Synspira; all in the past 36 months; and declares six patents. C.F. Vogelmeier declares institution grants from German Ministry of Education and Science (BMBF), AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Grifols and Novartis, consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis and Nuvaira, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini and Novartis, in the past 36 months. D.J. Rowlands is an employee of Novartis Pharma AG and retains Novartis stock. M. Schoenberger is a full-time employee of Novartis Pharma AG and retains Novartis stock. P. Altman is a full-time employee of Novartis Pharmaceutical Corporation.
Support statement: Financial support for medical editorial assistance was provided by Novartis Pharma AG, Basel, Switzerland. M.A. Mall has been supported by grants from the German Ministry for Education and Research (82DZL0098B1) and the German Research Foundation (SFB 1449–431232613 A01, C04, Z02 and SFB-TR84 B08). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009; 4: 435–459. doi: 10.1146/annurev.pathol.4.110807.092145 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez Fernandez E, De Santi C, De Rose V, et al. CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev Respir Med 2018; 12: 483–492. doi: 10.1080/17476348.2018.1475235 [DOI] [PubMed] [Google Scholar]
- 3.Raju SV, Solomon GM, Dransfield MT, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med 2016; 37: 147–158. doi: 10.1016/j.ccm.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J 2014; 44: 1042–1054. doi: 10.1183/09031936.00228013 [DOI] [PubMed] [Google Scholar]
- 5.Dransfield MT, Wilhelm AM, Flanagan B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest 2013; 144: 498–506. doi: 10.1378/chest.13-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clunes LA, Davies CM, Coakley RD, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 2012; 26: 533–545. doi: 10.1096/fj.11-192377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raju SV, Jackson PL, Courville CA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 2013; 188: 1321–1330. doi: 10.1164/rccm.201304-0733OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çolak Y, Nordestgaard BG, Afzal S. Morbidity and mortality in carriers of the cystic fibrosis mutation CFTR Phe508del in the general population. Eur Respir J 2020; 56: 2000558. doi: 10.1183/13993003.00558-2020 [DOI] [PubMed] [Google Scholar]
- 9.Martin C, Burgel P-R. Carriers of a single CFTR mutation are asymptomatic: an evolving dogma? Eur Respir J 2020; 56: 2002645. doi: 10.1183/13993003.02645-2020 [DOI] [PubMed] [Google Scholar]
- 10.Barry PJ, Mall MA, Álvarez A, et al. Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med 2021; 385: 815–825. doi: 10.1056/NEJMoa2100665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veit G, Avramescu RG, Chiang AN, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016; 27: 424–433. doi: 10.1091/mbc.e14-04-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCague AF, Raraigh KS, Pellicore MJ, et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med 2019; 199: 1116–1126. doi: 10.1164/rccm.201901-0145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farinha CM, Canato S. From the endoplasmic reticulum to the plasma membrane: mechanisms of CFTR folding and trafficking. Cell Mol Life Sci 2017; 74: 39–55. doi: 10.1007/s00018-016-2387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato F, Scudieri P, Musante I, et al. Two CFTR mutations within codon 970 differently impact on the chloride channel functionality. Hum Mutat 2019; 40: 742–748. doi: 10.1002/humu.23741 [DOI] [PubMed] [Google Scholar]
- 15.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006; 173: 1139–1144. doi: 10.1164/rccm.200508-1330OC [DOI] [PubMed] [Google Scholar]
- 16.Sloane PA, Shastry S, Wilhelm A, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 2012; 7: e39809. doi: 10.1371/journal.pone.0039809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dransfield M, Rowe S, Vogelmeier CF, et al. Cystic fibrosis transmembrane conductance regulator: roles in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2022; 205: 170–179. doi: 10.1164/rccm.202109-2064TR [DOI] [PubMed] [Google Scholar]
- 18.Le Gars M, Descamps D, Roussel D, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med 2013; 187: 170–179. doi: 10.1164/rccm.201205-0875OC [DOI] [PubMed] [Google Scholar]
- 19.McKelvey MC, Brown R, Ryan S, et al. Proteases, mucus, and mucosal immunity in chronic lung disease. Int J Mol Sci 2021; 22: 5018. doi: 10.3390/ijms22095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csanády L, Vergani P, Gadsby DC. Structure, gating, and regulation of the CFTR anion channel. Physiol Rev 2019; 99: 707–738. doi: 10.1152/physrev.00007.2018 [DOI] [PubMed] [Google Scholar]
- 21.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med 2020; 201: 1193–1208. doi: 10.1164/rccm.201910-1943SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65–124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda K, Dang H, Kobayashi Y, et al. Secretory cells dominate airway CFTR expression and function in human airway superficial epithelia. Am J Respir Crit Care Med 2021; 203: 1275–1289. doi: 10.1164/rccm.202008-3198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plasschaert LW, Žilionis R, Choo-Wing R, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018; 560: 377–381. doi: 10.1038/s41586-018-0394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018; 560: 319–324. doi: 10.1038/s41586-018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci 2017; 74: 93–115. doi: 10.1007/s00018-016-2391-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt JF, Yankaskas JR, Ernst SA, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992; 2: 240–248. doi: 10.1038/ng1192-240 [DOI] [PubMed] [Google Scholar]
- 28.Okuda K, Shaffer KM, Ehre C. Mucins and CFTR: their close relationship. Int J Mol Sci 2022; 23: 10232. doi: 10.3390/ijms231810232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 2014; 307: L917–L923. doi: 10.1152/ajplung.00326.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura K, Nakamura H, Trapnell BC, et al. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res 1991; 19: 5417–5423. doi: 10.1093/nar/19.19.5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorio C, Buffelli M, Angiari C, et al. Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One 2011; 6: e22212. doi: 10.1371/journal.pone.0022212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Porto P, Cifani N, Guarnieri S, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 2011; 6: e19970. doi: 10.1371/journal.pone.0019970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di A, Brown ME, Deriy LV, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 2006; 8: 933–944. doi: 10.1038/ncb1456 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep 2018; 8: 17066. doi: 10.1038/s41598-018-35151-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkert K, Hedtfeld S, Burhop A, et al. Rescue from Pseudomonas aeruginosa airway infection via stem cell transplantation. Mol Ther 2021; 29: 1324–1334. doi: 10.1016/j.ymthe.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Shrestha CL, Wisniewski BL, et al. Consequences of CRISPR-Cas9-mediated CFTR knockout in human macrophages. Front Immunol 2020; 11: 1871. doi: 10.3389/fimmu.2020.01871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnaby R, Koeppen K, Nymon A, et al. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2018; 314: L432–L438. doi: 10.1152/ajplung.00461.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hisert KB, Birkland TP, Schoenfelt KQ, et al. CFTR modulator therapy enhances peripheral blood monocyte contributions to immune responses in people with cystic fibrosis. Front Pharmacol 2020; 11: 1219. doi: 10.3389/fphar.2020.01219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarosz-Griffiths HH, Scambler T, Wong CH, et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. eLife 2020; 9: e54556. doi: 10.7554/eLife.54556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabillard-Lefort C, Casey M, Glasgow AMA, et al. Trikafta rescues CFTR and lowers monocyte P2X7R-induced inflammasome activation in cystic fibrosis. Am J Respir Crit Care Med 2022; 205: 783–794. doi: 10.1164/rccm.202106-1426OC [DOI] [PubMed] [Google Scholar]
- 41.Hiemstra PS. Altered macrophage function in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013; 10: Suppl., S180–S185. doi: 10.1513/AnnalsATS.201305-123AW [DOI] [PubMed] [Google Scholar]
- 42.Hey J, Paulsen M, Toth R, et al. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat Commun 2021; 12: 6520. doi: 10.1038/s41467-021-26777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jundi B, Pohl K, McElvaney NG, et al. The importance of CFTR expression for neutrophil function in patients with cystic fibrosis. BMC Proc 2015; 9: Suppl. 1, A36. doi: 10.1186/1753-6561-9-S1-A36 [DOI] [Google Scholar]
- 44.Painter RG, Valentine VG, Lanson NA Jr, et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006; 45: 10260–10269. doi: 10.1021/bi060490t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng HP, Valentine VG, Wang G. CFTR targeting during activation of human neutrophils. J Leukoc Biol 2016; 100: 1413–1424. doi: 10.1189/jlb.4A0316-130RR [DOI] [PubMed] [Google Scholar]
- 46.Hardisty GR, Law SM, Carter S, et al. Ivacaftor modifies cystic fibrosis neutrophil phenotype in subjects with R117H residual function CFTR mutations. Eur Respir J 2021; 57: 2002161. doi: 10.1183/13993003.02161-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moriceau S, Lenoir G, Witko-Sarsat V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbance. J Innate Immun 2010; 2: 260–266. doi: 10.1159/000295791 [DOI] [PubMed] [Google Scholar]
- 48.Jasper AE, McIver WJ, Sapey E, et al. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res 2019; 8: 557. doi: 10.12688/f1000research.18411.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler A, Walton GM, Sapey E. Neutrophilic inflammation in the pathogenesis of chronic obstructive pulmonary disease. COPD 2018; 15: 392–404. doi: 10.1080/15412555.2018.1476475 [DOI] [PubMed] [Google Scholar]
- 50.Tang XX, Fok KL, Chen H, et al. Lymphocyte CFTR promotes epithelial bicarbonate secretion for bacterial killing. J Cell Physiol 2012; 227: 3887–3894. doi: 10.1002/jcp.24101 [DOI] [PubMed] [Google Scholar]
- 51.Polverino F, Lu B, Quintero JR, et al. CFTR regulates B cell activation and lymphoid follicle development. Respir Res 2019; 20: 133. doi: 10.1186/s12931-019-1103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frija-Masson J, Martin C, Regard L, et al. Bacteria-driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis. Eur Respir J 2017; 49: 1601873. doi: 10.1183/13993003.01873-2016 [DOI] [PubMed] [Google Scholar]
- 53.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 54.Faner R, Cruz T, Casserras T, et al. Network analysis of lung transcriptomics reveals a distinct B-cell signature in emphysema. Am J Respir Crit Care Med 2016; 193: 1242–1253. doi: 10.1164/rccm.201507-1311OC [DOI] [PubMed] [Google Scholar]
- 55.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009; 2: 103–121. doi: 10.1038/mi.2008.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huaux F, Noel S, Dhooghe B, et al. Dysregulated proinflammatory and fibrogenic phenotype of fibroblasts in cystic fibrosis. PLoS One 2013; 8: e64341. doi: 10.1371/journal.pone.0064341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazio C, Scognamiglio LS, De Cegli R, et al. Intrinsic abnormalities of cystic fibrosis airway connective tissue revealed by an in vitro 3D stromal model. Cells 2020; 9: 1371. doi: 10.3390/cells9061371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Togo S, Holz O, Liu X, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med 2008; 178: 248–260. doi: 10.1164/rccm.200706-929OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong J, Jiang X, Zhang X, et al. Dynamically regulated CFTR expression and its functional role in cutaneous wound healing. J Cell Physiol 2015; 230: 2049–2058. doi: 10.1002/jcp.24931 [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Chen Y, Chen Y, et al. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell Physiol Biochem 2016; 39: 2262–2274. doi: 10.1159/000447919 [DOI] [PubMed] [Google Scholar]
- 61.Chiu WT, Tran TV, Pan SC, et al. Cystic fibrosis transmembrane conductance regulator: a possible new target for photodynamic therapy enhances wound healing. Adv Wound Care 2019; 8: 476–486. doi: 10.1089/wound.2018.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandey KC, De S, Mishra PK. Role of proteases in chronic obstructive pulmonary disease. Front Pharmacol 2017; 8: 512. doi: 10.3389/fphar.2017.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaeloudes C, Kuo C-H, Haji G, et al. Metabolic re-patterning in COPD airway smooth muscle cells. Eur Respir J 2017; 50: 1700202. doi: 10.1183/13993003.00202-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook DP, Rector MV, Bouzek DC, et al. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am J Respir Crit Care Med 2016; 193: 417–426. doi: 10.1164/rccm.201508-1562OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang JH, Panariti A, O'Sullivan MJ, et al. Characterization of cystic fibrosis airway smooth muscle cell proliferative and contractile activities. Am J Physiol Lung Cell Mol Physiol 2019; 317: L690–L701. doi: 10.1152/ajplung.00090.2019 [DOI] [PubMed] [Google Scholar]
- 66.Adam RJ, Hisert KB, Dodd JD, et al. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight 2016; 1: e86183. doi: 10.1172/jci.insight.86183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Ribeuz H, To L, Ghigna MR, et al. Involvement of CFTR in the pathogenesis of pulmonary arterial hypertension. Eur Respir J 2021; 58: 2000653. doi: 10.1183/13993003.00653-2020 [DOI] [PubMed] [Google Scholar]
- 68.Wells JM, Farris RF, Gosdin TA, et al. Pulmonary artery enlargement and cystic fibrosis pulmonary exacerbations: a cohort study. Lancet Respir Med 2016; 4: 636–645. doi: 10.1016/S2213-2600(16)30105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabeling C, Yu H, Wang L, et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci USA 2015; 112: E1614–E1623. doi: 10.1073/pnas.1421190112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green CE, Turner AM. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir Res 2017; 18: 20. doi: 10.1186/s12931-017-0505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hisata S, Racanelli AC, Kermani P, et al. Reversal of emphysema by restoration of pulmonary endothelial cells. J Exp Med 2021; 218: e20200938. doi: 10.1084/jem.20200938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khalaf M, Scott-Ward T, Causer A, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) in human lung microvascular endothelial cells controls oxidative stress, reactive oxygen-mediated cell signaling and inflammatory responses. Front Physiol 2020; 11: 879. doi: 10.3389/fphys.2020.00879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Totani L, Plebani R, Piccoli A, et al. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 3243–3253. doi: 10.1016/j.bbadis.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Chen W, Yao Y, et al. Upregulation of CFTR protects against palmitate-induced endothelial dysfunction by enhancing autophagic flux. Oxid Med Cell Longev 2020; 2020: 8345246. doi: 10.1155/2020/8345246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Declercq M, de Zeeuw P, Conchinha NV, et al. Transcriptomic analysis of CFTR-impaired endothelial cells reveals a pro-inflammatory phenotype. Eur Respir J 2021; 57: 2000261. doi: 10.1183/13993003.00261-2020 [DOI] [PubMed] [Google Scholar]
- 76.Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis 2016; 11: 637–648. doi: 10.2147/COPD.S79638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Henaff C, Faria Da Cunha M, Hatton A, et al. Genetic deletion of keratin 8 corrects the altered bone formation and osteopenia in a mouse model of cystic fibrosis. Hum Mol Genet 2016; 25: 1281–1293. doi: 10.1093/hmg/ddw009 [DOI] [PubMed] [Google Scholar]
- 78.Stalvey MS, Clines KL, Havasi V, et al. Osteoblast CFTR inactivation reduces differentiation and osteoprotegerin expression in a mouse model of cystic fibrosis-related bone disease. PLoS One 2013; 8: e80098. doi: 10.1371/journal.pone.0080098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumortier C, Danopoulos S, Velard F, et al. Bone cells differentiation: how CFTR mutations may rule the game of stem cells commitment? Front Cell Dev Biol 2021; 9: 611921. doi: 10.3389/fcell.2021.611921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braux J, Jourdain ML, Guillaume C, et al. CFTR-deficient pigs display alterations of bone microarchitecture and composition at birth. J Cyst Fibros 2020; 19: 466–475. doi: 10.1016/j.jcf.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 81.Stalvey MS, Havasi V, Tuggle KL, et al. Reduced bone length, growth plate thickness, bone content, and IGF-I as a model for poor growth in the CFTR-deficient rat. PLoS One 2017; 12: e0188497. doi: 10.1371/journal.pone.0188497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Velard F, Delion M, Henaff CL, et al. Cystic fibrosis and bone disease: defective osteoblast maturation with the F508del mutation in cystic fibrosis transmembrane conductance regulator. Am J Respir Crit Care Med 2014; 189: 746–748. doi: 10.1164/rccm.201312-2144LE [DOI] [PubMed] [Google Scholar]
- 83.Diwakar A, Adam RJ, Michalski AS, et al. Sonographic evidence of abnormal tracheal cartilage ring structure in cystic fibrosis. Laryngoscope 2015; 125: 2398–2404. doi: 10.1002/lary.25255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banks C, Freeman L, Cho DY, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction. World J Otorhinolaryngol Head Neck Surg 2018; 4: 193–199. doi: 10.1016/j.wjorl.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreindler JL, Jackson AD, Kemp PA, et al. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 2005; 288: L894–L902. doi: 10.1152/ajplung.00376.2004 [DOI] [PubMed] [Google Scholar]
- 86.Raju SV, Lin VY, Liu L, et al. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol 2017; 56: 99–108. doi: 10.1165/rcmb.2016-0226OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin VY, Fain MD, Jackson PL, et al. Vaporized e-cigarette liquids induce ion transport dysfunction in airway epithelia. Am J Respir Cell Mol Biol 2019; 61: 162–173. doi: 10.1165/rcmb.2017-0432OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCormick LL, Phillips SE, Kaza N, et al. Maternal smoking induces acquired CFTR dysfunction in neonatal rats. Am J Respir Crit Care Med 2018; 198: 672–674. doi: 10.1164/rccm.201805-0827LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirtz S, Gonska T, Seydewitz HH, et al. CFTR Cl− channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology 2004; 127: 1085–1095. doi: 10.1053/j.gastro.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 90.Wilschanski M, Dupuis A, Ellis L, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med 2006; 174: 787–794. doi: 10.1164/rccm.200509-1377OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dahl M, Tybjaerg-Hansen A, Wittrup HH, et al. Cystic fibrosis ΔF508 heterozygotes, smoking, and reproduction: studies of 9141 individuals from a general population sample. Genomics 1998; 50: 89–96. doi: 10.1006/geno.1998.5272 [DOI] [PubMed] [Google Scholar]
- 92.Raju SV, Tate JH, Peacock SK, et al. Impact of heterozygote CFTR mutations in COPD patients with chronic bronchitis. Respir Res 2014; 15: 18. doi: 10.1186/1465-9921-15-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang XQ, Mei XD, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis 2016; 8: E31–E40. doi: 10.3978/j.issn.2072-1439.2015.11.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boschetto P, Quintavalle S, Miotto D, et al. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J Occup Med Toxicol 2006; 1: 11. doi: 10.1186/1745-6673-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Molen HF, de Groene GJ, Hulshof CTJ, et al. Association between work and chronic obstructive pulmonary disease (COPD). J Clin Med 2018; 7: 335. doi: 10.3390/jcm7100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassan F, Xu X, Nuovo G, et al. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir Res 2014; 15: 69. doi: 10.1186/1465-9921-15-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hassan F, Nuovo GJ, Crawford M, et al. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS One 2012; 7: e50837. doi: 10.1371/journal.pone.0050837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Viart V, Bergougnoux A, Bonini J, et al. Transcription factors and miRNAs that regulate fetal to adult CFTR expression change are new targets for cystic fibrosis. Eur Respir J 2015; 45: 116–128. doi: 10.1183/09031936.00113214 [DOI] [PubMed] [Google Scholar]
- 99.Mazumdar M, Christiani DC, Biswas SK, et al. Elevated sweat chloride levels due to arsenic toxicity. N Engl J Med 2015; 372: 582–584. doi: 10.1056/NEJMc1413312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu F, Qin XQ, Cui YR, et al. Ozone stress down-regulates the expression of cystic fibrosis transmembrane conductance regulator in human bronchial epithelial cells. Chem Biol Interact 2009; 179: 219–226. doi: 10.1016/j.cbi.2008.10.059 [DOI] [PubMed] [Google Scholar]
- 101.Qu F, Liu H-J, Xiang Y, et al. Activation of CFTR trafficking and gating by vasoactive intestinal peptide in human bronchial epithelial cells. J Cell Biochem 2011; 112: 902–908. doi: 10.1002/jcb.22999 [DOI] [PubMed] [Google Scholar]
- 102.Pesce E, Gorrieri G, Sirci F, et al. Evaluation of a systems biology approach to identify pharmacological correctors of the mutant CFTR chloride channel. J Cyst Fibros 2016; 15: 425–435. doi: 10.1016/j.jcf.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 103.Laube M, Thome UH. CFTR is negatively regulated by glucocorticoids in alveolar epithelial cells. Pediatric Research 2011; 70: 522. doi: 10.1038/pr.2011.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laube M, Bossmann M, Thome UH. Glucocorticoids distinctively modulate the CFTR channel with possible implications in lung development and transition into extrauterine life. PLoS One 2015; 10: e0124833. doi: 10.1371/journal.pone.0124833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caohuy H, Jozwik C, Pollard HB. Rescue of ΔF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem 2009; 284: 25241–25253. doi: 10.1074/jbc.M109.035345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prota LF, Cebotaru L, Cheng J, et al. Dexamethasone regulates CFTR expression in Calu-3 cells with the involvement of chaperones HSP70 and HSP90. PLoS One 2012; 7: e47405. doi: 10.1371/journal.pone.0047405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bomberger JM, Coutermarsh BA, Barnaby RL, et al. Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval. PLoS One 2014; 9: e89599. doi: 10.1371/journal.pone.0089599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bossmann M, Ackermann BW, Thome UH, et al. Signaling cascade involved in rapid stimulation of cystic fibrosis transmembrane conductance regulator (CFTR) by dexamethasone. Int J Mol Sci 2017; 18: 1807. doi: 10.3390/ijms18081807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galietta LJ, Folli C, Marchetti C, et al. Modification of transepithelial ion transport in human cultured bronchial epithelial cells by interferon-γ. Am J Physiol Lung Cell Mol Physiol 2000; 278: L1186–L1194. doi: 10.1152/ajplung.2000.278.6.L1186 [DOI] [PubMed] [Google Scholar]
- 110.Pankow S, Bamberger C, Yates JR 3rd. A posttranslational modification code for CFTR maturation is altered in cystic fibrosis. Sci Signal 2019; 12: eaan7984. doi: 10.1126/scisignal.aan7984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma M, Pampinella F, Nemes C, et al. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 2004; 164: 923–933. doi: 10.1083/jcb.200312018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramachandran S, Karp PH, Osterhaus SR, et al. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am J Respir Cell Mol Biol 2013; 49: 544–551. doi: 10.1165/rcmb.2012-0430OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bardin P, Sonneville F, Corvol H, et al. Emerging microRNA therapeutic approaches for cystic fibrosis. Front Pharmacol 2018; 9: 1113. doi: 10.3389/fphar.2018.01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sonneville F, Ruffin M, Guillot L, et al. New insights about miRNAs in cystic fibrosis. Am J Pathol 2015; 185: 897–908. doi: 10.1016/j.ajpath.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 115.Sun H, Harris WT, Kortyka S, et al. TGF-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS One 2014; 9: e106842. doi: 10.1371/journal.pone.0106842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lutful Kabir F, Ambalavanan N, Liu G, et al. MicroRNA-145 antagonism reverses TGF-β inhibition of F508del CFTR correction in airway epithelia. Am J Respir Crit Care Med 2018; 197: 632–643. doi: 10.1164/rccm.201704-0732OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther 1995; 6: 445–455. doi: 10.1089/hum.1995.6.4-445 [DOI] [PubMed] [Google Scholar]
- 118.Johannesson B, Hirtz S, Schatterny J, et al. CFTR regulates early pathogenesis of chronic obstructive lung disease in βENaC-overexpressing mice. PLoS One 2012; 7: e44059. doi: 10.1371/journal.pone.0044059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guimbellot JS, Fortenberry JA, Siegal GP, et al. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol 2008; 39: 514–521. doi: 10.1165/rcmb.2007-0452OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32: 1371–1385. doi: 10.1183/09031936.00015608 [DOI] [PubMed] [Google Scholar]
- 121.MacEachran DP, Ye S, Bomberger JM, et al. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun 2007; 75: 3902–3912. doi: 10.1128/IAI.00338-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rubino R, Bezzerri V, Favia M, et al. Pseudomonas aeruginosa reduces the expression of CFTR via post-translational modification of NHERF1. Pflugers Arch 2014; 466: 2269–2278. doi: 10.1007/s00424-014-1474-6 [DOI] [PubMed] [Google Scholar]
- 123.Trinh NT, Bilodeau C, Maillé É, et al. Deleterious impact of Pseudomonas aeruginosa on cystic fibrosis transmembrane conductance regulator function and rescue in airway epithelial cells. Eur Respir J 2015; 45: 1590–1602. doi: 10.1183/09031936.00076214 [DOI] [PubMed] [Google Scholar]
- 124.Maillé É, Ruffin M, Adam D, et al. Quorum sensing down-regulation counteracts the negative impact of Pseudomonas aeruginosa on CFTR channel expression, function and rescue in human airway epithelial cells. Front Cell Infect Microbiol 2017; 7: 470. doi: 10.3389/fcimb.2017.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwarzer C, Fischer H, Kim EJ, et al. Oxidative stress caused by pyocyanin impairs CFTR Cl− transport in human bronchial epithelial cells. Free Radic Biol Med 2008; 45: 1653–1662. doi: 10.1016/j.freeradbiomed.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Londino JD, Lazrak A, Jurkuvenaite A, et al. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am J Physiol Lung Cell Mol Physiol 2013; 304: L582–L592. doi: 10.1152/ajplung.00314.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brand JD, Lazrak A, Trombley JE, et al. Influenza-mediated reduction of lung epithelial ion channel activity leads to dysregulated pulmonary fluid homeostasis. JCI Insight 2018; 3: e123467. doi: 10.1172/jci.insight.123467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Z, Leir S-H, Harris A. Oxidative stress regulates CFTR gene expression in human airway epithelial cells through a distal antioxidant response element. Am J Respir Cell Mol Biol 2015; 52: 387–396. doi: 10.1165/rcmb.2014-0263OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanssens LS, Duchateau J, Casimir GJ. CFTR protein: not just a chloride channel? Cells 2021; 10: 2844. doi: 10.3390/cells10112844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strandvik B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids 2010; 83: 121–129. doi: 10.1016/j.plefa.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 131.Elbehairy AF, Raghavan N, Cheng S, et al. Physiologic characterization of the chronic bronchitis phenotype in GOLD grade IB COPD. Chest 2015; 147: 1235–1245. doi: 10.1378/chest.14-1491 [DOI] [PubMed] [Google Scholar]
- 132.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010; 363: 2233–2247. doi: 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miravitlles M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir Med 2011; 105: 1118–1128. doi: 10.1016/j.rmed.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 134.Dunican EM, Elicker BM, Henry T, et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med 2021; 203: 957–968. doi: 10.1164/rccm.202006-2248OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv 2008; 21: 13–24. doi: 10.1089/jamp.2007.0659 [DOI] [PubMed] [Google Scholar]
- 136.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 228–237. doi: 10.1164/rccm.201210-1843CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 2011; 140: 626–633. doi: 10.1378/chest.10-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim V, Davey A, Comellas AP, et al. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res 2014; 15: 52. doi: 10.1186/1465-9921-15-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stanford D, Kim H, Bodduluri S, et al. Airway remodeling in ferrets with cigarette smoke-induced COPD using µCT imaging. Am J Physiol Lung Cell Mol Physiol 2020; 319: L11–L20. doi: 10.1152/ajplung.00328.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 2015; 50: Suppl. 40, S24-S30. doi: 10.1002/ppul.23247 [DOI] [PubMed] [Google Scholar]
- 141.Kunzelmann K, Schreiber R, Hadorn HB. Bicarbonate in cystic fibrosis. J Cyst Fibros 2017; 16: 653–662. doi: 10.1016/j.jcf.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 142.Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012; 209: 1263–1272. doi: 10.1084/jem.20120562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Downs CA, Kreiner LH, Trac DQ, et al. Acute effects of cigarette smoke extract on alveolar epithelial sodium channel activity and lung fluid clearance. Am J Respir Cell Mol Biol 2013; 49: 251–259. doi: 10.1165/rcmb.2012-0234OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mall MA. ENaC inhibition in cystic fibrosis: potential role in the new era of CFTR modulator therapies. Eur Respir J 2020; 56: 2000946. doi: 10.1183/13993003.00946-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Keown K, Brown R, Doherty DF, et al. Airway inflammation and host responses in the era of CFTR modulators. Int J Mol Sci 2020; 21: 6379. doi: 10.3390/ijms21176379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatr Pulmonol 2019; 54: Suppl. 3, S84–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Puchelle E, Bajolet O, Abély M. Airway mucus in cystic fibrosis. Paediatr Respir Rev 2002; 3: 115–119. doi: 10.1016/S1526-0550(02)00005-7 [DOI] [PubMed] [Google Scholar]
- 148.Yang L, Jelsbak L, Molin S. Microbial ecology and adaptation in cystic fibrosis airways. Environ Microbiol 2011; 13: 1682–1689. doi: 10.1111/j.1462-2920.2011.02459.x [DOI] [PubMed] [Google Scholar]
- 149.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 2017; 9: a028241. doi: 10.1101/cshperspect.a028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cendra MDM, Blanco-Cabra N, Pedraz L, et al. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci Rep 2019; 9: 16284. doi: 10.1038/s41598-019-52726-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Françoise A, Héry-Arnaud G. The microbiome in cystic fibrosis pulmonary disease. Genes 2020; 11: 536. doi: 10.3390/genes11050536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dicker AJ, Huang JTJ, Lonergan M, et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2021; 147: 158–167. doi: 10.1016/j.jaci.2020.02.040 [DOI] [PubMed] [Google Scholar]
- 153.Leung JM, Tiew PY, Mac Aogáin M, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology 2017; 22: 634–650. doi: 10.1111/resp.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matamouros S, Hayden HS, Hager KR, et al. Adaptation of commensal proliferating Escherichia coli to the intestinal tract of young children with cystic fibrosis. Proc Natl Acad Sci USA 2018; 115: 1605–1610. doi: 10.1073/pnas.1714373115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manor O, Levy R, Pope CE, et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci Rep 2016; 6: 22493. doi: 10.1038/srep22493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zemanick ET, Wagner BD, Robertson CE, et al. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur Respir J 2017; 50: 1700832. doi: 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yi B, Dalpke AH, Boutin S. Changes in the cystic fibrosis airway microbiome in response to CFTR modulator therapy. Front Cell Infect Microbiol 2021; 11: 548613. doi: 10.3389/fcimb.2021.548613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 2012; 109: 5809–5814. doi: 10.1073/pnas.1120577109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Coburn B, Wang PW, Diaz Caballero J, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 2015; 5: 10241. doi: 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meeker SM, Mears KS, Sangwan N, et al. CFTR dysregulation drives active selection of the gut microbiome. PLoS Pathog 2020; 16: e1008251. doi: 10.1371/journal.ppat.1008251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schippa S, Iebba V, Santangelo F, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLoS One 2013; 8: e61176. doi: 10.1371/journal.pone.0061176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hisert KB, Heltshe SL, Pope C, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 2017; 195: 1617–1628. doi: 10.1164/rccm.201609-1954OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Agarwal R, Khan A, Aggarwal AN, et al. Link between CFTR mutations and ABPA: a systematic review and meta-analysis. Mycoses 2012; 55: 357–365. doi: 10.1111/j.1439-0507.2011.02130.x [DOI] [PubMed] [Google Scholar]
- 164.Heltshe SL, Mayer-Hamblett N, Burns JL, et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 2015; 60: 703–712. doi: 10.1093/cid/ciu944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. doi: 10.1164/rccm.201404-0703OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muthu V, Prasad KT, Sehgal IS, et al. Obstructive lung diseases and allergic bronchopulmonary aspergillosis. Curr Opin Pulm Med 2021; 27: 105–112. doi: 10.1097/MCP.0000000000000755 [DOI] [PubMed] [Google Scholar]
- 167.Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J 2014; 43: 64–71. doi: 10.1183/09031936.00162912 [DOI] [PubMed] [Google Scholar]
- 168.Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J 2020; 56: 2000418. doi: 10.1183/13993003.00418-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Everaerts S, Lagrou K, Dubbeldam A, et al. Sensitization to Aspergillus fumigatus as a risk factor for bronchiectasis in COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2629–2638. doi: 10.2147/COPD.S141695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giacalone VD, Margaroli C, Mall MA, et al. Neutrophil adaptations upon recruitment to the lung: new concepts and implications for homeostasis and disease. Int J Mol Sci 2020; 21: 851. doi: 10.3390/ijms21030851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2014; 306: L591–L603. doi: 10.1152/ajplung.00335.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McKelvey MC, Weldon S, McAuley DF, et al. Targeting proteases in cystic fibrosis lung disease. Paradigms, progress, and potential. Am J Respir Crit Care Med 2020; 201: 141–147. doi: 10.1164/rccm.201906-1190PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017; 97: 529–552. doi: 10.1152/physrev.00025.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Usmani OS, Dhand R, Lavorini F, et al. Why we should target small airways disease in our management of chronic obstructive pulmonary disease. Mayo Clin Proc 2021; 96: 2448–2463. doi: 10.1016/j.mayocp.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 175.Higham A, Quinn AM, Cançado JED, et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res 2019; 20: 49. doi: 10.1186/s12931-019-1017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pisi R, Aiello M, Zanini A, et al. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 1191–1197. doi: 10.2147/COPD.S82509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Day K, Ostridge K, Conway J, et al. Interrelationships among small airways dysfunction, neutrophilic inflammation, and exacerbation frequency in COPD. Chest 2021; 159: 1391–1399. doi: 10.1016/j.chest.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 178.Young AL, Bragman FJS, Rangelov B, et al. Disease progression modeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2020; 201: 294–302. doi: 10.1164/rccm.201908-1600OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan F, Gao H, Zhao H, et al. Roles of airway smooth muscle dysfunction in chronic obstructive pulmonary disease. J Transl Med 2018; 16: 262. doi: 10.1186/s12967-018-1635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Puchelle E, Zahm J-M, Tournier J-M, et al. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 726–733. doi: 10.1513/pats.200605-126SF [DOI] [PubMed] [Google Scholar]
- 181.Sauler M, McDonough JE, Adams TS, et al. Characterization of the COPD alveolar niche using single-cell RNA sequencing. Nat Commun 2022; 13: 494. doi: 10.1038/s41467-022-28062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dupuit F, Kälin N, Brézillon S, et al. CFTR and differentiation markers expression in non-CF and delta F 508 homozygous CF nasal epithelium. J Clin Invest 1995; 96: 1601–1611. doi: 10.1172/JCI118199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lopes-Pacheco M. CFTR modulators: shedding light on precision medicine for cystic fibrosis. Front Pharmacol 2016; 7: 275. doi: 10.3389/fphar.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gaggar A, Hector A, Bratcher PE, et al. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur Respir J 2011; 38: 721–727. doi: 10.1183/09031936.00173210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McCuaig S, Martin JG. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir Med 2013; 1: 137–147. doi: 10.1016/S2213-2600(12)70058-9 [DOI] [PubMed] [Google Scholar]
- 186.Rowe SM, Verkman AS. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med 2013; 3: a009761. doi: 10.1101/cshperspect.a009761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106: 18825–18830. doi: 10.1073/pnas.0904709106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu H, Burton B, Huang C-J, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros 2012; 11: 237–245. doi: 10.1016/j.jcf.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 191.Lin VY, Kaza N, Birket SE, et al. Excess mucus viscosity and airway dehydration impact COPD airway clearance. Eur Respir J 2020; 55: 1900419. doi: 10.1183/13993003.00419-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lambert JA, Raju SV, Tang LP, et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol 2014; 50: 549–558. doi: 10.1165/rcmb.2013-0228OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ooi CY, Syed SA, Rossi L, et al. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci Rep 2018; 8: 17834. doi: 10.1038/s41598-018-36364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johns JD, Rowe SM. The effect of CFTR modulators on a cystic fibrosis patient presenting with recurrent pancreatitis in the absence of respiratory symptoms: a case report. BMC Gastroenterol 2019; 19: 123. doi: 10.1186/s12876-019-1044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Angyal D, Bijvelds MJC, Bruno MJ, et al. Bicarbonate transport in cystic fibrosis and pancreatitis. Cells 2021; 11: 54. doi: 10.3390/cells11010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. doi: 10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sermet-Gaudelus I, Delion M, Durieu I, et al. Bone demineralization is improved by ivacaftor in patients with cystic fibrosis carrying the p.Gly551Asp mutation. J Cyst Fibros 2016; 15: e67-e69. doi: 10.1016/j.jcf.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 198.Putman MS, Greenblatt LB, Bruce M, et al. The effects of ivacaftor on bone density and microarchitecture in children and adults with cystic fibrosis. J Clin Endocrinol Metab 2021; 106: e1248–e1261. doi: 10.1210/clinem/dgaa890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Raju SV, Rasmussen L, Sloane PA, et al. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir Res 2017; 18: 173. doi: 10.1186/s12931-017-0656-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rennard SI, Calverley PM, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res 2011; 12: 18. doi: 10.1186/1465-9921-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Patel SD, Bono TR, Rowe SM, et al. CFTR targeted therapies: recent advances in cystic fibrosis and possibilities in other diseases of the airways. Eur Respir Rev 2020; 29: 190068. doi: 10.1183/16000617.0068-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Solomon GM, Hathorne H, Liu B, et al. Pilot evaluation of ivacaftor for chronic bronchitis. Lancet Respir Med 2016; 4: e32–e33. doi: 10.1016/S2213-2600(16)30047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Van Goor F, Yu H, Burton B, et al. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros 2014; 13: 29–36. doi: 10.1016/j.jcf.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 204.Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci USA 2013; 110: 4404–4409. doi: 10.1073/pnas.1215982110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kaza N, Lin VY, Stanford D, et al. Evaluation of a novel CFTR potentiator in COPD ferrets with acquired CFTR dysfunction. Eur Respir J 2022; 60: 2101581. doi: 10.1183/13993003.01581-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kazani S, Rowlands DJ, Bottoli I, et al. Safety and efficacy of the cystic fibrosis transmembrane conductance regulator potentiator icenticaftor (QBW251). J Cyst Fibros 2021; 20: 250–256. doi: 10.1016/j.jcf.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rowe SM, Jones I, Dransfield MT, et al. Efficacy and safety of the CFTR potentiator icenticaftor (QBW251) in COPD: results from a phase 2 randomized trial. Int J Chron Obstruct Pulmon Dis 2020; 15: 2399–2409. doi: 10.2147/COPD.S257474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sergeev V, Chou FY, Lam GY, et al. The extrapulmonary effects of cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Ann Am Thorac Soc 2020; 17: 147–154. doi: 10.1513/AnnalsATS.201909-671CME [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01307-2022.Shareable (379.5KB, pdf)